ABSTRACT
HIV-1 infection of noncycling cells, such as dendritic cells (DCs), is impaired due to limited availability of deoxynucleoside triphosphates (dNTPs), which are needed for HIV-1 reverse transcription. The levels of dNTPs are tightly regulated during the cell cycle and depend on the balance between dNTP biosynthesis and degradation. SAMHD1 potently blocks HIV-1 replication in DCs, although the underlying mechanism is still unclear. SAMHD1 has been reported to be able to degrade dNTPs and viral nucleic acids, which may both hamper HIV-1 reverse transcription. The relative contribution of these activities may differ in cycling and noncycling cells. Here, we show that inhibition of HIV-1 replication in monocyte-derived DCs (MDDCs) is associated with an increased expression of p21cip1/waf, a cell cycle regulator that is involved in the differentiation and maturation of DCs. Induction of p21 in MDDCs decreases the pool of dNTPs and increases the antiviral active isoform of SAMHD1. Although both processes are complementary in inhibiting HIV-1 replication, the antiviral activity of SAMHD1 in our primary cell model appears to be, at least partially, independent of its dNTPase activity. The reduction in the pool of dNTPs in MDDCs appears rather mostly due to a p21-mediated suppression of several enzymes involved in dNTP synthesis (i.e., RNR2, TYMS, and TK-1). These results are important to better understand the interplay between HIV-1 and DCs and may inform the design of new therapeutic approaches to decrease viral dissemination and improve immune responses against HIV-1.
IMPORTANCE DCs play a key role in the induction of immune responses against HIV. However, HIV has evolved ways to exploit these cells, facilitating immune evasion and virus dissemination. We have found that the expression of p21, a cyclin-dependent kinase inhibitor involved in cell cycle regulation and monocyte differentiation and maturation, potentially can contribute to the inhibition of HIV-1 replication in monocyte-derived DCs through multiple mechanisms. p21 decreased the size of the intracellular dNTP pool. In parallel, p21 prevented SAMHD1 phosphorylation and promoted SAMHD1 dNTPase-independent antiviral activity. Thus, induction of p21 resulted in conditions that allowed the effective inhibition of HIV-1 replication through complementary mechanisms. Overall, p21 appears to be a key regulator of HIV infection in myeloid cells.
KEYWORDS: cellular factors, HIV replication, HIV-1, SAMHD1, dNTPs, dendritic cells, p21
INTRODUCTION
Myeloid dendritic cells (mDCs) play a critical role in the pathogenesis of HIV-1 infection (1). On one hand, mDCs are professional antigen-presenting cells that orchestrate innate and adaptive immune responses against human immunodeficiency virus type 1 (HIV-1). On the other hand, mDCs can capture and retain infectious viral particles and transmit them to CD4+ T cells over extended periods of time, undoubtedly contributing to HIV-1 dissemination (2–5). Moreover, mDCs are directly susceptible to HIV-1 infection (6), which alters their function and impairs their maturation (1, 5, 7). However, HIV-1 replication in mDCs is less efficient than that in CD4+ T cells. In particular, similar to the case in macrophages, HIV-1 reverse transcription is much slower in mDCs than in activated CD4+ T cells (5, 8). In addition, although HIV-1 can replicate in immature monocyte-derived DCs (MDDCs), HIV-1 replication is strongly inhibited in mature MDDCs (9). The mechanisms underlying this inhibition are not completely clear. Some reasons that could explain the overall modest HIV infection of dendritic cells include low expression levels of the HIV receptor and coreceptors, rapid and extensive degradation of incoming HIV-1 particles in the intracellular compartments of DCs, and the expression of host factors that block HIV replication (5).
SAMHD1 (sterile alpha motif and histidine-aspartate-domain-containing protein 1) has been shown to strongly block HIV-1 reverse transcription in dendritic cells, macrophages, and resting T cells (10–18). SAMHD1 potentially can block HIV replication through two distinct enzymatic functions. SAMHD1 can degrade viral nucleic acids through its exonuclease activity (19, 20) and deplete the intracellular deoxynucleoside triphosphate (dNTP) pool through its dNTP triphosphatase activity (17). The relative contribution of each activity to the inhibition of HIV replication is the subject of intense debate (21, 22). Phosphorylation of threonine 592 has been shown to reduce SAMHD1 antiviral activity while not affecting its dNTPase activity (23). On the other hand, degradation of SAMHD1 by the HIV-2/simian immunodeficiency virus (SIV) Vpx protein is accompanied by a sharp increase in the intracellular pool of dNTPs, which reach the levels necessary for efficient reverse transcription (17, 24).
The cellular dNTP pool is a critical limiting factor for HIV-1 replication (25, 26) that is sustained by various cellular pathways (27): the salvage pathway recovers nucleotide intermediates from extracellular media and intracellular DNA degradation, and the de novo pathway synthetizes new dNTP molecules. The expression of the cellular factors involved in both pathways is strongly regulated by the cell cycle (28, 29). Consequently, dividing cells have much higher dNTP levels than nondividing cells (24). Our team has previously shown that the induction of p21Waf1/Cip1 (referred to here as p21), a cyclin-dependent kinase (CDK) inhibitor that mediates cell cycle arrest, potently blocks HIV-1 replication in macrophages (30, 31). p21 blocks dNTP biosynthesis in macrophages by downregulating the expression of the RNR2 subunit of ribonucleotide reductase (30), an enzyme essential for the reduction of ribonucleotides to dNTP. On the other hand, the capacity of p21 to inhibit cyclin/CDK activity could regulate SAMHD1 antiviral activity by impairing the CDK-mediated phosphorylation of SAMHD1 Thr592 (23, 32–34). In line with these findings, p21 has been shown to regulate SAMHD1 phosphorylation in human macrophages (35, 36).
As a cell cycle inhibitor, p21 plays a critical role in not only the control of cell growth but also monocyte differentiation and survival (37–39). p21 has been shown to participate in dendritic cell differentiation and maturation, and expression of p21 in mature mDCs has been associated with improved function of these cells (40). Therefore, we used the model of dendritic cells and the greater capacity of mature dendritic cells to block HIV-1 infection in order to explore the role of p21-mediated HIV-1 inhibition. These studies can shed light on the intermingled antiviral activities of p21 and SAMHD1 and their impact on the intracellular dNTP pool.
RESULTS
Strong inhibition of HIV-1 reverse transcription in mature MDDCs.
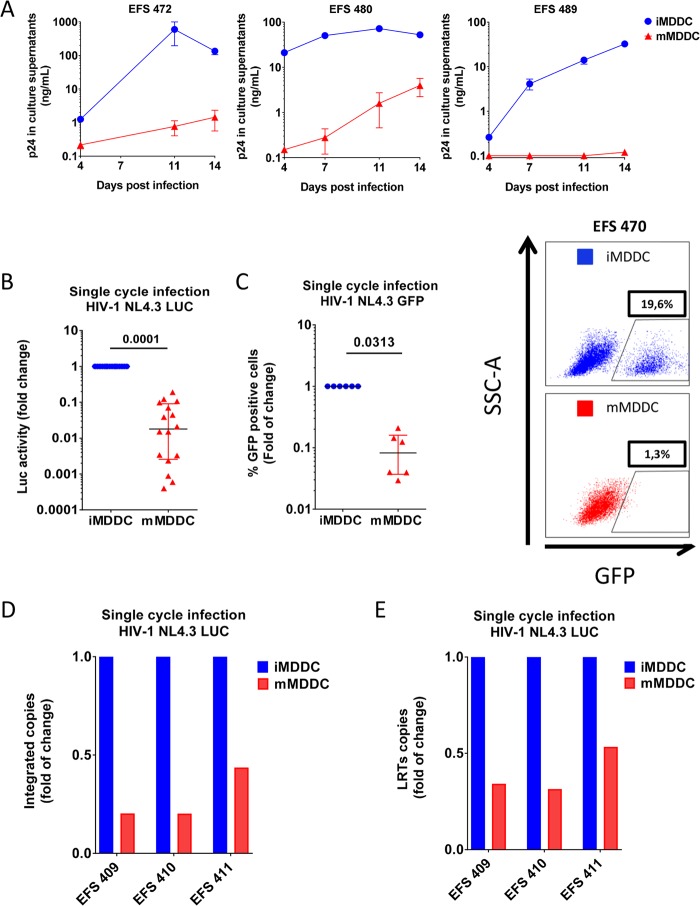
We implemented an MDDC model to study HIV replication and the strong decrease in HIV susceptibility that has been reported to occur upon maturation of these cells (9). MDDCs were obtained from purified CD14+ cells from blood donors and cultured for 24 h in the absence or presence of gamma interferon (IFN-γ) and CD40L to induce MDDC maturation. Maturation was evidenced by morphological changes and strong upregulation of CD40, CD80, and CD86 (data not shown), as previously described (41, 42). We challenged immature and mature MDDCs with replicative HIV-1 BaL, and we monitored the p24 levels in the culture supernatants for 14 days. As expected, viral replication was strongly impaired in mature MDDCs compared with that in immature MDDCs from the earliest time points after infection, reaching 2.8 logs of inhibition in the supernatants of mature MDDCs at day 11 (P < 0.001; n = 3) (Fig. 1A). To identify the stage of the viral cycle at which the impairment occurred, we used single-cycle HIV-1 NL4.3Δenv particles carrying a luciferase (Luc) (Fig. 1B) or GFP (Fig. 1C) reporter gene and pseudotyped with the pantropic vesicular stomatitis virus G (VSV-G) protein. Our results showed a strong reduction in the luciferase activity (57× [fold] [11.5 to 397×] decrease in luciferase activity; P = 0.0001, n = 16) and the proportion of GFP-expressing cells (16.5× [6.4 to 27.5×] decrease in the percentage of GFP+ cells; P = 0.003, n = 6) in mature MDDCs 72 h postinfection. Reverse transcription-quantitative PCR (RT-qPCR) analysis of HIV replication intermediates at that time revealed a substantial decrease in the number of integrated proviruses (4.9× [2.3 to 4.9×] decrease in the number of HIV-1 DNA copies/106 cells; P = 0.012; n = 3) and U5-gag reverse transcripts (2.9× [1.8 to 3.1×]; P = 0.013 n = 3) in mature MDDCs (Fig. 1D and E). Overall, our results showed a strong impairment of HIV-1 replication in mature MDDCs, with reverse transcription being one of the steps affected.
FIG 1.
Mature MDDCs have low susceptibility to HIV-1 infection, which is blocked at the reverse transcription level. (A) p24 measured in culture supernatants after the infection of immature MDDCs (iMDDCs; blue) and mature MDDCs (mMDDCs; red) with HIV-1 BaL in 3 independent experiments. The means and standard deviations for three replicates are shown at each time point. (B and C) Luciferase activity (B) or percentage of GFP+ cells (C) in mature MDDCs compared with that in immature MDDCs after infection with single-cycle HIV-1 NL4.3Δenv VSV-G particles carrying luciferase (n = 16) or GFP (n = 6) reporter genes. Each symbol represents results with cells from different donors. The median and IQR values are shown. (Right) One representative example of GFP production in immature and mature MDDCs is shown. (D and E) Relative number of integrated Alu-long terminal repeat (LTR) copies (D) or U5-Gag copies (E) quantified by RT-qPCR in mature MDDCs compared with that in immature MDDCs 72 h after infection with single-cycle HIV-1 NL4.3ΔenvLuc VSV-G particles. Viral cDNAs were normalized to the albumin gene content in each sample. Experiments with cells from three different donors are shown. Bars represent the means from 2 replicates.
Induction of p21 in mature MDDCs.
We have previously shown that the cellular factor p21 blocks HIV-1 reverse transcription in human macrophages (30). We wondered whether p21 was involved in the inhibition observed in mature MDDCs. The maturation of MDDCs promoted strong induction of p21 mRNA (6.25 × [2.9 to 21×] increase in p21 mRNA levels in mature MDDCs over immature cells; P = 0.0002; n = 13) (Fig. 2A). The increase in p21 mRNA levels in mature MDDCs was accompanied by an increase in p21 protein levels, as shown by Western blotting (1.6× [1.5 to 2×] increase in p21 protein levels; P = 0.031; n = 5) (Fig. 2B). A significant negative correlation was found between the relative induction of p21 mRNA upon MDDC maturation and the relative reduction of HIV-1 replication in the matured cells compared to immature MDDCs (Spearman, −0.77; P = 0.0006; n = 14 independent donors) (Fig. 2C). These results showed that p21 was induced upon the maturation of MDDCs and revealed an association between p21 and the inhibition of viral replication in MDDCs.
FIG 2.
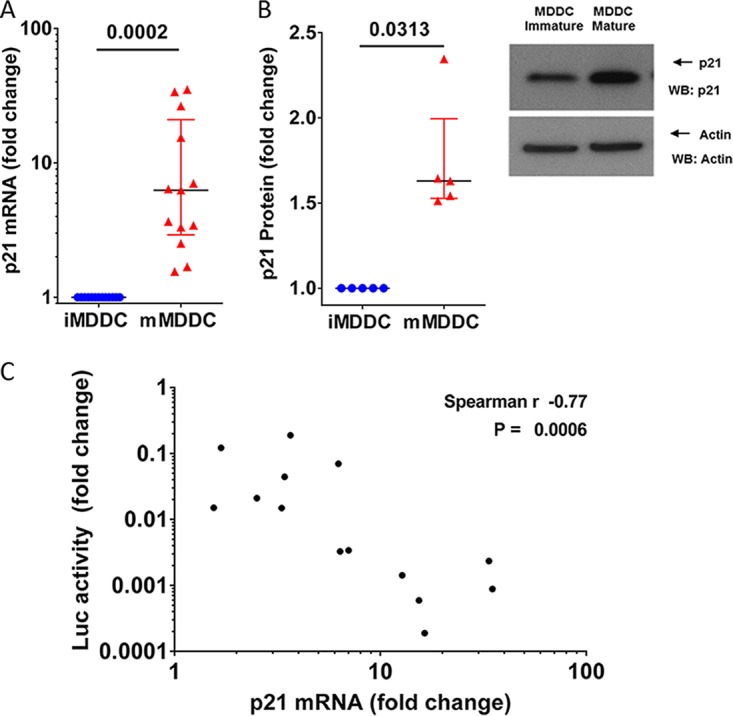
p21 induction in mature MDDCs correlates with viral replication levels. (A) p21 mRNA expression was quantified by qRT-PCR in immature (blue) and mature (red) MDDCs. The data are expressed as the fold change in the number of p21 copies in mature MDDCs compared to that in immature MDDCs. Each symbol represents the results obtained with cells from one donor (n = 13). The median and IQR values are shown. (B) Analysis of protein expression in immature and mature MDDCs by Western blotting (WB). (Left) p21 protein expression quantification are expressed as the relative change in p21 protein expression in mature MDDCs compared with that in immature MDDCs in the same experiments. The median and IQR values of independent experiments (n = 5 donors) are depicted. An example of p21 protein analyzed by Western blotting is shown. (C) Correlation between relative changes in the number of p21 mRNA copies and relative changes in luciferase activity after infection with HIV-1 NL4.3ΔenvLuc VSV-G in mature MDDCs compared with those in immature MDDCs from 14 different donors.
Restoration of HIV-1 replication in MDDCs after p21 silencing.
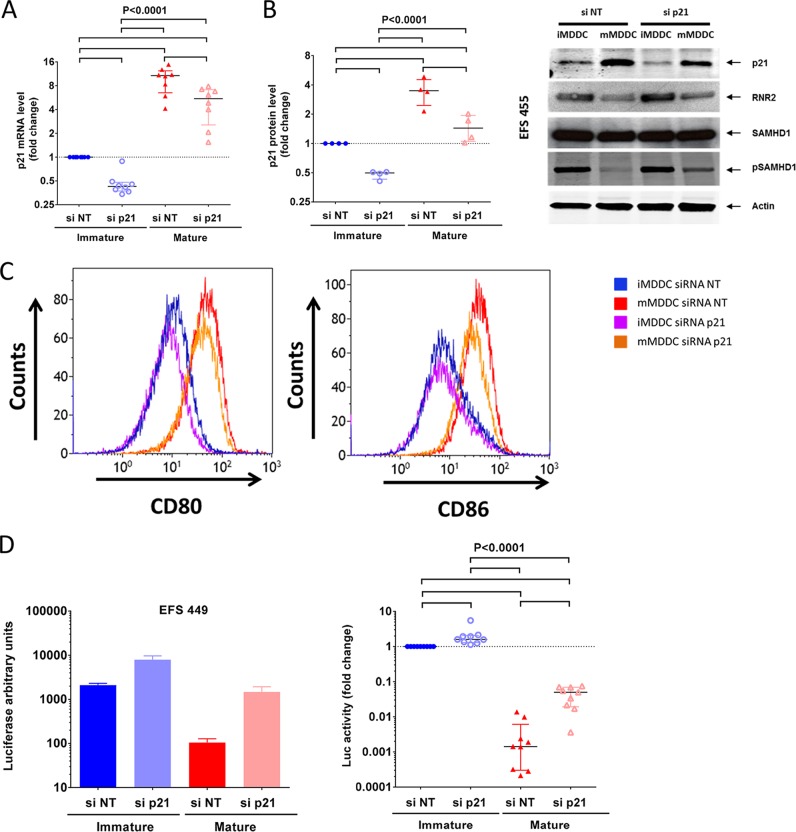
We examined whether p21 downregulation could modulate HIV-1 replication in MDDCs. We treated MDDCs with p21-specific small interfering RNA (siRNA) and achieved a consistent and significant reduction in p21 mRNA levels in immature (2.3× [2.1 to 2.7×] decrease in p21 mRNA levels; P = 0.008; n = 8) and mature (2.2× [1.6 to 2.8×]; P = 0.0078; n = 8) MDDCs (Fig. 3A). Similar reductions were observed at the protein level by Western blot analyses in immature (2.2× [1.6 to 2.8×] decrease in p21 protein levels; n = 4) and mature (2.5 × [1.8 to 3×]; P = 0.02, n = 4) MDDCs (P < 0.0001) (Fig. 3B). Importantly, the downregulation of p21 expression did not affect the maturation of MDDCs (Fig. 3C). In contrast, the downregulation of p21 in mature MDDCs resulted in a substantial increase in viral replication (17× [9 to 51×] increase in luciferase activity; n = 8) (Fig. 3D). A significant, although more modest, increase in viral replication (1.8× [1.2 to 2×]; n = 8) was also observed after the downregulation of p21 in immature MDDCs (Fig. 3D). Overall, these results confirmed a link between p21 expression and the inhibition of viral replication in MDDCs.
FIG 3.
Downregulation of p21 expression levels by siRNA in mature MDDCs restores viral replication. (A and B) The relative number of copies of p21 mRNA (A) and the relative p21 protein levels (B) in immature and mature MDDCs after transfection with siRNA targeting p21 (siRNA p21) or a pool of nontargeting siRNA (siRNA NT). The data are expressed in relation to the number of mRNA copies or protein levels in immature MDDCs transfected with nontargeting siRNA. Symbols represent experiments with cells from different donors (n = 8 for mRNA and n = 4 for protein). The median and IQR values are shown. An example of Western blot analysis of the different proteins studied is shown on the right. (C) Level of expression of CD86, CD80, and CD40 on the surface of MDDCs transfected with siRNA NT or siRNA p21 and cultured in the absence or presence of CD40L and IFN-γ. (D) Changes in luciferase activity in immature and mature MDDCs transfected with siRNA NT or siRNA p21 after infection with HIV-1 NL4.3ΔenvLuc VSV-G. The results are shown as the fold change relative to the luciferase activity in immature MDDCs transfected with siRNA NT. Symbols represent independent experiments (n = 8 donors). The median and IQR values are shown. One representative experiment is shown on the left (means and standard deviations from three replicates). P values of ANOVA are shown. Pairwise significant differences (P < 0.05) in post hoc analyses are identified by horizontal lines.
dNTP levels in MDDCs are affected by changes in p21 expression.
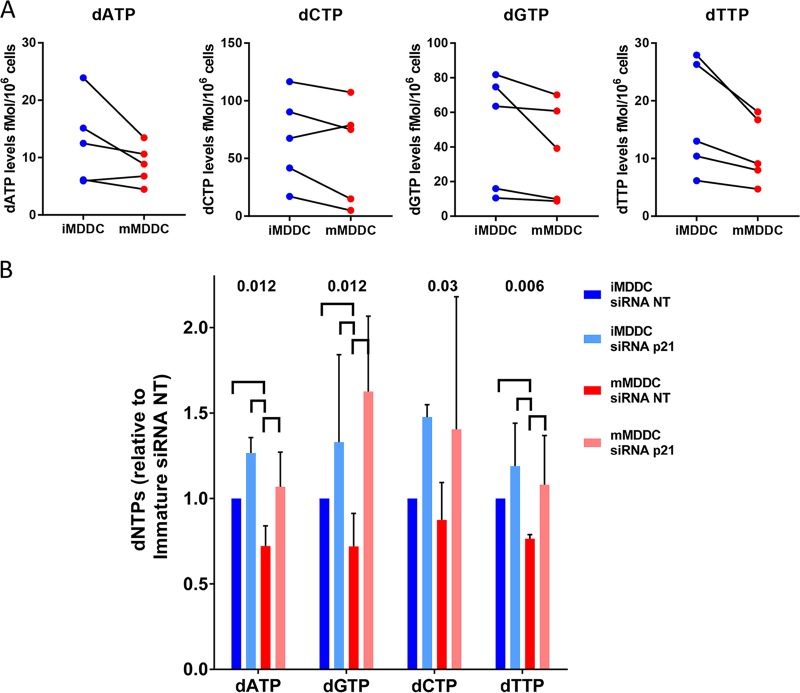
We then searched for the mechanisms by which p21 inhibited HIV-1 replication in MDDCs. Because we have already shown that the induction of p21 in macrophages reduces the intracellular pool of dNTPs (30), we explored whether differences could be observed in the dNTP levels in immature and mature MDDCs. The absolute concentrations of dATP, dGTP, dCTP, and dTTP were determined in MDDCs from 5 blood donors by a highly sensitive single-nucleotide incorporation assay (25). As previously reported (24, 43, 44), dNTP levels in immature MDDCs were very weak, close to the limit of detection of the technique. Despite these low levels, a further decrease in dNTPs was observed upon maturation of the MDDCs (in 5/5 experiments for dGTP and dTTP and 4/5 experiments for dATP and dCTP) (Fig. 4A). Treatment of MDDCs with p21-siRNA increased the levels of all dNTPs until the levels were equal in immature and mature cells (n = 4) (Fig. 4B). Thus, p21 seemed to negatively regulate the intracellular concentrations of the dNTP pools in MDDCs.
FIG 4.
Maturation of MDDCs decreases the size of the intracellular dNTP pools, but the pool sizes recover upon p21 knockdown. (A) Intracellular levels of dATP, dCTP, dGTP, and dTTP quantified by single-nucleotide primer extension gel analysis in immature and mature MDDCs. Independent experiments with cells from 5 donors are shown. (B) Changes in the dNTP levels in immature and mature MDDCs transfected with siRNA NT or siRNA p21. The results are shown as fold change relative to each dNTP level in immature MDDCs transfected with siRNA NT. The median and IQR values of the results obtained with cells from 4 different donors are shown (n = 4 donors). P values of ANOVA are shown. Significant differences (P < 0.05) between conditions in post hoc analyses are identified by horizontal lines.
p21 participates in the regulation of enzymes involved in the synthesis of dNTPs.
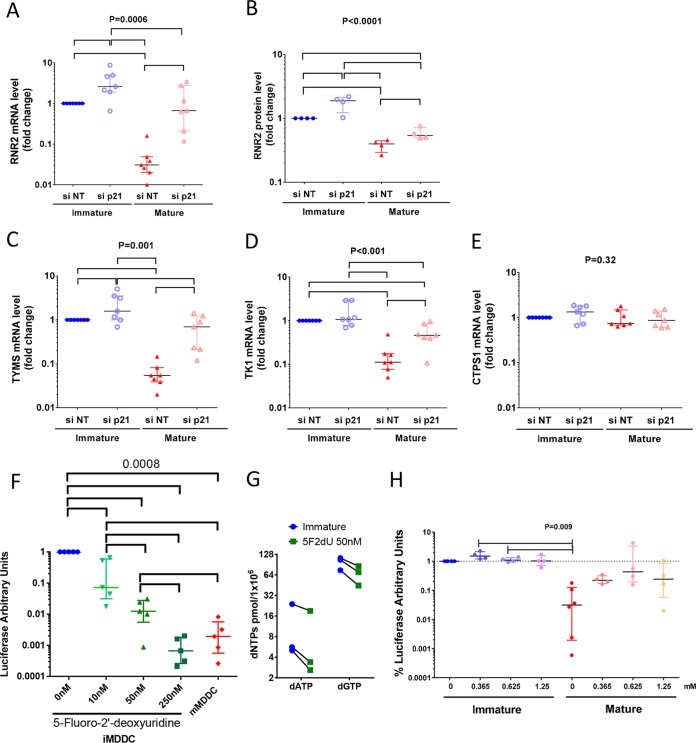
We analyzed the expression of RNR2, a key enzyme in the de novo synthesis of dADP, dGDP, and dCDP, whose expression levels are influenced by p21 and whose downregulation affects HIV-1 replication (30). RNR2 mRNA levels strongly decreased after MDDC maturation (32× [20.5 to 50×] decrease in RNR2 mRNA levels; n = 7) (Fig. 5A). This decrease corresponded to a drop in RNR2 protein levels in mature MDDCs (2.5× [2.2 to 3.5×]; n = 4) (Fig. 3B and 5B). Downregulation of p21 led to a significant increase in RNR2 mRNA in both immature and mature MDDCs (2.6× [1.9 to 4.9×] and 17.3× [13 to 57×] increase in RNR2 mRNA upon p21 silencing in immature and mature MDDCs, respectively; n = 7). An increase in RNR2 protein levels was also observed (1.9× [1.2 to 2.1×] and 1.5× [1.2 to 2×] in immature and mature MDDCs, respectively; n = 4) (Fig. 3B and 5A and B).
FIG 5.
p21 regulates the de novo and salvage dNTP synthesis pathways. (A to E) Relative levels of RNR2 mRNA (A), relative RNR2 protein levels (B), relative levels of thymidylate synthase (TYMS) mRNA (C), relative levels of thymidine kinase 1 (TK-1) mRNA (D), and relative levels of CTP synthase 1 (CTPS1) mRNA (E) in immature and mature MDDCs after transfection with siRNA p21 or siRNA NT. The data are expressed relative to the number of mRNA copies or protein levels in immature MDDCs transfected with nontargeting siRNA. Symbols represent independent experiments (n = 7 donors for mRNA and n = 4 donors for RNR2 protein). (F) Changes in luciferase activity after infection with HIV-1 NL4.3ΔenvLuc VSV-G in immature MDDCs cultured in the presence of increasing amounts of 5-fluoro-2′-deoxyuridine. The results with cells from five donors are shown. (G) Changes in intracellular levels of dATP and dGTP quantified by single-nucleotide primer extension gel analysis in immature MDDCs after treatment with 50 nM 5-fluoro-2′-deoxyuridine. Independent experiments with cells from 3 donors are shown. (H) Changes in luciferase activity after infection with HIV-1 NL4.3ΔenvLuc VSV-G in immature and mature MDDCs cultured in the presence of increasing amounts of exogenous dNTPs. The results are shown as fold change relative to the luciferase activity in immature MDDCs cultured in the absence of exogenous dNTPs. The symbols represent independent experiments (n = 5 donors). The median and IQR values are shown. P values of ANOVA are shown. Significant differences (P < 0.05) between conditions in post hoc analyses are identified by horizontal lines.
The synthesis of dTTPs, which was systematically reduced in mature MDDCs in our experiments (Fig. 4), follows different pathways from those of the other dNTPs. Thus, we evaluated the expression of thymidylate synthase (TYMS) and thymidine kinase 1 (TK1), key enzymes in the de novo and salvage pathways of dTTP synthesis. The maturation of MDDCs significantly decreased the mRNA expression of TYMS (18.4× [12.2 to 25.9×] decrease in TYMS mRNA levels; n = 7) (Fig. 5C) and TK1 (9× [5.8 to 13×]; n = 7) (Fig. 5D). Downregulation of p21 expression led to a significant recovery of the mRNA levels of both enzymes in mature MDDCs (8× [4.5 to 26.2×] and 2.4× [2 to 7.7×] increases in TYMS and TK-1 mRNA, respectively, upon the silencing of p21; n = 7). No significant changes for TK1 and only a modest increase for TYMS (1.6× [1 to 3.5×]) were observed upon p21 silencing in immature MDDCs (Fig. 5C and D). No differences were observed in the levels of the control, CTPS1, an enzyme involved in the synthesis of CTP and whose expression level may unbalance dTTP synthesis (45), either between immature and mature MDDCs or after p21 silencing (Fig. 5E). Inhibition of TYMS activity with 5-fluoro-2′-deoxyuridine in immature MDDCs induced strong, dose-dependent inhibition of HIV-1 replication (up to 3 logs of inhibition), confirming the importance of this enzyme for the HIV-1 cycle (Fig. 5F). The inhibition of TYMS by 5-fluoro-2′-deoxyuridine was accompanied by a decrease in the levels of dNTPs (n = 3 independent experiments) (Fig. 5G), which is in agreement with the existence of several cellular feedback mechanisms to contain the imbalance of individual dNTPs (46). Overall, our results show that induction of p21 was accompanied by decreased expression of several enzymes playing a key role in dNTP biosynthesis. The inhibition of one of these enzymes was sufficient to decrease intracellular dNTP levels and block HIV-1 infection of iMDDCs.
We next explored whether the replenishment of intracellular dNTP levels affected HIV-1 infection. The addition of exogenous dNTPs to the culture media during single-cycle HIV-1 infection strongly increased HIV-1 replication in mature MDDCs in a dose-dependent manner (82 × [21 to 228×] increase in viral replication in mature MDDCs cultured in the presence of 0.625 nM dNTPs, n = 4), while the effect was modest at best in immature MDDCs (Fig. 5H). Of note, the viral replication level in mature MDDCs did not quite reach the levels observed in immature MDDCs, even at the highest concentrations of exogenous dNTPs (Fig. 5H). Overall, these results support that the p21-related downregulation of enzymes involved in dNTP synthesis and the consequent decrease in the size of the dNTP pools contribute to the restriction of HIV-1 replication in mature MDDCs but that additional mechanisms might also be involved.
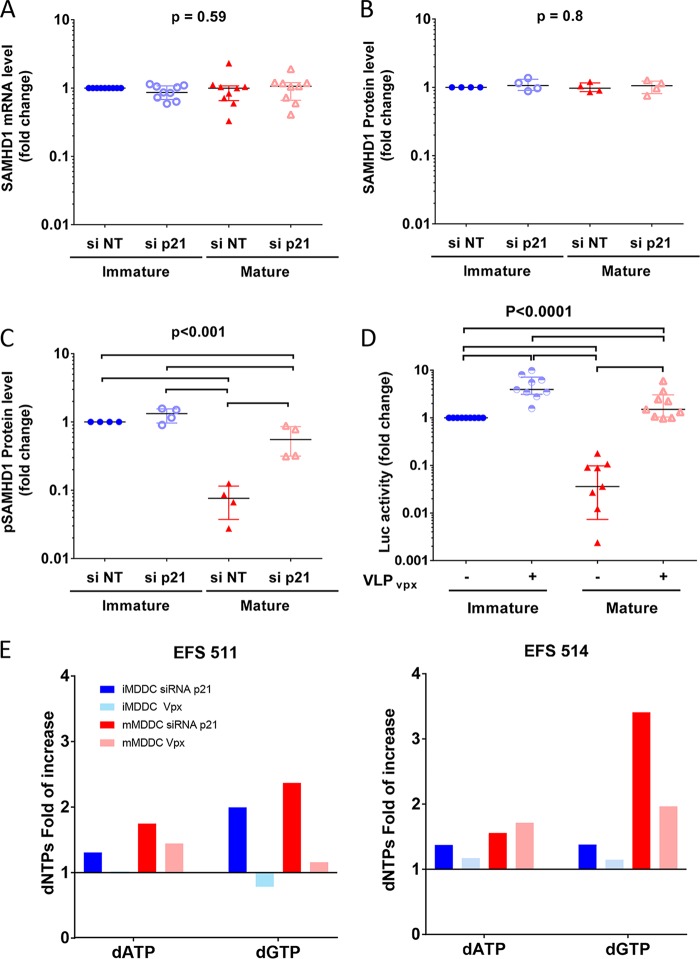
Induction of p21 in mature MDDCs alters the balance between active antiviral and inactive forms of SAMHD1.
p21, through its cyclin-dependent kinase inhibitory activity, might control the kinases involved in the phosphorylation of SAMHD1 (30, 32, 36). Maturation of MDDCs did not have any impact on the expression of SAMHD1 mRNA (P = 0.59, n = 9) (Fig. 6A) or on total protein levels (P = 0.8, n = 4) (Fig. 3B and 6B). In contrast, a strong decrease in the phosphorylated form of SAMHD1 (pSAMHD1) was observed after MDDC maturation (13× [9 to 31×] decrease in pSAMHD1 levels) (Fig. 3B and 6C). Downregulation of p21 by siRNA was associated with an increase in the levels of pSAMHD1, particularly in the mature MDDCs (7× [3 to 26×]) (Fig. 3B and 6C), whereas the mRNA and total protein levels remained unchanged (Fig. 3B and 6A and B).
FIG 6.
p21 expression modulates SAMHD1 phosphorylation levels in MDDCs. (A to C) Relative copies of SAMHD1 mRNA (A), total SAMHD1 protein levels (B), and pSAMHD1 protein levels (C) in immature and mature MDDC after transfection with siRNA p21 or siRNA NT. The data are expressed relative to the number of mRNA copies or protein levels in immature MDDCs transfected with nontargeting siRNA. The symbols represent independent experiments (n = 9 donors for mRNA and n = 4 donors for proteins). (D) Changes in luciferase activity after infection with HIV-1 NL4.3ΔenvLuc VSV-G in immature and mature MDDCs cultured in the presence of virus-like particles with or without the SIV VPX protein. The results are shown as the fold change relative to the luciferase activity in immature MDDCs cultured with VLPs not carrying VPX. The symbols represent independent experiments (n = 9). The median and IQR values are shown. P values of ANOVA are shown. Significant differences (P < 0.05) between conditions in post hoc analyses are identified by horizontal lines. (E) Changes in the dNTP levels in immature and mature MDDCs transfected with siRNA p21 or treated with VLP-VPX particles. The results are shown as fold change in dNTP levels relative to those of control nontreated immature or mature MDDCs, respectively.
Because dephosphorylation has been shown to increase the antiviral activity of SAMHD1 (23), the p21-dependent shift in the balance between the phosphorylated and dephosphorylated forms of SAMHD1 upon MDDC maturation suggested that SAMHD1 plays a role in the HIV-1 block observed in mature MDDCs. MDDCs were transduced with virus-like particles containing VPX to degrade SAMHD1 without affecting p21 expression (data not shown). SAMHD1 degradation had a strong impact on HIV-1 replication, increasing it 4× (3 to 7×) and 56× (16 to 577×) in immature and mature MDDCs, respectively (P < 0.0001, n = 9) (Fig. 6D). These results were evidence of the involvement of SAMHD1 in the impairment of HIV-1 replication in mature MDDCs, likely due to upstream regulation of its antiviral activity by p21. Intriguingly, degradation of SAMHD1 in immature and mature MDDCs appeared to have a milder effect on dNTP levels than silencing of p21 (Fig. 6E), which is in agreement with p21 regulating dNTP levels through the additional mechanisms discussed above.
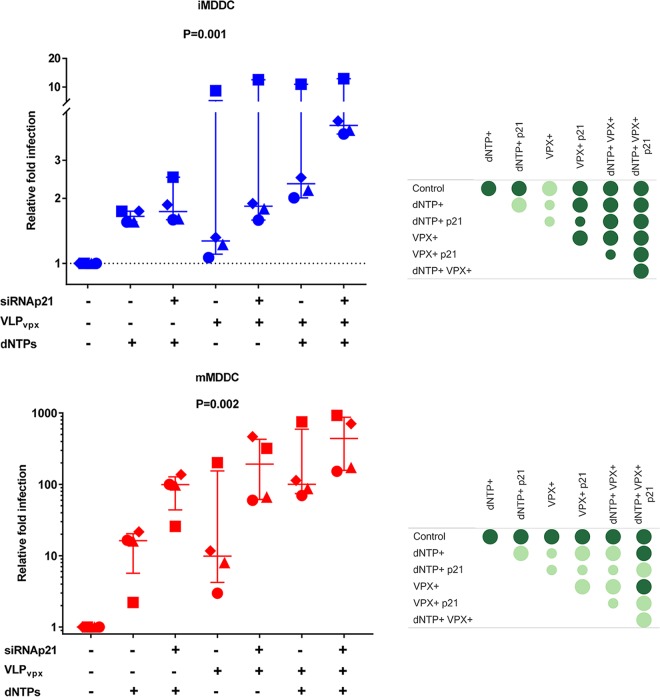
p21 can regulate distinct and synergistic anti-HIV mechanisms in MDDCs.
Our results showed that p21 could block HIV-1 replication in MDDCs by reducing the synthesis of dNTPs to below the levels needed for reverse transcription and by shifting the balance between the phosphorylated and nonphosphorylated forms of SAMHD1. However, it was unclear whether these activities were independent or whether they might act complementarily to block HIV-1. To evaluate this, we infected immature and mature MDDCs with HIV-1 after the degradation of SAMHD1 with virus-like particles (VLPs) carrying VPX in the presence or absence of exogenous dNTPs. Although some variation was observed in the magnitude of the effects of the treatments on cells from different individuals, the effects were very consistent in all experiments performed. As in the previous experiments, SAMHD1 degradation or exogenous dNTPs strongly increased HIV-1 replication, but the presence of exogenous dNTPs in cultures of VPX-treated cells had an additional and significant enhancing effect in both immature and mature MDDCs (Fig. 7). On the other hand, the concomitant silencing of p21 and the degradation of SAMHD1 in immature and mature MDDCs consistently led to higher viral replication than the degradation of SAMHD1 alone (Fig. 7). Similarly strong enhancement (median 100× increase compared to control conditions) of HIV replication in mature MDDCs was achieved when any combination of two conditions among p21 silencing, VLP-VPX, or exogenous dNTPs were used in the cultures (Fig. 7). It should be noticed that the effect of VLP-VPX or exogenous dNTPs on sip21-treated immature and mature MDDCs was significantly diminished compared to their effect on cells in which p21 had not been silenced (data not shown), suggesting that partial p21 silencing was sufficient to recapitulate much of the effect caused by either VPX or dNTPs. Finally, a further increment of HIV replication (median 2.5× increase compared to cultures combining two culture conditions) was observed when all three conditions were combined together, which suggests that p21, besides regulation of SAMHD1 activity and dNTP pool levels, controls additional mechanisms to block HIV-1 infection.
FIG 7.
p21 controls the HIV-1 inhibition in mature MDDCs through two synergistic mechanisms. Changes in luciferase activity after infection with HIV-1 NL4.3ΔenvLuc VSV-G in immature (top) or mature (bottom) MDDCs transfected with siRNA p21 or siRNA NT, pretreated with VLPs with or without VPX, and cultured in the absence or presence of exogenous dNTPs (0.625 nM). The data are expressed as the fold change in luciferase activity relative to the control condition for immature or mature MDDCs. The median and IQR values of independent experiments with cells from 4 different donors are shown. Each donor is identified with a different symbol. Panels on the right summarize the following comparisons between the different conditions: statistically significant differences in post hoc analyses are identified by dark green circles, and big circles indicate consistent differences between the conditions tested in all 4 experiments.
DISCUSSION
Here, we show that expression of p21 was associated with a potent block of HIV-1 replication in MDDCs, potentially through several complementary mechanisms. Among them are (i) p21 suppression of the expression of several enzymes involved in the de novo (RNR2 and TYMS) and salvage (TK-1) routes of dNTP biosynthesis and (ii) p21-impaired SAMHD1 phosphorylation, which enhances its antiviral activity. Of note, under our conditions SAMHD1 antiviral activity appeared to be, at least partially, independent of its dNTPase activity, as it was still observed in the presence of saturating levels of dNTPs. Both mechanisms of p21-mediated HIV-1 restriction were observed in immature MDDCs, but the effects were exacerbated in mature cells.
HIV-1 replication was indeed strongly inhibited in mature MDDCs in our experiments. In parallel, p21 was strongly induced upon MDDC maturation. This result is in agreement with previous reports showing that p21 induction is involved in monocyte differentiation and DC development and maturation (38, 40, 47). Moreover, DC maturation is governed by cell cycle-associated processes (48–50). The magnitude of p21 induction in mature MDDCs compared to immature MDDCs correlated tightly with the extent of relative HIV-1 restriction in mature MDDCs. The pivotal role of p21 in the impairment of HIV-1 replication in MDDCs was comforted by p21 knockdown experiments that strongly increased viral replication in mature MDDCs (and more modestly in immature MDDCs), even though only partial silencing of p21 was achieved.
We have previously shown that p21 induction also potently blocked HIV-1 replication in human macrophages (30, 31). This blockage was associated with p21-mediated inhibition of dNTP biosynthesis through the suppression of the RNR2 subunit of the ribonucleotide reductase. Here, we further explored the role of p21 in the dNTP biosynthesis pathways and the relationship between these pathways and HIV-1 infection. We found that the RNR2 and dNTP levels, already low in immature MDDCs, were further reduced in mature MDDCs. However, these levels recovered and matched those observed in immature MDDCs when p21 was silenced. Among the dNTPs, the dTTP levels showed the most consistent changes under the different conditions assayed. dTTP is synthesized by the methylation of dUMP by thymidylate synthase in the de novo pathway or after thymidine phosphorylation by thymidine kinase 1 in the salvage pathway (29). The expression of both enzymes was strongly reduced upon MDDC maturation, and their levels increased sharply in p21-silenced cells. Overall, our results showed that p21 expression in MDDCs was accompanied by suppression of several key enzymes involved in dNTP synthesis.
We have shown before that specific downmodulation of RNR2 inhibits HIV-1 replication in macrophages (30). We show here that chemical inhibition of thymidylate synthase function in immature MDDCs decreases dNTP levels and efficiently blocks HIV-1 replication to an extent similar to that seen in mature MDDCs. Thus, the suppression of one of the enzymes involved in dNTP synthesis, whose expression is regulated by p21, appeared sufficient to block HIV-1 infection in myeloid cells. Because equilibrium among nucleotide levels is critical for cell survival and function (27), it is likely that changes in the level of one dNTP would lead to changes in that of the others. For instance, dTTP mediates allosteric regulation of RNR, and variations in dTTP levels may affect de novo synthesis of the other dNTPs (46, 51). Supplementation of cells with exogenous dNTPs increased viral replication slightly in immature MDDCs and much more decisively in mature MDDCs, confirming that dNTP availability is a critical determinant of HIV-1 inhibition in mature MDDC.
The dNTP pool is regulated by the cell cycle and, in particular, through the control of enzymes involved in dNTP biosynthesis (27). Whether changes in RNR2, TYMS, and TK-1 expression levels are directly regulated by p21 or are the indirect consequence of cellular changes provoked by p21 expression remains to be determined. It is worth noting that expression of CTPS1, an enzyme critical for dCTP synthesis, was not affected by either MDDC maturation or p21 expression, which suggests that p21 induction was accompanied by specific regulation of some genes rather than a general alteration of dNTP metabolic pathways. We have previously shown that p21 inhibits RNR2 transcription in macrophages by suppressing its transcriptional activator, E2F1 (30). E2F1 is a key transcription factor in the retinoblastoma/CDK pathway regulating cell proliferation and differentiation (52, 53). Moreover, E2F1 is a suppressor of DC maturation, and its downregulation promotes the activation and maturation of these cells in the absence of additional stimulation (48). Although we did not address this question here, a previous study has reported that TYMS and TK1 correlate tightly with E2F1 (54).
The size of the dNTP pool is also regulated by nucleotide degradation. SAMHD1, which has been shown to block HIV-1 replication in quiescent CD4+ T cells, macrophages, and dendritic cells (12, 15, 17, 44, 55), possess nucleoside triphosphatase activity and can mediate the depletion of dNTP pools. Although SAMHD1 is expressed in both cycling and noncycling cells, its antiviral activity only occurs in the latter (10, 12, 14, 16, 23, 56). This difference was explained when two different teams reported that SAMHD1 antiviral activity was regulated by the phosphorylation state of threonine 592 (23, 33). CDK1, CDK2, and CDK6 all have been proposed to mediate SAMHD1 phosphorylation. Because p21 is a promiscuous CDK inhibitor (57), it was possible that induction of p21 in MDDCs affected the balance between the active antiviral and inactive forms of SAMHD1. We found that SAMHD1 mRNA and total protein levels were not altered by MDDC maturation. In contrast, maturation of MDDCs caused a strong decrease in phosphorylated (inactive) SAMHD1 levels. Silencing of p21 reversed this effect, confirming the capacity of p21 to regulate SAMHD1 phosphorylation (35, 36). The degradation of SAMHD1 in the presence of virus-like particles containing SIV VPX rescued HIV-1 replication in mature MDDCs. Therefore, these results suggest that HIV-1 inhibition in mature MDDCs also could be associated with an increased antiviral potential of SAMHD1 linked to p21 induction.
Thus, p21 might regulate at least two different mechanisms that could interfere with HIV-1 replication in MDDCs: (i) downregulation of key enzymes involved in dNTP synthesis and (ii) countering the phosphorylation (inactivation) of SAMHD1. We further explored whether these mechanisms are intertwined. Both supplementation with exogenous dNTPs and degradation of SAMHD1 independently increased HIV-1 replication in MDDCs, especially in mature MDDCs. The simultaneous treatment of immature or mature dendritic cells with VPX and exogenous dNTP enhanced infection to levels above those obtained with either of the two treatments alone. Therefore, endogenous dNTP levels present in the absence of SAMHD1 did not allow optimal viral replication, which suggests that low dTNP levels in MDDCs were not due to SAMHD1 dNTP catabolic activity only. Conversely, SAMHD1 still imposed a limit to HIV replication in the presence of saturating amounts of dNTPs, suggesting that SAMHD1 action was not due solely to dNTP degradation.
It has been suggested that, in addition to its dNTP triphosphohydrolase activity, SAMHD1 exerts its antiviral function by degrading HIV-1 RNA from incoming particles or single-stranded HIV-1 DNA through exonuclease activity (22). Along these lines, SAMHD1 phosphorylation appears to eliminate its antiviral activity without affecting its dNTPase activity (22, 23). However, the relative contribution of each of these two alternative mechanisms in SAMHD1 antiviral activity is controversial (21, 58, 59). While the ability of SAMHD1 to regulate the size of the dNTP pool is well established in cycling cells, this role is much less clear in noncycling cells, where dNTPs levels are very low. Recent reports have shown that the dNTPase activity but not the RNase activity of SAMHD1 depends on its tetramerization (60, 61). SAMHD1 oligomerization is allosterically regulated by a combined action of GTP and dNTP levels (60, 62). The levels of dNTPs necessary to trigger SAMHD1 tetramerization and activation of its dNTPase activity are similar to those found in cycling cells (63), which implies that in noncycling cells, such as dendritic cells, SAMHD1 would preferentially exist in monomeric form and that its antiviral activity would not be preferentially mediated by its dNTPase activity. In contrast, p21-associated downregulation of the enzymes involved in dNTP biosynthesis might decisively contribute to the low levels of the dNTP pools in mMDDC. Of note, a recent study has shown that while SAMHD1 did not have an antiviral effect in transduced HeLa cells, treatment of these cells with hydroxyurea, which, as with p21, interferes with RNR2 activity and inhibits dNTP synthesis (64), triggered SAMHD1-dependent antiviral activity (65).
p21 silencing strongly increased HIV-1 replication even when SAMHD1 was degraded, which supports the hypothesis that the inhibition caused by p21 induction was not limited to the modulation of SAMHD1 activity. Induction of p21 therefore might provide the optimal conditions for SAMHD1 antiviral activity by decreasing the synthesis of dNTPs and inhibiting the CDKs responsible for SAMHD1 phosphorylation (Fig. 8). This would reconcile the apparently conflicting results about the role of p21 in HIV-1 inhibition in macrophages (35, 36). Our experiments suggest that these activities accounted for most of the p21-induced HIV-1 block in mature MDDCs. However, silencing p21 upon degradation of SAMHD1 and in the presence of exogenous dNTPs further increased levels of infection, suggesting that additional effects mediated by p21, independent of SAMHD1 or dNTP regulation, contribute to inhibit HIV-1 infection in MDDC. Although we have focused here on mechanisms that could interfere with reverse transcription and, more particularly, with the regulation of intracellular dNTP levels, it has been reported that p21 also directly interferes with HIV-1 reverse transcriptase and affects postintegration steps of viral replication by impairing HIV transcription in CD4+ T cells through inhibition of CDK2 and CDK9 (66, 67).
FIG 8.
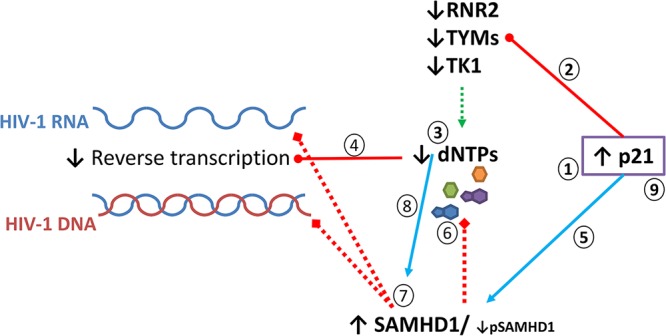
Proposed model for p21-mediated HIV-1 restriction in MDDCs. The expression of p21 is induced during MDDC maturation (step 1). The increase in p21 expression leads to the downregulation of several enzymes (RNR2, TYMS, and TK1) involved in dNTP biosynthesis (step 2), which would decrease the dNTP pool size (step 3), impairing HIV-1 reverse transcription (step 4). In parallel, p21 induction could increase the active isoform of SAMHD1 (step 5), which would further block viral replication through the degradation of dNTPs (step 6) or exonuclease activity (step 7). Low levels of dNTP synthesis as a consequence of p21 induction might further facilitate the exonuclease activity of SAMHD1 (step 8). Although not explored here, induction of p21 might also directly block reverse transcriptase activity and impair HIV transcription, as has been shown in CD4+ T cells (step 9) (66, 67).
Overall, our results suggest that p21 is a key regulator of HIV-1 replication in primary myeloid cells. p21 does not appear to exert a direct antiviral effect but rather would be able to control HIV-1 replication indirectly through processes that are related to the role of p21 in governing cell fate. Because of the complexity of p21 interactions and the indirect nature of its antiviral activity, the establishment of definitive mechanistic links between p21 expression and HIV replication will require further experiments, possibly in simpler cellular models. The relative contribution to the block of HIV-1 infection of the different mechanisms potentially regulated by p21 is unclear, but it is likely that this will be heterogeneous and vary in function of individual factors or the context of the target cell. Although we did not have the opportunity to analyze p21 expression in MDDCs from HIV controllers in this study, it is interesting that enhanced expression of p21 has been found in CD4+ T cells and macrophages from these patients (66, 68). The potential role of the mechanisms described here in the establishment and maintenance of natural control of HIV-1 infection deserves further exploration.
MATERIALS AND METHODS
Production of immature and mature monocyte-derived dendritic cells.
Buffy coats were obtained from the French blood bank (Etablissement Français du Sang) through a collaboration agreement with the Institut Pasteur and in accordance with French law. CD14+ cells were purified (>90%) from freshly isolated peripheral blood mononuclear cells (PBMCs) by positive selection with antibody-coated magnetic beads in a Robosep instrument (Stemcell Technologies, Canada). Monocytes were differentiated into monocyte-derived dendritic cells (MDDCs) by culturing them in the presence of interleukin-4 (IL-4; 1,450 IU/ml) and granulocyte-macrophage colony-stimulating factor (GM-CSF; 750 UI/ml) (all from R&D Systems, USA) for 5 to 6 days. At this time, 96% of the cells expressed CD11c and HLA-DR. MDDCs were cultured in the absence (immature) or the presence (mature) of IFN-γ (1,000 U/ml) (R&D Systems, USA), CD40L with a His tag (1 μg/ml), and His6× (10 μg/ml) for 24 h (69). MDDC maturation was controlled by the enhanced expression of CD40, CD80, and CD86. MDDCs were harvested and suspended in RPMI medium with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) and 1% (vol/vol) antibiotics. MDDCs were seeded at 106 cells/ml of media.
Flow cytometry.
For MDDC phenotyping, the following antibodies were used: CD11c-V450 (B-ly6), CD40-phycoerythrin (PE)-Cy5 (RUO), CD80-PE (MAB104), CD86-allophycocyanin (FUN-1), and HLA-DR-ECD (Immu-357), from BD Biosciences. Cells were incubated with the antibodies for 15 min, washed with phosphate-buffered saline (PBS) with 1% FBS, and fixed in 1% paraformaldehyde for flow cytometry with an LSRII device (BD Biosciences). The data were analyzed with Kaluza software (Beckman Coulter). Infected cells expressing GFP were incubated with a Live/Dead stain (Invitrogen), and then they were washed in PBS with 1% FBS and fixed in 1% paraformaldehyde for flow cytometry with an LSRII device (BD Biosciences).
Infection with replication-competent viruses.
MDDCs were infected with HIV-1 BaL (R5). Viral stocks were amplified in phytohemagglutinin-activated human primary CD4+ T cells. MDDCs (7.5 × 105) were infected in triplicate in 96-well plates with 1 ng of p24 from HIV-1 BaL using a spinoculation protocol (1 h, 1,200 × g at room temperature) (68, 70). Culture supernatants were harvested at days 4, 7, 10, and 14 after infection, and p24 was measured by enzyme-linked immunosorbent assay (ELISA) (Zeptometrix).
Single-round infections.
Single-round infections were performed with NL4.3Δenv-Luc HIV-1 and NL4.3Δenv-GFP HIV-1 (kind gifts from Nathaniel Landau) (71, 72), pseudotyped with the VSV-G envelope protein as previously described (31, 73). MDDCs were infected in triplicate (2 × 105 cells per well) with 3 to 4 ng/1 × 106 cells of HIV-1.luc/VSV-G or 35 ng/1 × 106 HIV-1.GFP/VSV-G with spinoculation (1,200 × g at room temperature for 1 h), followed by incubation for 1 h at 37°C. The cells were then washed with PBS and incubated at 37°C in fresh medium supplemented with 10% FBS. After 72 to 96 h, HIV-1 replication was estimated as a function of the percentage of GFP-expressing cells by either flow cytometry or luciferase activity in cell lysates (Luciferase reporter 1000 assay system [Promega, Charbonnieres, France] and a Veritas microplate luminometer [Turner BioSystems, Sunnyvale, CA]) as described previously (74).
Treatment of MDDCs with dNTPs and virus-like particles.
MDDCs were cultured in the presence of dNTPs and/or virus-like particles (VLPs) with or without the SIV VPX protein. A mix of the deoxynucleosides dATP (NTATP100), dCTP (NTCTP100), dGTP (NTGTP100), and dTTP (NTTTP100) (all from MP Biomedicals) was added to the cell cultures at a final concentration of 0.325, 0.650, or 1.25 mM (for each dNTP) for 4 h before infection and maintained for the duration of the experiments. VLPs with or without the SIV-VPX protein and pseudotyped with the VSV-G protein were produced as described previously (56). VLPs were added to cell cultures for 4 h before infection and maintained for the duration of the experiments.
Quantification of HIV-1 DNA intermediates.
HIV-1 late reverse transcripts (U5-Gag) and integrated DNA were quantified by real-time PCR with an Applied Biosystems 7500 real-time PCR system 72 h after infection of the MDDCs with VSV-G-pseudotyped HIV-1 NL4.3-Luc particles. MDDCs were washed in PBS, and total DNA was extracted with the DNeasy tissue kit (Qiagen). The U5-Gag PCR and Alu-Gag-nested PCR were conducted as described elsewhere (73). Amplification of the albumin gene was quantified relative to control human genomic DNA (Roche) and used to control for DNA loading. U5-Gag copies were quantified relative to a standard curve of serial dilutions of 8E5 cells containing one integrated copy of HIV-1 per cell (75) and integrated copies relative to a standard of DNA from HIV-1 R7Neo-infected HeLa cells (31).
Quantification of mRNA gene expression.
Total RNA from 5 × 105 MDDCs was extracted with the NucleoSpin RNA kit (Macherey-Nagel) according to the manufacturer's instructions. RNA was reverse transcribed with SuperScript II RT (Invitrogen) using a Gene Amp PCR system 9700. PCR amplification of cDNAs was carried out as previously described (30) on a 7500 real-time PCR system using the TaqMan gene expression premade mix (Applied Biosystems) to amplify the following genes: RRM2 (Hs00357247_g1), TYMS (Hs00426586_m1), SAMHD1 (Hs00210019_m1), CTPS1 (Hs01041858_m1), TK1 (Hs01062125_m1), CDKN1A (Hs00355782_m1), and GAPDH (Hs02758991_g1). The data were analyzed by the cycle threshold (CT) method, and the amount of target mRNA in each sample was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as an endogenous reference.
Western blot and antibodies.
MDDCs (1 × 106 to 2 × 106) were lysed in 100 to 200 μl of lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100) containing an EDTA-free protease inhibitor (Roche). A phosphatase inhibitor mixture (PhosSTOP; 04906837001; Roche) and phosphatase inhibitor cocktail 3 (P5726; Sigma) also were added for pSAMHD1 analysis. Cell extracts (30 to 50 μg) were run on 4 to 12% Mini-PROTEAN TGX stain-free precast gels (Bio-Rad) and transferred into polyvinylidene difluoride (PVDF) Hybond 0.45-μm membranes (Amersham). The membranes then were hybridized with primary antibodies anti-β-actin (A5316; Sigma) and anti-RNR2 (Sc10844; Santa Cruz) and used at a 1:200 dilution in 1% skimmed milk in PBS–0.05% Tween; anti-p21 (CP74-05-655; Millipore) used at a 1:1,000 dilution in 1% skimmed milk in PBS–0.05% Tween, SAMHD1 (ab 67820; Abcam), used at 1:1,000 dilution in 1% skimmed milk in PBS–0.05% Tween), followed by secondary horseradish peroxidase anti-goat, anti-rabbit (Sigma), and anti-mouse antibodies (Cell Signaling). Proteins were visualized on Hyperfilms (Amersham) using the SuperSignal West Pico chemiluminescent substrate (Pierce), and films were digitalized with a scanner.
For pSAMHD1 analysis, the proteins were blotted onto PVDF-Immobilon-FL 0.45-μm fluorescence membranes, and then the membranes were blocked with 5 to 10% milk for 30 min and 1% bovine serum albumin (BSA) for 30 min; the membranes then were washed three times with 1× PBS for 10 min each. For immunodetection of pSAMHD1, a pSAMHD1 rabbit monoclonal antibody generated in-house was used (33). A fluorescent secondary anti-rabbit IgG antibody labeled with Dylight 680 (Thermo Fisher) was used, and protein expression was revealed by infrared laser scan for fluorescent antibodies in a LI-COR Odyssey system.
All Western blot films or digital images were analyzed with the Image studio lite software (LI-COR Technologies) for band semiquantification.
siRNA transfection.
All small interfering RNAs (siRNAs) were obtained from Dharmacon (ON-TARGETplus). To knock down p21, a prevalidated combination of two siRNAs (sip21) was used (31): p21 target sequence 9, 5′ CGA CUG UGA UGC GCU AAU G 3′, and p21 target sequence 12, 5′ AGA CCA GCA UGA CAG AUU U 3′. Control siRNAs were a pool of four nontargeting siRNAs (D-001810-04-20). siRNAp21 or nontargeting siRNAs were diluted (final concentration of 50 nM) in 1 ml of Opti-MEM (30) with 20 μl of INTERFERin (Polyplus Transfection). The transfection mix was left at room temperature for 10 min. Immature MDDCs then were incubated with the transfection mix at 37°C for 8 h. The medium was replaced with fresh medium supplemented with 10% FBS, and the cells were kept in the incubator for 16 h. A second siRNA transfection then was performed using the same conditions. Forty-eight hours after the first siRNA transfection, the medium was replaced with fresh medium supplemented with 10% FBS (immature MDDCs) or with maturation medium (mature MDDCs) for 24 h before infection. The efficacy of p21 downregulation was evaluated at the time of infection by Western blotting to measure protein levels and by RT-qPCR to measure mRNA.
dNTP quantification.
For each experiment, 2 × 106 MDDCs were collected, washed with cold PBS, and lysed in 2 ml of 60% (vol/vol) aqueous methanol. Lysates were heated at 95°C for 7 min, clarified by centrifugation at 16,000 × g for 15 min at 4°C, and dried under vacuum. Extracted dNTPs then were resuspended and quantified using a primer extension assay, as previously described (25). The linear range of the dNTP assay is between 2% and 32% of the primer extension. The calculation of intracellular dNTP concentrations (picomolars) was based on the reported number of MDDCs.
Statistical analysis.
Values are presented in the text as medians and interquartile ranges (IQR). Differences among culture conditions were analyzed with a nonparametric paired analysis of variance (ANOVA). P values from these analyses are indicated in the figures. When a significant difference was detected, post hoc multiple comparisons were performed with the Student-Newman-Keuls method. Significant differences in these pairwise analyses are identified by horizontal bars in the figures. For correlations, the Spearman's rank coefficient was calculated. The data were analyzed with SigmaPlot 12.5 (Systat Software, Inc.).
ACKNOWLEDGMENTS
This study was funded by grants from Sidaction (France) to A.S.-C. and the National Institutes of Health (NIH), grants R01 GM104198 and R01 AI049781-01AI049781, to B.K. J.C.V.-C. received postdoctoral financial support from Sidaction and the Roux-Cantarini program at the Institut Pasteur. A.A. received a postdoctoral fellowship from Sidaction.
We are grateful to Alexandra Cribier for kindly providing the pSAMHD1 antibody.
J.-C.V.-C., A.A., A.D., G.M.L., and L.S. performed the research; J.C.V.-C., A.A., F.B.-S., M.M.-T., B.K., G.P., and A.S.-C. analyzed and interpreted the data; J.C.V.-C., G.P., and A.S.-C. designed the study; and J.C.V.-C. and A.S.-C. wrote the paper.
REFERENCES
- 1.Manches O, Frleta D, Bhardwaj N. 2014. Dendritic cells in progression and pathology of HIV infection. Trends Immunol 35:114–122. doi: 10.1016/j.it.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burleigh L, Lozach PY, Schiffer C, Staropoli I, Pezo V, Porrot F, Canque B, Virelizier JL, Arenzana-Seisdedos F, Amara A. 2006. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol 80:2949–2957. doi: 10.1128/JVI.80.6.2949-2957.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman CM, Wu L. 2009. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology 6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nobile C, Petit C, Moris A, Skrabal K, Abastado JP, Mammano F, Schwartz O. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J Virol 79:5386–5399. doi: 10.1128/JVI.79.9.5386-5399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu L, Kewal Ramani VN. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol 6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald D. 2010. Dendritic cells and HIV-1 trans-infection. Viruses 2:1704–1717. doi: 10.3390/v2081704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. 2004. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci U S A 101:7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM, Granelli-Piperno A, Pope M, Trumpfheller C, Ignatius R, Arrode G, Racz P, Tenner-Racz K. 2003. The interaction of immunodeficiency viruses with dendritic cells. Curr Top Microbiol Immunol 276:1–30. [DOI] [PubMed] [Google Scholar]
- 9.Izquierdo-Useros N, Naranjo-Gomez M, Erkizia I, Puertas MC, Borras FE, Blanco J, Martinez-Picado J. 2010. HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse? PLoS Pathog 6:e1000740. doi: 10.1371/journal.ppat.1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med 18:1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. 2011. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Descours B, Cribier A, Chable-Bessia C, Ayinde D, Rice G, Crow Y, Yatim A, Schwartz O, Laguette N, Benkirane M. 2012. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology 9:87. doi: 10.1186/1742-4690-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LP, Stoye JP, Crow YJ, Taylor IA, Webb M. 2011. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 14.Hrecka K, Hao CL, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim B, Nguyen LA, Daddacha W, Hollenbaugh JA. 2012. Tight interplay among SAMHD1 protein level, cellular dNTP levels, and HIV-1 proviral DNA synthesis kinetics in human primary monocyte-derived macrophages. J Biol Chem 287:21570–21574. doi: 10.1074/jbc.C112.374843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. 2012. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13:621–621. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell RD, Holland PJ, Hollis T, Perrino FW. 2011. Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J Biol Chem 286:43596–43600. doi: 10.1074/jbc.C111.317628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beloglazova N, Flick R, Tchigvintsev A, Brown G, Popovic A, Nocek B, Yakunin AF. 2013. Nuclease activity of the human SAMHD1 protein implicated in the Aicardi-Goutieres syndrome and HIV-1 restriction. J Biol Chem 288:8101–8110. doi: 10.1074/jbc.M112.431148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J, Ryoo J, Oh C, Hwang S, Ahn K. 2015. SAMHD1 specifically restricts retroviruses through its RNase activity. Retrovirology 12:46. doi: 10.1186/s12977-015-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonucci JM, St Gelais C, de Silva S, Yount JS, Tang CX, Ji XY, Shepard C, Xiong Y, Kim B, Wu L. 2016. SAMHD1-mediated HIV-1 restriction in cells does not involve ribonuclease activity. Nat Med 22:1072–1074. doi: 10.1038/nm.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryoo J, Choi J, Oh C, Kim S, Seo M, Kim SY, Seo D, Kim J, White TE, Brandariz-Nunez A, Diaz-Griffero F, Yun CH, Hollenbaugh JA, Kim B, Baek D, Ahn K. 2014. The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat Med 20:936–941. doi: 10.1038/nm.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White TE, Brandariz-Nunez A, Valle-Casuso JC, Amie S, Nguyen LA, Kim B, Tuzova M, Diaz-Griffero F. 2013. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell Host Microbe 13:441–451. doi: 10.1016/j.chom.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amie SM, Noble E, Kim B. 2013. Intracellular nucleotide levels and the control of retroviral infections. Virology 436:247–254. doi: 10.1016/j.virol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond TL, Roshal M, Jamburuthugoda VK, Reynolds HM, Merriam AR, Lee KY, Balakrishnan M, Bambara RA, Planelles V, Dewhurst S, Kim B. 2004. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J Biol Chem 279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy EM, Amie SM, Bambara RA, Kim B. 2012. Frequent incorporation of ribonucleotides during HIV-1 reverse transcription and their attenuated repair in macrophages. J Biol Chem 287:14280–14288. doi: 10.1074/jbc.M112.348482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews CK. 2015. Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat Rev Cancer 15:528–539. doi: 10.1038/nrc3981. [DOI] [PubMed] [Google Scholar]
- 28.D'Angiolella V, Donato V, Forrester FM, Jeong YT, Pellacani C, Kudo Y, Saraf A, Florens L, Washburn MP, Pagano M. 2012. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell 149:1023–1034. doi: 10.1016/j.cell.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane AN, Fan TW. 2015. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res 43:2466–2485. doi: 10.1093/nar/gkv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allouch A, David A, Amie SM, Lahouassa H, Chartier L, Margottin-Goguet F, Barre-Sinoussi F, Kim B, Saez-Cirion A, Pancino G. 2013. p21-mediated RNR2 repression restricts HIV-1 replication in macrophages by inhibiting dNTP biosynthesis pathway. Proc Natl Acad Sci U S A 110:E3997–E4006. doi: 10.1073/pnas.1306719110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergamaschi A, David A, Le Rouzic E, Nisole S, Barre-Sinoussi F, Pancino G. 2009. The CDK inhibitor p21Cip1/WAF1 is induced by FcgammaR activation and restricts the replication of human immunodeficiency virus type 1 and related primate lentiviruses in human macrophages. J Virol 83:12253–12265. doi: 10.1128/JVI.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badia R, Pujantell M, Riveira-Munoz E, Puig T, Torres-Torronteras J, Marti R, Clotet B, Ampudia RM, Vives-Pi M, Este JA, Ballana E. 2016. The G1/S specific cyclin D2 is a regulator of HIV-1 restriction in non-proliferating cells. PLoS Pathog 12:e1005829. doi: 10.1371/journal.ppat.1005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cribier A, Descours B, Valadao AL, Laguette N, Benkirane M. 2013. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 3:1036–1043. doi: 10.1016/j.celrep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Welbourn S, Dutta SM, Semmes OJ, Strebel K. 2013. Restriction of virus infection but not catalytic dNTPase activity is regulated by phosphorylation of SAMHD1. J Virol 87:11516–11524. doi: 10.1128/JVI.01642-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allouch A, David A, Amie SM, Lahouassa H, Chartier L, Margottin-Goguet F, Barre-Sinoussi F, Kim B, Saez-Cirion A, Pancino G. 2014. Reply to Pauls et al.: p21 is a master regulator of HIV replication in macrophages through dNTP synthesis block. Proc Natl Acad Sci U S A 111:E1325–E1326. doi: 10.1073/pnas.1322699111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauls E, Ruiz A, Riveira-Munoz E, Permanyer M, Badia R, Clotet B, Keppler OT, Ballana E, Este JA. 2014. p21 regulates the HIV-1 restriction factor SAMHD1. Proc Natl Acad Sci U S A 111:E1322–E1324. doi: 10.1073/pnas.1322059111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. 1999. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J 18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M, Iavarone A, Freedman LP. 1996. Transcriptional activation of the human p21(WAF1/CIP1) gene by retinoic acid receptor. Correlation with retinoid induction of U937 cell differentiation. J Biol Chem 271:31723–31728. [DOI] [PubMed] [Google Scholar]
- 39.Xaus J, Cardo M, Valledor AF, Soler C, Lloberas J, Celada A. 1999. Interferon gamma induces the expression of p21(waf-1) and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity 11:103–113. doi: 10.1016/S1074-7613(00)80085-0. [DOI] [PubMed] [Google Scholar]
- 40.Kramer JL, Baltathakis I, Alcantara OSF, Boldt DH. 2002. Differentiation of functional dendritic cells and macrophages from human peripheral blood monocyte precursors is dependent on expression of p21 (WAF1/CIP1) and requires iron. Br J Haematol 117:727–734. doi: 10.1046/j.1365-2141.2002.03498.x. [DOI] [PubMed] [Google Scholar]
- 41.Han TH, Jin P, Ren JQ, Slezak S, Marincola FM, Stroncek DF. 2009. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J Immunother 32:399–407. doi: 10.1097/CJI.0b013e31819e1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Kim HO, Kim HJ, Lee K, Kim HS. 2008. Generation of functionally mature dendritic cells from elutriated monocytes using polyinosinic: polycytidylic acid and soluble CD40 ligand for clinical application. Clin Exp Immunol 154:365–374. doi: 10.1111/j.1365-2249.2008.03757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhard C, Bottinelli D, Kim B, Luban J. 2014. Vpx rescue of HIV-1 from the antiviral state in mature dendritic cells is independent of the intracellular deoxynucleotide concentration. Retrovirology 11:12. doi: 10.1186/1742-4690-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St Gelais C, de Silva S, Amie SM, Coleman CM, Hoy H, Hollenbaugh JA, Kim B, Wu L. 2012. SAMHD1 restricts HIV-1 infection in dendritic cells (DCs) by dNTP depletion, but its expression in DCs and primary CD4(+) T-lymphocytes cannot be upregulated by interferons. Retrovirology 9:105. doi: 10.1186/1742-4690-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meuth M, Trudel M, Siminovitch L. 1979. Selection of Chinese-hamster cells auxotrophic for thymidine by 1-beta-D-arabinofuranosyl cytosine. Somatic Cell Genet 5:303–318. doi: 10.1007/BF01538844. [DOI] [Google Scholar]
- 46.Rampazzo C, Miazzi C, Franzolin E, Pontarin G, Ferraro P, Frangini M, Reichard P, Bianchi V. 2010. Regulation by degradation, a cellular defense against deoxyribonucleotide pool imbalances. Mutat Res 703:2–10. doi: 10.1016/j.mrgentox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Gazitt Y, Reddy SV, Alcantara O, Yang J, Boldt DH. 2001. A new molecular role for iron in regulation of cell cycling and differentiation of HL-60 human leukemia cells: iron is required for transcription of p21(WAF1/CIP1) in cells induced by phorbol myristate acetate. J Cell Physiol 187:124–135. doi:. [DOI] [PubMed] [Google Scholar]
- 48.Fang F, Wang Y, Li R, Zhao Y, Guo Y, Jiang M, Sun J, Ma Y, Ren ZJ, Tian ZG, Wei F, Yang D, Xiao WH. 2010. Transcription factor E2F1 suppresses dendritic cell maturation. J Immunol 184:6084–6091. doi: 10.4049/jimmunol.0902561. [DOI] [PubMed] [Google Scholar]
- 49.Olex AL, Hiltbold EM, Leng XY, Fetrow JS. 2010. Dynamics of dendritic cell maturation are identified through a novel filtering strategy applied to biological time-course microarray replicates. BMC Immunol 11:41. doi: 10.1186/1471-2172-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. 1998. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med 188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofer A, Crona M, Logan DT, Sjoberg BM. 2012. DNA building blocks: keeping control of manufacture. Crit Rev Biochem Mol Biol 47:50–63. doi: 10.3109/10409238.2011.630372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giacinti C, Giordano A. 2006. RB and cell cycle progression. Oncogene 25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 53.van den Heuvel S, Dyson NJ. 2008. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 9:713–724. doi: 10.1038/nrm2469. [DOI] [PubMed] [Google Scholar]
- 54.Vuaroqueaux V, Urban P, Labuhn M, Delorenzi M, Wirapati P, Benz CC, Flury R, Dieterich H, Spyratos F, Eppenberger U, Eppenberger-Castori S. 2007. Low E2F1 transcript levels are a strong determinant of favorable breast cancer outcome. Breast Cancer Res 9:R33. doi: 10.1186/bcr1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laguette N, Bregnard C, Hue P, Basbous J, Yatim A, Larroque M, Kirchhoff F, Constantinou A, Sobhian B, Benkirane M. 2014. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell 156:134–145. doi: 10.1016/j.cell.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 56.Berger G, Durand S, Goujon C, Nguyen XN, Cordeil S, Darlix JL, Cimarelli A. 2011. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat Protoc 6:806–816. doi: 10.1038/nprot.2011.327. [DOI] [PubMed] [Google Scholar]
- 57.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. 1993. P21 is a universal inhibitor of cyclin kinases. Nature 366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 58.Ballana E, Este JA. 2015. SAMHD1: at the crossroads of cell proliferation, immune responses, and virus restriction. Trends Microbiol 23:680–692. doi: 10.1016/j.tim.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Ryoo J, Hwang SY, Choi J, Oh C, Ahn K. 2016. Reply to SAMHD1-mediated HIV-1 restriction in cells does not involve ribonuclease activity. Nat Med 22:1074–1075. doi: 10.1038/nm.4164. [DOI] [PubMed] [Google Scholar]
- 60.Ji XY, Tang CX, Zhao Q, Wang W, Xiong Y. 2014. Structural basis of cellular dNTP regulation by SAMHD1. Proc Natl Acad Sci U S A 111:E4305–E4314. doi: 10.1073/pnas.1412289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koharudin LM, Wu Y, DeLucia M, Mehrens J, Gronenborn AM, Ahn J. 2014. Structural basis of allosteric activation of sterile alpha motif and histidine-aspartate domain-containing protein 1 (SAMHD1) by nucleoside triphosphates. J Biol Chem 289:32617–32627. doi: 10.1074/jbc.M114.591958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansen EC, Seamon KJ, Cravens SL, Stivers JT. 2014. GTP activator and dNTP substrates of HIV-1 restriction factor SAMHD1 generate a long-lived activated state. Proc Natl Acad Sci U S A 111:E1843–E1851. doi: 10.1073/pnas.1401706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang ZH, Bhattacharya A, Villacorta J, Diaz-Griffero F, Ivanov DN. 2016. Allosteric activation of SAMHD1 protein by deoxynucleotide triphosphate (dNTP)-dependent tetramerization requires dNTP concentrations that are similar to dNTP concentrations observed in cycling T cells. J Biol Chem 291:21407–21413. doi: 10.1074/jbc.C116.751446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shao J, Zhou B, Chu B, Yen Y. 2006. Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets 6:409–431. doi: 10.2174/156800906777723949. [DOI] [PubMed] [Google Scholar]
- 65.Welbourn S, Strebel K. 2016. Low dNTP levels are necessary but may not be sufficient for lentiviral restriction by SAMHD1. Virology 488:271–277. doi: 10.1016/j.virol.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H, Li C, Huang J, Cung T, Seiss K, Beamon J, Carrington MF, Porter LC, Burke PS, Yang Y, Ryan BJ, Liu R, Weiss RH, Pereyra F, Cress WD, Brass AL, Rosenberg ES, Walker BD, Yu XG, Lichterfeld M. 2011. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J Clin Investig 121:1549–1560. doi: 10.1172/JCI44539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leng J, Ho HP, Buzon MJ, Pereyra F, Walker BD, Yu XG, Chang EJ, Lichterfeld M. 2014. A cell-intrinsic inhibitor of HIV-1 reverse transcription in CD4(+) T cells from elite controllers. Cell Host Microbe 15:717–728. doi: 10.1016/j.chom.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saez-Cirion A, Hamimi C, Bergamaschi A, David A, Versmisse P, Melard A, Boufassa F, Barre-Sinoussi F, Lambotte O, Rouzioux C, Pancino G, Cohort AC. 2011. Restriction of HIV-1 replication in macrophages and CD4(+) T cells from HIV controllers. Blood 118:955–964. doi: 10.1182/blood-2010-12-327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castiello L, Sabatino M, Jin P, Clayberger C, Marincola FM, Krensky AM, Stroncek DF. 2011. Monocyte-derived DC maturation strategies and related pathways: a transcriptional view. Cancer Immunol Immunother 60:457–466. doi: 10.1007/s00262-010-0954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Doherty U, Swiggard WJ, Malim MH. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol 74:10074–10080. doi: 10.1128/JVI.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human-immunodeficiency-virus type-1 in mononuclear phagocytes. Virology 206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 72.He JL, Choe S, Walker R, Dimarzio P, Morgan DO, Landau NR. 1995. Human-immunodeficiency-virus type-1 viral-protein-R (Vpr) arrests cells in the G(2) phase of the cell-cycle by inhibiting P34(Cdc2) activity. J Virol 69:6705–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.David A, Saez-Cirion A, Versmisse P, Malbec O, Iannascoli B, Herschke F, Lucas M, Barre-Sinoussi F, Mouscadet JF, Daeron M, Pancino G. 2006. The engagement of activating Fc gamma Rs inhibits primate lentivirus replication in human macrophages. J Immunol 177:6291–6300. doi: 10.4049/jimmunol.177.9.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saez-Cirion A, Versmisse P, Truong LX, Chakrabarti LA, Carpentier W, Barre-Sinoussi F, Scott-Algara D, Pancino G. 2006. Persistent resistance to HIV-1 infection in CD4 T cells from exposed uninfected Vietnamese individuals is mediated by entry and post-entry blocks. Retrovirology 3:81. doi: 10.1186/1742-4690-3-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Folks TM, Powell D, Lightfoote M, Koenig S, Fauci AS, Benn S, Rabson A, Daugherty D, Gendelman HE, Hoggan MD, Venkatesan S, Martin MA. 1986. Biological and biochemical-characterization of a cloned Leu-3− cell surviving infection with the acquired-immune-deficiency-syndrome retrovirus. J Exp Med 164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]