Abstract
Objective:
We conducted a systematic review and meta-analysis of studies that evaluated the effect of hormonal therapy [estrogen therapy including oral contraceptive pills (OCP)] and bisphosphonates in preventing bone loss in patients with functional hypothalamic amenorrhea (FHA).
Methods:
We searched several electronic databases for controlled and noncontrolled studies that enrolled females of any age presenting with FHA (including athletic, weight loss, and stress-associated amenorrhea/oligomenorrhea) through 9 January 2017. The outcomes of interest were fractures and bone mineral density (BMD). Random effects meta-analysis was used to pool outcomes across studies expressed as weighted mean difference and 95% confidence interval (CI).
Results:
Nine studies reporting on 280 patients that received different hormonal therapies were included. We did not identify studies that evaluated bisphosphonates. Meta-analysis demonstrated a statistically significant increase in BMD of the lumbar spine in patients receiving hormonal therapy after a median follow-up of 12 months (weighted mean difference, 0.032 g/cm2; 95% CI, 0.017 to 0.047; percentage change in BMD, 3.30%; 95% CI, 1.74 to 4.86). There was no substantial effect of receiving hormonal therapy on BMD of the femoral neck, trochanteric region, Ward triangle, or total body BMD. The quality of evidence was low because of the high risk of bias, imprecision (small sample size), and indirectness (as BMD is a surrogate outcome). None of the studies reported the incidence of fractures.
Conclusion:
The current evidence does not support using hormonal therapy for the sole purpose of improving bone health in patients with FHA. There are no data about bisphosphonates in this population.
Keywords: amenorrhea, female athletes, bone density, functional hypothalamic amenorrhea, bisphosphonates, oral contraceptives
The current evidence does not support hormonal therapy for the sole purpose of improving bone health in functional hypothalamic amenorrhea. There are no data about bisphosphonates in this population.
Functional hypothalamic amenorrhea (FHA) is defined as the absence of menstruation because of an interruption of the hypothalamic -pituitary-ovarian axis with no apparent anatomic or organic disease [1]. Severe stressors such as excessive exercise, dieting, and psychological stress affect the hypothalamic -pituitary-ovarian axis, leading to FHA [2]. A previous systematic review by Gibbs et al. showed that the prevalence of primary and secondary amenorrhea from FHA in exercising women ranged from 0% to 56.0% and from 1.0% to 60%, respectively [3].
A study by Drinkwater et al. was conducted in 1984 and compared lumbar spine bone mineral density (BMD) between eumenorrheic athletes and amenorrheic athletes [4]. Vertebral mineral density was significantly lower in the amenorrheic group (mean, 1.12 g/cm2) than in the eumenorrheic group (mean, 1.30 g/cm2) [4]. Gibbs et al. reported that the prevalence of low BMD combined with menstrual disturbances, including FHA and oligomenorrhea, in exercising women ranged from 0% to 15.9% [3]. Davies et al. compared women age 16 to 40 years with past or current history of amenorrhea from various causes and of a median duration of 3 years with a control group of age-matched normal volunteers with no history of menstrual disorder. They found that the amenorrheic group had a mean reduction in BMD of 15% compared with controls [5]. Bone loss was related to the duration of amenorrhea and the severity of estrogen deficiency. Ten patients had a history of atraumatic fracture [5]. Another study of young ballet dancers showed that amenorrheic intervals were marked by prolonged hypoestrogenism and that a delay in menarche and prolonged intervals of amenorrhea were associated with scoliosis and stress fractures [6].
There are many suggested interventions to mitigate bone loss associated with FHA. These include, but not limited to, hormonal therapies [often in the form of oral contraceptive pills (OCPs)] and bisphosphonates [7]. In 2008, Vescovi et al. published a systematic review evaluating multiple treatment strategies, including hormonal therapy and bisphosphonates; however, meta-analysis was not performed because of the small number of available studies at that time [8].
To support the development of clinical practice guidelines by the Endocrine Society, we conducted a systematic review and meta-analysis to evaluate hormonal replacement therapy (estrogen therapy including OCPs) and bisphosphonates compared with placebo or no intervention in preventing bone loss in patients with FHA.
1. Methods
A protocol was developed in advance by the investigators to specify eligibility criteria, outcomes of interest, and analysis methods. The methodology and reporting of this systematic review comply with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [9].
A. Eligibility Criteria
We included randomized controlled trials (RCTs) and observational studies that enrolled female patients of any age presenting with FHA, including athletic, weight loss, and stress-associated amenorrhea/oligomenorrhea, as defined by studies’ authors. We included controlled and uncontrolled cohort studies designed to evaluate hormonal replacement therapy (estrogen therapy including OCPs) and bisphosphonates. Included studies should evaluate the efficacy of the intervention measured as change in the number of fractures or change in BMD, reported as a z score or absolute BMD in grams per square centimeter.
We excluded studies that enrolled patients with amenorrhea resulting from anovulation (e.g., polycystic ovarian syndrome), bona fide psychiatric disorders [e.g., bulimia, anorexia nervosa (AN), depression], and organic forms of hypothalamic amenorrhea (e.g., pituitary tumors, adenomas, Kallmann syndrome).
B. Search Methods
A comprehensive search of several databases from each database’s earliest inception through 9 January 2017 without language restrictions was designed and conducted by an expert reference librarian (L.J.P.) with input from the investigators. Databases included Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus (Appendix). To identify additional candidate studies, we also searched previous systematic and narrative reviews for relevant studies [8, 10–15]. A Google search was also used to capture unpublished or gray literature.
C. Study Selection
Two reviewers (A.A.L. and B.G.C.L.) assessed the eligibility of candidate studies for inclusion in a blinded and independent manner by screening titles and abstracts followed by reviewing the retrieved full text publications of the included studies. Disagreements between the two reviewers were solved by meeting with a third reviewer (O.A.) and establishing consensus.
D. Data Collection Process
Data were abstracted from each study using a standardized, piloted, and Web-based data extraction form. Three independent reviewers (A.A.N., B.G.C.L., and O.A.) performed the abstraction in duplicate; disagreements were resolved by discussion among the three reviewers. The following data were abstracted: full description of enrolled patients (age, inclusion criteria, gynecological history, and lifestyle), detailed description of intervention received and control, follow-up monitoring, measures of outcomes, and source of funding.
E. Assessment of the Risk of Bias
Three independent reviewers (A.A.N., B.G.C.L., and O.A.) evaluated the methodological quality of included studies in duplicates. For RCTs, we used the Cochrane Risk of Bias tool in terms of adequacy of randomization, allocation concealment, blinding (patients, health care providers, data collectors, and outcome assessors), baseline imbalance, and extent of loss to follow-up [16]. For observational studies, we used the Newcastle-Ottawa quality assessment tool after adding a question to assess the method used to allocate patients. We evaluated allocation method, representativeness of the cohort, selection of the control cohort (if present), baseline imbalance, outcome assessment method, and adequacy of follow-up [17].
F. Statistical Analysis
For each of the included studies, we calculated the absolute and percentage change of the outcomes from the baseline to the end of the interventions for the intervention and the control group. We pooled the weighted mean difference (WMD) across studies using the DerSimonian and Laird random effects method [18]. A positive WMD implies improvement in BMD favoring the intervention.
We assessed heterogeneity across the studies using the Cochran’s Q test and the I squared statistic, where P value < 0.10 or I2 > 50% suggest substantial heterogeneity that is due to real differences in study populations, protocols, interventions, and/or outcomes. Although we planned to assess potential publication bias, we were not able to conduct formal tests because of the small number of studies included in the analysis [19]. All statistical analyses were conducted using STATA, version 12 (StataCorp, College Station, TX).
2. Results
A. Search Results
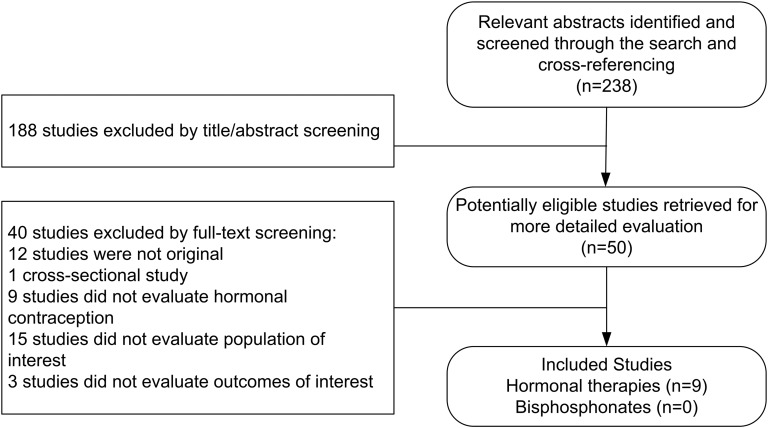
The search strategy identified 238 publications, of which nine were included (reported in 10 manuscripts) (Fig. 1). Three were RCTs [20–22], two were prospective comparative studies [23, 24], one was a retrospective comparative study [25], and three were prospective single-arm studies [26–29]. All of these studies evaluated hormonal therapy. The search did not identify any study that evaluated bisphosphonates in treating patients with FHA.
Figure 1.
The process of study selection.
B. Evidence on Hormonal Therapies
The included studies evaluated 280 patients. The mean follow-up period ranged from 8 to 48 months, with a median of 12 months. The inclusion criteria, characteristics of the included patients, and the interventions they received are detailed in Table 1.
Table 1.
Characteristics of Included Studies
| Study and F/U | Inclusion/Exclusion Criteria, Demographics, and Risk Factors | Interventions (No. of Patients) |
|---|---|---|
| Castelo-Branco, 2001 [20] | Inclusion criteria: amenorrhea (no menstruation for 6 mo) or oligomenorrhea (6 or fewer menstrual periods during the previous year). Depressed and anxiety prone. Exclusion: AN, current use of any drug that may affect bone mass, renal or GI disease, thyroid dysfunction, DM, moderate to severe osteopenia (T score ≤1.5) or osteoporosis (T score ≤2.5) | OCP (0.03 EE + desogestrel) [24] |
| Length of F/U: 12 mo | Demographics: age, 24.4 y ± 7.9; race, NR; BMI, 23.7 kg/cm2; length of amenorrhea, 16.7 ± 10.4 mo | EE 0.030 mg and desogestrel 0.15 mg |
| Risk factor: stress (patients were depressed and anxiety prone) | OCP (0.02 EE + desogestrel) [22] | |
| EE 0.020 mg and desogestrel 0.15 mg | ||
| Control [18] | ||
| No treatment | ||
| Cumming, 1996 [25] | Inclusion criteria: Confirmation of the pathophysiology of the amenorrhea and bone density measurement by DXA if amenorrheic for longer than 2 y. Bone density >1.0 SD below the mean value. Characteristic picture of exercise-associated amenorrhea (low/low-normal LH, FSH, estradiol, and prolactin) | HRT (CE/estradiol + MPA) [8] |
| Length of F/U: 24-30 months | Demographics: age, 23-34 y; race, NR; BMI, 17.1 kg/cm2; length of amenorrhea, 2-8 y | Conjugated estrogen 0.625 mg (n = 6), or estradiol transdermal patch 50 μg daily with cyclic medroxyprogesterone acetate 10 mg daily for 14 d monthly (n = 2) for at least 2 y |
| Risk factor: exercise (run 35-75 miles/wk) | Control [5] | |
| No treatment | ||
| De Cree, 1998 [23] | Inclusion criteria: Sportswomen aged 18-29 y. Athletic amenorrhea with osteoporosis. Free for at least 3 months of any medical drugs known to interfere with calcium or sex hormone metabolism. Amenorrhea (no menstruation for at least 6 mo) or oligomenorrhea (decreased frequency of menstrual cycles in the previous year with only 1 menstrual period during last 3 mo) | OCP (EE + CPA) [7] |
| Length of F/U: 8 mo | Demographics: age, 21.9 ± 3.9 y; race, NR; BMI, NR; length of amenorrhea, NR (as defined previously) | EE 0.05 mg and cyproterone acetate 2 mg |
| Risk factor: exercise (run 21.7 ± 9.3 miles/wk). Stress (27% had stressful life) | Control [4] | |
| No treatment | ||
| Gibson, 1999 [21] | Inclusion criteria: subjects had to have trained for 3 y and currently training for a minimum of 3 h/wk and to run at least 40 km/wk. Exclusion: history of respiratory disease, DM, metabolic bone disorders, rheumatoid arthritis, thyroid or parathyroid disease, malignancy, cardiac, renal or inflammatory bowel disease. Patients taking oral or inhaled steroids | Hormone replacement (estriol, estradiol, and norethisterone) + calcium supplements [10] |
| Length of F/U: 9 mo (18 mo, but outcomes were reported for 9 mo of F/U) | Demographics: age, 27.5 ± 9.3 y; race, 100% white; BMI, 19.21 kg/cm2; length of amenorrhea, 6.9 y | Daily oral treatment with Trisequens tablets (estriol 1 mg and estradiol 2 mg for 12 d; estriol 1 mg, estradiol 2 mg, and norethisterone acetate 1 mg for 10 d; estriol 0.5 mg and estradiol 1 mg for 6 d) + 1000 mg calcium carbonate |
| Risk factor: exercise (run 53.1 ± 13.8 miles/wk) | Calcium supplements [14] | |
| 1000 mg calcium carbonate per day | ||
| Control [10] | ||
| No treatment | ||
| Hergenroeder, 1997 [24] | Inclusion criteria: hypothalamic amenorrhea (no menstrual bleeding in the past 6 mo) and oligomenorrhea (6 or fewer menstrual periods in the past 12 mo, with 1 or more menstrual periods in the past 6 mo) associated with a lifestyle that included exercise, weight loss, dieting, or stress. Exclusion: evidence of disease that would affect bone mineral, the use of medications that affect bone mineral, tobacco use, and obesity (>120% of estimated ideal weight) | OCP (EE + norethindrone) [5] |
| Demographics: age, 20.1 ± 7.4 y; race, 100% white; BMI, 18 kg/cm2; length of amenorrhea, NR (as defined previously) | EE 0.035 mg and norethindrone 0.5-1.0 mg/d on 21 d of each 28-d cycle | |
| Risk factor: exercise (80% of patients exercised as a part of a team or independently), dieting, stress | Medroxyprogesterone [5] | |
| Medroxyprogesterone 10 mg/d on the last 12 d of the calendar month | ||
| Placebo [5] | ||
| One placebo tablet daily for the last 12 d of the calendar month | ||
| Warren, 2003 [22] | Inclusion criteria: elite ballet dancers solicited from national and regional schools and dance companies, via advertisements in college publications, and by physician referrals for hormonal problems and interest in bone density measurements. Amenorrheics (no menstruation for at least 5 mo). Exclusion: patients taking hormones or oral contraceptives for 6 mo before the study | Hormone replacement (CE + MPA) [13] |
| Length of F/U: 24 months | Demographics: age 20.1 ± 4.6 y; race, 100% white; BMI, 18.87 kg/cm2; length of amenorrhea, NR (as defined previously) | CE 0.635 mg on d 1-25 + medroxyprogesterone 10 mg on d 16-25, in a 30-d circle + calcium supplements |
| Risk factor: exercise (dancing 24.0 ± 10.8 h/w) | Control [11] | |
| Placebo + calcium supplements | ||
| Rickenlund, 2004 [26] | Inclusion criteria: female athletes in endurance sports. 16-35 y, BMI 18-24, nulliparous, healthy, nonsmoking. Endurance training defined as a minimum of 6 h of aerobic weight-bearing training or a minimum of 70 km of running or 6 h of specific endurance training weekly. Amenorrhea (no menstruation for at least 3 mo) or oligomenorrhea (menstrual periods at intervals exceeding 6 wk) | OCP (EE + levonorgestrel) [13] |
| Length of F/U: 10 months | Demographics: age, 19.8 ± 4.6 y; race, NR; BMI, 19.4 kg/cm2; length of amenorrhea, NR (as defined previously) | EE 0.03 mg + levonorgestrel 0.15 mg on d 1-21, followed by a hormone- and tablet-free interval on d 22-28 |
| Risk factor: exercise (train 8.1 ± 1.6 h/wk) | ||
| Sowińska-Przepiera, 2011/2011 [27, 28] | Inclusion criteria: girls 16-17 y with FHA. (1) At least 6 mo of amenorrhea preceded by at least 3 y of oligomenorrhea; (2) psychological problems (learning disabilities and/or family problems). Exclusion: PCOS, CAH, premature ovarian failure, low birth weight or preterm birth, confirmed episode of an eating disorder, poor dieting during childhood or puberty, episodes of impaired growth and body mass gain, extensive participation in sports, metabolic disorders associated with decreased bone mineralization, prolonged use of stimulants or drugs that may affect bone metabolism, familial history of osteoporosis, incomplete 4-y F/U | OCP (17B-estradiol + dydrogesterone) |
| Length of F/U: 24 mo | Demographics: age, 16.7 ± 1.2 y; race, NR; BMI, 18.7 ± 2.2 kg/cm2; length of amenorrhea NR (as defined previously) | 17-b estradiol (2 mg from the second to 25th day of the menstrual cycle) and dydrogesterone (10 mg from the 16th to the 25th day of the menstrual cycle) |
| Risk factor: stress (psychological problems, defined previously) | ||
| Warren, 2005 [29] | Inclusion criteria: age, 18-40 y, with lumbar spine BMD ≥0.937 g/cm2 (DXA scan) and BMI 16-24 kg/m2. No menstrual periods during the last 3 months and 2 or fewer periods during the previous 12 mo or women with previously diagnosed FHA with no spontaneous menses during the previous 3 mo. Less than 6 months of hormonal therapy in the previous year. Women age 35-40 y required to be nonsmokers and those ages 18-34 y could not smoke more than 10 cigarettes per day. Exclusion: Pregnant, nursing, a contraindication for steroid hormonal therapy, evidence of cervical dysplasia, undiagnosed breast lesion, medical condition that could contribute to osteopenia, history of severe migraines, hypertension, hormonal therapy within 3-6 mo of screening, high prolactin, FSH, testosterone, LH/FSH ratio or LH >11, hirsutism, AN within 12 mo, or recent history of alcohol or other substance abuse. Medications that may interact with OCPs | OCP (EE + NGM) [27] |
| Length of F/U: 9 months | Demographics: age, 26.7 ± 6.64 y; race, 92.6% white and 7.4% other; BMI, 20.54 kg/cm2; length of amenorrhea 18.5 mo | EE 0.035 mg and norgestimate 0.180-0.250 mg on 21 d of each 28-d cycle. 10-13 cycles |
| Risk factor: NR |
Abbreviations: BMI, bone mass index; CAH, congenital adrenal hypoplasia; CE, conjugated estrogen; CPA, cyproterone acetate; DM, diabetes mellitus; DXA, dual energy x-ray absorptiometry; EE, ethinyl estradiol; FSH, follicle-stimulating hormone; F/U, follow-up; HRT, hormone replacement therapy; GI, gastrointestinal; LH, luteinizing hormone; MPA, medroxyprogesterone acetate; NR, not reported; PCOS, polycystic ovarian syndrome; SD, standard deviation.
C. Types of Hormonal Therapy
The studies evaluated a variety of hormonal therapies: different combinations of oral contraceptive pills (ethinyl estradiol/desogestrel [20], ethinyl estradiol/cyproterone acetate [23], ethinyl estradiol/norethindrone [24], ethinyl estradiol/levonorgestrel [26], 17B-estradiol/dydrogesterone [27, 28], ethinyl estradiol/norgestimate [29]); transdermal patches of conjugated estrogen or estriol with oral medroxyprogesterone acetate [25]; oral conjugated estrogens and medroxyprogesterone [22]; and oral estriol, estradiol, and norethisterone acetate with calcium supplements [21]. The control groups received no treatment [20, 21, 23, 25], calcium supplements [21], placebo [24], or placebo with calcium supplements [22].
D. Etiology of FHA
Castelo-Branco and Sowińska-Przepiera included patients with amenorrhea/oligomenorrhea associated with stress (depression, anxiety, learning disabilities, and/or family problems). Cumming [25], Gibson et al. [21], De Cree et al. [23], Rickenlund et al. [26], and Warren et al. [22] included patients with amenorrhea/oligomenorrhea associated with exercise (athletic, endurance sportswomen and elite ballet dancers). Hergenroeder et al. [24] and Warren et al. [29] included patients with hypothalamic amenorrhea/oligomenorrhea with no specific risk factor.
D-1. Risk of bias (methodological quality)
For the three included RCTs, there was a high risk of bias. All of them reported that their studies were randomized but they did not provide details regarding the method of randomization or if the allocation was concealed. Gibson et al. [21] reported that they did not blind the OCP group, although they did blind the other control groups. Castelo-Branco et al. reported that 24% of their sample was lost to follow-up [20]. Although the included observational studies were well performed in terms of quality and reducing bias, their observational nature puts them at a high risk for bias [23–29]. Detailed description of the quality indicators of each study are presented in Tables 2 and 3.
Table 2.
Randomized Controlled Trials
| Study | Randomization | Allocation Concealment | Blinding | Baseline Imbalance | % Lost to F/U | Source of Funding |
|---|---|---|---|---|---|---|
| Castelo-Branco, 2001 [20] | Yes, method NR | NR | NR | NR | 24 | NR or unclear |
| Gibson, 1999 [21] | Yes, method NR | NR | OCP group had no blinding, the other 2 groups had double blinding | NR | NR | NR or unclear |
| Warren, 2003 [22] | Yes, method NR | NR | NR | NR | NR | NIH funded, but pharmaceutical company donated medications |
Table 3.
Nonrandomized Controlled/Single-Arm Trials
| Study | Time and Design | Allocation | Representativeness of Population | Selection of Control | Baseline Imbalance | Assessment of Outcome | Adequacy of F/U | Source of Funding |
|---|---|---|---|---|---|---|---|---|
| Cumming, 1996 [25] | Retrospective (comparative) | Patient preference | Truly representative | Drawn from the same community of the exposed cohort | Study reported no substantial difference between groups in height, weight, BMI, serum estradiol levels, clinical record, or training level | Independent assessment | No LFU | NR or unclear |
| De Cree, 1998 [23] | Prospective (comparative) | Physicians allocated patients | Truly representative | Drawn from the same community of the exposed cohort | Study reported no substantial difference between groups in clinical record and training level | Independent assessment | No LFU | NR or unclear |
| Hergenroeder, 1997 [24] | Prospective (comparative) | Patients were alternately allocated in each group. Patients who did not want to receive hormones constituted the control group | Truly representative | Drawn from the same community of the exposed cohort | Study controlled for confounders: age, mean amenorrhea time, weight, and height | Independent assessment | 6% LFU | Only not-for-profit source (e.g., university, research center, NIH) |
| Rickenlund, 2004 [26] | Prospective (single arm) | According to inclusion/exclusion criteria | Truly representative | NA | NA | Independent assessment | No LFU | Only not-for-profit source |
| Sowińska-Przepiera, 2011/2012 [27, 28] | Prospective (single arm) | According to inclusion/exclusion criteria | Truly representative | NA | NA | Independent assessment | No LFU | NR or unclear |
| Warren, 2005 [29] | Prospective (single arm) | According to inclusion/exclusion criteria | Truly representative | NA | NA | Independent assessment | 29% LFU | Includes for-profit source (pharmaceutical company) |
Abbreviations: LFU, lost to follow up; NA, not available; NIH, National Institutes of Health.
D-2. Reported outcomes
None of the studies reported fractures or z score. Studies reported BMD measured in grams per square centimeter at different areas of the skeleton. All of the studies reported BMD of the lumbar spine [20–29]. Cumming [25], Gibson et al. [21], and Hergenroeder et al. [24] reported BMD of the femoral neck; only Gibson et al. reported BMD of the trochanteric region. Cumming [25] and Gibson et al. [21] reported BMD of Ward triangle. Hergenroeder et al. and Rickenlund et al. reported total body BMD [24, 26]; only Rickenlund et al. reported BMD of the legs. Only Warren et al. reported BMD of the wrist and foot [22]. Detailed results of individual studies are presented in Supplemental Table 1 (94.8KB, docx) .
D-3. Change and percentage change in BMD measured in grams per square centimeter
Random-effects meta-analysis demonstrated a statistically significant change in BMD of the lumbar spine in patients receiving hormonal therapy (n = 213) compared with patients receiving control (n = 67) with WMD = 0.032 g/cm2 favoring hormonal therapy [95% confidence interval (CI), 0.017 to 0.047] and WMD of percentage change in BMD = 3.30% (95% CI, 1.74 to 4.86). The heterogeneity P value = 0.14.
Meta-analysis showed no statistically significant difference between patients receiving hormonal therapy (n = 23) and patients receiving control (n = 34) in BMD of the femoral neck (WMD = 0.002 g/cm2, 95% CI, −0.048 to 0.052; WMD of percentage change = 0.20%, 95% CI, −5.93 to 6.33; heterogeneity P value = 0.96). No statistically significant difference was shown in BMD of trochanteric region (total n = 34; WMD = 0.019 g/cm2 and WMD of percentage change = 2.8%; heterogeneity P value = 0.76), Ward triangle (total n = 47; WMD = 0.016 g/cm2 and WMD of percentage change = 2.4%; heterogeneity P value = 0.80), or total body (total n = 23; WMD = 0.02 g/cm2 and WMD of percentage change = 1.8%; heterogeneity P value = 0.61).
E. Evidence on Bisphosphonates
The search did not identify any study that evaluated bisphosphonates in treating patients with FHA. Nevertheless, we were able to identify four studies (Supplemental Table 2 (94.8KB, docx) ) that evaluated the effects of bisphosphonate on BMD in patients with AN and amenorrhea (a likely related population to FHA) by reviewing previous systematic reviews [30–32].
Golden et al. compared alendronate with placebo in a trial of 32 patients. After a year of follow-up, they found no statistically significant difference between the two groups in the percent increase in BMD of the lumbar spine or the femoral neck [31]. Nakahara et al. conducted a trial to compare etidronate, calcium supplements and vitamin D, and placebo. The trial included 41 patients and demonstrated that etidronate increased tibial BMD (measured by speed of sound technique) more than placebo, but they found no substantial difference between etidronate compared with calcium supplements and vitamin D [30]. Miller et al. compared 10 anorexic women receiving risedronate with published data of 14 anorexic women whom they studied prospectively in the past. At 9 months, they found that both lumbar spine BMD and total body BMD increased significantly in patients receiving risedronate compared with patients who received no treatment [32]. In another trial by Miller et al., compared with placebo, risedronate significantly increased BMD in the spine and hip, but not in the radius after 1-year follow-up in patients with AN. The presence of menses was found not to be a predictor for response to bisphosphonates in this cohort [33]. In terms of the clinical importance of BMD change in response to bisphosphonate, the magnitude of BMD increase in the spine with 5 mg of risedronate was 4.1 ± 1.6% at 6 months and 4.9 ± 1.0% at 9 months [32].
3. Discussion
We conducted a systematic review and meta-analysis of studies evaluating the preventive effect of hormonal therapy and bisphosphonates on BMD in females with FHA. We found nine small studies evaluating hormonal therapy, none of which reported fractures. This body of evidence is very small (280 patients with a median follow-up of 12 months). Hormonal therapy increased BMD in the lumbar region only. The quality of this evidence according the Grades of Recommendation, Assessment, Development, and Evaluation approach was low [34, 35], rated down because of the high risk of bias, imprecision (very small sample size), and indirectness of BMD is a surrogate outcome. The changes in BMD of the femoral neck, trochanteric region, Ward triangle, and total body were not statistically significant. In terms of bisphosphonates, we found no studies evaluating its effectiveness in patients with FHA. Indirect evidence from populations with potentially related condition (AN and amenorrhea) was sparse and inconsistent with low quality.
In terms of the clinical implications of this meta-analysis, it seems that evidence of effectiveness of hormonal therapy (primarily oral contraceptives and estrogens, with a very small number of patients using transdermal patches) is very limited. This may be due to hypercortisolism, decreased thyroid function, or other reasons such as the persistent low body weight of many of these individuals. Data on transdermal estrogen use in FHA are also very limited, and evidence may have to be extrapolated from patients with AN. A randomized trial in patients with AN compared 100 µg of 17β-estradiol (with cyclic progesterone) or placebo transdermally for 18 months. Spine and hip BMD z scores increased over time in those receiving physiologic transdermal estradiol replacement compared with the placebo group [36]. Therefore, a rationale for prescribing hormonal therapy to women with FHA for the sole purpose of bone protection is not supported by high-quality evidence.
Similarly, evidence supporting bisphosphonates is also very limited in patients with FHA. This means that the primary approach for the treatment of FHA depends on correcting the energy imbalance to improve hypothalamic-pituitary-gonadal axis function. This can be done by changing caloric consumption and exercise habits, and requires a multidisciplinary approach by physicians with expertise in this disorder, dieticians, psychotherapists, and other health professionals.
A. Limitations
This evidence synthesis has several limitations. The definition of amenorrhea and oligomenorrhea varied across studies. Hormonal therapy regimens and length of follow-up were also quite variable. The included studies had methodological limitations and were small. Some of the comparisons we conducted were across studies and subject to ecological bias. We were unable to assess publication bias because of the small number of included studies. Although we were interested in summarizing evidence on FHA excluding patients with bona fide psychiatric disorders such as AN, it is likely that some patients with AN may have been included in studies with populations labeled as having FHA.
B. Strengths
The strengths of this systematic review relate to measures undertaken by reviewers, such as screening studies and evaluating their quality in duplicate. The search strategy was comprehensive and included multiple databases and was supplemented by contacting experts and cross-referencing with existing documents. This systematic review is accompanied by a meta-analysis conducted to quantitatively evaluate the effectiveness of drug therapies for preventing bone loss in patients with FHA/oligomenorrhea.
4. Conclusion
The current evidence does not support using hormonal therapy for the sole purpose of improving bone health in patients with FHA. There are no data about bisphosphonates in this population.
Acknowledgments
Acknowledgments
This work was partially funded by a contract from the Endocrine Society.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AN
- anorexia nervosa
- BMD
- bone mineral density
- FHA
- functional hypothalamic amenorrhea
- OCP
- oral contraceptive pill
- RCT
- randomized controlled trial
- WMD
- weighted mean difference.
References and Notes
- 1.Liu JH, Bill AH. Stress-associated or functional hypothalamic amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:179–184. [DOI] [PubMed] [Google Scholar]
- 2.Meczekalski B, Podfigurna-Stopa A, Warenik-Szymankiewicz A, Genazzani AR. Functional hypothalamic amenorrhea: current view on neuroendocrine aberrations. Gynecol Endocrinol. 2008;24:4–11. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc. 2013;45:985–996. [DOI] [PubMed] [Google Scholar]
- 4.Drinkwater BL, Nilson K, Chesnut CH III, Bremner WJ, Shainholtz S, Southworth MB. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med. 1984;311:277–281. [DOI] [PubMed] [Google Scholar]
- 5.Davies MC, Hall ML, Jacobs HS. Bone mineral loss in young women with amenorrhoea. BMJ. 1990;301:790–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren MP, Brooks-Gunn J, Hamilton LH, Warren LF, Hamilton WG. Scoliosis and fractures in young ballet dancers. Relation to delayed menarche and secondary amenorrhea. N Engl J Med. 1986;314:1348–1353. [DOI] [PubMed] [Google Scholar]
- 7.Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med. 2010;363:365–371. [DOI] [PubMed] [Google Scholar]
- 8.Vescovi JD, Jamal SA, De Souza MJ. Strategies to reverse bone loss in women with functional hypothalamic amenorrhea: a systematic review of the literature. Osteoporos Int. 2008;19:465–478. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 10.Rackoff P, Honig S. Anorexia nervosa, athletics, and amenorrhea: The female athlete triad. Curr Opin Endocrinol Diabetes. 2006;13(6):491–496. [Google Scholar]
- 11.Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am. 2010;39:155–167 (x). [DOI] [PubMed] [Google Scholar]
- 12.Jayasinghe Y, Grover SR, Zacharin M. Current concepts in bone and reproductive health in adolescents with anorexia nervosa. BJOG. 2008;115:304–315. [DOI] [PubMed] [Google Scholar]
- 13.Khan A. Premenopausal women and low bone density. Can Fam Physician. 2006;52:743–747. [PMC free article] [PubMed] [Google Scholar]
- 14.Liu SL, Lebrun CM. Effect of oral contraceptives and hormone replacement therapy on bone mineral density in premenopausal and perimenopausal women: a systematic review. Br J Sports Med. 2006;40:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gourlay ML, Brown SA. Clinical considerations in premenopausal osteoporosis. Arch Intern Med. 2004;164:603–614. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O’Connell D, Peterson J, Losos M, Tugwell P, Welch V. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 28 April 2017.
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 19.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castelo-Branco C, Vicente JJ, Pons F, Martinez de Osaba MJ, Casals E, Vanrell JA. Bone mineral density in young, hypothalamic oligoamenorrheic women treated with oral contraceptives. J Reprod Med. 2001;46:875–879. [PubMed] [Google Scholar]
- 21.Gibson JH, Mitchell A, Reeve J, Harries MG. Treatment of reduced bone mineral density in athletic amenorrhea: a pilot study. Osteoporos Int. 1999;10:284–289. [DOI] [PubMed] [Google Scholar]
- 22.Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG, Hamilton L. Persistent osteopenia in ballet dancers with amenorrhea and delayed menarche despite hormone therapy: a longitudinal study. Fertil Steril. 2003;80:398–404. [DOI] [PubMed] [Google Scholar]
- 23.De Cree C, Lewin R, Ostyn M. Suitability of cyproterone acetate in the treatment of osteoporosis associated with athletic amenorrhea. Int J Sports Med. 1988;9:187–192. [DOI] [PubMed] [Google Scholar]
- 24.Hergenroeder AC, Smith EO, Shypailo R, Jones LA, Klish WJ, Ellis K. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months. Am J Obstet Gynecol. 1997;176(5):1017–1025. [DOI] [PubMed] [Google Scholar]
- 25.Cumming DC. Exercise-associated amenorrhea, low bone density, and estrogen replacement therapy. Arch Intern Med. 1996;156:2193–2195. [PubMed] [Google Scholar]
- 26.Rickenlund A, Carlstrom K, Ekblom B, Brismar TB, Von Schoultz B, Hirschberg AL. Effects of oral contraceptives on body composition and physical performance in female athletes. J Clin Endocrinol Metab. 2004;89(9):4364–4370. [DOI] [PubMed] [Google Scholar]
- 27.Sowinska-Przepiera E, Andrysiak-Mamos E, Syrenicz J, Jarzabek-Bielecka G, Friebe Z, Syrenicz A. Polymorphism of the vitamin D3 receptor gene and bone mineral density in girls with functional hypothalamic amenorrhea subjected to oestroprogestagen treatment. Endokrynol Pol. 2011;62(6):492–498. [PubMed] [Google Scholar]
- 28.Sowinska-Przepiera E, Syrenicz A, Friebe Z, Jarzabek-Bielecka G, Chelstowski K. PvuII and XbaI polymorphisms of estrogen receptor-alpha and the results of estroprogestagen therapy in girls with functional hypothalamic amenorrhea - Preliminary study. Arch Med Sci. 2012;8(5):841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren MP, Miller KK, Olson WH, Grinspoon SK, Friedman AJ. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in women with hypothalamic amenorrhea and osteopenia: an open-label extension of a double-blind, placebo-controlled study. Contraception. 2005;72:206–211. [DOI] [PubMed] [Google Scholar]
- 30.Nakahara T, Nagai N, Tanaka M, Muranaga T, Kojima S, Nozoe S, Naruo T. The effects of bone therapy on tibial bone loss in young women with anorexia nervosa. Int J Eat Disord. 2006;39:20–26. [DOI] [PubMed] [Google Scholar]
- 31.Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, Hertz S, Shenker IR. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:3179–3185. [DOI] [PubMed] [Google Scholar]
- 32.Miller KK, Grieco KA, Mulder J, Grinspoon S, Mickley D, Yehezkel R, Herzog DB, Klibanski A. Effects of risedronate on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2004;89:3903–3906. [DOI] [PubMed] [Google Scholar]
- 33.Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, Herzog D, Klibanski A. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. [DOI] [PubMed] [Google Scholar]
- 35.Murad MH, Montori VM, Ioannidis JP, Jaeschke R, Devereaux PJ, Prasad K, Neumann I, Carrasco-Labra A, Agoritsas T, Hatala R, Meade MO, Wyer P, Cook DJ, Guyatt G. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA. 2014;312:171–179. [DOI] [PubMed] [Google Scholar]
- 36.Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, Goldstein MA, Ebrahimi S, Clauss L, Weigel T, Mickley D, Schoenfeld DA, Herzog DB, Klibanski A. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]