Abstract
Known germline gene abnormalities cause one-fifth of the pituitary adenomas in children and adolescents, but, in contrast with other pituitary tumor types, the genetic causes of corticotropinomas are largely unknown. In this study, we report a case of Cushing disease (CD) due to a loss-of-function mutation in PRKAR1A, providing evidence for association of this gene with a corticotropinoma. A 15-year-old male presenting with hypercortisolemia was diagnosed with CD. Remission was achieved after surgical resection of a corticotropin (ACTH)-producing pituitary microadenoma, but recurrence 3 years later prompted reoperation and radiotherapy. Five years after the original diagnosis, the patient developed ACTH-independent Cushing syndrome, and a diagnosis of primary pigmented nodular adrenocortical disease was confirmed. A PRKAR1A mutation (c.671delG, p.G225Afs*16) was detected in a germline DNA sample from the patient, which displayed loss of heterozygosity in the corticotropinoma. No other germline or somatic mutations of interest were found. As corticotropinomas are not a known component of Carney complex (CNC), we performed loss of heterozygosity and messenger RNA stability studies in the patient’s tissues, and analyzed the effect of Prkar1a silencing on AtT-20/D16v-F2 mouse corticotropinoma cells. No PRKAR1A defects were found among 97 other pediatric CD patients studied. Our clinical case and experimental data support a role for PRKAR1A in the pathogenesis of a corticotroph cell tumor. This is a molecularly confirmed report of a corticotropinoma presenting in association with CNC. We conclude that germline PRKAR1A mutations are a novel, albeit apparently infrequent, cause of CD.
Keywords: Cushing disease, genetics, pituitary tumor, protein kinase A, Carney complex
We report an association between a PRKAR1A loss-of-function mutation and a corticotropinoma, in a patient with Carney complex.
Loss-of-function germline mutations of the PRKAR1A gene are the main genetic cause of primary pigmented nodular adrenocortical disease (PPNAD) and Carney complex (CNC) [1]. Pituitary disease is not a rare finding in CNC, but, although the majority of patients develop subclinical hypersomatotropinemia and/or hyperprolactinemia, only few develop tumors that require surgery; clinically relevant hyperprolactinemia is infrequent [2, 3]. No other types of pituitary adenomas have been detected to date in this context. We present a case of Cushing disease (CD) subsequently followed by corticotropin (ACTH)-independent Cushing syndrome in a patient carrying an inactivating PRKAR1A germline mutation, providing evidence for the role of this gene in corticotroph tumorigenesis.
1. Case Report
A 15.3-year-old African-American male presented with a 6-year history of progressive growth deceleration [height: 136.7 cm/−3.9 standard deviation (SD)] and weight gain (weight: 92.2 kg/+2.23 SD, body mass index 49.3 kg/m2/+2.99 SD). He had a history of nephrolithiasis diagnosed 18 months before. In the year before his admission, he developed striae; hyperpigmentation of the upper torso, arms, and face; excessive corporal hair; easy bruising; and headaches. During his initial workup, he was diagnosed with hypertension, central hypothyroidism, osteopenia, multiple vertebral compression fractures, bilateral avascular hip necrosis, and retroperitoneal and intraspinal lipomatosis. Sexual development was adequate for his age (Tanner V for pubic hair and genitalia).
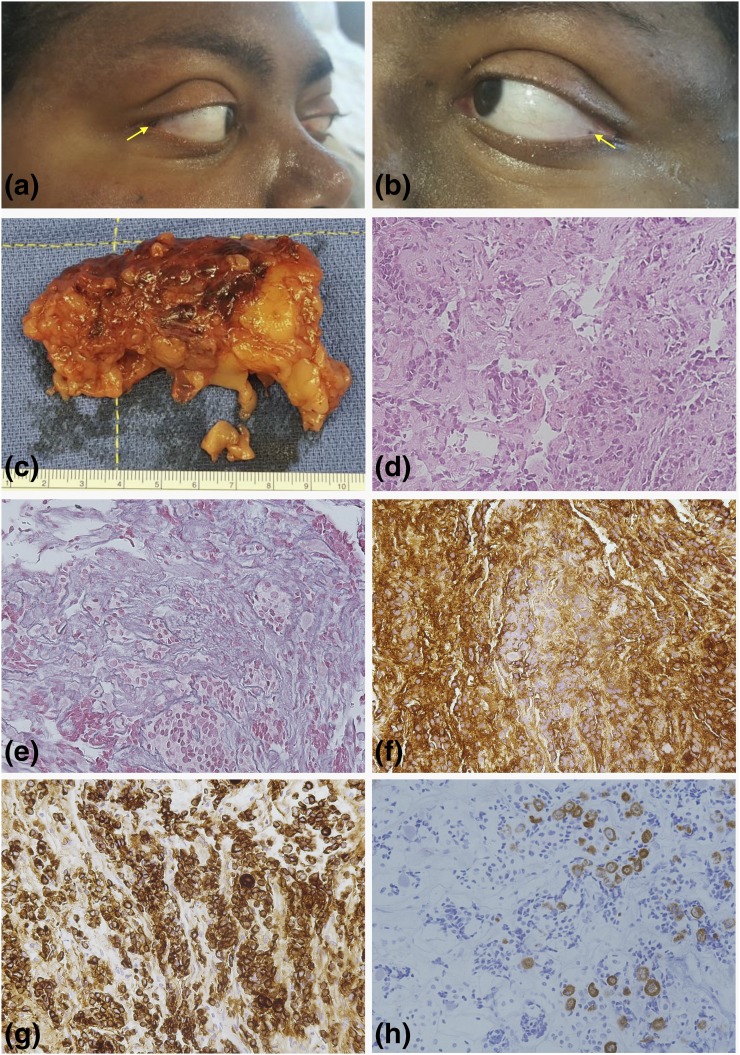
A diagnosis of ACTH-dependent hypercortisolemia was established on the basis of elevated midnight serum cortisol (27.5 μg/dL), 24-hour urinary free cortisol (306.8 μg/24 h), and ACTH (53.05 ng/mL) levels. This was confirmed by the response to CRH stimulation, although the patient failed to suppress to the high-dose dexamethasone test. Additional results are included in Table 1. No lesion was identified in the pituitary magnetic resonance imaging (MRI), but bilateral inferior petrosal sinus sampling demonstrated a high central-to-peripheral ACTH ratio, compatible with CD. The patient underwent transsphenoidal surgical exploration and resection of a pituitary microadenoma; the pathology report confirmed a corticotropinoma (Fig. 1). Remission was achieved, and, after discharge on glucocorticoid replacement therapy, the patient experienced substantial improvement of his symptoms. Recurrence of hypercortisolemia with a possible pituitary lesion by MRI prompted surgical reintervention 3 years later, but no adenomatous tissue was identified. Due to persistent hypercortisolemia, the patient was treated with radiotherapy and placed on ketoconazole and appeared in remission. He was lost to follow-up for 2 years; during that time his disease progressed, causing uncontrolled hypertension, headaches, and weight gain, as well as further complications (hypokalemia, nocturnal orthopnea, and urinary and fecal incontinence). A new diagnostic workup ruled out recurrent CD, but identified ACTH-independent hypercortisolemia with multiple small nodular bilateral adrenal lesions. Genetic testing identified a frameshift variant in the PRKAR1A gene. Careful clinical examination revealed numerous lentigines on the face, oral mucosa, and bulbar conjunctive [Fig. 1(a) and 1(b)], and calcifications compatible with large-cell calcifying Sertoli cell tumors by ultrasonography. No myxomas were identified in the echocardiogram or cardiac MRI. The patient underwent bilateral adrenalectomy, and PPNAD was confirmed [Fig. 1(c)]. Because CD is not a known component of CNC, we investigated a possible role for PRKAR1A loss-of-function in corticotroph cell tumorigenesis.
Table 1.
Additional Hormonal Measurements at Presentation
| Basal Hormones | ||
|---|---|---|
| Parameter | Result | Reference |
| Insulin | 15.4 μU/mL | 6–27 |
| TSH | 0.16 µU/mL | 0.4–4 |
| Free T4 | 0.9 ng/dL | 0.8–1.5 |
| T4 | 5.1 µg/dL | 4.5–12.5 |
| T3 | 88 ng/dL | 90–215 |
| FSH | <0.1 U/L | 1–11 |
| LH | 0.2 U/L | 1–8 |
| Free testosterone | 1.5 ng/dL | 7.4–22.6 |
| Androstendione | 186 ng/dL | 65–210 for Tanner V |
| Dehydroepiandrosterone | 2 ng/mL | <6.6 |
| Deydroepiandrosterone sulfate | 1.75 ng/dL | 0.8–5.6 |
| IGF1 | 120 ng/dL | 171–814 for Tanner V |
| Midnight salivary cortisol | 0.54 µg/dL | 0.01–0.09 |
| Dynamic Tests | ||
| 8 mg Dexamethasone Suppression Test | ||
| Time Point | Parameter | Result |
| Basal | Cortisol | 29.3 µg/dL |
| Final | 31.9 µg/dL | |
| CRH Stimulation Test | ||
| Time Point | Parameter | Result |
| −5 min | ACTH | 45.7 pg/mL |
| Cortisol | 28.8 µg/dL | |
| 0 | ACTH | 51.7 pg/mL |
| Cortisol | 28.2 µg/dL | |
| 15 min | ACTH | 58.5 pg/mL |
| Cortisol | 33.8 µg/dL | |
| 30 min | ACTH | 66.5 pg/mL |
| Cortisol | 30.6 µg/dL | |
| 40 min | ACTH | 66.6 pg/mL |
| Cortisol | 27.9 µg/dL | |
| Bilateral Inferior Petrosal Sinus Sampling | ||
| Time Point | Parameter | Result |
| −5 min | ACTH, RPV | 40.7 pg/mL |
| ACTH, LPV | 4446 pg/mL | |
| ACTH, peripheral | 38.8 pg/mL | |
| 0 | ACTH, RPV | 34.9 pg/mL |
| ACTH, LPV | 3545 pg/mL | |
| ACTH, peripheral | 35.7 pg/mL | |
| 3 min | ACTH, RPV | 476 pg/mL |
| ACTH, LPV | 2958 pg/mL | |
| ACTH, peripheral | 35.5 pg/mL | |
| 5 min | ACTH, RPV | 300 pg/mL |
| ACTH, LPV | 2737 pg/mL | |
| ACTH, peripheral | 33.3 pg/mL | |
| 10 min | ACTH, RPV | 359 pg/mL |
| ACTH, LPV | 2905 pg/mL | |
| ACTH, peripheral | 31.8 pg/mL | |
Abbreviations: FSH, follicle-stimulating hormone; IGF1, insulin-like growth factor1; LH, luteinizing hormone; LVP, left petrosal vein; RPV, right petrosal vein; TSH, thyrotropin.
Figure 1.
Clinical and histopathological presentation. (a and b) Small epicanthal lentigines were observed in this patient. (c) The surgical specimens of bilateral adrenalectomy displayed the characteristics of PPNAD, and such diagnosis was later confirmed by histopathological examination. (d) Hematoxylin–eosin staining (20×) of the corticotropinoma tissue. The tumor was a microadenoma measuring approximately 6 × 4 × 2 mm, with Crooke’s cells surrounding the neoplastic tissue. (e) Breakdown of the reticulin network (20×), (f) as well as strong and diffusely positive ACTH staining (20×), was demonstrated. (g) Extensive positive immunostaining for CAM5.2 was identified (20×). (h) Keratin 20 immunostaining was found in some areas containing Crooke’s cells (20×). These images were compatible with a diagnosis of Crooke’s cell adenoma.
2. Materials and Methods
Materials and Methods are presented as Supplemental Material (114.5KB, docx) .
3. Results
A. A PRKAR1A Variant Is Associated With a Corticotropinoma in the Setting of CNC
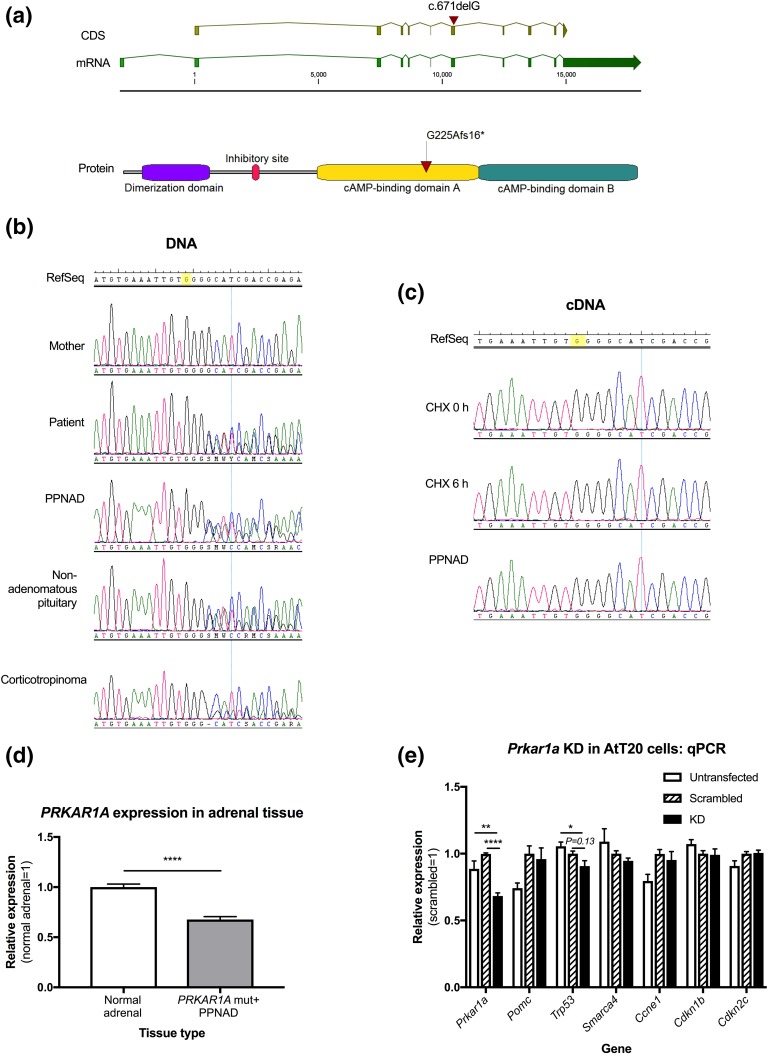
Genetic testing demonstrated a heterozygous frameshift variant of the PRKAR1A gene (c.671delG, p.G225Afs*16), not previously reported in the public databases or in CNC and/or PPNAD [Fig. 2(a)]. No other variants of interest in PRKAR1A were identified among 97 other pediatric CD patients screened. Mutations in known pituitary disease-causative genes (AIP, MEN1, CDKN1B, GPR101) and in selected genes involved in the pituitary–adrenal axis (POMC, GR, MC2R, MC3R, BRG1, CRH, CRHR1, and CRHR2) were ruled out by manual check of whole-exome sequencing raw data. Previous Sanger sequencing and deletion testing had excluded AIP and MEN1 gene defects in this and additional patients with CD [4].
Figure 2.
Role of PRKAR1A in corticotroph cell tumorigenesis, and in PPNAD. (a) The frameshift PRKAR1A gene (NG_007093.3) variant c.671delG, p.G225Afs*16 affects the exon 7 of the reference transcript (NM_002734.4, the first exon is not translated). The surrounding region encodes the first of two cyclic adenosine monophosphate–binding domains in the protein, which are crucial for its regulatory function. (b) The germline mutation identified in the patient was not present in the mother. As a sample from the father was not available, we could not determine whether the mutation was inherited from the father or if it appeared as a de novo event. The PPNAD and nonadenomatous pituitary (obtained from the second surgery) tissues were heterozygous for such mutation. However, loss of heterozygosity was identified in the corticotropinoma tissue, with a 72% to 82% predominance of mutant DNA in the chromatogram peaks measured. (c) In samples from lymphoblastoid cells before and after the treatment with cycloheximide and in the PPNAD tissue, only the wild-type allele was detected. Given that these tissues did not display loss of heterozygosity at the DNA level, the homozygosity for the wild-type allele should be explained by nonsense-mediated messenger RNA decay. Unfortunately, we did not achieve rescue of the mutant allele during the cycloheximide experiment performed. (d) Compared with a normal adrenal tissue sample, the PPNAD specimen displayed significantly reduced Prkar1a expression (mean: 1 ± 0.02 vs 0.68 ± 0.01, P < 0.0001). (e) We achieved 30% Prkar1a KD compared with the scrambled control (mean: 0.68 ± 0.02 vs 1 ± 0.01, P < 0.0001 KD). Compared with the untransfected cells, Trp53 expression was reduced in the KD experiment (mean: 1.05 ± 0.03 vs 0.91 ± 0.1, P = 0.0130), and there was a trend for lower Trp53 in the KD cells compared with the scrambled control (mean: 1 ± 0.05 vs 0.91 ± 0.1, P = 0.13). No other significant differences in the expression of cell cycle markers were found among experimental conditions.
B. Loss-of-Function of PRKAR1A p.G225Afs*16 Is Due to Messenger RNA Instability
Compared with the peripheral blood-extracted DNA, the corticotropinoma presented loss of heterozygosity at PRKAR1A, with clear predominance of the mutant allele [Fig. 2(b)]. At the messenger RNA level, only the wild-type allele was detected in the PPNAD tissue and lymphoblastoid cells, supporting messenger RNA instability, and cycloheximide treatment was insufficient to rescue the expression of the mutant allele [Fig. 2(c)]. PRKAR1A expression was significantly reduced in the PPNAD tissue, compared with normal adrenal [Fig. 2(d)]. As expected from other PRKAR1A mutations in CNC, our data support nonsense-mediated RNA decay as the mechanism for loss-of-function.
C. Effects of Prkar1a Gene Silencing on Corticotroph Cell Function and Proliferation
Under 30% Prkar1a knockdown (KD), Pomc expression was increased in both the scrambled control and the KD experiment; only the former reached statistical significance [Fig. 2(e)]. A trend for lower Trp53 expression was found in the KD compared with the scrambled control. No significant changes were observed in the expression of other markers of cell cycle progression.
4. Discussion
Since its first description by Carney and collaborators in 1985, ∼750 cases of CNC have been reported [5, 6]. This infrequent, autosomal dominant syndrome (Mendelian Inheritance in Man: 160980 and 605244) of multiple endocrine neoplasia and cardiocutaneous manifestations is caused by inactivating mutations in the PRKAR1A gene (17q24.2) in 73% of the cases, and by deletions of the 17q24.2-q24.3 region in 6% of the patients [1, 7]. A triplication of the PRKACB gene at the somatic level was identified as the cause of disease in a single patient, whereas other cases are linked to an uncharacterized defect in 2p16 [8, 9]. More than half of the cases display familial presentation, with almost full penetrance [10]. No germline or somatic PRKAR1A mutations have been identified in sporadic pituitary adenomas [4, 11, 12].
Pituitary disease in CNC consists of single or multiple somatotroph or mammosomatotroph adenomas, occasionally surrounded by areas of hyperplasia [13–15]. However, a number of such adenomas in patients with CNC are histologically pleomorphic [16, 17], and mice with Prkar1a and Rb1 haploinsufficiency develop intermediate lobe tumors [18]. Thus, although Prkar1a complete deficiency in mouse growth hormone-releasing hormone receptor–expressing pituitary cells undoubtedly leads to tumors expressing growth hormone, prolactin, and thyrotropin [19, 20], the data point to the possibility of PRKAR1A deficiency predisposing to other pituitary tumors as well.
A single patient with CNC possibly having CD has previously been reported [21]. She was first seen at the age of 3 years with Cushing syndrome and high ACTH levels, although a pituitary tumor was never proven. She was treated with metyrapone and mitotane and eventually cured of her disease. Twenty-three years later, she was reported as a patient with CNC with apparently normal circadian rhythm and cortisol secretion, although biochemical data were not presented; the patient had the most commonly identified germline PRKAR1A mutation (c.491_492delTG, p.V164Dfs*5) [1, 21].
The consequence of loss-of-function of the PRKAR1A gene is deregulated activation of the cyclic adenosine monophosphate pathway due to uncontrolled catalytic subunit activity [6, 22]. This is true for all PRKAR1A mutations causing CNC, even those that are expressed [3, 10, 23, 24]. The mutation described in this patient appears to act the same way, and loss of heterozygosity in the pituitary tumor further strengthens its causative association.
In conclusion, we describe the association between a new PRKAR1A-inactivating mutation and a corticotropinoma in a patient with CNC. This is a documented case with a clinical–genetic association. No other PRKAR1A defects were found in a large cohort of patients with CD screened previously [4] and now. Germline PRKAR1A mutations are a notable, although infrequent, cause of CD may now be included among the genetic defects that predispose to CD, albeit rarely.
Acknowledgments
Acknowledgments
This work was supported by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health.
Clinical trial registry: ClinicalTrials.gov no. NCT00001595 (registered 3 November 1999).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CD
- Cushing disease
- CNC
- Carney complex
- KD
- knockdown
- MRI
- magnetic resonance imaging
- PPNAD
- primary pigmented nodular adrenocortical disease
- SD
- standard deviation.
References and Notes
- 1.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26(1):89–92. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DK, Irvine AD, Handley JM, Walsh MY, Hadden DR, Bingham EA. Carney complex: report of a kindred with predominantly cutaneous manifestations. Br J Dermatol. 1997;136(4):578–582. [PubMed] [Google Scholar]
- 3.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94(6):2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratakis CA, Tichomirowa MA, Boikos S, Azevedo MF, Lodish M, Martari M, Verma S, Daly AF, Raygada M, Keil MF, Papademetriou J, Drori-Herishanu L, Horvath A, Tsang KM, Nesterova M, Franklin S, Vanbellinghen JF, Bours V, Salvatori R, Beckers A. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78(5):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore). 1985;64(4):270–283. [DOI] [PubMed] [Google Scholar]
- 6.Correa R, Salpea P, Stratakis CA. Carney complex: an update. Eur J Endocrinol. 2015;173(4):M85–M97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salpea P, Horvath A, London E, Faucz FR, Vetro A, Levy I, Gourgari E, Dauber A, Holm IA, Morrison PJ, Keil MF, Lyssikatos C, Smith ED, Sanidad MA, Kelly JC, Dai Z, Mowrey P, Forlino A, Zuffardi O, Stratakis CA. Deletions of the PRKAR1A locus at 17q24.2-q24.3 in Carney complex: genotype-phenotype correlations and implications for genetic testing. J Clin Endocrinol Metab. 2014;99(1):E183–E188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratakis CA, Carney JA, Lin JP, Papanicolaou DA, Karl M, Kastner DL, Pras E, Chrousos GP. Carney complex, a familial multiple neoplasia and lentiginosis syndrome: analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest. 1996;97(3):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forlino A, Vetro A, Garavelli L, Ciccone R, London E, Stratakis CA, Zuffardi O. PRKACB and Carney complex. N Engl J Med. 2014;370(11):1065–1067. [DOI] [PubMed] [Google Scholar]
- 10.Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, Libé R, Remmers E, René-Corail F, Faucz FR, Clauser E, Calender A, Bertagna X, Carney JA, Stratakis CA. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 2010;31(4):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaltsas GA, Kola B, Borboli N, Morris DG, Gueorguiev M, Swords FM, Czirják S, Kirschner LS, Stratakis CA, Korbonits M, Grossman AB. Sequence analysis of the PRKAR1A gene in sporadic somatotroph and other pituitary tumours. Clin Endocrinol (Oxf). 2002;57(4):443–448. [DOI] [PubMed] [Google Scholar]
- 12.Sandrini F, Kirschner LS, Bei T, Farmakidis C, Yasufuku-Takano J, Takano K, Prezant TR, Marx SJ, Farrell WE, Clayton RN, Groussin L, Bertherat J, Stratakis CA. PRKAR1A, one of the Carney complex genes, and its locus (17q22-24) are rarely altered in pituitary tumours outside the Carney complex. J Med Genet. 2002;39(12):e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pack SD, Kirschner LS, Pak E, Zhuang Z, Carney JA, Stratakis CA. Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex). J Clin Endocrinol Metab. 2000;85(10):3860–3865. [DOI] [PubMed] [Google Scholar]
- 14.Stergiopoulos SG, Abu-Asab MS, Tsokos M, Stratakis CA. Pituitary pathology in Carney complex patients. Pituitary. 2004;7(2):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonser RR, Mehta GU, Kindzelski BA, Ray-Chaudhury A, Vortmeyer AO, Dickerman R, Oldfield EH. Surgical management of Carney complex-associated pituitary pathology [published online ahead of print August 9, 2016]. Neurosurgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossis I, Voutetakis A, Matyakhina L, Pack S, Abu-Asab M, Bourdeau I, Griffin KJ, Courcoutsakis N, Stergiopoulos S, Batista D, Tsokos M, Stratakis CA. A pleiomorphic GH pituitary adenoma from a Carney complex patient displays universal allelic loss at the protein kinase A regulatory subunit 1A (PRKARIA) locus. J Med Genet. 2004;41(8):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boikos SA, Stratakis CA. Pituitary pathology in patients with Carney complex: growth-hormone producing hyperplasia or tumors and their association with other abnormalities. Pituitary. 2006;9(3):203–209. [DOI] [PubMed] [Google Scholar]
- 18.Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, Cheadle C, Watkins T, Wen F, Starost MF, Bossis I, Nesterova M, Stratakis CA. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/- or Rb1+/- backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet. 2010;19(8):1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Z, Williams-Simons L, Parlow AF, Asa S, Kirschner LS. Pituitary-specific knockout of the Carney complex gene Prkar1a leads to pituitary tumorigenesis. Mol Endocrinol. 2008;22(2):380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschner LS. PRKAR1A and the evolution of pituitary tumors. Mol Cell Endocrinol. 2010;326(1-2):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basson CT, Aretz HT. Case records of the Massachusetts General Hospital: Weekly clinicopathological exercises: Case 11-2002: a 27-year-old woman with two intracardiac masses and a history of endocrinopathy. N Engl J Med. 2002;346(15):1152–1158. [DOI] [PubMed] [Google Scholar]
- 22.Meoli E, Bossis I, Cazabat L, Mavrakis M, Horvath A, Stergiopoulos S, Shiferaw ML, Fumey G, Perlemoine K, Muchow M, Robinson-White A, Weinberg F, Nesterova M, Patronas Y, Groussin L, Bertherat J, Stratakis CA. Protein kinase A effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res. 2008;68(9):3133–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene EL, Horvath AD, Nesterova M, Giatzakis C, Bossis I, Stratakis CA. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat. 2008;29(5):633–639. [DOI] [PubMed] [Google Scholar]
- 24.Patronas Y, Horvath A, Greene E, Tsang K, Bimpaki E, Haran M, Nesterova M, Stratakis CA. In vitro studies of novel PRKAR1A mutants that extend the predicted RIα protein sequence into the 3′-untranslated open reading frame: proteasomal degradation leads to RIα haploinsufficiency and Carney complex. J Clin Endocrinol Metab. 2012;97(3):E496–E502. [DOI] [PMC free article] [PubMed] [Google Scholar]