Abstract
In this work, an electrochemical sensor was fabricated for determination of an anthracycline, doxorubicin (DOX) as a chemotherapy drug in plasma based on multi-walled carbon nanotubes modified platinum electrode (Pt/MWCNTs). DOX was effectively accumulated on the surface of modified electrode and generated a pair of redox peaks at around 0.522 and 0.647 V (vs. Ag/AgCl) in Britton Robinson (B-R) buffer (pH 4.0, 0.1 M). The electrochemical parameters including pH, type of buffer, accumulation time, amount of modifier and scan rate were optimized. Under the optimized conditions, there was a linear correlation between cathodic peak current and concentration of DOX in the range of 0.05–4.0 µg/mL with the detection limit of 0.002 µg/mL. The number of electron transfers (n) and electron transfer-coefficient (α) were estimated as 2.0 and 0.25, respectively. The constructed sensor displayed excellent precision, sensitivity, repeatability and selectivity in the determination of doxorubicin in plasma. Moreover, cyclic voltammetry studies of DOX in the presence of DNA showed an intercalation mechanism with binding constant (Kb) of 1.12×105 L/mol.
Keywords: Doxorubicin, MWCNTs, Electrochemical sensor, Human plasma, Doxorubicin-DNA interaction
1. Introduction
In recent years, particular attention has been devoted to the development of nanomaterials for amplification of signal in electrochemical sensors [1], [2]. Modification of working electrodes with nanomaterials increases electroactive surface area and as a result enhances accumulation and sensitivity. This can be explained in terms of increasing number of binding sites which are employed to characterize a specific chemical analyte [1]. In this context, more attention has recently been paid to the use of carbon nanotubes (CNTs) as promising nanomaterials with unique chemical, mechanical, conductivity, geometrical, and surface characteristics which also make them entirely appropriate for electrochemical detection [2], [3]. Moreover, due to the fact that CNTs have a large surface area and show efficient catalytic activities, they enhance charge transfer reaction and can be used for fabrication of electrochemical sensors [2], [4], [5], [6], [7], [8], [9], [10], [11], [12]. Electrochemical sensors can be modified with CNTs to promote electron-transfer reactions and sensitivity [11], [12], [13].
As an anthracycline and anti-cancer chemotherapy drug, doxorubicin (DOX) is used in the treatment of various forms of sarcoma and cancer, including bladder cancer, breast cancer, leukemia, liver cancer, head and neck cancer, and lung cancer [14]. In the 1960s, anthracyclines were first introduced and used as an antitumoral drug due to their cytostatic impact [15], [16]. Although chemotherapeutic drugs are efficient in the treatment of the diseases, they also show severe side effects such as skin and tissue toxicity [17], [18], [19]. Through the intercalation and inhibition of macromolecular biosynthesis, DOX can interact with DNA. As a result, after using DOX, the function of the enzyme topoisomerase II is inhibited, and DNA is unwound and transcribed. Besides, the planar aromatic chromophore part of the molecule intercalates between two base pairs of DNA [20].
Due to the prevalence cases of cancer in modern world, it is of great importance to take scrutiny and surveillance on chemotherapy medicines. Different anthracyclines such as DOX, idarubicin, and daunorubicin are generally detected using high-performance liquid chromatography with fluorescent detector [21], [22] as well as liquid chromatography coupled with tandom mass spectrometry [23], [24]. Since chromatographic techniques are highly sensitive and selective, long-run sample treatments (e.g., extraction and deproteinization) and skilled staffs are required, electrochemical techniques have been paid more attention to the sensors technology especially for the detection of anthracycline moiety due to their advantages including high sensitivity, good stability, inexpensive instrumentation, and portability for on-site monitoring [25], [26], [27], [28], [29], [30], [31]. Although some previous works on determination of DOX in literature have lower detection limits and higher sensitivity, nevertheless they have major disadvantages such as complicate sensor fabrication [29], long time analysis [27], [30], real sample analysis [25], [26], [27], [30] and exchange media during analysis [27]. Less attention has been paid to the interaction of anthracyclines with DNA upon cyclic voltammetry on the surface of modified electrodes.
In this work, an electrochemical sensor was presented based on multi-walled carbon nanotubes (MWCNTs) modified platinum electrode for determination of DOX in human plasma and study on the interaction of DOX with DNA using cyclic voltammetry. Moreover, diffusion coefficient (Do), transfer coefficient (α), Tafel slope and DNA binding constant were calculated. The proposed sensor was also used to study DNA binding with DOX. To the best of our knowledge, this research is the first to be reported on the electrochemical study of DOX in the presence of DNA to calculate binding constant upon cyclic voltammetry.
2. Experimental
2.1. Chemicals and reagents
All chemicals were of analytical grade and used without further purification. MWCNTs (diameter: 10–20 nm, length: 1–2 µm, purity >95%) were purchased from Sigma-Aldrich Chemicals (USA). DOX was purchased from Pharmacia limited (Italy) and used as received. The stock solution of DOX (200 μg/mL) was prepared by dissolving the required amount of doxorubicin hydrochloride in 10 mL with distilled water. Double stranded DNA (Sigma-Aldrich) was used without further purification and its stock solution was prepared by dissolving an appropriate amount of DNA strands in phosphate buffer (pH 7.4) and stored at 4 °C. The concentration of DNA in stock solution was determined by UV absorption at 260 nm using the molar absorption coefficient of 6600 L/mol cm [20].
2.2. Instruments
All the voltammetric measurements were carried out using Autolab 302 N electrochemical system (Metrohm Co., Ltd., Switzerland). A conventional three-electrode system was employed, including platinum electrode (Pt) modified with MWCNTs film as the working electrode, Ag/AgCl (3.0 M KCl) electrode as a reference electrode and a graphite bare electrode as an auxiliary electrode. A pH meter (Metrohm, model 827) was used for all the pH measurements.
2.3. Modification of Pt electrode with MWCNTs
The bare Pt electrode was pretreated carefully with 0.05 µm alumina slurry on a polishing cloth, rinsed thoroughly with HNO3–H2O (1:1, v/v), and then washed with pure ethanol and double-distilled water, respectively. 10 mg of the untreated MWCNTs was added to a large amount of concentrated nitric acid (assay 68%), and then sonicated for about 4 h. The mixture was filtered and washed with double-distilled water until the filtrate was neutral. The treated MWCNTs was dried in an oven at 50 °C for 2 h. Then 5.0 mg of the treated MWCNTs was sonicated in 10.0 mL N, N-dimethylformamide (DMF) for about 30 min to prepare a homogeneous suspension. The pretreated Pt electrode was coated evenly with 10.0 µL of MWCNTs suspension, and then the solvent was evaporated under an infrared lamp. Before using, the modified electrode was washed repeatedly with double-distilled water to remove the loosely bonded modifier [10].
2.4. Preparation of real samples
Blood samples were collected from healthy volunteers. The informed consent was obtained. Serum samples of volunteers were collected in the morning and frozen at −20 °C until analysis. For preparation of plasma samples, 20 mL of methanol was added to 2 mL of human serum and centrifuged at 4000 rpm for 20 min. The resultant sample was transferred into a 25 mL calibrated flask and completed to the mark with Britton Robinson buffer (B-R buffer, pH 4.0).
2.5. Analytical procedure for determination of DOX
10 mL of a solution containing 0.1 M B-R buffer (pH 4.0) was transferred into an electrochemical cell equipped with Pt/MWCNTs as working electrode. Then the solution was stirred for a period of 80 s under open circuit. Following the preconcentration, stirring was stopped and after equilibrium period of 10 s, cyclic voltammetry was recorded from 1.0 to 0.40 V with a potential scan rate of 0.1 V/s. The peak current was measured and recorded as a blank (Ib). Then, aliquot of a sample solution containing DOX was added into the cell and a cyclic voltammogram was recorded as described at above conditions to determine sample peak current. The peak current was measured and recorded as a sample (Is). The increase of the peak current at the above potential (ΔI) was calculated and considered as an analytical signal. Calibration graph was plotted by correlation between cathodic peak current against DOX concentration.
3. Results and discussions
3.1. Characterization of electrochemical sensor
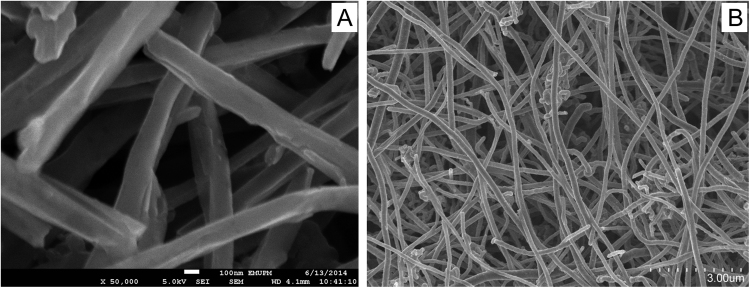
The surface morphology of Pt/MWCNTs was characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Fig. 1 displays some network-like structure homogeneously dispersed on the surface of electrode due to the dispersion of CNTs on the surface of Pt electrode.
Fig. 1.
(A) SEM image of pretreated MWCNTs and (B) TEM image of MWCNTs on Pt electrode after drying in oven at 50 °C.
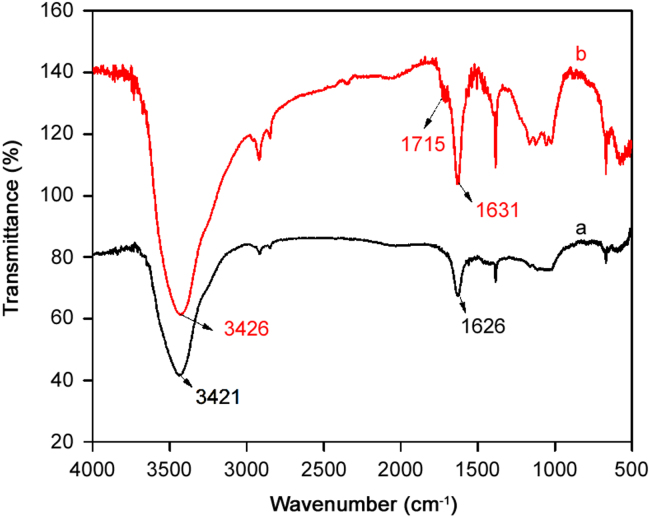
The treated and untreated CNTs were characterized by Fourier transform infrared spectroscopy (FTIR). The spectra of the acid treated CNTs give an absorption peak at 1628.12 cm−1 which was weak in the pristine CNTs before treatment ( Fig. 2). This peak at 1628.12 cm−1 belongs to C=O stretching vibration which arises due to the formation of carboxylic group while the peak at 3433 cm−1 is that of O–H stretching vibration.
Fig. 2.
FTIR spectra of (a) pristine MWCNTs and (b) pretreated MWCNTs with concentrated nitric acid.
The microscopic surface area of modified electrode (Pt/MWCNTs) was calculated by cyclic voltammetry using Randles-Sevcik equation (Eq. (1)) in K3[Fe(CN)]6 (1.0 mM) at different scan rates.
| (1) |
Where Ipa (A) refers to the anodic peak current, n is the electron transfer number, A (cm2) is the surface area of the electrode, DR (cm2/s) is diffusion coefficient, (mol/cm3) is the concentration of K3[Fe(CN)6] and ʋ (V/s) is scan rate. For 1.0 mM of K3[Fe(CN)6] in 0.1 M KCl, n is equal to 1.0 and DR is 7.6×10−6 cm2/s; then from the slope of the Ipa−ʋ1/2 plot, the microscopic surface area was calculated as 0.0258 cm2 while it was 0.0193 cm2 for bare electrode.
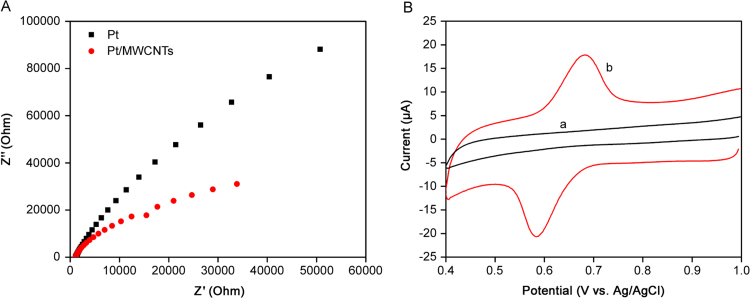
As a complementary study, electrochemical impedance spectroscopy (EIS) analysis was carried out to further investigate on the effect of MWCNTs on the electrochemical performance of electrode, because it is an effective way of assessing the features of surface modified electrodes. The electron transfer resistance (Ret) is an important parameter and it can be measured by the semicircle diameter of the Nyquist plots [32], [33]. The results (Fig. 3A) show that bare Pt electrode exhibits higher Ret, indicating lower conductivity due to sluggish electron transfer properties while the Pt/MWCNTs shows lower Ret, indicating an increase in conductivity which can be attributed to the increase in electron transfer of electrode by MWCNTs.
Fig. 3.
(A) EIS Nyquist plots of bare Pt electrode and Pt/MWCNTs in 1 mM Fe(CN)63−/4− solution containing 0.1 KCl, with frequency of 100 kHz to 0.1 Hz and amplitude of 0.01 V. (B) Cyclic voltammogram of 2.0 µg/mL doxorubicin on (a) bare Pt electrode and (b) Pt/MWCNTs in 0.1 M B-R buffer (pH 4.0) at scan rate of 0.1 V/s.
3.2. Electrochemical activity of DOX on Pt/MWCNTs
Cyclic voltammetric responses of DOX were recorded on Pt/MWCNTs electrode in 0.1 M B-R buffer (pH 4.0) and compared with bare electrode. DOX has no redox peak on bare Pt electrode while a pair of redox peaks appeared in the range of 0.5–0.8 V (vs. Ag/AgCl) on the Pt/MWCNTs (Fig. 3B). MWCNTs as a modifier enhance the electron-transfer rate and surface area due to their high conductivity and nanostructured properties.
3.3. Optimization of parameters
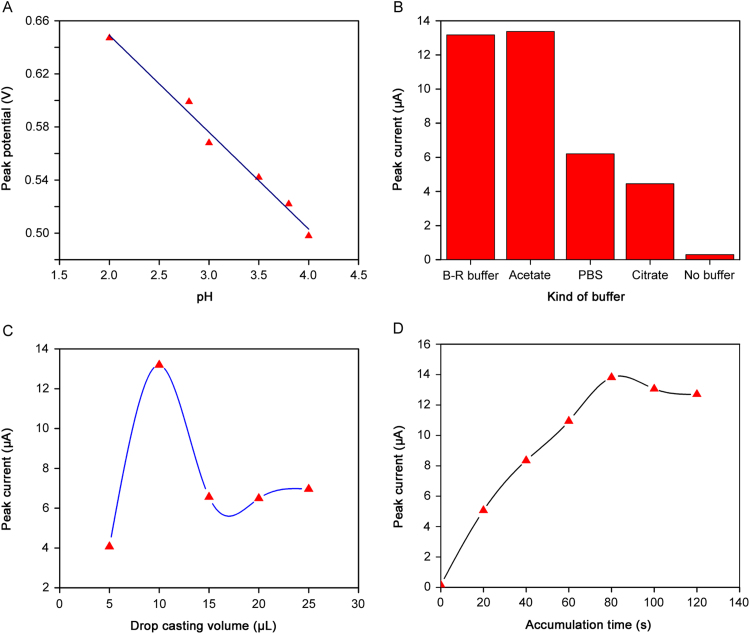
The effect of pH on the reduction peak current of DOX was studied in the presence of B-R buffer (0.1 M) in the range of 2.0–9.0. The cathodic peak current was increased from pH 2.0 to pH 4.0 and then decreased up to pH 9.0. No peak current was observed at higher pH values due to the electrostatic repulsion between CNTs and DOX with negative charges. The relationship between cathodic peak potential and pH values was Epc=−0.0704 pH+0.809 (r=0.985) (Fig. 4A) in the pH range of 2.0–4.0. The slope of this equation is close to the nernestian value of −0.059 V/pH, indicating that the number of electrons and protons transferred were equal in the electrochemical reaction. As a result, pH 4.0 was chosen as the appropriate pH for determination of DOX.
Fig. 4.
(A) The relationship between cathodic peak potential and pH value in 0.1 M B-R buffer in the pH range of 2–4 at scan rate of 0.1 V/s. (B) The effect of kind of buffer on the reduction peak current of 2.0 µg/mL doxorubicin in the presence of 0.1 M of different buffers at pH 4.0. (C) The effect of drop casting volume of MWCNTs on the reduction peak current of doxorubicin in B-R buffer (pH 4.0) at scan rate of 0.1 V/s. (D) Effect of accumulation time on the cyclic voltammogram cathodic peak currents of 2.0 µg/mL doxorubicin in B-R buffer (pH 4.0) solution at scan rate of 0.1 V/s.
The effect of kind of buffer on the reduction peak current of 2.0 μg/mL DOX was investigated in different types of buffer at pH 4.0. As can be seen in Fig. 4B, the cathodic peak current in B-R buffer and acetate buffer is the same; therefore, B-R buffer was selected as supporting electrolyte due to higher buffer capacity.
The amount of MWCNTs on Pt surface can enhance the sensitivity of fabricated sensor towards DOX. Fig. 4C shows that by increasing the volume of MWCNTs up to 10 µL, the peak height increased but at volumes more than 10 µL, the reduction peak current decreased due to the increase of thickness. Hence, 10 µL was chosen as the optimal volume of MWCNTs for fabrication of DOX electrochemical sensor. The effect of accumulation time on the cathodic peak current of DOX was studied under open circuit at pH 4.0. Increasing the accumulation time led to an increase in cathodic peak current up to 80 s and then leveled off due to the saturation of the electrode surface (Fig. 4D). Therefore, the solution of DOX was stirred in open circuit for 80 s before each analysis to enhance the sensitivity.
3.4. Tafel plot and chronoamperometry studies
The effect of scan rate on the electrochemical reduction of 2 µg/mL DOX was investigated in the range of 0.01−0.2 V/s in B-R buffer (0.1 M, pH 4.0). The peak current varied linearly with scan rate (ʋ) with the regression equation of Ipc=169.51ʋ−1.3294 (Ipc: µA, ʋ: V/s, r=0.9943), indicating that the electron transfer reaction of DOX on the Pt/MWCNTs was under adsorption controlled process [34]. The redox peak potentials shifted with increase of scan rate and also the peak-to-peak separation became larger, which indicated that the redox reaction gradually extended to more irreversibility. Therefore, scan rate of 0.1 V/s was selected for further studies.
According to Eq. (2) [35], [36], the slope of Ipc−ʋ plot is proportional to n. Where A (cm2) is surface area of the electrode, is the surface concentration and Q (C) is the quantity of charge. The value of Q was found to be 11.69 C as the average of integrated voltammograms at different scan rates. Finally, the number of electron transferred was determined as ≈2.0 based on the slope of Ipc−ν.
| (2) |
Moreover, Tafel equation (Eqs. (3), (4)) [37] was used for calculation of electron transfer coefficient (α) where i0 (A), η (V) and n refer to exchange current, over potential and number of electron transfers in rate determining step, respectively. According to the slope of Tafel plot for cathodic branch, (equal to −9.9835), α was estimated as 0.25 (if n=2).
| (3) |
| (4) |
The electrochemical reaction of DOX was proposed based on the reduction/oxidation of quinone/hydroquinone moiety [30]. In buffered aqueous media quinone-hydroquinone couples provide familiar single step two-electron redox systems in which peak potentials vary with pH in a straightforward Nernstian manner. This behavior was conveniently summarized in Ep−pH study. It was shown that in aqueous buffer, at acidic, neutral and alkaline pH, anthracyclines, anthraquinones, and other para-quinones are reduced by two electrons generating a pair of peaks in cyclic voltammetry. At acidic pH the reduction is a single step two-electron and two-proton process [28], [37], [38], [39].
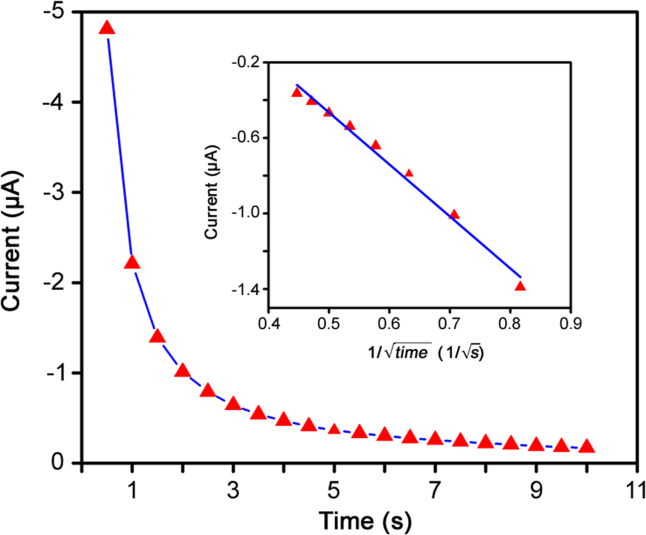
The amperometric response of Pt/MWCNTs in 100 μg/mL DOX at potential of 0.5 V in B-R buffer (pH 4.0) was studied to determine the diffusion coefficient (Do) of DOX (Fig. 5). Based on Cottrell equation (5) [34], the slope of the Ip versus t−1/2 plot (Fig. 5, inset) was 2.75×10−6, so diffusion coefficient (Do) for DOX was calculated as 2.60×10−5 cm2/s. Based on the results reported by Kaowumpai et al. [40] on development of a 3D mathematical model for a DOX controlled released system, the diffusion coefficient was predicted as 2.7×10−1 cm2/s in breast tissue using a sigmoid function.
| (5) |
Fig. 5.
Amperometry study of doxorubicin (100 μg/mL) in 0.1 M B-R buffer (pH 4.0) at applied potential of 0.5 V. Inset: Cottrell plot in the interval time from 1.5 s to 5.0 s.
3.5. Evaluation of the method
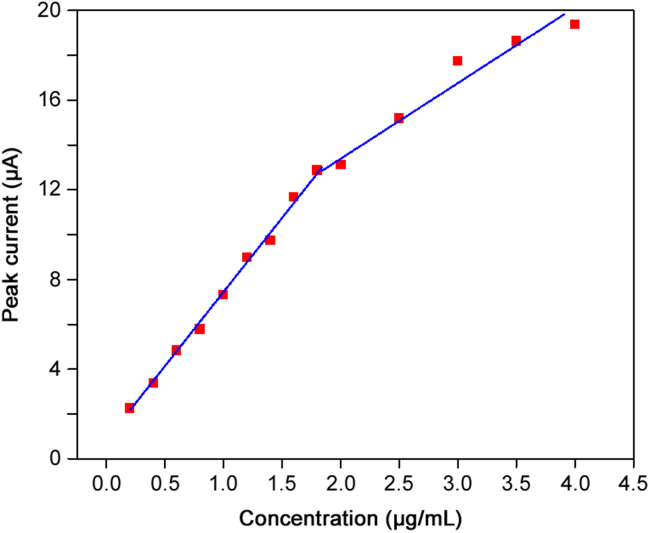
In order to study the feasibility of the fabricated sensor for quantitative analysis of DOX, the relationship between the cathodic peak current and the concentration of DOX was studied using cyclic voltammetry. Under the optimum conditions, the calibration curve (Fig. 6) showed two linear ranges including 0.2–2.0 and 2.0–4.0 μg/mL with equations of Ipc(μA)=6.43 C (μg/mL)+0.9316 (R=0.9924) and Ipc(μA)=3.20 C (μg/mL)+7.2362 (R=0.9416), respectively. The activated surface area for the modified electrode deceased due to the adsorption of DOX during accumulation step. As a result, the sensitivity decreased at higher DOX concentrations. The detection limit (3Sb/m) was found as 0.002 μg/mL.
Fig. 6.
Calibration curve for doxorubicin under optimized conditions in the concentration range of 0.2–2.0 and 2.0–4.0 μg/mL. Conditions: 0.1 M B-R buffer (pH 4.0); accumulation time, 80 s; scan rate, 0.1 V/s.
As is shown in Table 1, the analytical terms of the fabricated sensor such as limit of determination (LOD), accuracy and linear calibration range are better than or comparable with those of some previous reports in the literature for electrochemical analysis of DOX. Through some works on the determination of DOX in biological samples, the results show that the modified electrode (Pt/MWCNTs) can be used for determination of DOX without any extraction steps. In most cases, the resulting LODs were significantly higher than that of the proposed sensor. The electrochemical DNA sensor based on electropolymerization of Neutral Red on polycarboxylated thiacax [4] aren for determination of DOX [29] could be used for sensitive determination of DOX with LOD value of 0.1 nM. However, the fabrication process is too complicated and time consuming. Moreover, it can be stored for a short time with limited working range due to instability of DNA. Although dynamic linearity range of the proposed sensor is not as wide as the previous work [29], LOQ is capable of covering the therapeutic purposes. Herein, the fabricated sensor is rapid, simple, inexpensive, and sensitive enough for determination of DOX in spiked human plasma and can be compared in terms of reproducibility, applicability, fundamental electrochemical study (diffusion coefficient, transfer coefficient and Tafel plot) and DNA binding interaction.
Table 1.
Literature review on the electrochemical study of doxorubicin.
| Electrode | Modifier | Method | Linear range (μ M) | LODa (μM) | Application | Ref. |
|---|---|---|---|---|---|---|
| GCEb | MWCNTs/AgNPsc | CV, DPV | 0.0008–0.019 | 0.002 | – | [25] |
| GCE | PSd/Fe3O4-GO-SO3H | DPV | 0.043–3.5 | 0.0049 | Plasma | [26] |
| CPEe | Carbon paste | DPV | 0.1–10 | 0.01 | Urine | [27] |
| CPE | No modifier | CV, DPV | 0.01–100 | – | – | [28] |
| GCE | DNA sensorf | DPV, Impedimetric | 0.001–100 | 0.0001 | Drugs, artificial plasma | [29] |
| HMDEg | No modifier | SWVh | 0.5–10 | 0.1 | – | [30] |
| GCE | GODi | CV, DPV | 0.02–3.6 | 0.016 | Plasma | [31] |
| Pt | MWCNTs | CV | 0.09–7.36 | 0.003 | Plasma | This work |
Limit of detection.
Glassy carbon electrode.
Silver nanoparticles/Multi-walled carbon nanotubes.
Polystyrene.
Carbon paste electrode.
Electropolymerized Neutral Red/polycarboxylated thiacax[4]aren/DNA.
Hanging mercury drop electrode.
Square wave voltammetry.
Graphene quantum dots.
The reproducibility of the constructed sensor was studied under the optimum conditions by recording the reduction peak current of a sample containing DOX at 2.0 μg/mL. The relative standard deviation (RSD) for six measurements was 2.59%. After each measurement, the constructed sensor (Pt/MWCNTs) was polished with 0.05 mm alumina slurry on a polishing cloth, rinsed thoroughly with HNO3–H2O (1:1, v/v), and then washed with pure ethanol and redistilled water (each for 1 min). Finally, the MWCNTs suspension (10 µL) in DMF was drop casted on Pt electrode and dried in an oven at 50 °C for 2 h. Consequently, the RSD for six successive measurements was 2.16%, indicating good repeatability with the same sensor.
The influence of some common compounds found in plasma such as some ions and drugs on the determination of DOX was studied under the optimum conditions (Table 2). As is shown, all investigated compounds have no serious interference for determination of DOX excluding losartan at 2.5 (%, m/m), indicating that the constructed sensor is selective for determination of DOX in biological fluids.
Table 2.
The effect of some common compounds on the determination of doxorubicin using fabricated sensor.
| Species | Tolerance limit (%, m/m) |
|---|---|
| Caffeine, ascorbic acid, azithromycin, urea, Mg2+, Na+, Ca2+ | 10 |
| Fe2+, amoxicillin, propranolol, ibuprofen | 5 |
| Losartan | 2.5 |
Conditions: doxorubicin concentration, 2.0 μg/mL, 0.1 mol/L; B-R buffer (pH 4.0), 0.1 M scan rate, 0.1 V/s.
To investigate the applicability of the constructed sensor for practical analysis, some real samples including human plasma were analyzed using standard addition method (Table 3). As it can be seen, the recovery of the spiked DOX is in the range of 91.3%–105%, showing that the fabricated sensor based on Pt/MWCNTs is a reliable sensor for analysis of doxorubicin in blood samples.
Table 3.
Determination of doxorubicin in plasma samples of two healthy volunteers using Pt/MWCNTs (n=3) under optimum conditions.
| Sample | Added concentration (μg/mL) | Found concentration (μg/mL) | Recovery (%) |
|---|---|---|---|
| Plasma (1) | – | <LOD | – |
| 0.2 | 0.21 | 105.0 | |
| 0.4 | 0.39 | 97.5 | |
| Plasma (2) | – | <LOD | – |
| 1.5 | 1.37 | 91.3 | |
| 3.0 | 3.08 | 102.7 |
3.6. DOX-DNA interaction
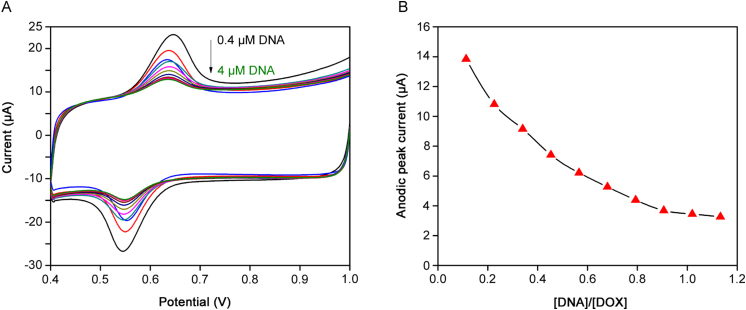
The electrochemical behavior of DOX in the presence of DNA at Pt/MWCNTs was also studied by cyclic voltammetry. The anodic and cathodic peaks appeared at 0.631 and 0.560 V (Ag/AgCl) with a formal potential (E°) of 0.595 V. The redox peak currents for DOX decreased significantly after successive addition of DNA up to 4.16 µM and the reduction peak potential shifted in the negative direction (Fig. 7A). The anodic peak potential shifted to cathode direction indicates that the reduced state of DOX is easier to oxidize in the presence of DNA because its oxidized form is more strongly bound to DNA than its reduced form. The binding of drugs to DNA leads to a significant decrease in peak current due to the formation of drug-DNA adduct with smaller diffusion coefficient. The diffusion coefficient for DOX in the presence of DNA was calculated as 1.09×10−5 cm2/s by chronoamperometry studies [20], [41], [42], [43].
Fig. 7.
(A) Cyclic voltammograms of doxorubicin after successive additions of double stranded DNA. CDNA=0.416, 0.832, 1.248, 1.664, 2.08, 2.496, 2.912, 3.328, 3.744, and 4.16 μM; Cdoxorubicin=2 µg/mL, scan rate=0.1 V/s. (B) The variation of reduction peak current for doxorubicin at different concentrations of DNA.
The mode of interaction between drugs and macromolecules can be well known from the variation in formal potential. In general, the positive shift (anodic shift) in formal potential is caused by the intercalation of the drug with DNA [44], while negative shift is observed for the electrostatic interaction of molecules with DNA [45]. Therefore, this is evident that negative shift in peak potential (cathodic shift) in the CV behavior of DOX by the addition of DNA (E0=0.559 V) is due to the intercalation of DOX with DNA.
Based upon the decrease in DOX peak current by the addition of DNA (Fig. 7B), the binding constant was calculated according to the following equation [46].
| (6) |
where Kb is binding constant, I0 and I are the peak currents of free DOX and DOX–DNA complex, respectively. The plot of log (1/[DNA]) vs. log (1/(I0−I)) was constructed and from the linear fitting, the binding constant (Kb) was estimated as 1.12×105 L/mol. The calculated binding constant is in agreement with the previous reports on the DNA binding studies of DOX and daunorubicin by spectrophotometry [20], [47]. The equilibrium concentration of free DOX in the presence of DNA decreases due to formation of DOX-DNA adduct with smaller diffusion coefficient. This work provides some significant information for clinical research on DOX and the theoretical basis for new drug design.
4. Conclusion
In this work, an electrochemical sensor was fabricated based on modification of Pt electrode with MWCNTs for determination of DOX as a chemotherapy drug. The unique structure of MWCNTs exhibited a suitable electroactive substrate for oxidation/reduction of DOX. The electrochemical parameters including pH, kind of buffer, amounts of deposited MWCNTs and scan rate have been optimized. Under the optimum conditions, there was a good linear relationship between cathodic peak current and concentration of DOX in the range of 0.05–4.0 μg/mL with a detection limit of 0.002 μg/mL. The proposed method displayed excellent characteristics as simplicity, economy, good sensitivity, selectivity, reproducibility and rapid response, so it is recommended as a suitable sensor for analysis of DOX in biological samples.
In preliminary study of interaction with macromolecules using fabricated sensor, it was observed that the presence of DNA in a solution of DOX reduces the equilibrium concentration of free DOX and produces a DOX-DNA adduct with smaller diffusion coefficient due to the intercalation mechanism. This work provides some significant information for clinical research on DOX and the theoretical basis for new drug design.
Acknowledgments
The authors thank gratefully the research council of Gachsaran Branch, Islamic Azad University, Iran for supporting this project under Grant no. 25518.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Padigi S.K., Reddy R.K.K., Prasad S. Carbon nanotube based aliphatic hydrocarbon sensor. Biosens. Bioelectron. 2007;22:829–837. doi: 10.1016/j.bios.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Baughman R.H., Zakhidov A.A., de Heer W.A. Carbon nanotubes – the route toward applications. Science. 2002;297:787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 3.Rao C.N., Satishkumar B.C., Govindaraj A. Nanotubes. Chemphyschem. 2001;2:78–105. doi: 10.1002/1439-7641(20010216)2:2<78::AID-CPHC78>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Ajayan P. Nanotubes from carbon. Chem. Rev. 1999;99:1787–1800. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- 5.Goyal R.N., Gupta V.K., Chatterjee S. Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sens. Actuators B-Chem. 2010;149:252–258. [Google Scholar]
- 6.Hu C., Feng B. Carbon nanotubes used in electroanalysis. Int. J. Mod. Phys. B. 2005;19:603–605. [Google Scholar]
- 7.Rochefort A., Avouris P., Lesage F. Electrical and mechanical properties of distorted carbon nanotubes. Phys. Rev. B. 1999;60:13824. [Google Scholar]
- 8.Sadik O.A., Land W.H., Wang J. Targeting chemical and biological warfare agents at the molecular level. Electroanalysis. 2003;15:1149–1159. [Google Scholar]
- 9.Wu K., Hu J.S. Simultaneous determination of dopamine and serotonin on a glassy carbon electrode coated with a film of carbon nanotubes. Anal. Biochem. 2003;318:100–106. doi: 10.1016/s0003-2697(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 10.Yang S., Yang R., Li G. Nafion/multi-wall carbon nanotubes composite film coated glassy carbon electrode for sensitive determination of caffeine. J. Electroanal. Chem. 2010;639:77–82. [Google Scholar]
- 11.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 12.Keyvanfard M., Ahmadi M., Karimi F. Voltammetric determination of cysteamine at multiwalled carbon nanotubes paste electrode in the presence of isoproterenol as a mediator. Chin. Chem. Lett. 2014;25:1244–1246. [Google Scholar]
- 13.Cesarino I., Galesco H.V., Machado S.A. Determination of serotonin on platinum electrode modified with carbon nanotubes/polypyrrole/silver nanoparticles nanohybrid. Mater. Sci. Eng. C. 2014;40:49–54. doi: 10.1016/j.msec.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Brayfield A. Vol. 15. Pharmaceutical Press; London, UK: 2014. Doxorubicin. Martindale: The Complete Drug Reference. [Google Scholar]
- 15.Bonadonna G., Monfardini S., de Lena M. Clinical evaluation of adriamycin, a new antitumour antibiotic. Br. Med. J. 1969;3:503–506. doi: 10.1136/bmj.3.5669.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Marco A., Gaetani M., Scarpinato B. Adriamycin (NSC-123,127): a new antibiotic with antitumor activity. Cancer Chemother. Rep. 1969;53:33–37. [PubMed] [Google Scholar]
- 17.Dantchev D., Balercia G., Bourut C. Comparative microscopic study of cardiotoxicity and skin toxicity of anthracycline analogs. Biomed. Pharmacother. 1984;38:322–328. [PubMed] [Google Scholar]
- 18.Dantchev D., Slioussartchouk V., Paintrand M. Vol. 74. Springer; Berlin, Heidelberg: 1980. Ultrastructural Study of the Cardiotoxicity and Light-microscopic Findings of the Skin after Treatment of Golden Hamsters with Seven Different Anthracyclines. Cancer Chemo-and Immunopharmacology; pp. 223–249. [DOI] [PubMed] [Google Scholar]
- 19.Israel M., Modest E.J., Frei E. N-Trifluoroacetyladriamycin-14-valerate, an analog with greater experimental antitumor activity and less toxicity than adriamycin. Cancer Res. 1975;35:1365–1368. [PubMed] [Google Scholar]
- 20.Hajian R., Shams N., Mohagheghian M. Study on the interaction between doxorubicin and deoxyribonucleic acid with the use of methylene blue as a probe. J. Braz. Chem. Soc. 2009;20:1399–1405. [Google Scholar]
- 21.Pérez-Blanco J.S., Fernandez de Gatta M. Del M., Hernández-Rivas J.M. Validation and clinical evaluation of a UHPLC method with fluorescence detector for plasma quantification of doxorubicin and doxorubicinol in haematological patients. J. Chromatogr. B. 2014;955–956:93–97. doi: 10.1016/j.jchromb.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Alhareth K., Vauthier C., Gueutin C. HPLC quantification of doxorubicin in plasma and tissues of rats treated with doxorubicin loaded poly (alkylcyanoacrylate) nanoparticles. J. Chromatogr. B. 2012;887–888:128–132. doi: 10.1016/j.jchromb.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Gómez-Canela C., Cortés-Francisco N., Ventura F. Liquid chromatography coupled to tandem mass spectrometry and high resolution mass spectrometry as analytical tools to characterize multi-class cytostatic compounds. J. Chromatogr. A. 2013;1276:78–94. doi: 10.1016/j.chroma.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Maudensa K.E., Stove C.P., Lambert W.E. Quantitative liquid chromatographic analysis of anthracyclines in biological fluids. J. Chromatogr. B. 2011;879:2471–2486. doi: 10.1016/j.jchromb.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K., Zhang Y. Electrochemical behavior of adriamycin at an electrode modified with silver nanoparticles and multi-walled carbon nanotubes, and its application. Microchim. Acta. 2010;169:161–165. [Google Scholar]
- 26.Soleymani J., Hasanzadeh M., shadjou N. A new kinetic-mechanistic approach to elucidate electrooxidation of doxorubicin hydrochloride in unprocessed human fluids using magnetic graphene based nanocomposite modified glassy carbon electrode. Mater. Sci. Eng. C. 2016;61:638–650. doi: 10.1016/j.msec.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Chaney E.N., Baldwin R.P. Electrochemical determination of adriamycin compounds in urine by preconcentration at carbon paste electrodes. Anal. Chem. 1982;54:2556–2560. doi: 10.1021/ac00251a034. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin R.P., Packett D., Woodcock T.M. Electrochemical behavior of adriamycin at carbon paste electrodes. Anal. Chem. 1981;53:540–542. [Google Scholar]
- 29.Evtugyn G., Porfireva A., Stepanova V. Electrochemical biosensors based on native DNA and nanosized mediator for the detection of anthracycline preparations. Electroanalysis. 2015;27:629–637. [Google Scholar]
- 30.Hahn Y., Lee H.Y. Electrochemical behavior and square wave voltammetric determination of doxorubicin hydrochloride. Arch. Pharm. Res. 2004;27:31–34. doi: 10.1007/BF02980041. [DOI] [PubMed] [Google Scholar]
- 31.Hashemzadeh N., Hasanzadeh M., Shadjou N. Graphene quantum dot modified glassy carbon electrode for the determination of doxorubicin hydrochloride in human plasma. J. Pharm. Anal. 2016;6:235–241. doi: 10.1016/j.jpha.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia L., Wang H. Electrochemical reduction synthesis of graphene/Nafion nanocomposite film and its performance on the detection of 8-hydroxy-2′-deoxyguanosine in the presence of uric acid. J. Electroanal. Chem. 2013;705:37–43. [Google Scholar]
- 33.Liu Y., Yin F., Long Y. Study of the immobilization of alcohol dehydrogenase on Au-colloid modified gold electrode by piezoelectric quartz crystal sensor, cyclic voltammetry, and electrochemical impedance techniques. J. Colloid Interface Sci. 2003;258:75–81. doi: 10.1016/s0021-9797(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 34.Bard A.J., Faulkner L.R. John Wiley and Sons; New York: 1980. Electrochemical Methods: Fundamentals and Applications; pp. 617–618. (pp. 267–268) [Google Scholar]
- 35.Laviron E. The use of linear potential sweep voltammetry and of a.c. voltammetry for the study of the surface electrochemical reaction of strongly adsorbed systems and of redox modified electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1979;100:263–270. [Google Scholar]
- 36.Tang Y.Y., Kao C.L., Chen P.Y. Electrochemical detection of hydrazine using a highly sensitive nanoporous gold electrode. Anal. Chim. Acta. 2012;711:32–39. doi: 10.1016/j.aca.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Rao G.M., Lown J.W., Plambeck J.A. Electrochemical studies of antitumor antibiotics III. Daunorubicin and adriamycin. J. Electrochem. Soc. 1978;125:534–539. [Google Scholar]
- 38.Molinier-Jumel C., Malfoy B., Reynaud J.A. Electrochemical study of DNA-anthracyclines interaction. Biochem. Biophys. Res. Commun. 1978;84:441–449. doi: 10.1016/0006-291x(78)90189-4. [DOI] [PubMed] [Google Scholar]
- 39.Guin P.S., Das S., Mandal P.C. Electrochemical reduction of sodium 1, 4-dihydroxy-9, 10-anthraquinone-2-sulphonate in aqueous and aqueous dimethyl formamide mixed solvent: a cyclic voltammetric study. Int. J. Electrochem. Sci. 2008;3:1016–1028. [Google Scholar]
- 40.Kaowumpai W., Koolpiruck D., Viravaidya K. Development of a 3D mathematical model for a doxorubicin controlled release system using pluronic gel for breast cancer treatment. World Acad. Sci. Eng. Technol. 2007;26:287–292. [Google Scholar]
- 41.Aslanoglu M., Ayne G. Voltammetric studies of the interaction of quinacrine with DNA. Anal. Bioanal. Chem. 2004;380:658–663. doi: 10.1007/s00216-004-2797-5. [DOI] [PubMed] [Google Scholar]
- 42.Pang D.W., Abruña H.D. Micromethod for the investigation of the interactions between DNA and redox-active molecules. Anal. Chem. 1998;70:3162–3169. doi: 10.1021/ac980211a. [DOI] [PubMed] [Google Scholar]
- 43.Shehatta I., Ibrahim M. Binding of anti-inflammatory drug indomethacin with cyclodextrin and DNA: solubility, spectroscopic, and voltammetric studies. Can. J. Chem. 2001;79:1431–1438. [Google Scholar]
- 44.Aslanoglu M. Electrochemical and spectroscopic studies of the interaction of proflavine with DNA. Anal. Sci. 2006;22:439–443. doi: 10.2116/analsci.22.439. [DOI] [PubMed] [Google Scholar]
- 45.Li N., Ma Y., Yang C. Interaction of anticancer drug mitoxantrone with DNA analyzed by electrochemical and spectroscopic methods. Biophys. Chem. 2005;116:199–205. doi: 10.1016/j.bpc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Jalali F., Dorraji P.S. Electrochemical and spectroscopic studies of the interaction between the neuroleptic drug, gabapentin, and DNA. J. Pharm. Biomed. Anal. 2012;70:598–601. doi: 10.1016/j.jpba.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Hajian R., Shams N., Parvin A. DNA-binding studies of daunorubicin in the presence of methylene blue by spectroscopy and voltammetry techniques. Chin. J. Chem. 2009;27:1055–1060. [Google Scholar]