Abstract
In this study, we developed and validated a fast, specific, sensitive, precise and stability-indicating high performance liquid chromatography (HPLC) method to determine the drug apocynin in bovine serum albumin (BSA) nanoparticles. Chromatographic analyses were performed on an RP C18 column and using a photodiode array detector at a wavelength of 276 nm. Mobile phase consisted of a mixture of acetonitrile and 1% acetic acid (60:40, v/v), and it was eluted isocratically at a flow rate of 0.8 mL/min. The retention time of apocynin chromatographic peak was 1.65 min. The method was linear, precise, accurate and specific in the range of 5–100 μg/mL. The intra- and inter-day precisions presented relative standard deviation (RSD) values lower than 2%. The method was robust regarding changes in mobile phase proportion, but not for flow rate. Limits of detection and quantitation were 78 ng/mL and 238 ng/mL, respectively. Apocynin was exposed to acid and alkali hydrolysis, oxidation and visible light. The drug suffered mild degradation under acid and oxidation conditions and great degradation under alkali conditions. Light exposure did not degrade the drug. The method was successfully applied to determine the encapsulation efficiency of apocynin in BSA nanoparticles.
Keywords: Apocynin, Nanoparticles, Bovine serum albumin, HPLC
1. Introduction
Apocynin (4-hidroxy-3-methoxyacetophenone) is a methoxy-catechol originally extracted from the roots of Picrorhiza kurroa Royle (Scrophulariaceae) native to the Himalaya [1], [2]. This drug presents potential anti-inflammatory and antioxidant properties [2], [3] and also is widely described as a specific NADPH oxidase inhibitor [4], [5], [6]. The inhibition of NADPH oxidase represents a therapeutic target to prevent various diseases such as cancer [7], [8], [9], atherosclerosis [10], vascular and neurodegenerative diseases [11], [12]. However, apocynin presents pharmacokinetics limitations such as low oral bioavailability and rapid elimination [13], and narrow dose-response relationship [14], thus, limiting its clinical application.
Nanostructured systems have been widely used to improve physicochemical and biological properties of compounds. The in vivo efficacy of nanoparticles as drug carrier systems depends upon particle size, surface properties and release pattern of the drug loaded. The nanoencapsulation of a drug is an interesting strategy to improve biopharmaceutical and pharmacokinetics properties of the drug, leading to an improvement of the biological effects [15], [16], [17], [18].
Nanoparticles can be composed of natural or synthetic polymers [16], [19], [20], [21]. Natural polymers commonly used originate from proteins such as albumin, collagen and gelatin or from polysaccharides such as alginate, chitosan, and dextran [22], [23]. Bovine serum albumin (BSA) has been used for the preparation of nanostructured materials due to its high stability and antigenicity, allowing to obtain nanoparticles with low toxicity and immunogenicity [24], [25], [26].
There are a few works in the literature about apocynin determination and quantification by analytical methods. Wang et al. [27] evaluated the bioavailability of apocynin in plasma, liver and brain tissues using liquid chromatography–mass spectrometry (LC–MS). Trumbull et al. [28] determined accumulation of apocynin and diapocynin in the brain and spinal cord tissue by high performance liquid chromatography (HPLC) with selective ion monitoring by mass spectrometry. Wang et al. [13] reported an HPLC method with ultraviolet (UV) detection for the pharmacokinetics evaluation of apocynin. Recently, Chandasana et al. [29] developed a sensitive and selective method by liquid chromatography–tandem mass spectrometry (LC–MS/MS) to determine apocynin in biological samples. However, no reports are available for determination of apocynin in nanoparticles, especially albumin BSA nanoparticles. The objective of the present study was to develop and validate a simple, fast and optimized HPLC method with photodiode array (PDA) detection to determine apocynin in BSA nanoparticles.
2. Material and methods
2.1. Chemicals and reagents
Apocynin (Acetovanillone 98%) and bovine serum albumin (BSA) (≥98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ethyl alcohol was obtained from FMaia (São Francisco, MG, Brazil). Glutaraldehyde (25%) and acetic acid were from Vetec (Rio de Janeiro, RJ, Brazil). HPLC-grade acetonitrile was from JTBaker (Philippsburg, NJ, USA). For all analyses, ultrapure water was obtained from Millipore Milli-Q Plus apparatus (Millipore, Rio de Janeiro, RJ, Brazil). All solvents and chemicals used were of analytical or HPLC grade.
2.2. Instruments and chromatographic conditions
The analytical method was developed and validated on a Waters 2695 Alliance HPLC apparatus (Milford, MA, USA) with photodiode array detector (Waters 2998-PDA). HPLC system was equipped with a column compartment with temperature control, on-line degasser, quaternary pump, auto sampler and auto injector.
Experiments were carried out on a RP C18 (Merk Millipore®) analytical reverse phase column (125 mm×4 mm, 5 µm), maintained at ±25 °C, with isocratic mode. The mobile phase consisted of a mixture of acetonitrile and water with acetic acid (60:40 , v/v), and was eluted at a flow rate of 0.8 mL/min. After filtering through a 0.22 µm pore size filter (Millipore, Bedford, USA), 20 µL of the sample was automatically injected and analyzed at 276 nm, and run time was about 3 min. The chromatographic data analysis was performed using Empower Chromatography Software (Milford, MA, USA).
2.3. Preparation of standards and samples
A stock solution of apocynin (1000 μg/mL) was prepared in acetonitrile. From this solution, subsequent dilutions were carried out to obtain eight concentration levels ranging from 5.0 to 100.0 μg/mL. The apocynin samples corresponded to supernatant originated after ultracentrifugation of nanoparticles containing apocynin, as described further. Standard samples were obtained from standard apocynin solution spiked in the blank nanoparticles supernatant. Standards and samples were appropriately diluted in acetonitrile to obtain the desired concentration and filtered through a 0.22 µm pore size filter (Millipore, Bedford, USA) prior to injection.
2.4. System suitability
System suitability (number of theoretical plates (N) and the tailing factor (T)) was evaluated by six replicate analyses of standard apocynin solution (50 µg/mL).
2.5. Method validation
HPLC method was validated according to the parameters described by the International Conference on Harmonization (ICH) guidelines [30]. The following characteristics were considered for validation: linearity, specificity, precision (intra-day and inter-day), accuracy, robustness, limit of detection (LOD) and limit of quantification (LOQ).
2.5.1. Linearity
Linearity was determined through the construction of three independent calibration curves using eight apocynin standards (5, 10, 20, 40, 50, 60, 80 and 100 μg/mL). Linear least squares methodology was applied to calculate the calibration equation and correlation coefficient. The statistical analysis to evaluate the linearity and the deviation from linearity was performed by analysis of variance (ANOVA).
2.5.2. Specificity
Specificity was determined by comparing the representative chromatograms of samples obtained from the supernatant of blank nanoparticles (without apocynin) and samples containing apocynin.
2.5.3. Precision and accuracy
For precision, the intra- and inter-day variations for the determination of apocynin were carried out at three different concentration levels of standard samples (10, 50 and 100 μg/mL) and were expressed in terms of the relative standard deviation (% RSD) and standard deviation (SD). Accuracy was tested by calculating the percent recovery (% recovery) of the mean concentration of apocynin in standard samples at three different concentration levels (10, 50 and 100 µg/mL).
2.5.4. Robustness
Robustness was evaluated by deliberately varying two parameters: (i) flow rate of the mobile phase (0.70 and 0.90 mL/min) and (ii) mobile phase proportion (59:41 and 61:39, v/v), using the standard samples at low (10 µg/mL), medium (50 µg/mL) and high (100 µg/mL) concentration in triplicate. Assessment of change in these parameters was based on the percent recovery and RSD.
2.5.5. LOD and LOQ
LOD and LOQ were determined based upon the slope (b) of the calibration curve and least standard deviation obtained from the response (σ), according to Eqs. (1) and (2).
| (1) |
| (2) |
2.5.6. Forced degradation of apocynin
Apocynin stock solutions (10, 50 and 100 µg/mL) were used for forced degradation to provide an indication of the stability property and specificity of the proposed method.
For acid- and base-induced degradation, 10 mL of 1 M HCL and 10 mL of 1 M NaOH were added separately into 10 mL of stock solution of apocynin at room temperature and the mixtures were maintained for 1 h before HPLC analysis. For hydrogen peroxide (H2O2)–induced degradation, 10 mL of H2O2 (30%, v/v) was added into 10 mL of stock solution of apocynin and the mixtures were maintained for 1 h before HPLC analysis. For photodegradation product, the drug stock solutions were directly exposed to visible light for 24 h.
2.6. Determination of apocynin encapsulation efficiency in BSA nanoparticles
BSA nanoparticles containing apocynin were obtained by desolvation method as described by Khalil et al. [31]. Mean particle size was determined by laser light scattering.
Apocynin content in nanoparticles was determined indirectly. Supernatant obtained from the ultracentrifugation process of formed nanoparticles was diluted in the mobile phase (1:10) and filtered through a 0.22 µm pore-size filter, and the drug concentration was obtained by the HPLC method developed and validated. The amount of apocynin encapsulated (EE – encapsulation efficiency) was determined in triplicate, according to Eq. (3).
| (3) |
where AI is the amount of apocynin initially added to the formulation and AF is the amount of the free apocynin quantified in the supernatant after ultracentrifugation.
3. Results and discussion
3.1. Chromatography
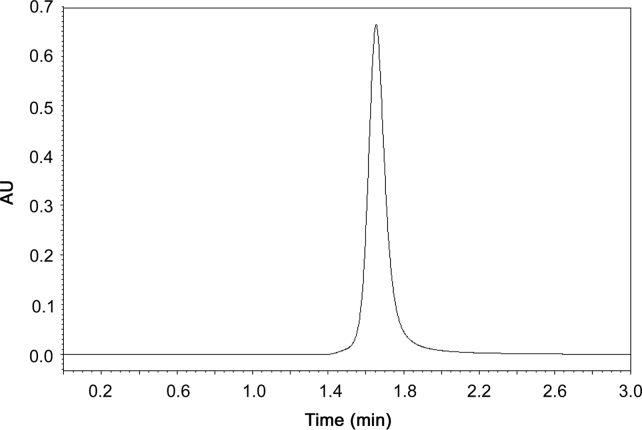
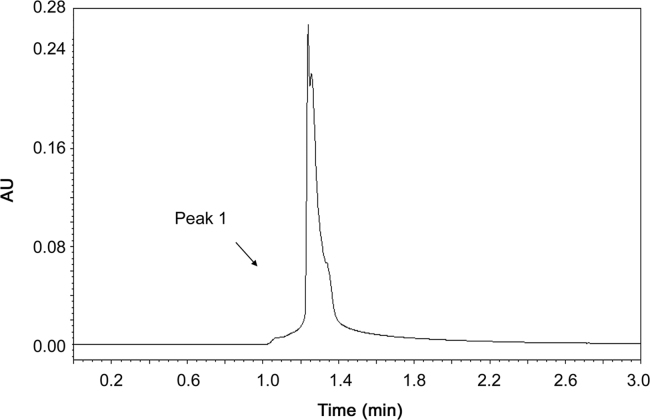
Initial tests were performed using mixtures of methanol and water, based on existing methods for the determination of apocynin in plasma [13], [27]. Several ratios of these solvents were tested and none of them resulted in a symmetric peak, in our attempts. Posteriorly, acetonitrile and water in various proportions were tested, but a favorable peak was not obtained. The addition of acetic acid in water promoted an improvement in the peak characteristics. Thus, acetonitrile and the acidified water mixture in proportions of 60:40 (v/v) eluted at a flow rate of 0.8 mL/min associated with other parameters such as column temperature (25 °C), sample temperature (25 °C), injection volume (20 µL) and wavelength (276 nm), showed the regular and symmetrical apocynin peak. In these conditions, apocynin was detected in approximately 1.65 min (Fig. 1). The system suitability was analyzed, and T value was 0.937±0.024 and N value was 6388±64.32. The parameters analyzed followed the specified limits (T<2; N>2000).
Fig. 1.
Chromatogram of apocynin standard solution (50 µg/mL).
3.2. Method validation
3.2.1. Linearity
Linearity of the HPLC analytical method was evaluated at eight concentration levels (5, 10, 20, 40, 50, 60, 80 and 100 μg/mL) by calculation of regression equation (y=93,500× +2340) and correlation coefficient (r=0.999) using the method of least squares. The correlation coefficient near one indicates linearity in the proposed range. The validity of the test was confirmed by ANOVA, which showed that the regression was significant and the linearity deviation was not significant (p<0.01).
3.2.2. Specificity
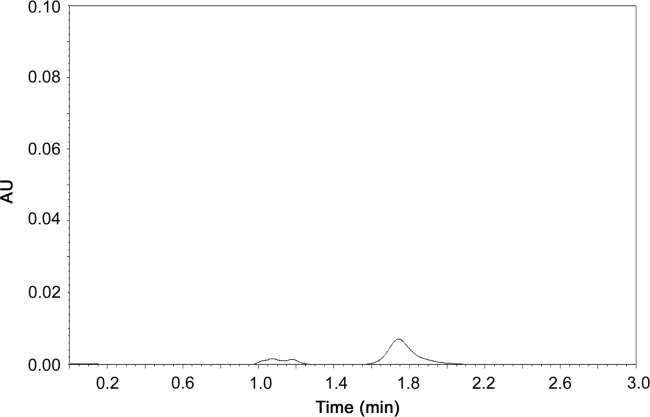
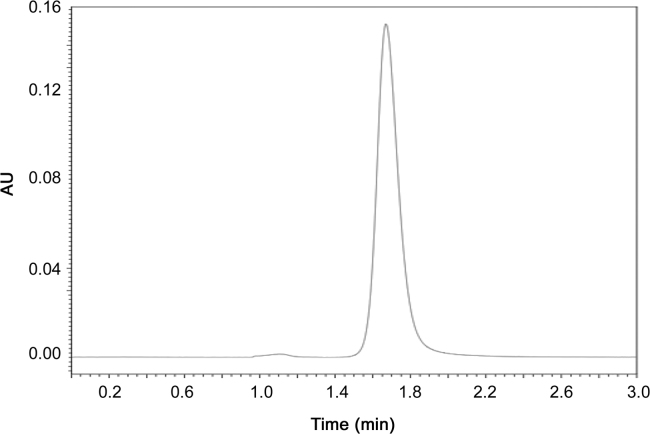
To evaluate specificity, interferences of nanoparticles components were examined in the chromatographic peak of apocynin. Blank nanoparticles (without apocynin) were prepared and the recovered supernatant was diluted in acetonitrile and analyzed by the described HPLC method and the resulting chromatogram (Fig. 2) was compared with that of apocynin sample (Fig. 3) and apocynin standard (Fig. 1). A peak near apocynin retention time was observed in the chromatogram of the blank nanoparticles, but the peak is so small that it does not interfere with quantitative determination of apocynin in nanoparticles. To verify it, a standard sample (supernatant of blank nanoparticles spiked with standard apocynin solution) was analyzed and compared to the apocynin standard solution, at the same concentration. The recovery of apocynin was 99.98%.
Fig. 2.
Chromatogram of supernatant from blank nanoparticles.
Fig. 3.
Chromatogram of supernatant from apocynin nanoparticles (apocynin sample).
3.2.3. Precision and accuracy
Apocynin standard samples (10, 50 and 100 µg/mL) were prepared in triplicate, and analyzed under the same conditions/day (repeatability) and on three different days (intermediate precision). Neither of the precisions do not exceeded the required RSD value (Table 1). The maximum RSD value was 1.32%.
Table 1.
Precision and accuracy of the HPLC method to determine apocynin (n=3).
| Nominal concentration (μg/mL) | Precision (% RSD) |
Accuracy (% recovery) | |
|---|---|---|---|
| Intra-day | Inter-day | ||
| 10 | 0.17 | 0.83 | 104.43 |
| 50 | 0.23 | 1.32 | 101.22 |
| 100 | 0.17 | 1.22 | 100.37 |
Accuracy was assessed as the percent recovery at three different concentration levels and is described in Table 1, indicating the accuracy of the method.
3.2.4. Robustness
Robustness was assessed by calculating the percent recovery and RSD of the mean concentration of apocynin standard samples at three different concentrations obtained using different parameters for mobile phase flow rate and ratio of mobile phase (Table 2). The method was proved to be robust considering changes in mobile phase volume ratio, but sensible with changes in mobile phase flow rate.
Table 2.
Robustness of the HPLC method to determine apocynin.
| Nominal apocynin concentration (μg/mL) | % Recovery±% RSD |
|||
|---|---|---|---|---|
| Acetonitrile:0.1% | Flow rate (mL/min) | |||
| Acetic acid | ||||
| 59:41 | 61:39 | 0.7 | 0.9 | |
| 10 | 99.9±0.3 | 105.4±0.3 | 113.1±0.1 | 90.2±0.1 |
| 50 | 99.1±0.3 | 99.4±0.4 | 107.2±0.7 | 89.4±0.2 |
| 100 | 99.7±0.3 | 100.0±0.1 | 108.3±0.8 | 90.1±0.1 |
3.2.5. LOD and LOQ
LOD and LOQ were calculated from the values obtained from linear regression of a calibration curve, with information on the average standard deviation of the y-intercept (σ) and the slope (b) of the analytical curve, using the Eqs. (1) and (2). The values for LOD and LOQ were 78 ng/mL and 238 ng/mL, respectively.
3.2.6. Stability-indicating property
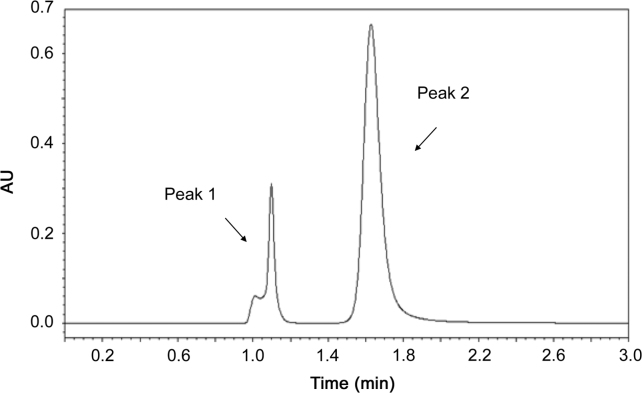
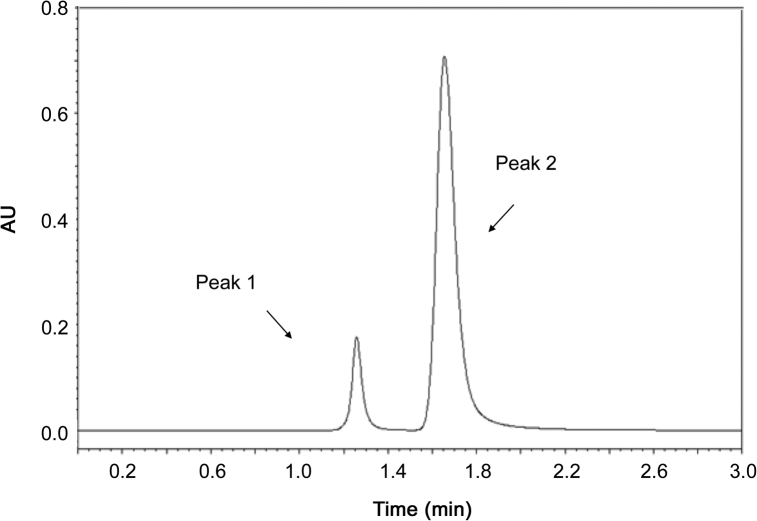
The chromatograms of the samples treated with acid, base, H2O2 and visible light showed well separated peak of apocynin standard as well as some additional peaks at different retention times of apocynin. The chromatograms of the apocynin samples were compared with the chromatogram of apocynin standard solution. The chromatogram of apocynin standards exposed to visible light for 24 h showed no additional peaks (data not shown), suggesting the stability of apocynin in visible light. However, acid, base and H2O2 induced apocynin degradation, as can be shown in Fig. 4, Fig. 5, Fig. 6. The chromatogram of the acid degraded the apocynin sample showed one additional peak at retention time of 1.10 min (Fig. 4). The chromatogram of the base degraded sample showed only one peak at 1.25 min (Fig. 5), and apocynin peak was not detected. The chromatogram of the sample of apocynin treated with 30% (v/v) H2O2 showed an additional peak at 1.25 min (Fig. 6). Also, percent recovery of apocynin after stress conditions was calculated and listed in Table 3. The exposure of apocynin standards to acid and H2O2 conditions leads to a mild degradation since the percent recovery was close to 96%–97%. When treated with 1 M NaOH, apocynin was not detected at a same retention time and, thereby, it was not possible to calculate percent of recovery, suggesting drug degradation. The apocynin percent recovery of apocynin after exposure to visible light did not exceed the variation of 2%, indicating drug stability in this condition.
Fig. 4.
Chromatogram of acid (1 M HCl) treated apocynin: peak 1, degradant (1.10 min); peak 2, apocynin (1.65 min).
Fig. 5.
Chromatogram of base (1 M NaOH) treated apocynin: peak 1, degradant (1.25 min).
Fig. 6.
Chromatogram of H2O2 (30%, v/v) treated apocynin: peak 1, degradant (1.25 min); peak 2, apocynin (1.65 min).
Table 3.
Results of apocynin forced degradation.
| Exposure conditions | % recovery*±% RSD* |
|---|---|
| UV light | 100.9 ± 0.55 |
| NaOH | – |
| HCl | 96.0 ± 1.60 |
| H2O2 | 96.8 ± 1.34 |
Mean of the three concentrations (10 μg/mL, 50 μg/mL and 100 μg/mL, n=3).
3.3. Method applicability
BSA nanoparticles containing apocynin were obtained by the desolvation method and presented particle size in the range of 110–230 nm. The encapsulation efficiency of apocynin in nanoparticles was determined by the HPLC method validated in this work, using an indirect method based on the quantitation of free drug present in the supernatant of nanoparticles centrifugation. Specificity assay demonstrated no interference of supernatant products in drug quantitation. The encapsulation efficiency was 80–90%, showing that nanoparticles present high ability to encapsulate the drug.
After preparation of nanoparticles, characterization is required and an indispensable step is the determination of the drug content within the nanoparticles. This parameter must be properly assessed since the drug must be efficiently loaded into the nanoparticles to reach its therapeutic goal. Therefore, a suitable quantification method needs to be adequately validated to ensure a reliable quantification of the analyte [32], [33].
In this study, we described for the first time an HPLC method for the quantification of the apocynin in BSA . The obtaining of apocynin-loaded nanoparticles is not described in the literature nanoparticles and thus, there are no chromatographic methods to determine apocynin in nanoparticles matrix. The method was proved to be simple, efficient, sensitive and fast, which allows analyzing a large number of samples in a short period; being capable of application to the quantification of apocynin in BSA nanoparticles. Also, as the method separated the drug from its degradation products, it can be employed as a stability-indicating method.
4. Conclusion
The developed HPLC method was precise, accurate, specific and stability-indicating for the determination of apocynin. The method was reproducible and selective for the analysis of apocynin as bulk drug and from BSA nanoparticles. The method was found to be suitable for the determination of apocynin encapsulation efficiency in BSA nanoparticles. It could be further extended and applied to in vitro apocynin release assay and study of degradation kinetics.
Acknowledgments
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/Brazil (CAPES)for the scholarship.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Engels F., Renirie B.F., Hart B.A. Effects of apocynin, a drug isolated from the roots of Picrorhiza kurroa, on arachidonic acid metabolism. FEBS Lett. 1992;305:254–256. doi: 10.1016/0014-5793(92)80680-f. [DOI] [PubMed] [Google Scholar]
- 2.Hougee S., Hartog A., Sanders A. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur. J. Pharmacol. 2006;531:264–269. doi: 10.1016/j.ejphar.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Houser K.R., Johnson D.K., Ishmael F.T. Anti-inflammatory effects of methoxyphenolic compounds on human airway cells. J. Inflamm. 2012;9:1–12. doi: 10.1186/1476-9255-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J.M., Gall N.P., Grieve D.J. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 5.El-Sawalhi M.M., Ahmed L.A. Exploring the protective role of apocynin, a specific NADPH oxidase inhibitor, in cisplatin-induced cardiotoxicity in rats. Chem. Biol. Interact. 2014;207:58–66. doi: 10.1016/j.cbi.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Miller D.K., Oelrichs C.E., Sun G.Y. Subchronic apocynin treatment attenuates methamphetamine-induceddopamine release and hyperactivity in rats. Life Sci. 2014;98:6–11. doi: 10.1016/j.lfs.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Klees R.F., De Marco P.C., Salasznyk R.M. Apocynin derivatives interrupt intracellular signaling resulting in decreased migration in breast cancer cells. J. Biomed. Biotechnol. 2006;2006:1–10. doi: 10.1155/JBB/2006/87246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lirdprapamongkol K., Kramb J.P., Suthiphongchai T. Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J. Agric. Food Chem. 2009;57:3055–3063. doi: 10.1021/jf803366f. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S., Pitchakarn P., Sato S. Apocynin, an NADPH oxidase inhibitor, suppresses progression of prostate cancer via Rac1 dephosphorylation. Exp. Toxicol. Pathol. 2013;65:1035–1041. doi: 10.1016/j.etp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita H., Matsumura T., Ishii N. Apocynin suppresses the progression of atherosclerosis in apoE-deficient mice by inactivation of macrophages. Biochem. Biophys. Res. Commun. 2013;431:124–130. doi: 10.1016/j.bbrc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Tang X.N., Cairns B., Cairns N. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambeth J.D., Krause K.H., Clark R.A. NOX enzymes as novel targets for drug development. Semin. Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang K., Li L., Song Y. Improvement of pharmacokinetics behavior of apocynin by nitrone derivatization: comparative pharmacokinetics of nitrone-apocynin and its parent apocynin in rats. PLoS One. 2013;8:1–6. doi: 10.1371/journal.pone.0070189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connell B.J., Saleh M.C., Khan B.V. Apocynin may limit total cell death following cerebral ischemia and reperfusion by enhancing apoptosis. Food Chem. Toxicol. 2011;9:43063–43069. doi: 10.1016/j.fct.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Mohanraj V.J., Chen Y. Nanoparticles – a review. Trop. J. Pharm. Res. 2006;5:561–573. [Google Scholar]
- 16.Khalil N.M., Carraro E., Cótica L.F. Potential of polymeric nanoparticles in AIDS treatment and prevention. Expert Opin. Drug Deliv. 2011;8:95–112. doi: 10.1517/17425247.2011.543673. [DOI] [PubMed] [Google Scholar]
- 17.Khalil N.M., do Nascimento T.C., Casa D.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B. 2013;101:353–360. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues C.D., Meza Casa D., Dalmolin L.F. Amphotericin b-loaded poly(lactide)-poly(ethylene glycol)-blend nanoparticles: characterization and in vitro efficacy and toxicity. Curr. Nanosci. 2013;9:594–598. [Google Scholar]
- 19.Frank A., Pridgen E., Molnar L.K. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalil N.M., Mainardes R.M. Colloidal polymeric nanoparticles and brain drug delivery. Curr. Drug Deliv. 2009;6:261–273. doi: 10.2174/156720109788680912. [DOI] [PubMed] [Google Scholar]
- 21.Kumari A., Yadav S.K., Yadav S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Barratt G. Colloidal drug carriers: achievements and perspectives. Cell. Mol. Life Sci. 2003;60:21–37. doi: 10.1007/s000180300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulbricht M. Advanced functional polymer membranes. Polymer. 2006;47:2217–2262. [Google Scholar]
- 24.Dongmei Z., Xiuhua Z., Yuangang Z. Preparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles. Int. J. Nanomed. 2010;5:669–677. doi: 10.2147/ijn.s12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elzoghby A.O., Samy W.M., Elgindy N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release. 2012;157:168–182. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Rahimnejad M., Najafpour G., Bakeri G. Investigation and modeling effective parameters influencing the size of BSA protein nanoparticles as colloidal carriers. Colloids Surf. A. 2012;412:96–100. [Google Scholar]
- 27.Wang Q., Smith R.E., Luchtefeldc R. Bioavailability of apocynin through its conversion to lycoconjugate but not to diapocynin. Phytomedicine. 2008;15:496–503. doi: 10.1016/j.phymed.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trumbull K.A., McAllister D., Gandelman M.M. Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice. Neurobiol. Dis. 2012;45:137–144. doi: 10.1016/j.nbd.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandasana H., Chhonker Y.S., Bala V. Pharmacokinetic, bioavailability, metabolism and plasma protein binding evaluation of NADPH-oxidase inhibitor apocynin using LC–MS/MS. J. Chromatogr. B. 2015;985:180–188. doi: 10.1016/j.jchromb.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 30.ICH–International Conference on Harmonization of Technical Requeriments for Registration of Pharmaceuticals for Human Use: Q2B–Validation of Analytical Procedures: Methodology, 2005.
- 31.N.M. Khalil, J. Ascari, D.V. Ronik, et al., Processo de formulação de nanopartículas de apocinina revestidas com o polímero natural BSA e reticuladas com glutaraldeído pelo método de coacervação e fármaco 2014, Patente: Número Do registro: BR 10 2014 029625-5 A2, 2014, Brasil
- 32.da Rocha Lindner G., Khalil N.M., Mainardes R.M. Resveratrol-loaded polymeric nanoparticles: validation of an HPLC-PDA method to determine the drug entrapment and evaluation of its antioxidant activity. ScientificWorldJournal. 2013;2013:1–9. doi: 10.1155/2013/506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.das Neves J., Sarmento B., Amiji M.M., Bahia M.F. Development and validation of a rapid reversed-phase HPLC method for the determination of the non-nucleoside reverse transcriptase inhibitor dapivirine from polymeric nanoparticles. J. Pharm. Biomed. Anal. 2010;52:167–172. doi: 10.1016/j.jpba.2010.01.007. [DOI] [PubMed] [Google Scholar]