Abstract
Premna integrifolia Linn. is a medicinal plant used in “Dhasamula” drug preparation of Ayurvedic systems of medicine in the treatment of various ailments like bronchitis, dyspepsia, liver disorders, piles, constipation, hyperlipidemia and fever. The anti-atherosclerotic activity of hydroalcoholic extract (HAE) of root bark of P. integrifolia was evaluated in high fat diet induced atherosclerosis rats. Sixty Wistar rats were divided into six groups: the first group served as control, the second group was fed with high fat diet and the other three groups were fed with high fat diet along with various concentrations of HAE and the last group was treated with atorvastatin for 30 days. Lipid and lipoprotein profile, atherogenic index, and cardiac markers and histopathological evaluation of aorta were determined in high fat diet induced atherosclerosis rats. HAE of P. integrifolia produced a significant and dose-dependent anti-atherosclerotic activity in terms of reduction in lipids and lipoprotein profile, atherogenic index, HMG-CoA reductase activity, marker enzymes such as lactate dehydrogenase (LDH), creatine phosphokinase (CPK), aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP), alteration in collagen and calcium contents, mild mineralization and focal rupture of intima and media of aorta was noticed in treated groups as compared to the control. The results suggested that anti-atherosclerotic activity of HAE of P. integrifolia Linn. was due to its modulatory activity on metabolic pathway of lipid. The results contribute to the validation of the traditional use of Agnimantha in high fat diet induced atherosclerosis rats.
Keywords: P. integrifolia, HMG-CoA reductase, Atherogenic index, Biomarkers, Atherosclerosis
1. Introduction
Atherosclerosis is one of the major risk factors for coronary artery disease. It is a complex, multifactorial inflammatory disease, characterized by the presence of lesions due to the accumulation of lipids in the walls of arteries. There are a number of genetic, metabolic, and environmental factors involved in the formation and evolution of the atherosclerotic plaque. A well-known risk factor in humans is hypercholesterolemia, i.e., elevated total cholesterol (TC) and low-density lipoprotein cholesterol (LDLc) [1], and other important contributors to this disease includes inflammation, oxidative stress and insulin resistance [2], [3]. Foods rich in saturated fat and cholesterol have been linked to elevations in circulating cholesterol levels [4]. Lipid-enriched diets are often used to induce or accelerate the rate of atherosclerotic lesion in murine models of atherosclerosis [5]. Therefore, using high fat diet for promoting atherosclerosis is a valuable tool for understanding the disease and treatment effect. Lipoprotein oxidation and oxidative processes in general play an important role in the pathogenesis of atherosclerosis. Disorders of lipid metabolism are manifested by elevation of plasma lipids and lipoprotein fractions, which in turn results in cardiovascular diseases.
The alternative systems of medicines like Ayurveda, Siddha, Unani and other tribal folklore medicines which are herbal based have significantly contributed to the health care of the population in India. Today, these systems are not only complementary but also competitive in the treatment of various diseases. Herbs and herb-based compounds have always been an important source of medicines for various diseases and have been paid considerable attention to in recent years due to their diverse pharmacological actions. All over the world, the genus Premna contains 200 species under the family Verbenaceae, out of which approximately 30 species are present in India. The genus Premna can be used traditionally in treating various ailments like rheumatism, asthma, dropsy, cough, fever, and boils. The different parts of this plant have been used for the treatment of various diseases. Hypolipidemic [6], anti-diabetic [7], [8], anti-inflammatory [9], [10], immuno-modulatory [11], anti-obesity [12], anti-bacterial and analgesic [13], anti-arthritic [10], antioxidant [14], [15], antiparasitic [16], hepatoprotective and in vitro cytotoxic [17] activities and beneficial effect of P. integrifolia root extract on human leucocytes and erythrocytes against H2O2 induced oxidative damage [18] were reported. In Ayurveda, the roots are used in the treatment of chyluria, bronchitis, dyspepsia, liver disorders, piles, constipation and fever [19]. P. integrifolia is a medicinal herb and is popularly known as “Agnimantha” in Ayurvedic system of medicine. Their roots are important ingredient of well-known Ayurvedic preparations like Dasamula kwatha, Chyanprashavleh, Haritakiavleh, Ayushavardhaak tel, and Narayan tel, valued for the treatment of variety of ailments [20]. Hence the present study was aimed to demonstrate the anti-atherosclerotic activity of hydroalcoholic extract (HAE) of P. integrifolia in high fat diet induced atherosclerosis rats.
2. Materials and methods
2.1. Preparation of plant extract from root bark
The root bark of P. integrifolia was procured from Indian Medical Practitioners Co-operative Pharmacy and Stores (IMPCOPS), Chennai, Tamil Nadu, India, and authenticated by Shri. C. Arunachalam, Research Officer (Botany), Captain Srinivasa Murthy Regional Ayurveda Drug Development Institute, Chennai and the voucher specimen (00641/2014) was deposited in the Botany Department. The coarse powder of root bark of 500 g was soaked in 5 L of hydro alcohol (60:40, v/v) for 72 h with intermittent shaking at room temperature. The extract was filtered through Whatmann No. 1 filter paper; the filtrate was evaporated to dryness and stored in an air-tight container. The percentage yield was higher (7.6%) at 60:40 (v/v) than that at 50:50 (v/v) and 70:30 (v/v). Therefore, the ratio of 60:40 was chose for the following study.
2.2. Animals
Wistar rats of 6–8 weeks of age were purchased from King's Institute of Preventive Medicine, Guindy, Chennai-600032, India. Experiment was conducted in 60 (30 males+30 females) rats. The rats were maintained in an institutional animal house facility with 12 h light and dark cycles. Temperature was maintained at 25±3 °C and feeding schedule consisted of rat pellet diet and water ad libitum. Daily intake of food was quantitated precisely. The proposal was duly approved by Institutional Animal Ethical Committee as per CPCSEA guidelines (Registration No. IAEC/CSMDRIAS/10/2014) prior to initiation of experiments.
2.3. Establishment of atherosclerosis model
Atherosclerosis model was established by feeding atherogenic diet consisting of 2.0 g of cholesterol, 8.0 g of saturated fat (groundnut oil) and 0.1 g of calcium (BYCALVIT-500; Biochem Pharmaceutical Industries Ltd., Mumbai, India) and mixing thoroughly with 90 g of powdered standard commercial pellet diet. The rats were fed with high fat diet along with weekly challenge of oral vitamin D3 (3,00,000 IU) through per oral route [21]. Feed was prepared daily and intake was recorded.
2.4. Grouping and dosing methods
Sixty rats were randomly divided into 6 groups with 10 animals (5M+5F) in each group. Group I served as control and was fed with standard pellet diet; Group II was fed with high fat diet; Group III, IV and V were fed with high fat diet and administered 100, 200, 500 mg/kg HAE orally, respectively; Group VI was fed with high fat diet and atorvastatin for 30 days. HAE and vehicle (0.5% SCMC) were administered for 30 days by intragastric route using appropriate graduated syringe.
2.5. Biochemical analysis
At the end of experimental period (i.e. 31st day) 4 mL of blood sample was collected from retro-orbital plexus under mild ether anesthesia. Serum was separated using cooling centrifuge (Remi C-24) at 3000 rpm for 10 min at 4 °C. Cross sections of 5 µm thickness of paraffin embedded aorta were stained with hematoxylin and eosin. TC, triglycerides (TG), high density lipoprotein cholesterol (HDLc), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), creatine phosphokinase (CPK) and calcium were estimated by clinical chemistry fully autoanalyser (XL-640; Transasia). Instrument was calibrated with XL-Multical (Erba XL SysPack-Level 1-14441) using kits from ERBA. Quality control (Biorad) was run before analysis.
HMG-CoA/mevalonate ratio [22], collagen [23] and total protein [24] were estimated in the liver by standard procedures. Free fatty acids (FFA) were measured in serum [25] with the color reagent of Itaya [26] using UV–Visible Spectrophotometer (Perkin-Elmer EZ-201). LDLc and very low density lipoprotein cholesterol (VLDLc) were calculated by standard method and atherogenic index was calculated using the following equation [27]:
2.6. Statistical analysis
Statistical analysis was carried out using graph pad prism software, version 5. All the values were expressed as mean±SD (n=10). Analysis of variance (ANOVA) was used for multiple comparison of treatment groups with the vehicle control and disease control followed by Dunnett's test. p<0.05 was considered to be statistically significant.
3. Results and discussion
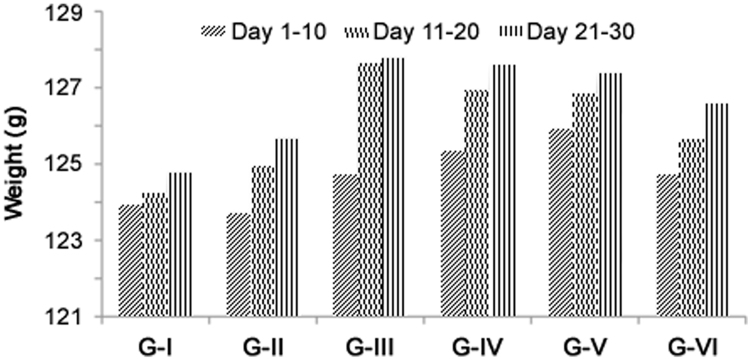
Hypercholesterolemia and atherogenic dyslipidemia have been closely implicated in the pathogenesis of coronary artery disease. Changes in body weight are used to access the course of disease and response to therapy, and also indicate the adverse effects of drugs. Changes in body weight during the experiment are shown in Table 1. Results indicated that body weight of rats was increased from the beginning to the end of experiment in all six groups. On day 30, Group II showed an increase of 44.76% in body weight when compared to day 1 of the same group and an increase of 1.21 fold when compared to Group I. On treatment with HAE the body weight was maintained to near normal values and there was a decrease in body weight of 1.51 fold in Group III, 1.55 fold in Group IV, 1.62 fold in Group V and 1.32 fold in Group VI when compared to Group II (Table 1). There were no significant changes in the feed consumption and organ weight such as liver, heart and kidney between the control and experimental groups (Fig. 1 and Table 2). Reduction in body weights of experimental rats treated with HAE might be due to the anti-obesity potential of HAE in a dose-dependent manner. The result of the present study was in agreement with that of aqueous extract of root of P. integrifolia reported [12], [28].
Table 1.
Body weight (gm) changes in experimental rats.
| Group | Day 1 | Day 7 | Day 14 | Day 21 | Day 30 |
|---|---|---|---|---|---|
| Group I | 107.20±8.31 | 112.90±9.05 | 127.40±5.46 | 132.90±5.76 | 136.80±8.27 |
| (5.32)a | (18.84)a | (23.97)a | (27.61)a | ||
| Group II | 105.00±9.23 | 116.10±8.91 | 129.70±7.59 | 140.00±6.73 | 152.00±6.69 |
| (10.57)a | (23.52)a | (33.33)a | (44.76)a | ||
| (11.11)c | |||||
| Group III | 104.70±8.89 | 113.30±9.97 | 121.90 ±13.55 | 129.40±17.08 | 138.70±21.57 |
| (8.21)a | (16.43)a | (23.59)a | (29.61)a | ||
| (9.59)b | |||||
| Group IV | 106.00±8.31 | 115.70±9.89 | 123.90±13.95 | 129.90±16.18 | 136.60±20.96 |
| (9.15)a | (16.89)a | (22.55)a | (28.87)a | ||
| (11.27)b | |||||
| Group V | 103.90±5.88 | 117.00±6.13 | 124.22±12.66 | 129.67±13.83 | 134.67±18.43 |
| (12.61)a | (19.56)a | (24.80)a | (27.69)a | ||
| (12.87)b | |||||
| Group VI | 105.50±8.93 | 114.60±10.18 | 122.80±10.10 | 134.06±16.05 | 141.33±19.05 |
| (8.63)a | (16.39)a | (27.07)a | (33.96)a | ||
| (7.55)b |
Values are expressed as mean±SD of respective group of rats. (n=10).
Percentage change in body weight with respect to day 1.
Percentage change in body weight with respect to group II of 30 days.
Percentage change in body weight with respect to group I of 30 days.
Fig. 1.
Feed consumption of experimental rats (values are expressed as mean of respective group of rats (n=10)).
Table 2.
Organ weights (g) of experimental rats.
| Group |
|||
|---|---|---|---|
| Liver | Heart | Kidney | |
| Group I | 7.319±0.744 | 0.792±0.019 | 2.134±0.118 |
| Group II | 8.098±0.336 | 0.764±0.030 | 2.128±0.089 |
| Group III | 7.711±0.289 | 0.758±0.024 | 2.090±0.056 |
| Group IV | 7.413±0.453 | 0.777±0.042 | 2.135±0.117 |
| Group V | 7.178±0.499 | 0.790±0.077 | 2.249±0.145 |
| Group VI | 7.304±0.567 | 0.771±0.044 | 2.144±0.088 |
Values are expressed as mean±SD of 10 rats; ANOVA was used for multiple comparisons.
3.1. Lipid profile
The levels of TC, TG and FFA in serum of control and experimental groups are shown in Table 3. The levels of TC and TG, FFA were significantly increased in group II when compared to group I. The levels of TC, TG and FFA were significantly decreased in groups III, IV, V and VI, respectively, when compared to group II. No significant changes in lipid profile were noticed in groups III, IV, V and VI when compared to group I. Lipid metabolism normally maintains the synthesis and degradation of fat in biological tissues. Cholesterol is an essential structural element of the biological membranes and the precursor for synthesis of many compounds such as bile acids, steroidal hormones and vitamin D. High concentration of serum cholesterol increases the risk of coronary heart diseases [29]. There was a significant increase in the levels of plasma TC, TG and FFA in high fat diet fed. Cholesterol-enriched diet resulted in significant increase of TC and TG levels accompanied by increased serum LDLc and decreased circulating HDLc levels [29].
Table 3.
Estimation of TC, TG and FEA in serum.
| Group | Total cholesterol (mg/L) | Triglycerides (mg/L) | Free fatty acids (mg/L) |
|---|---|---|---|
| Group I | 8.23±1.52 | 6.48±1.64 | 6.48±1.64 |
| Group II | 12.69±1.16## | 11.41±1.90## | 11.41±1.90## |
| Group III | 8.87±1.41** | 8.2±1.77** | 8.22±1.75** |
| Group IV | 8.22±2.83** | 7.99±1.60** | 7.99±1.61** |
| Group V | 8.19±2.18** | 7.14±1.28** | 7.14±1.29** |
| Group VI | 9.68±2.71* | 7.16±1.79** | 7.16±1.79** |
Values are expresses as mean±SD of 10 rats; ANOVA was used for multiple comparison; compared with group I, ##p<0.01; compared with group II, *p<0.05, **p<0.01.
3.2. Lipoprotein status
The levels of HDLc, LDLc and VLDLc in serum of control and experimental groups are shown in Table 4. The level of HDLc was significantly decreased in group II when compared to group I and significantly increased in groups IV and V when compared to group II. The level of LDLc was significantly increased in group II when compared to group I and significantly decreased in groups IV, V and VI when compared to group II. The level of VLDLc was significantly increased in group II when compared to group I and significantly decreased in groups III, IV, V and VI when compared to group II. No significant change in HDLc was noticed in group VI treated with atorvastatin when compared to group II. No significant changes of LDLc, VLDLc or HDLc were noticed in groups III, IV, V and VI when compared to group I. Dyslipidaemia, one of the major risk factors for coronary artery diseases like atherosclerosis, promotes accumulation of oxidised LDL in the arterial wall. Oxidised LDL plays an immense role in the initiation and progression of the cardiovascular dysfunction associated with atherosclerosis or atherogenesis; hence amelioration of oxidative stress is equally important in controlling or decreasing dyslipidaemia. High oxidative stress plays an important role in atherogenesis [30]. The oxidative modification of LDLc is considered to be an initial step in the conversion of LDLc into atherogenic form [31]. A strong positive association between plasma levels of oxidants and atherogenic lipoproteins in patients with cardiovascular diseases was reported [32], [33]. Atorvastatin treated rats showed a significant reduction in serum levels of TC, TG and HDLc when compared to HAE.
Table 4.
Determination of LDLc, VLDLc and HDLc in serum.
| Group | LDLc (mg/L) | VLDLc (mg/L) | HDLc (mg/L) |
|---|---|---|---|
| Group I | 3.22±0.89 | 1.29±0.33 | 5.55±1.12 |
| Group II | 4.82±0.51## | 2.28±0.38## | 3.75±0.54## |
| Group III | 3.91±0.75 | 1.64±0.36** | 4.69±0.99 |
| Group IV | 3.65±0.88* | 1.59±0.32** | 5.33±1.22* |
| Group V | 3.49±0.56** | 1.43±0.26** | 5.46±0.75* |
| Group VI | 3.63±0.54* | 1.43±0.36** | 5.04±1.28 |
Values are expresses as mean±SD of 10 rats; ANOVA was used for multiple comparison; compared with group I, ##p<0.01; compared with group II, *p<0.05, **p<0.01.
Atherosclerosis is associated with high concentrations of TC, LDLc, and TG and low concentration of HDLc in serum. HDLc is inversely related to total body cholesterol, and a reduction in plasma HDLc concentration may accelerate the development of atherosclerosis which leads to ischaemic heart diseases, by impairing the cholesterol clearance from the arterial wall [34]. HDLc is largely attributed to reserve cholesterol transport, a process by which excess tissue cholesterol is taken up and subsequently processed by HDLc particles for further delivery to the liver for metabolism. Therefore, an increase in HDLc level helps to lower the risk of atherosclerosis [35]. No significant changes in HDLc level were noticed in all other groups when compared to the control, indicating that the levels of HDLc were brought back to near normalcy after treatment with HAE in rats fed with high fat diet. Several lines of evidence support the hypothesis that the oxidation of LDL may play a significant role in early stage of atherosclerosis, while thrombosis is one of the fatal clinical consequences of this disease [36]. The imbalance in lipid metabolism further causes lipid peroxidation which was observed in the present study. It is widely accepted that the elevated levels of plasma LDLc and VLDLc are the major risk factor for coronary heart disease. Direct correlation between LDLc level and atherosclerosis as well as the reversibility of related pathological events by lowering the serum LDLc has been reported [37]. The high level of LDLc was significantly reduced in atorvastain treated group when compared to high fat diet fed rats [38]. Results of the present study indicated that the high level of LDLc was significantly reduced in rats treated with HAE. Flavonoids are reported to increase HDLc concentration and decrease LDLc and VLDLc levels in hypercholesterolemic rats [39]. Flavonoids and phenols are found to be present in HAE which might be the reason for increasing HDLc and decreasing LDLc and VLDLc levels in rats on HAE treatment. An enzyme, lecithin cholesterol-O-acyltransferase (LCAT), is involved in the trans-esterification of cholesterol, the maturation of HDL and the flux of cholesterol from cell membranes into HDL [40]. HAE may involve in increasing HDLc level, which is attributed to the mobilization of cholesterol from peripheral cells to the liver by the action of LCAT which plays a key role in lipoprotein metabolism. The rats treated with atorvastatin showed no significant variation in the level of HDLc.
Atherogenic index, TC/HDLc and LDLc/HDLc in serum of control and experimental groups are shown in Table 5. Atherogenic index was significantly higher in group II than in group I, and significantly lower in groups III, IV, V and VI than in group II. The ratio of TC/HDLc was significantly higher in group II than in group I. The ratio was significantly less in groups III, IV, V and VI than in group II. The ratio of LDLc/HDLc was significantly higher in group II than in group I. The ratio was significantly reduced in groups IV, V and VI than that in group II. No significant changes in atherogenic indexes, TC/HDLc and LDLc/HDLc, were noticed in groups III, IV, V and VI compared with those that in group I. High atherogenic index is believed to be an important factor for detection of atherosclerosis. Our data clearly indicated that HAE potentially decreased the atherogenic index. High fat diet-induced atherosclerosis has a relation with vascular damage and oxidative stress and in turn leads to pathogenesis of various diseases [41]. TC/HDLc and LDLc/HDLc ratios were significantly high in high fat diet fed rats when compared to the control. These atherogenic factors are the better indexes reflecting the abnormality of lipid metabolism [42]. Increase in antioxidants and decrease in pro-oxidants upon HAE treatment were observed in the present study.
Table 5.
Determination of atherogenic index, TC/HDLc and LDLc/HDLc in serum.
| Group | Atherogenic index | TC/HDLc | LDLc/HDLc |
|---|---|---|---|
| Group I | 0.55±0.49 | 1.55±0.49 | 0.612±0.265 |
| Group II | 2.46±0.65## | 3.11±1.25## | 1.184±0.492## |
| Group III | 0.96±0.50** | 1.96±0.50* | 0.869±0.239 |
| Group IV | 0.54±0.39** | 1.54±0.39** | 0.719±0.220** |
| Group V | 0.52±0.46** | 1.37±0.64** | 0.591±0.269** |
| Group VI | 0.96±0.54** | 1.77±0.80** | 0.684±0.328** |
Values are expresses as mean±SD of 10 rats; ANOVA was used for multiple comparison; compared with group I, ##p<0.01; compared with group II, *p<0.05, **p<0.01.
3.3. Status of atherosclerotic markers
The activities of serum ALT, AST and ALP in control and experimental groups are shown in Table 6. The activity of ALT was significantly increased in group II compared with group I and significantly decreased in groups IV, V and VI and were not changed in group III compared with group II. The activities of AST and ALP were significantly increased in group II compared with group I and significantly decreased in groups V and IV and no significant changes were noticed in other groups when compared to group II. No significant changes in ALT, AST and ALP were noticed in groups III, IV, V and VI compared with group I. The activities of LDH and CPK in serum of control and experimental groups are depicted in Table 7. The activity of LDH was significantly increased in groups II, III and IV compared with group I and significantly decreased in groups III, IV, V, and VI compared with group II. Treatment with 200 and 500 mg/kg HAE showed a drastic reduction in LDH activity when compared to group III (100 mg/kg). The results showed that the activity was inversely proportional to the concentration of HAE on treatment. Also atorvastatin treated group showed a major decline in LDH activity. The activity of CPK was significantly increased in group II compared with group I and significantly decreased in groups IV, V and VI compared with group II. No significant increase in the activities of LDH and CPK was noticed in groups IV, V and VI compared with group I. Measurement of LDH activity in blood is considered to be a diagnostic marker for certain cardiovascular diseases and it is a sensitive marker of myocardial infarction [43]. AST level is used as a diagnostic marker for myocardial infarction. In pathological conditions, the enzymes such as CPK, LDH, AST and ALT leak from the necrotic heart cells to blood, which are important measuring markers of cardiac injury. These enzymes are not specific for myocardial injury individually; however, evaluation of these enzymes together may be an indicator of myocardial damage [44], [45]. CPK, another important enzyme in energy metabolism in the body, maintains the high concentration of intracellular ATP through phosphorylation and is considered as another important index for atherosclerosis [46]. The results of the present study showed that serum AST, ALT, LDH and CPK levels were significantly increased in high fat diet fed rats when compared to the control. Higher activities of these enzymes in serum have been found in response to oxidative stress induced by high fat diet [30]. There was a significant restoration of these enzymes on administration with different doses of HAE. Markable decrease in AST, ALT, CKP and LDH enzyme activities demonstrated the cardio-protective effect of the drug. Also atorvastatin treated rats showed its protective potential on atherosclerosis.
Table 6.
Activities of ALT, AST and ALP in serum.
| Group | ALT (U/L) | AST (U/L) | ALP (U/L) |
|---|---|---|---|
| Group I | 101.07±23.00 | 118.50±31.15 | 470.10±73.80 |
| Group II | 143.34±10.60## | 162.44±9.14## | 619.11±55.70## |
| Group III | 120.11±25.90 | 132.28±21.80 | 514.60±62.60 |
| Group IV | 109.18±20.40** | 130.89±23.05 | 504.22±90.40* |
| Group V | 107.18±16.80** | 127.32±22.71* | 495.22±64.00* |
| Group VI | 110.74±19.70* | 134.37±27.09 | 531.33±120.20 |
Values are expresses as mean±SD of 10 rats; ANOVA was used for multiple comparison; compared with group I, ##p<0.01; compared with group II, *p<0.05, **p<0.01.
Table 7.
Activities of LDH and CPK in serum.
| Group | LDH (IU/L) | CPK (IU/L) |
|---|---|---|
| Group I | 249.90±42.46 | 166.50±44.40 |
| Group II | 539.78±89.82## | 282.43±35.50## |
| Group III | 432.70±78.02## | 231.28±58.70 |
| Group IV | 374.20±63.54## | 188.48±85.70** |
| Group V | 273.56±58.79** | 165.49±35.50** |
| Group VI | 309.22±61.24** | 195.09±57.00* |
Values are expresses as mean±SD of 10 rats; ANOVA was used for multiple comparison; compared with group I, ##p<0.01; compared with group II, *p<0.05, **p<0.01.
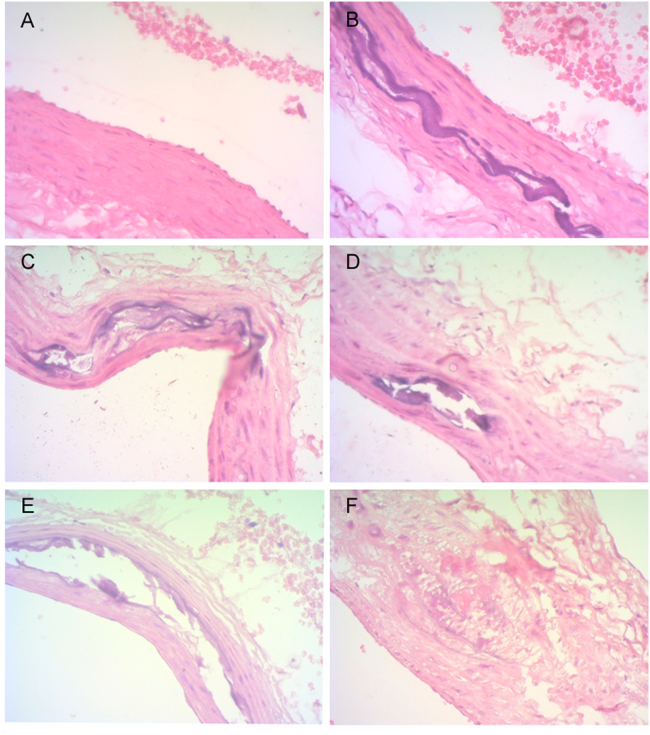
Levels of HMG CoA/mevalonate ratio, collagen, calcium and total protein in liver of control and experimental groups are depicted in Table 8. HMG CoA/mevalonate ratio was increased in group II compared with group I. A significant improvement was observed in groups IV, V and VI compared with group II. The collagen content was significantly increased in groups II and III compared with group I, and significantly decreased in groups IV, V, and VI compared with group II. The calcium level in the liver was significantly increased in group II compared with group I, and significantly decreased in groups III, IV and V compared with group II. The level of total protein was significantly increased in group II when compared to group I and significantly decreased in groups IV, V, and VI compared with group II. No significant change in the level of total protein was observed in groups III, IV, V and VI compared with group I. HMG-CoA reductase activity was indirectly measured in terms of HMG CoA/mevalonate ratio in the liver tissue. Inhibitors of HMG-CoA reductase are used to lower the serum cholesterol levels and improve the survival of individuals at the risk of atherosclerotic vascular disease [47]. Their primary site of action is in the liver, where they inhibit HMG-CoA reductase, the metabolic pathway that produces cholesterol and isoprenoids [48]. Group V rats showed a significant reduction in the ratio of HMG-CoA/mevalonate, which might be due to the inhibition of HMG-CoA reductase activity by HAE; hence it is evident that the drug possesses the hypolipidimic activity. The liver is an important organ in which all metabolic reactions take place. One of the most effective and widely used drugs for the treatment of hyperlipidemia is the atorvastatin which competitively inhibits HMG-CoA reductase enzyme in the liver that converts HMG-CoA to mevalonate, an early precursor for cholesterol biosynthesis. Hence, this enzyme might be competitively inhibited by HAE, which was evident from the lipid and lipoprotein status of the present study. The unique distribution of hydroxy proline within the collagen can be easily studied [49]. It is a family of proteins which consists of several genetically distinct molecular species and is closely involved in tissue organization, function, differentiation and development. HAE treated group showed the reversibility of the changes when compared to high fat diet fed rats. Histopathology study revealed that alteration in collagen and calcium content, mild mineralization and focal rupture of intima and media of aorta were noticed in treated groups as compared to the control (Fig. 2). These parameters were significantly reverted to near normalcy on treatment with HAE of P. integrifolia in a dose-dependent manner.
Table 8.
Determination of HMG-CoA reductase, collagen, calcium and total protein in the liver.
| Group | HMG CoA/Mevalonate ratio | Collagen | Calcium | Total protein |
|---|---|---|---|---|
| (µg/mg protein) | (mg/g tissue) | (g/mg tissue) | ||
| Group I | 1.161±0.110 | 38.83±16.25 | 12.02±2.28 | 198.6±32.2 |
| Group II | 1.404±0.144## | 92.51±24.67## | 14.66±1.64# | 254.7±39.9# |
| Group III | 1.204±0.105 | 82.07±26.17** | 12.34±1.34* | 208.3±35.3 |
| Group IV | 1.158±0.150** | 76.52±41.40* | 11.87±1.64** | 200.2±51.1* |
| Group V | 1.056±0.139** | 38.95±22.94** | 11.02±1.83** | 197.3±17.6* |
| Group VI | 1.108±0.130** | 40.07±29.66** | 13.30±1.04 | 203.5±23.6* |
Values are expresses as mean±SD of 10 rats; ANOVA was used for multiple comparison; compared with group I, #p<0.05, ##p<0.01; compared with group II, *p<0.05, **p<0.01.
Fig. 2.
Histopathology of experimental rats. (A): No changes (Group I). (B): Multifocal severe fragmentation of elastic and collagen fibres in media with marked mineralization (Group II). (C): Moderate multifocal splitting of media with marked peripheral mineralization (Group III). (D): Moderate multifocal marked splitting of tunica media with vacuolation and mineralization (Group IV). (E): Mild diffuse thickening and fragmentation of media with mild mineralization; focal rupture of intima and media (Group V). (F): Mild multifocal fragmentation of elastic and collagen fibres in media with mineralization; focal rupture of intima & media (Group VI).
4. Conclusion
The data of present study showed significant increases in serum TC, TG, LDLc, VLDLc, atherogenic index (TC/HDLc and LDLc/HDLc ratio), HMG-CoA reductase activity and a significant decrease in HDLc in rats fed with high fat diet. The present study revealed that this plant individually maintains the lipid and lipoprotein status through a metabolic key enzyme HMG-CoA reductase along with the maintenance of collagen and calcium contents, which plays an integral role in atherosclerosis. The efficacy of the drug was further proved through histopathological study. No data is available on the efficacy of root bark of P. integrifolia in high fat diet induced atherosclerosis model. Further study is required on the activity guided fractionation and exact molecular mechanisms involved in the anti-atherosclerotic activity of P. integrifolia.
Acknowledgments
The authors gratefully acknowledge the Central Council for Research in Ayurvedic Sciences, M/o AYUSH, Govt. of India for financial support and Dr. Hemalatha, Associate Professor, Central University Laboratory, Tamil Nadu and Animal Sciences University, Chennai-600 051 for histopathological work.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Pearson T.A., Blair S.N., Daniels S.R. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 2.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Van Gaal L.F., Mertens I.L., De Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 4.Hegsted D.M., McGandy R.B., Myers M.L. Quantitative effects of dietary fat on serum cholesterol in man. Am. J. Clin. Nutr. 1965;17:281–295. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- 5.Getz G.S., Reardon C.A. Diet and murine atherosclerosis. Aterio. Thromb. Vasc. Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- 6.Khanna A.K., Chander R., Kapoor N.K. Hypolipidemic activity of P. integrifolia in rats. Fitoterapia. 1991;62:271–274. [Google Scholar]
- 7.Kar A., Choudhary B.K., Bandyopadhyay N.G. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol. 2003;84:105–108. doi: 10.1016/s0378-8741(02)00144-7. [DOI] [PubMed] [Google Scholar]
- 8.Alamgir M., Rokeya B., Hannan J.M. The effect of Premna integrifolia Linn. (Verbenaceae) on blood glucose in streptozotocin induced type 1 and type 2 diabetic rats. Pharmazie. 2001;56:903–904. [PubMed] [Google Scholar]
- 9.Barik B.R., Bhowmik A.K., Patra A. Premnazole, an isooxazole alkaloid of Premna integrifolia and Gmelina arborea with anti-inflammatory activity. Fitoterapia. 1992;63:295–299. [Google Scholar]
- 10.Rathore R.S., Prakash A., Singh P.P. Preliminary study of anti-inflammatory and anti-arthritic activity. Rheumatism. 1997;12:130–138. [Google Scholar]
- 11.Gokani R.H., Lahiri S.K., Santani D.D. Evaluation of immune-modulatory activity of Clerodendrum phlomidis and Premna integrifolia root. Int. J. Pharmacol. 2007;3:352–356. [Google Scholar]
- 12.Mali P.Y., Bigoniya P., Panchal S.S. Anti-obesity activity of chloroform-ethanol extract of Premna integrifolia in mice fed with cafeteria diet. J. Pharm. Bioallied. Sci. 2013;5:229–236. doi: 10.4103/0975-7406.116825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmakar U.K., Pramanik S., Sadhu S.K. Assessment of analgesic and anti-bacterial activity of Premna integrifolia Linn. (family:Verbenaceae) leaves. Int. J. Pharm. Sci. Res. 2011;2:1430–1435. [Google Scholar]
- 14.Jain S., Singh M., Barik R. In-vitro antioxidant activity of Premna integrifolia Linn. roots. Res. J. Pharmacol. Pharmacodyn. 2013;5:293–296. [Google Scholar]
- 15.Majumder R., Akter S., Naim Z. Antioxidant and anti-diabetic activities of the methanolic extract of Premna integrifolia bark. Adv. Biol. Res. 2014;8:29–36. [Google Scholar]
- 16.Desrivot J., Waikedre J., Cabalion P. Antiparasitic activity of some New Caledonian medicinal plants. J. Ethnopharmacol. 2007;112:7–12. doi: 10.1016/j.jep.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Vadivu R., Suresh A.J., Girinath K. Evaluation of hepatoprotective and in-vitro cytotoxic activity of leaves of Premna serratifolia Linn. J. Sci. Res. 2009;1:145–152. [Google Scholar]
- 18.Mali P.Y. Beneficial effect of extracts of Premna integrifolia root on human leucocytes and erythrocytes against hydrogen peroxide induced oxidative damage. Chron. Young Sci. 2014;5:53–58. [Google Scholar]
- 19.Anonymous, Indian Medicinal Plants . In: A compendium of 500 species. Warrier P.K., Nambiyar V.P., Ramankutty C., editors. Orient Longman Ltd.; Madras: 1997. pp. 348–352. [Google Scholar]
- 20.Anonymous, The Ayurvedic Pharmacopoeia of India, Part-I, Vol. III, Ist Ed. Department of ISM and Homeopathy, Ministry of Health and Family Welfare, Government of India, Delhi, 2001, 3–4.
- 21.Altman R.F. A simple method for rapid production of atherosclerosis in rats. Experientia. 1973;29:256. doi: 10.1007/BF01945508. [DOI] [PubMed] [Google Scholar]
- 22.Rao A.V., Ramakrishnan S. Indirect assessment of hydroxylmethylglutaryl-CoA reductase (NADPH) activity in liver. Clin. Chem. 1975;21:1523–1525. [PubMed] [Google Scholar]
- 23.Edwards C.A., O’Brien W.D., Jr. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin. Chim. Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O.H., Rosebrough N.J., Farr A.L. Protein measurements with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Horn W.T., Menahan L.A. A sensitive method for the determination of free fatty acids in plasma. J. Lipid Res. 1981;22:377–381. [PubMed] [Google Scholar]
- 26.Itaya K. A more sensitive and stable colorimetric determination of free fatty acids in blood. J. Lipid Res. 1977;18:663–665. [PubMed] [Google Scholar]
- 27.Onat A., Can G., Kaya H. Atherogenic index of plasma (log10 (triglyceride/high density lipoprotein cholesterol) predicts high blood pressure, diabetes, and vascular events. J. Clin. Lipidol. 2010;4:89–98. doi: 10.1016/j.jacl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Mali P.Y. Effect of aqueous enriched fraction of Premna integrifolia root against cafeteria diet induced obesity in Swiss albino mice. Int. J. Green. Pharm. 2013;7:315–321. [Google Scholar]
- 29.Shrivastava A., Chaturvedi U., Singh S.V. Lipid lowering and antioxidant effect of miglitol in triton treated hyperlipidemic and high fat diet induced obese rats. Lipids. 2013;48:597–607. doi: 10.1007/s11745-012-3753-3. [DOI] [PubMed] [Google Scholar]
- 30.Demori I., Voci A., Fugassa E.B. Combined effects of high-fat diet and ethanol induce oxidative stress in rat liver. Alcohol. 2006;40:185–191. doi: 10.1016/j.alcohol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Stocker R., Keaney J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 32.Albertini R., Moratti R., De Luca G. Oxidation of low-density lipoprotein in atherosclerosis from basic biochemistry to clinical studies. Curr. Mol. Med. 2002;2:579–592. doi: 10.2174/1566524023362177. [DOI] [PubMed] [Google Scholar]
- 33.Matsuura E., Hughes G.R., Khamashta M.A. Oxidation of LDL and its clinical implication. Autoimmun. Rev. 2008;7:558–566. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Kanungo S.K., Panda D.S., Swain S.R. Comparative evaluation of hypolipidimic activity of some marketed herbal formulations in triton induced hyperlipidemic rats. Pharmacol. Online. 2007;3:211–221. [Google Scholar]
- 35.Barter P. The role of HDL-cholesterol in preventing atherosclerotic disease. Eur. Soc. Cardiol. 2005;7:F4–F8. [Google Scholar]
- 36.Lusis A.J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammed M.H. Mini review experimental atherosclerosis: a historical overview. Life Sci. 2002;70:855–865. doi: 10.1016/s0024-3205(01)01479-5. [DOI] [PubMed] [Google Scholar]
- 38.Marz W., Genser B., Drechsler C. Atorvastatin and low-density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2011;6:1316–1325. doi: 10.2215/CJN.09121010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel D.K., Patel K.A., Patel U.K. Assessment of lipid lowering effect of Sida rhomboidea. Roxb. methanolic extract in experimentally induced hyper lipidemia. J. Young Pharm. 2009;3:233–238. [Google Scholar]
- 40.Faheemuddin M.D., Janarthan M., Durraivel S. Evaluation of protective effect of Cleome viscosa extract on diet induced atherosclerosis in diabetic rats. J. Chem. Pharm. Sci. 2013;6:238–242. [Google Scholar]
- 41.Pamidiboina V., Razdan R., Hariprased M.G. Evaluation of the antihyperlipidemic, cardioprotective activity of a polyherbal formulation. Int. J. Pharm. Pharm. Sci. 2010;2:86–91. [Google Scholar]
- 42.Otunola G.A., Oloyede O.B., Oladiji A.T. Effects of diet-induced hypercholesterolemia on the lipid profile and some enzyme activities in female Wistar rats. Afr. J. Biochem. Res. 2010;4:149–154. [Google Scholar]
- 43.Stegink L.D., Vestling C.S. Rat liver lactate dehydrogenase. Amino-termin and acetylation status. J. Biol. Chem. 1966;241:4923–4930. [PubMed] [Google Scholar]
- 44.Al-Shabanah O., Mansour M., El-Kashef H. Captopril ameliorates myocardial and hematological toxicities induced by adriamycin. Biochem. Mol. Biol. Int. 1998;45:419–427. doi: 10.1080/15216549800202802. [DOI] [PubMed] [Google Scholar]
- 45.Chopra S., Pillai K.K., Husain S.Z. Propolis protects against doxorubicin-induced myocardiopathy in rats. Exp. Mol. Pathol. 1995;62:190–198. doi: 10.1006/exmp.1995.1021. [DOI] [PubMed] [Google Scholar]
- 46.Brancacci P., Maffulli N., Limongelli F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007;81–82:209–230. doi: 10.1093/bmb/ldm014. [DOI] [PubMed] [Google Scholar]
- 47.Endo A. The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 1992;33:1569–1582. [PubMed] [Google Scholar]
- 48.Adnan F., Sadiq M., Jehangir A. Anti-hyperlipidemic effect of acacia honey (desi kikar) in cholesterol-diet induced hyperlipidemia in rats. Biomedica. 2011;27:62–67. [Google Scholar]
- 49.Pihlajaniemi T., Myllya R., Krivirikko K.I. Poly 4-hydroxylase and its role in collagen synthesis. J. Hepatol. 1991;13:S2–S7. doi: 10.1016/0168-8278(91)90002-s. [DOI] [PubMed] [Google Scholar]