Abstract
Methylene blue (MB) is a hydrophobic drug molecule, having importance both as a staining reagent and pharmaceutical agent. MB is strongly fluorescent, with an emission peak at 686 nm (λex 665 nm). In the study, the possibility of MB as an extrinsic fluorophore to study the micellization behavior of bile salts (BSs) was carried out. Since BSs are drug delivery systems, the solubilization of hydrophobic MB drug molecule by BSs was achieved and the nature of association of MB with BS media, namely sodium cholate (NaC) and sodium deoxycholate (NaDC) was evaluated. Change in the photophysical properties of MB is monitored through fluorescence intensity and fluorescence anisotropy at emission peak, 686 nm of MB. Molecular mechanics calculations were carried out to evaluate the MB–BS association. The estimated heat of formation, ΔHf values are –625.19 kcal/mol for MB–NaC and –757.48 kcal/mol for MB–NaDC. The photophysical study also revealed that MB reports the step-wise aggregation pattern of BSs media, as an extrinsic fluorescence probe.
Keywords: Methylene blue, Sodium deoxycholate, Sodium cholate, Absorption, Fluorescence spectroscopyMolecular mechanics
1. Introduction
Methylene blue (MB) has a long history of diverse applications both as a staining reagent and pharmaceutical agent. MB has been used as a redox indicator in catalytic oxidation reactions, a sensitizer in solar energy conversion, an antioxidant and antiseptic agent and stain for fixed and living tissues, an antidote for cyanide and also a drug in photodynamic therapy for anticancer treatment [1], [2], [3]. MB has recently been reported to be effective in arresting the progress of Alzheimer's disease and other neurodegenerative diseases [4]. MB undergoes spontaneous aggregation in aqueous media [5], [6], [7], [8]. The aggregation pattern of MB follows the formation of dimer first and then larger aggregates and it is strongly dependent on the concentration, structure, pH, temperature and nature of solvents. Owing to the structure and aggregation pattern of MB, the exciton band splitting theory was proposed to explain its photophysical property [9]. MB exhibits two major absorption bands at 293 nm (π-π* transition) and 665 nm (n-π* transition) in dilute aqueous solutions, with a shoulder at 610 nm corresponding to the 0–1 vibronic transition [10], [11]. Spectral properties of MB have been studied in both homogenous and organized media like human serum albumin, bovine serum albumin, sodium lauryl sulfate, etc. [12], [13], [14], [15]. MB is a well-known staining dye, whose absorption spectral behavior is widely studied. The literature reports on fluorescence properties of MB are found to be minimal [15]. Hence it will be interesting to use MB as an extrinsic fluorescence probe to study the micellization behavior of bile salts (BSs) media. MB has also been used as a pharmaceutical agent and since the molecule is hydrophobic, the solubilization of MB poses a disadvantage in formulating as a drug molecule. To increase MB's solubility and enhance the bioavailability, it might be suitable to use BS media to serve the purpose.
The chosen organized media BS are ionic surfactants and well known drug delivery media/pharmaceutical excipients. BSs are biological compounds that are synthesized from cholesterol in the liver. The BS molecules exhibit facial polarity; the hydrophilic groups are situated on the concave face of the molecule and most of the steroidal skeleton with its protruding methyl groups lying on the convex face [16]. BSs are widely used as solubilization and disaggregating agents in the pharmaceutical industry [17], [18]. In aqueous media, BSs undergo spontaneous aggregation, leading to primary and secondary aggregates involving a step-wise aggregation model [19]. The primary aggregates are formed due to hydrophobic interaction between the steroidal back-bone. Secondary micelles are held together by hydrogen bond between the primary aggregates [20]. Among the various BSs, sodium cholate (NaC) and sodium deoxycholate (NaDC), primary unconjugated BSs, are chosen for the studies. NaDC is a dihydroxy BS and NaC is a trihydroxy BS [21]. With less number of hydroxyl groups, the dihydroxy BSs are more hydrophobic and attain micellization with lower concentrations than that of the trihydroxy BSs [22]. The unique structural features of BSs and the step-wise aggregation pattern suggest that there is no sharp critical micellar concentration (cmc) for these BSs. Only a cmc range is always reported in the literature [19], [20], [21], [22]. The cmc range for NaDC is 4–8 mM and it is 6–16 mM for NaC. The cmc range starts from a concentration at which dimer is formed to the concentration where the secondary aggregates appear.
The two important aspects of MB, viz. being a drug and fluorescent molecule, make it a versatile molecule to categorize it even as an intrinsic fluorescence drug molecule along with using MB as an extrinsic fluorescence probe. The current article discusses the use of intrinsic fluorescence property of MB to understand the association of MB and BS media. While BS is a simple drug delivery medium, this study can be adopted to explore the association of MB with more complicated binary/ternary/bulky macromolecular/ heterogeneous systems with the help of MB fluorescence. The aggregation pattern of BSs is to be studied using photophysical properties of MB such as absorbance, fluorescence intensity and fluorescence anisotropy . The effect of temperature on the aggregation of BSs is also to be explored by MB fluorescence. The study is extended further to understand the nature of interaction between MB and both the BSs through molecular mechanics (MM) calculations.
2. Materials and methods
2.1. Materials
Methylene blue, sodium cholate and sodium deoxycholate were purchased from Sisco Research Laboratories Private Limited (India) and were used without any further purification. A sodium phosphate buffer of pH 7.4 with 50 mM concentration was used for all experiments. The solvent ethanol used was of spectroscopy grade. The stock solutions of MB were prepared by dissolving it in ethanol and the required concentration of MB solution was prepared by further dilution with pH buffer. Triply distilled water was used to prepare the buffer solution.
2.2. Methods
UV–visible spectrophotometric experiments were recorded on a Shimadzu UV 1800 double beam spectrophotometer, using 10 mm quartz cuvettes. A Fluoromax-4 (Horiba Jobin Yvon, Germany) spectrofluorimeter was used to record the fluorescence spectra. Excitation and emission spectra were measured with 5 nm band width. Temperature control was achieved by circulating water through the jacketed cuvette holder from a refrigerated bath (Julabo, Germany). The steady state is defined as [23],
where IVV and IVH are the fluorescence intensities and the subscript indicates the vertical (V) and horizontal (H) orientations of the excitation and emission polarizer. G is the instrumental correction factor,
2.3. Preparation of MB in BS media
The stock solutions of BSs were prepared in phosphate buffer pH 7.4 with concentrations higher than their cmc range, namely [NaC]=48 mM and [NaDC]=20 mM. A range of different concentrations of BS solutions were prepared by diluting these stock solutions using buffer solution. MB concentration was maintained at 5 µM for all the experiments with less than 2% ethanol contamination. The concentration range of BS was varied from 0 to 43.2 mM for NaC and 0 to 18 mM for NaDC. The solutions were incubated for 2 h to achieve equilibrium.
2.4. Molecular mechanics calculations
The MM calculations were performed using Hyperchem 8.0 (Hypercube, Inc.). MB and BSs, both NaC and NaDC, were built by using Hyperchem builder module. All the three molecules were subjected to complete energy minimization using MM with an AMBER force field and Polak-Ribiere Conjugate Gradient Optimizer. The optimized geometries of MB and both NaC and NaDC were allowed to interact, within a periodic boundary of dimension 15–10–20 Å (x-y-z), with 51 water molecules as solvent molecules. The cutoff condition for complete optimization of the molecules was an RMS gradient equal to 0.1 kcal (Å mol)−1.
3. Results and discussion
3.1. Absorption and fluorescence spectra of MB-BS systems
MB in aqueous medium exhibits a peak at 665 nm, a shoulder at 610 nm and a peak at 293 nm. MB is expected to undergo self-aggregation at higher concentrations [5], [6], [7], [8] and hence the concentration of MB in this study was maintained at 5 µM, at which the self-aggregation of MB is avoided. In this way, the current study focuses only on exploring the fluorescence property of MB in probing an organized media. With the addition of NaC and NaDC, the absorption spectra of MB show a gradual increase. The MB absorbance shows a prominent increase at 665 nm along with an increase at 610 nm and 293 nm as shown in Fig. 1. The absorption study reveals possible solubilization of MB by BS media.
Fig. 1.
Absorption spectra of MB (5 µM) in (A) NaC (0, 4.8, 6, 9.2, 14.4, 16, 19.6, 24, 28.8, 33.6, 38.4, 43.2 mM) and (B) NaDC (0, 2, 4, 6, 8, 10, 12, 14, 16, 18 mM). T=25 °C; pH=7.4.
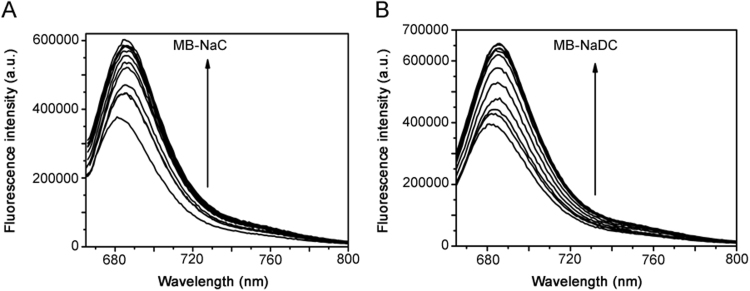
The fluorescence spectra of MB in the presence of BSs show an emission peak at 686 nm (λex 665 nm) exhibited at Fig. 2. The results indicate that there was an increase in the fluorescence intensity of MB with increasing concentration of both NaC and NaDC. There was a minimal shift in the emission wavelength towards the red region. The further studies were carried out using MB fluorescence with λex 665 nm and λem 686 nm.
Fig. 2.
Emission spectra of MB (5 µM; λex 665 nm) in (A) NaC (0, 4.8, 6, 9.2, 14.4, 16, 19.6, 24, 28.8, 33.6, 38.4, 43.2 mM) and (B) NaDC (0, 2, 4, 6, 8, 10, 12, 14, 16, 18 mM). T=25 °C; pH=7.4.
3.2. Steady state fluorescence anisotropy measurement for MB-BS media
The steady state rss was measured at 686 nm emission peak (λex 665 nm). The rss of MB–NaC increased from 0.09 to 0.16 and for MB–NaDC, the rss value increased from 0.09 to 0.20 as shown in Fig. 3. The increase in rss value showed that the MB molecule may get incorporated within the hydrophobic core of the BS aggregates. MB is located within larger secondary aggregates of the BSs, experiences a restricted motion and thereby imparts larger rss value.
Fig. 3.
Variation of (A) absorbance (λmax 665 nm), (B) fluorescence intensity (λex 665 nm, λem 686 nm) and (C) fluorescence anisotropy (λex 665 nm, λem 686 nm) of MB (5 µM) against NaC (0, 4.8, 6, 9.2, 14.4, 16, 19.6, 24, 28.8, 33.6, 38.4, 43.2 mM) and NaDC (0, 2, 4, 6, 8, 10, 12, 14, 16, 18 mM). T=25 °C; pH=7.4.
Analyzing the absorbance, fluorescence intensity and rss values of MB in the presence of NaC and NaDC generally show that there is an increase in all the three photophysical parameters of MB as shown in Fig. 3. The data reveals that the MB molecule was associated with both the BSs.
3.3. Temperature dependence of MB-BS media
The studies conducted at room temperature were further extended to understand the temperature dependence of MB–BS systems presented in Fig. 4. The photophysical parameters, fluorescence intensity and rss of MB (λex 665 nm; λem 686 nm) were used and temperature was varied from 15 °C to 45 °C. The results show that there was a decrease in both fluorescence intensity and rss of MB–BS system with increasing temperature. The magnitude of decrease in rss values of MB–NaC systems with increasing temperature was greater than that of MB–NaDC systems. On comparing the rss values of MB–NaC and MB–NaDC systems, it can be proposed that the temperature dependence of MB–NaC micelles is found to be more than that of the MB–NaDC micelles. This may be due to the higher facial hydrophobicity of NaDC than that of NaC. With increase in temperature, the data obtained indicated that MB gets incorporated into lesser hydrophobic region of NaC and NaDC aggregates. Conversely the MB fluorescence sensed the disaggregation of BSs aggregates with increased temperature.
Fig. 4.
Variation of (A) fluorescence intensity (λex 665 nm, λem 686 nm) and (B) fluorescence anisotropy (λex 665 nm, λem 686 nm) of MB (5 µM) in NaC (0, 4.8, 6, 9.2, 14.4, 16, 19.6, 24, 28.8, 33.6, 38.4, 43.2 mM) and variation of (C) fluorescence intensity (λex 665 nm, λem 686 nm) and (D) fluorescence anisotropy (λex 665 nm, λem 686 nm) of MB (5 µM) in NaDC (0, 2, 4, 6, 8, 10, 12, 14, 16, 18 mM) against different temperatures. T=15 °C, 25 °C, 35 °C and 45 °C; pH=7.4.
3.4. Molecular association of MB–BS media
The molecular association of MB with BSs media was observed from the changes in the photophysical properties of MB as reflected by Fig. 1, Fig. 2, Fig. 3. The absorption (λex 665 nm) and fluorescence property of MB (λex 665 nm; λem 686 nm), with the addition of BS media, show a positive association. Generally, there was an increase in absorbance, fluorescence intensity and rss of MB, under the influence of BS media. This clearly shows that the solubilization of MB was achieved by both the BSs. The trend in the data suggests that there is a possible hydrophobic nature of association between the molecules. Fig. 3 shows a saturation behavior at higher concentrations. Saturation intensities were attained at much lower concentrations of NaDC (at 18 mM) than NaC (at 43.2 mM). Enhancement of fluorescence intensity of MB with increase in BS concentration reflects an increase in the population of MB within the hydrophobic core of BS media; in contrast, the steady state rss reflecting the local rigidity of the immediate environment around MB increases with the increase in BS concentration. Owing to the structural variations of the two different BSs, the greater hydrophobicity of NaDC appears to be a more important factor for greater solubilization.
Another important aspect of the results obtained here reveals the aggregation pattern of BS media. The changes in the photophysical properties of MB in the presence of two BSs proceed along with the progressive micellization of BSs. As the concentration of BSs increases, the aggregation pattern of BSs initially forms a dimeric primary aggregates followed by formation of larger secondary aggregates. Fig. 3 shows that MB can sense the micellization behavior of BSs. It is well known that the BS aggregates do not possess a sharp cmc, rather they possess a cmc range, which is indicative of the transition from dimer to larger secondary aggregates. The data observed here presents a cmc range for NaC as 6–16 mM and for NaDC as to be 4–8 mM, which is in agreement with the literature data [15], [16], [17], [18], [19], [20]. The temperature dependence of MB-BS system was studied and showed a decrease in both fluorescence intensity and rss with increased temperature. The data obtained ascertains the disaggregation of BSs with increasing temperature and thereby provides a lesser hydrophobic core for accommodating MB molecule.
3.5. Molecular mechanics calculations
The interaction between MB and BS was analyzed for their hydrophobic nature by using MM calculations in-built within Hyperchem software. An AMBER force field and Polak-Ribiere Conjugate Gradient Optimizer were used. The heterocyclic aromatic ring of one molecule of MB is allowed to interact with the steroidal face of one molecule of NaC or NaDC within a periodic boundary conditions of dimensions 15–10–20 Å (x-y-z), filled with 51 water molecules. Both the molecules are allowed to interact with their hydrophobic moiety, while their hydrophilic moieties interact with the surrounding water molecules. The energy minimized molecular structures are provided by the heat of formation, with ΔHf values of −625.19 kcal/mol for MB–NaC system and −757.48 kcal/mol for MB–NaDC system.
Fig. 5 shows the feasible hydrophobic interactions between MB and BS molecules. The negative ΔHf values for both MB–BS systems clearly indicate the stable hydrophobic interactions. The ΔHf values for MB–NaDC system, being more negative than the MB–NaC system, further authenticate the hydrophobic nature of their interaction, since NaDC is more hydrophobic than NaC. Thus the minimized energies for the interaction of MB with both the BS molecules further support the experimental results from Fig. 1, Fig. 2, Fig. 3, indicating that the hydrophobicity of BS plays a major role in associating with MB.
Fig. 5.
Energy minimized geometries of (A) MB-NaC and (B) MB-NaDC systems. Atoms indicated in different colors are: cyan – carbon; white – hydrogen; red – oxygen; blue – nitrogen and yellow – sulfur.
4. Conclusions
The association of MB with two BS media, namely NaC and NaDC, was monitored by using the photophysical properties of MB. Generally, the results show that there is an increase in absorbance, fluorescence intensity and rss of MB with increasing BS concentrations. The results obtained here suggest that the nature of association between MB and BS media is hydrophobic, accompanied through an association of the heterocyclic aromatic face of MB with the hydrophobic steroidal face of BS. This observation was further verified and proved with the MM calculations. Thus BSs media can act as a solubilizing agent or a drug delivery medium in formulating MB as a drug. The study also establishes that MB fluorescence property can be used in probing the micellization behavior of NaC and NaDC, where the cmc range predicted for each of the BS molecules is in collaboration with the literature data. The photophysical property of MB also reveals the disaggregation of BSs with increasing temperature. Hence the current study proposes two results viz., (i) the association of MB with BS is hydrophobic in nature and thus leads to solubilization of MB and (ii) MB can be promoted as an extrinsic fluorescence probe for probing the physio-chemical properties of organized media.
Acknowledgments
The author, Dr. Susithra Selvam, is thankful to DST–SERB, India (SB/FT/CS-032/2012) for the financial support and Prof. Ashok Kumar Mishra, Department of Chemistry, Indian Institute of Technology Madras, India for unrestricted usage of spectrofluorometer.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Kochius S., Magnusson A.O., Hollmann F. Immobilized redox mediators for electrochemical NAD(P)+ regeneration. Appl. Microbiol. Biotechnol. 2012;93:2251–2264. doi: 10.1007/s00253-012-3900-z. [DOI] [PubMed] [Google Scholar]
- 2.Bhati K.K., Gangotri K.M. Photogalvanic conversion of solar energy into electrical energy by using NaLS-xylose-methylene blue system. Int. J. Electr. Power Energy Syst. 2011;33:155–158. [Google Scholar]
- 3.Tardivo J.P., Giglio A.D., Oliveira C.S. Methylene blue in photodynamic therapy: from basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther. 2005;2:175–191. doi: 10.1016/S1572-1000(05)00097-9. [DOI] [PubMed] [Google Scholar]
- 4.Oz M., Lorke D.E., Petroianu G.A. Methylene blue and Alzheimer's disease. Biochem. Pharmacol. 2009;78:927–932. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Ghanadzadeh A., Zeini A., Kashef A. Concentration effect on the absorption spectra of oxazinel and methylene blue in aqueous and alcoholic solutions. J. Mol. Liq. 2008;138:100–106. [Google Scholar]
- 6.Singhal G.S., Rabinowitch E. Changes in the absorption spectrum of methylene blue with pH. J. Phys. Chem. 1967;71:3347–3349. [Google Scholar]
- 7.Mukerjee P., Ghosh A.K. The “Isoextraction” method and the study of self-association of methylene blue in aqueous solutions. J. Am. Chem. Soc. 1970;92:6403–6407. [Google Scholar]
- 8.Golz E.K., Griend D.A.V. Modeling methylene blue aggregation in acidic solution to the limits of factor analysis. Anal. Chem. 2013;85:1240–1246. doi: 10.1021/ac303271m. [DOI] [PubMed] [Google Scholar]
- 9.Mcrae E.G., Kasha M. Enhancement of phosphorescence ability upon aggregation of dye molecules. J. Chem. Phys. 1958;28:721–722. [Google Scholar]
- 10.Rabinowitch E., Epstein L.F. Polymerization of dyestuffs in solution. Thionine and methylene blue. J. Am. Chem. Soc. 1941;63:69–78. [Google Scholar]
- 11.Heger D., Jirkovsky J., Klan P. Aggregation of methylene blue in frozen aqueous solutions studied by absorption spectroscopy. J. Phys. Chem. A. 2005;109:6702–6709. doi: 10.1021/jp050439j. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y.J., Li W., Liu Y. Fluorometric investigation of the interaction between methylene blue and human serum albumin. J. Pharm. Biomed. Anal. 2005;39:740–745. doi: 10.1016/j.jpba.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y.J., Liu Y., Zhao R.M. Spectroscopic studies on the interaction between methylene blue and bovine serum albumin. J. Photochem. Photobiol. A. 2006;179:324–329. [Google Scholar]
- 14.Goswami A., Pal M.K. Spectroscopic probes of the interactions of the dye stains-all with deoxycholate and cholate. Colloids Surf. B. 1998;10:149–159. [Google Scholar]
- 15.Patil K., Pawar R., Talap P. Self-aggregation of methylene blue in aqueous medium and aqueous solutions of Bu4NBr and urea. Phys. Chem. Chem. Phys. 2000;2:4313–4317. [Google Scholar]
- 16.Mukhopadhyay S., Maitra U. Chemistry and biology of bile acids. Curr. Sci. 2004;87:1666–1683. [Google Scholar]
- 17.Selvam S., Mishra A.K. Disaggregation of amphotericin B by sodium deoxycholate micellar aggregates. J. Photochem. Photobiol. B. 2008;93:66–70. doi: 10.1016/j.jphotobiol.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Selvam S. A fluorescence parameter based analysis on the solubilization of carvedilol by bile salt media. J. Photochem. Photobiol. B. 2012;116:105–113. doi: 10.1016/j.jphotobiol.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Selvam S., Andrews M.E., Mishra A.K. A photophysical study on the role of bile salt hydrophobicity in solubilizing Amphotericin B aggregates. J. Pharm. Sci. 2009;98:4153–4160. doi: 10.1002/jps.21718. [DOI] [PubMed] [Google Scholar]
- 20.Carey M.C., Small D.M. Micellar properties of dihydroxy and trihydroxy bile salts: effects of counterion and temperature. J. Colloid Interface Sci. 1969;31:382–396. doi: 10.1016/0021-9797(69)90181-7. [DOI] [PubMed] [Google Scholar]
- 21.Santhanalakshmi J., Lakshmi G.S., Aswal V.K. Small-angle neutron scattering study of sodium cholate and sodium deoxycholate interacting micelles in aqueous medium. Proc. Indian Acad. Sci. 2001;113:55–62. [Google Scholar]
- 22.Chae S.Y., Son S., Lee M. Deoxycholic acid-conjugated chitosan oligosaccharide nanoparticles for efficient gene carrier. J. Controll. Release. 2005;109:330–344. doi: 10.1016/j.jconrel.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 23.Lakowicz J.R. 3rd edition. Springer; New York: 2006. Principles of Fluorescence Spectroscopy. [Google Scholar]