Abstract
The Roux-en-Y gastric bypass is one of the most common procedures currently performed for surgical treatment of patients with severe obesity. Gastric cancer after bariatric surgery is not common, with most of them arising in the excluded stomach. Gastric mixed adenoneuroendocrine carcinomas are a rare type of stomach malignancy, composed of both adenocarcinoma and neuroendocrine tumor-cell components, with the latter comprising at least 30% of the whole neoplasm. In this article, we report a unique case of a mixed adenoneuroendocrine carcinoma with a mixed adenocarcinoma (tubular and poorly cohesive) component arising in the gastric pouch of a patient who underwent previous Roux-en-Y gastric bypass for glycemic control. Since stomach cancer is not usual in patients who have formerly undergone bariatric surgery and symptoms tend to be nonspecific, such diagnosis is often rendered at an advanced stage. Full assessment of these patients when presenting such vague symptoms is critical for an early cancer diagnosis.
Keywords: gastric cancer, mixed tumor, MANEC, mixed adenoneuroendocrine carcinomas, neuroendocrine tumors, gastric bypass
Introduction
In spite of a steadily declining incidence over the past 5 decades, gastric cancer is the fifth most common malignancy worldwide. The incidence of these neoplasms after bariatric surgery, however, is uncommon, with few cases reported in the literature, most of them arising in the excluded stomach. Gastric mixed adenoneuroendocrine carcinomas (MANECs) are a rare type of gastric malignancy, composed of both adenocarcinoma and neuroendocrine tumor-cell components. In this article, we report a unique case of a MANEC with a mixed adenocarcinoma component arising in the gastric pouch of a patient who underwent previous gastric bypass for glycemic control.
Case Report
A 61-year-old male, former cigarette smoker, with diabetes mellitus and a past medical history of Fobi-Capella Roux-en-Y gastric bypass for glycemic control in an outside institution, developed an incisional hernia 2 months after the operation. Nineteen months after the procedure, the patient was then submitted to an upper gastrointestinal endoscopy in our service for further evaluation of the hernia before repair surgery. At the time, he was asymptomatic with no significant findings in the clinical examination besides a 10.0-cm hernia over the abdominal midline incision. The endoscopic exam revealed a vegetating, friable lesion in the gastric pouch, near the gastroesophageal junction, measuring approximately 2.0 cm in the greatest diameter. A biopsy of the lesion was performed.
The hematoxylin-eosin slides of the endoscopic biopsy revealed a neuroendocrine neoplasm. Ancillary immunohistochemistry (IHC) studies showed positivity for cytokeratin pool AE1/AE3, cytokeratins 8/18, CD56, synaptophysin, chromoganin A, and Ki-67 index of 30% of the neoplastic cells. Based on those findings, a diagnosis of neuroendocrine carcinoma according to the 2010 World Health Organization (WHO) classification1 was rendered. For staging purposes, computed tomography and an endoscopic ultrasound were performed, which showed a paraesophageal adenomegaly and no signs of invasion of the muscularis propria (cT1 cN1 cM0—TNM 8th edition).2 The patient was then submitted to total gastrectomy with D2 lymph node (LN) dissection and hernia repair.
Grossly, the gastrectomy product showed a 2.0 × 1.5 cm elevated, vegetating lesion. Microscopic examination revealed a MANEC (WHO 20101), constituted by a mixed adenocarcinoma (tubular and poorly cohesive) in association with a neuroendocrine carcinoma (Figure 1), constituting 40% and 60% of the lesion, respectively. The tumor infiltrated on the submucosa, and lymphovascular invasion was detected. The surgical margins were free of tumor cells. The LN dissection revealed 24 LNs, with 2 of them from the lesser curvature compromised by the neoplasia. Surprisingly, one of the LNs was infiltrated by a pure neuroendocrine carcinoma whereas the other was compromised by a pure tubular adenocarcinoma component (Figure 2). The IHC panel findings from the surgical specimen were similar to the biopsy findings. The hernia sac showed no histologic abnormalities.
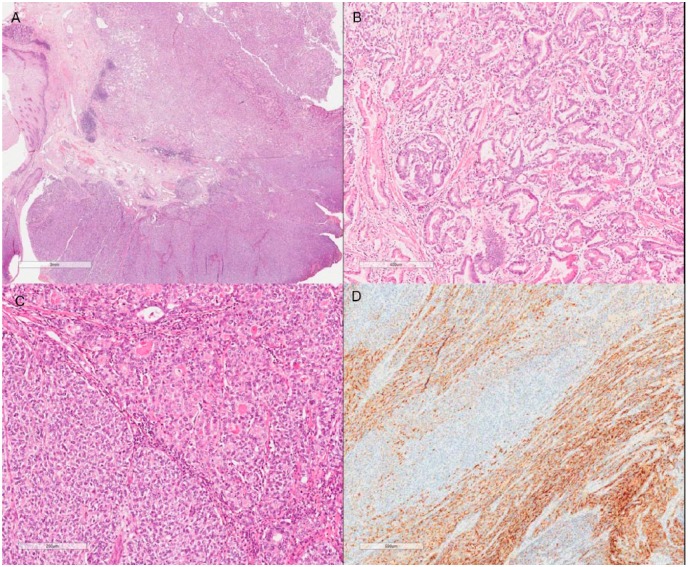
Figure 1.
Mixed adenoneuroendocrine carcinoma (A; hematoxylin-eosin [HE], original magnification 100×) constituted by an adenocarcinoma component (B; HE, original magnification 200×) in association with a neuroendocrine carcinoma (C; HE, original magnification 200×). Chromoganin immunohistochemical stain is positive in the neuroendocrine component (D; original magnification 200×).
Figure 2.
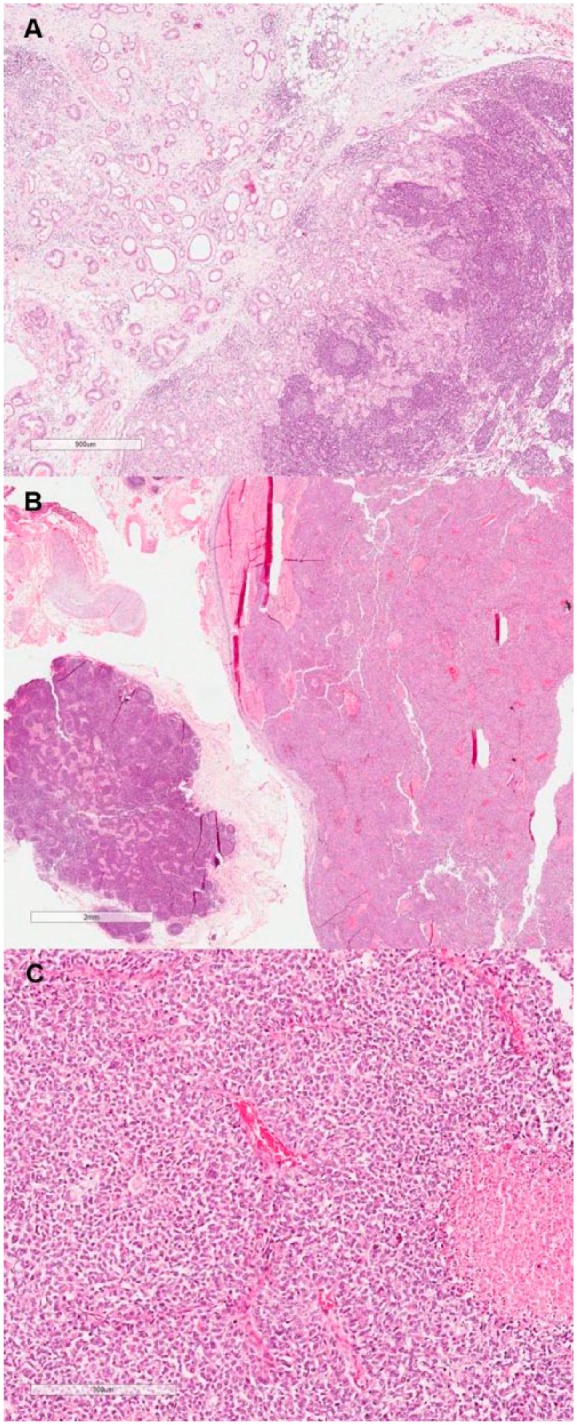
Lymph node metastasis of the adenocarcinoma component (A; hematoxylin-eosin [HE], original magnification 100×) in contrast to another lymph node infiltrated by the neuroendocrine component (B; HE, original magnification 100×). High-power view of the neuroendocrine carcinoma metastatic component (C; HE, original magnification 200×).
Laboratory and imaging postoperative examinations did not reveal residual or metastatic disease. A 12-cycle course of adjuvant chemotherapy including folinic acid, fluorouracil, and oxaliplatin (FOLFOX) was initiated, started at nearly 3 months after the surgery, and the patient tolerated it well.
Almost 3 years after the gastrectomy, the patient had to undergo a small bowel resection due to an acute obstruction caused by adhesions. Three years later, he presented with an expansive retroperitoneal formation on imaging follow-up exams. A computed tomography–guided biopsy of this lesion revealed infiltration of nodal and soft tissues by a pure adenocarcinoma, confirming the recurrence of the disease, this time as a single-component neoplasm. At the time of writing, the patient is now about to start a new course of systemic treatment.
Discussion
Gastric cancer is the fifth most common malignancy worldwide. In spite of a steadily declining incidence over the past 5 decades, nearly 1 million new cases of these cancers were estimated to have happened in 2012, with more than 70% of cases occurring in developing countries.3,4
High body mass index and obesity have been associated with increased risk for cancer.5 Surgery is a treatment option in morbidly obese patients known for effective weight loss and beneficial long-term results. The Roux-en-Y gastric bypass (RYGBP) is one of the most common procedures currently performed for surgical treatment of severe obesity.6 The incidence of gastric cancer after bariatric surgery is rare, with few cases reported in the literature,7 most of adenocarcinomas arising in the excluded stomach,8 in contrast to our patient’s tumor location in the gastric pouch.6,9-14
Gastric MANEC is a rare type of gastric cancer.15 As defined by the 2010 WHO classification, MANECs are composed of both adenocarcinoma and neuroendocrine tumor-cell components, with the latter comprising at least 30% of the whole neoplasm.1 According to the WHO, a minor (<30%) neuroendocrine component can often be present within gastric adenocarcinomas and these tumors should not be classified as MANECS. As stated by Stojsic et al,16 these neoplasms should be regarded as adenocarcinomas with focal neuroendocrine differentiation. Traditionally, mixed epithelial and endocrine cell type tumors were first classified into 3 groups (composite, collision, and amphicrine tumors) based on the 2 components’ relation and distribution.17 The use of different names in the literature to describe these neoplasms led to confusion among clinicians and pathologists.18 In 2000, the WHO classification defined them as mixed exocrine-endocrine carcinomas, and 10 years later, in the current classification, the WHO renamed them as MANECs.1,19
Due to its rarity, scarce specific epidemiological data are available for MANECs in the literature. Furthermore, few aspects are known about their etiology. It is likely that these neoplasms may originate from either the simultaneous proliferation of distinct lines of cells or the proliferation of stem cells able to differentiate into multiple cell lineages.17,20,21
Mixed adenoneuroendocrine carcinomas may occur at any site in the stomach.22-24 Although there are rare descriptions of these tumors arising after gastric surgery,25,26 there have been no reports in the English literature so far of gastric MANECs occurring after RYGBP, particularly in the gastric pouch, as depicted herein.
Clinically, no specific symptoms differing from conventional gastric malignancies have been described to be related to MANECs,27 and some authors indicate that their clinical behavior depends mostly on the neuroendocrine component.28,29 In patients who underwent gastric bypass, stomach cancers tend to present with vague manifestations, including abdominal discomfort, bloating, nausea, and weight loss. Such nonspecific symptoms may be easily confused with the ones secondary to the bypass itself. As a result, a malignancy diagnosis is often rendered at an advanced stage. Full assessment of these patients when presenting analogous symptoms is critical for an early cancer diagnosis.7,8,30
On gross examination, MANECs usually present the same features as conventional gastric cancer.1 Microscopically, the neuroendocrine component is normally composed of a high-grade neuroendocrine carcinoma, and uncommonly a well-differentiated neuroendocrine tumor.22,23 The non-neuroendocrine component is commonly an adenocarcinoma with variable degrees of differentiation.29,31 On exceedingly rare reports in the literature, as illustrated by our case, the exocrine component is formed by a mixed adenocarcinoma.32 As with pure neuroendocrine tumors, MANECs display positivity for neuroendocrine markers on IHC studies restricted to the neuroendocrine component, with expression of carcinoembryonic antigen in some of these cases as well.15,22 There are no data supporting differences in outcome regarding the proportion of each adenocarcinoma and neuroendocrine component of the neoplasm, and so there is no specific guideline in the literature suggesting the necessity to specify the proportion of each neoplasm component in the pathology report of an MANEC.
Genetic studies on gastric MANECs are scarce. Evidence on these indicates a rather higher frequency of chromosomal abnormalities in the neuroendocrine carcinoma in comparison to the adenocarcinoma component. Nonetheless, shared loss of heterozygosity at specific chromosomes proposed a close genetic relation and a potential multistep evolution from a common precursor lesion.23,33
The prognosis for patients with gastric MANECs is usually poor.15 The 5-year survival rate is lower for these patients than for those with conventional stomach adenocarcinomas, and the neuroendocrine component may have a critical role in the prognosis.34 A good prognosis of gastric MANEC is rare and normally restricted to tumors detected in their early stages.35
There is no optimal treatment strategy to date in the management of MANECs.18 Some authors suggest that the most aggressive component should be taken into account when considering the best treatment option.36-38 Surgical resection is mostly indicated and is usually followed by adjuvant therapy.39,40
Previous studies have shown that in MANECs the adenocarcinoma component was mostly located in the mucosa and submucosa, whereas the neuroendocrine component is in the deeper portions of the gastric wall,23,41,42 making a definitive diagnosis of MANEC difficult on preoperative endoscopic biopsy.
In conclusion, we report a unique case gastric cancer arising in a patient who underwent previous Roux-en-Y gastric bypass for glycemic control with 3 very unusual presentations: being localized in the gastric pouch instead of the excluded stomach and histologically present as an MANEC with the additional finding of harboring a mixed adenocarcinoma component. Though gastric cancer is not usual in patients who have formerly undergone bariatric surgery, it is crucial to monitor this population for the development of such malignancy, performing a full and thorough medical evaluation if they develop vague symptoms.
Acknowledgments
We thank Marina França for scanning the slides. We thank Fundacao Antonio Prudente for institutional support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Ethical Approval: Our institution does not require ethical approval for reporting individual cases or case series.
References
- 1. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System.4th ed. Lyon, France: IARC Press; 2010. [Google Scholar]
- 2. Amin MB, Edge SB, Byrd GF, et al. AJCC cancer staging manual. http://www.springer.com/us/book/9783319406176. Accessed September 25, 2017.
- 3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 4. Odze RD, Goldblum JR. Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. 3rd ed. Philadelphia, PA: Saunders; 2014. [Google Scholar]
- 5. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [DOI] [PubMed] [Google Scholar]
- 6. Ribeiro MCB, Lopes LR, de Souza Coelho Neto J, Tericoti V, Jr, Andreollo NA. Gastric adenocarcinoma after gastric bypass for morbid obesity: a case report and review of the literature. Case Rep Med. 2013;2013:609727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuruba R, Jawad M, Karl RC, Murr MM. Technique of resection of esophageal adenocarcinoma after Roux-en-Y gastric bypass and literature review of esophagogastric tumors after bariatric procedures. Surg Obes Relat Dis. 2009;5:576-581. [DOI] [PubMed] [Google Scholar]
- 8. Magge D, Holtzman MP. Gastric adenocarcinoma in patients with Roux-en-Y gastric bypass: a case series. Surg Obes Relat Dis. 2015;11:e35-e38. [DOI] [PubMed] [Google Scholar]
- 9. Raijman I, Strother SV, Donegan WL. Gastric cancer after gastric bypass for obesity. Case report. J Clin Gastroenterol. 1991;13:191-194. [DOI] [PubMed] [Google Scholar]
- 10. Lord RV, Edwards PD, Coleman MJ. Gastric cancer in the bypassed segment after operation for morbid obesity. Aust N Z J Surg. 1997;67:580-582. [DOI] [PubMed] [Google Scholar]
- 11. Khitin L, Roses RE, Birkett DH. Cancer in the gastric remnant after gastric bypass: a case report. Curr Surg. 2003;60:521-523. [DOI] [PubMed] [Google Scholar]
- 12. Watkins BJ, Blackmun S, Kuehner ME. Gastric adenocarcinoma after Roux-en-Y gastric bypass: access and evaluation of excluded stomach. Surg Obes Relat Dis. 2007;3:644-647. [DOI] [PubMed] [Google Scholar]
- 13. Escalona A, Guzmán S, Ibáñez L, Meneses L, Huete A, Solar A. Gastric cancer after Roux-en-Y gastric bypass. Obes Surg. 2005;15:423-427. [DOI] [PubMed] [Google Scholar]
- 14. Harper JL, Beech D, Tichansky DS, Madan AK. Cancer in the bypassed stomach presenting early after gastric bypass. Obes Surg. 2007;17:1268-1271. [DOI] [PubMed] [Google Scholar]
- 15. Kwok CM. Mixed adenoneuroendocrine carcinoma of the stomach. Case Rep Gastroenterol. 2015;9:241-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stojsic Z, Brasanac D, Stojiljkovic M, Babic D, Randjelovic T, Terzic T. Composite carcinoma of the stomach associated with sarcoid-like granulomas. Pathol Oncol Res. 2009;15:503-510. [DOI] [PubMed] [Google Scholar]
- 17. Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11(suppl 1):71-86. [DOI] [PubMed] [Google Scholar]
- 18. La Rosa S, Marando A, Sessa F, Capella C. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers (Basel). 2012;4:11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours: Pathology and Genetics: Tumours of the Digestive System. Lyon, France: IARC Press; 2000. [Google Scholar]
- 20. Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine I. Columnar cell. Am J Anat. 1974;141:461-479. [DOI] [PubMed] [Google Scholar]
- 21. Lee HH, Jung CK, Jung ES, Song KY, Jeon HM, Park CH. Mixed exocrine and endocrine carcinoma in the stomach: a case report. J Gastric Cancer. 2011;11:122-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caruso ML, Pilato FP, D’Adda T, et al. Composite carcinoid-adenocarcinoma of the stomach associated with multiple gastric carcinoids and nonantral gastric atrophy. Cancer. 1989;64:1534-1539. [DOI] [PubMed] [Google Scholar]
- 23. Kim KM, Kim MJ, Cho BK, Choi SW, Rhyu MG. Genetic evidence for the multi-step progression of mixed glandular-neuroendocrine gastric carcinomas. Virchows Arch. 2002;440:85-93. [DOI] [PubMed] [Google Scholar]
- 24. Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168-172. [DOI] [PubMed] [Google Scholar]
- 25. Cazzo E, Saito HPA. Mixed adenoneuroendocrine carcinoma of the gastric stump following Billroth II gastrectomy: case report and review of the literature [in Portuguese]. São Paulo Med J. 2016;134:84-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nemoto H, Tate G, Yokomizo K, et al. Gastric mixed adenoneuroendocrine carcinoma occurring 50 years after a gastroenterostomy with Braun anastomosis. Case Rep Oncol. 2014;7:330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Juanmartiñena JF, Fernández-Urien I, Córdoba A, Miranda MC, Borda A. Mixed adenoneuroendocrine carcinoma (MANEC) of the gastroesophageal junction: a case report and review of the literature. Rev Esp Enferm Dig. 2016;109:160-162. [DOI] [PubMed] [Google Scholar]
- 28. Kim TY, Chae HD. Composite neuroendocrine carcinoma with adenocarcinoma of the stomach misdiagnosed as a giant submucosal tumor. J Gastric Cancer. 2011;11:126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pericleous M, Toumpanakis C, Lumgair H, et al. Gastric mixed adenoneuroendocrine carcinoma with a trilineage cell differentiation: case report and review of the literature. Case Rep Oncol. 2012;5:313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Korswagen LA, Schrama JG, Bruins Slot W, Hunfeld MAJM. Adenocarcinoma of the lower esophagus after placement of a gastric band. Obes Surg. 2009;19:389-392. [DOI] [PubMed] [Google Scholar]
- 31. Rayhan N, Sano T, Qian ZR, Obari AK, Hirokawa M. Histological and immunohistochemical study of composite neuroendocrine-exocrine carcinomas of the stomach. J Med Investig. 2005;52:191-202. [DOI] [PubMed] [Google Scholar]
- 32. Gurzu S, Kadar Z, Bara T, et al. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: Report of two cases. World J Gastroenterol. 2015;21:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furlan D, Cerutti R, Genasetti A, et al. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003;83:963-971. [DOI] [PubMed] [Google Scholar]
- 34. Sandri LGB, Carboni F, Valle M, Visca P, Garofalo A. Mixed adenoneuroendocrine gastric carcinoma: a case report and review of the literature. J Gastric Cancer. 2014;14:63-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fukuba N, Yuki T, Ishihara S, et al. Gastric mixed adenoneuroendocrine carcinoma with a good prognosis. Intern Med. 2014;53:2585-2588. [DOI] [PubMed] [Google Scholar]
- 36. Yang GC, Rotterdam H. Mixed (composite) glandular-endocrine cell carcinoma of the stomach. Report of a case and review of literature. Am J Surg Pathol. 1991;15:592-598. [DOI] [PubMed] [Google Scholar]
- 37. Fujiyoshi Y, Kuhara H, Eimoto T. Composite glandular-endocrine cell carcinoma of the stomach. Report of two cases with goblet cell carcinoid component. Pathol Res Pract. 2005;200:823-829. [DOI] [PubMed] [Google Scholar]
- 38. Ronellenfitsch U, Ströbel P, Schwarzbach MHM, Staiger WI, Gragert D, Kähler G. A composite adenoendocrine carcinoma of the stomach arising from a neuroendocrine tumor. J Gastrointest Surg. 2007;11:1573-1575. [DOI] [PubMed] [Google Scholar]
- 39. Kubota T, Ohyama S, Hiki N, Nunobe S, Yamamoto N, Yamaguchi T. Endocrine carcinoma of the stomach: clinicopathological analysis of 27 surgically treated cases in a single institute. Gastric Cancer. 2012;15:323-330. [DOI] [PubMed] [Google Scholar]
- 40. Hervieu V, Scoazec JY. Mixed endocrine tumors [in French]. Ann Pathol. 2005;25:511-528. [DOI] [PubMed] [Google Scholar]
- 41. Matsui K, Kitagawa M, Miwa A, Kuroda Y, Tsuji M. Small cell carcinoma of the stomach: a clinicopathologic study of 17 cases. Am J Gastroenterol. 1991;86:1167-1175. [PubMed] [Google Scholar]
- 42. Wheeler DA, Chandrasoma P, Carriere CA, Schwinn CP. Cytologic diagnosis of gastric composite adenocarcinoma-carcinoid. Acta Cytol. 1984;28:706-708. [PubMed] [Google Scholar]