Abstract
Background and Aims:
The aim of this study was to estimate the detoxification status of serum and saliva by assessing the serum and salivary Vitamin C in oral potentially malignant disorders and oral cancer.
Subjects and Methods:
A total of 90 subjects, 30 subjects with oral potentially malignant disorders, 30 subjects with oral cancer, and 30 healthy subjects (controls) were included in the study. Serum and saliva were collected and levels of Vitamin C were assessed. Data obtained was analyzed using ANOVA test for the comparison between the groups. Post hoc Tukey's analysis was used for the comparison of the two study groups to the control group. Correlation between the groups was done using Pearson's correlation coefficient test.
Results:
The mean serum and salivary Vitamin C levels were decreased significantly in potentially malignant disorders and oral cancer when compared to healthy subjects.
Conclusions:
As significant reduction of Vitamin C is seen in saliva, it can be stated that saliva can be used as a reliable, noninvasive biomarker in diagnosis and management of potentially malignant disorders and oral cancer.
Keywords: Ascorbic acid, leukoplakia, oral cancer, oral submucous fibrosis, saliva, serum
Introduction
Carcinogenesis is a complex multisequential mechanism which directs a cell from healthy to a precancerous state and finally to an early cancerous stage. Three different stages of carcinogenesis have been defined which include initiation, promotion, and progression.[1] Lipid Peroxidation may perhaps be involved in the process of oral cancer. The essential nutrients which can scavenge free radicals, such as Vitamin E and C comprise a powerful line of defense, which in turn delays free radical induced cellular damage. An increase in lipid peroxidation and a decline in antioxidant activity has been reported in cancer when compared to normal. Antioxidant nutrients can withstand disturbances due to free radicals. Hence, they protect cell membranes against free radical mediated lipid peroxidation.[2]
Ascorbic acid is a chief extracellular antioxidant which has the capacity to entirely restrain oxidative modification of lipids by aqueous peroxy radicals. Being a water-soluble antioxidant, it acts against free radicals before lipid-soluble antioxidants.[3] In comparison to other antioxidants, it disappears quickly, thus sparing other antioxidants. Significant reduction in ascorbic acid levels in the patients compared to healthy individuals may be associated with increased oxidative stress. Vitamin C also influences the immune system, thus intensifying tumor immune surveillance in promotion and progression of cancer. Vitamin C reacts directly with superoxide singlet oxygen. In the process, tocopherol is regenerated from the tocopheroxy radical.[4]
The defenses of the human body against oxidative stress along with free radical induced damage essentially constitutes antioxidant enzymes and nutrients. The antioxidant enzymes are catalase, superoxide dismutase, and glutathione peroxidase which are produced in the body. Their concentrations cannot be influenced. In contrast, dietary or pharmacologic supplementation can influence levels of antioxidant nutrients.[2,5]
The information obtained from the present study would be useful to assess the detoxification status of serum and saliva by means of evaluating Vitamin C levels in potentially malignant disorders and oral cancer.
Subjects and Methods
The present study is observational study conducted in the Department of Oral Medicine and Maxillofacial Radiology, of a dental college in South India. The study was approved by the ethics committees of the University. Participants were provided with a written informed consent before data collection. The study sample comprised 90 subjects in the age group of 30–60 years and included 27 females and 63 males. They were divided into 3 groups of 30 patients each.
Control group (Group I): 30 healthy subjects without any oral lesions
Study group (Group II): 30 healthy subjects histopathologically confirmed with potentially malignant disorders
Study group (Group III): 30 subjects histopathologically confirmed with oral cancer.
Method of collection of data
Sample collection
Informed consent was obtained from the patients included in the study. Ethical clearance was obtained from the concerned authorities. Detailed case history was recorded along with thorough examination of the oral cavity.
Saliva collection
All salivary samples were collected from patients 2 hours after food using spit technique. The patient was asked to sit on the dental chair tilting the head forward and instructed neither to speak nor swallow any saliva. The patient was then instructed to spit in a sterile graduated container every minute for 10 min. The salivary sample represents whole mouth fluid (Major and minor salivary glands and gingival crevicular fluid).
Blood collection
A volume of 5 ml of venous blood was collected from the antecubital vein with syringe and placed in vials containing 3% citric acid. The serum is then extracted and stored at temperature of +4°C in glass vials.
Estimation of Vitamin C by 2,4-dinitrophenyl hydrazine (DNPH) Method
Vitamin C or Ascorbic acid is a good reducing agent. It undergoes conversion to its oxidized form dehydroascorbic acid which is reversible. Both these forms coupled with DNPH to yield an osazone which in turn gives a yellow color with sulfuric acid. Copper in the DNPH reagent functions as a catalyst and the intensity of the color is read at 520 nm.[6]
Method of analysis
The data obtained from this study was analyzed using SPSS version 18.0 Chicago, SPSS inc. Data obtained was analyzed using ANOVA test for the comparison between the groups. Post hoc Tukey's analysis was used for the comparison of the two study groups to the control group. Correlation between the groups was done using Pearson's correlation coefficient test.
Results
The mean age in Group I was 35.2 years. Females comprised 53.33% (16/30) while males comprised 46.67% (14/30). The mean age in Group II was 41.23 years. Males comprised 86.7% (26/30) of the cases, whereas females formed 13.3% (4/30). The mean age of the subjects in Group III was 50.87 years. 76.7% (23/30) were males, whereas females comprised 23.3% (7/30) [Tables 1, 2 and Figure 1].
Table 1.
Distribution of gender within the three groups
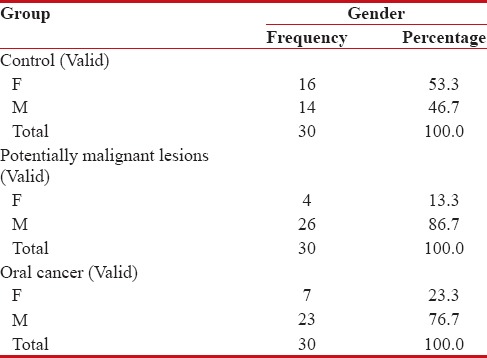
Table 2.
Comparison of age in three groups
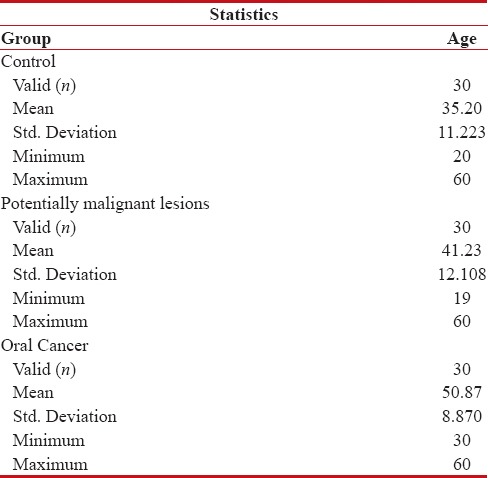
Figure 1.
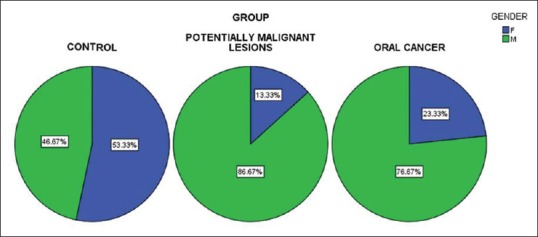
Gender distribution among the three groups
The mean serum Vitamin C levels in Group I was 1.379 mg/dl, whereas the mean serum Vitamin C levels of Group II and Group III were 1.1 mg/dl and 0.7833 mg/dl, respectively. The mean salivary Vitamin C levels in Group I was 0.925 mg/dl, Group II had mean salivary Vitamin C levels as 0.7027 mg/dl and in Group III, it was 0.4787 mg/dl, respectively [Table 3 and Figure 2].
Table 3.
Comparison of serum and saliva levels of vitamin c using one way anova
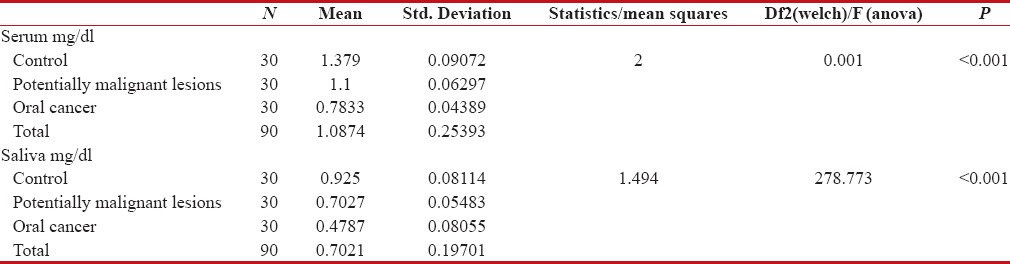
Figure 2.
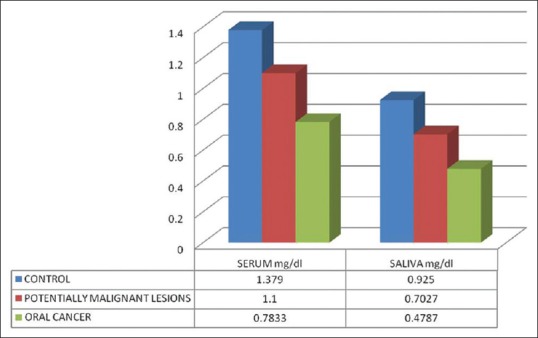
Mean serum and salivary Vitamin C
When serum levels of Vitamin C were compared between Group I (1.379 mg/dl) and Group II (1.1 mg/dl), the difference was statistically highly significant (P < 0.001). Similarly, when the serum levels of Group I was compared with Group III, highly significant difference was obtained (P < 0.001). Statistical comparison between serum levels of Group II and Group III (0.7833 mg/dl) showed highly significant differences (P < 0.001) [Table 4].
Table 4.
Post hoc tukey's test
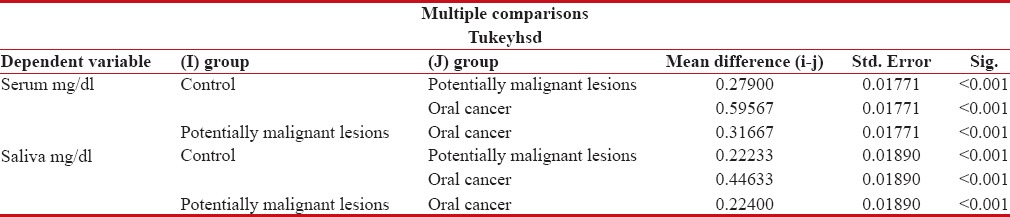
On comparing salivary Vitamin C levels of Group I (0.925 mg/dl) and Group II (0.7027 mg/dl), the difference was highly significant (P < 0.001). Similarly when salivary Vitamin C levels of Group I were compared with levels of Group III (0.4787 mg/dl), highly significant differences were obtained (P < 0.001). When a statistical comparison between salivary levels of Vitamin C levels of Group II and Group III was done, highly significant differences were obtained (P < 0.001) [Table 4]. Very good positive correlation was observed between serum and salivary Vitamin C in all the groups [Figures 3–5].
Figure 3.
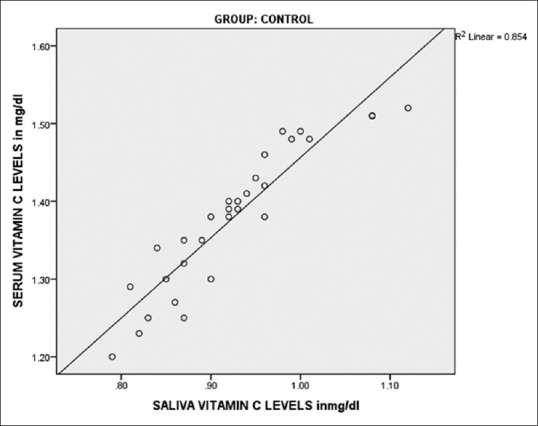
Correlation of serum and salivary Vitamin C in controls
Figure 5.
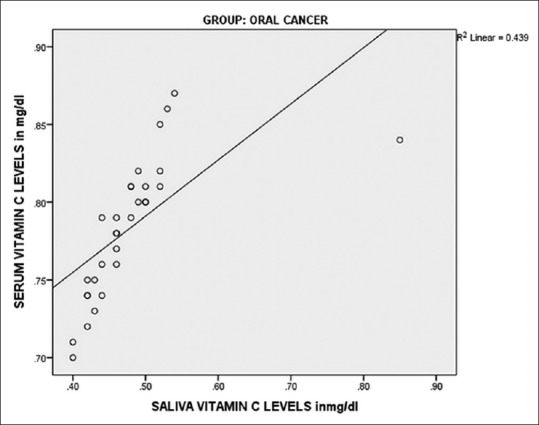
Correlation of serum and salivary Vitamin C in oral cancer
Figure 4.
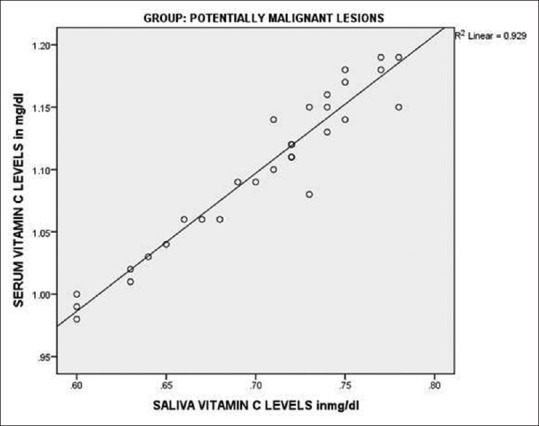
Correlation of serum and salivary Vitamin C in oral potentially malignant disorders
Discussion
The radical scavenging antioxidants have a chief role in the defense systems in vivo which is against oxidative stress induced by free radicals and active oxygen species. Vitamin C is the major water-soluble antioxidant and functions as the primary line of defense against free radicals in whole blood and plasma.[7] Epidemiological studies have revealed that the risk of certain cancers has been inversely proportional to the fruit and vegetable consumption containing essential antioxidant micronutrients, other essential micronutrients along with phytochemicals and fiber. Inverse risk has been associated with cancers of the oral cavity. Animal and cell culture studies have shown that Vitamin E, Vitamin C, beta-carotene, and the mineral selenium prevent the transformation of normal cells to cancer.[8] A reduced intake of fruits and vegetables have been associated with an increased risk of oral and other cancers. The factors conferring protective action from a diet rich in fruits and vegetables are naturally occurring antioxidants present in plant products. The most favorable diet to decrease the risk of oral cancer is frequent consumption of fruits and yellow/green and orange vegetables.[9]
Most of the oral cancer patients undergoing therapy take antioxidant supplements in an effort to alleviate toxicity associated with treatment, thus improving long-term outcome. Antioxidant protection of normal cells occurs in the principle of all treatments even though the mechanism of the chemotherapeutic drug is independent of free radical action. They help in maintaining the health of the normal tissues and protection from toxic effects of free radical producing cytokines which circulate in cancer patients increasing with the disease severity.
Antioxidants compete with oxygen binding along with oxidation of free radicals, thus chemically decreasing the free radicals and repairing the damage. This factor helps in maintenance of the integrity of the normal cells and affects tumor cells to a lesser extent. Tumor cells are exposed in a targeted way which consider variations in the cell cycle and growth patterns to high doses of radiation along with hypoxic cell sensitizers that simulate the effect of oxygen.[8]
Vitamin C reduces the degradation of Vitamin E thus enhancing chemotaxis, phagocytosis and collagen synthesis. It inhibits the formation of nitrosamines and causes reduction in oncogene expression. Vitamin E maintains the integrity of membranes thus inhibiting the growth of cancer cell and differentiation. It also inhibits mutagenicity and formation of nitrosamines. Synergistic action between Vitamin E, selenium and ascorbate hinders DNA and RNA protein synthesis in the cells.[10]
In the present study, the mean serum Vitamin C levels in healthy controls were 1.379 mg/dl, while in potentially malignant conditions, it was reduced to 1.1 mg/dl. This was in accordance with the studies conducted by Shetty et al. and Anuradha and Devi where they stated that since the tissue collagen increased in Oral submucous fibrosis, Vitamin C levels were reduced due to their utilization in the collagen synthesis. Further, the present study is consistent with studies by Tuovinen et al.[12,13] and Barikbin et al. who conducted the study in leukoplakia and lichen planus respectively, where a highly significant reduction in the levels of Vitamin C was observed. The primary role of this antioxidant is detoxification of free radicals, and due to its solubility in water, it can have an effect both extracellularly and intracellularly. Lower serum levels of Vitamin C and the resulting oxidative damage might be important in the pathogenesis of the condition.[13,14]
In the present study, mean serum level of Vitamin C in Oral cancer was 0.7833 mg/dl which was significantly reduced compared to controls, which was in accordance with the studies conducted by Marakala et al. and Gupta et al., thus establishing the role of Vitamin C in antioxidant defense. It acts as a scavenger of free radicals which hinders the detrimental chain reactions triggered by the free radicals which would otherwise result in tissue damage leading to increased oxidative stress.[15,16]
Salivary studies which have been documented in literature are few. In the present study, the mean salivary values of Vitamin C in healthy controls were 0.925 mg/dl, while in potentially malignant disorders it was significantly reduced to 0.7027 mg/dl, which was similar to a study conducted by Shetty et al. in OSMF cases. The reduction was due to the increased utilization of Vitamin C in the collagen production. Vitamin C has the potential to protect both cytosolic and membrane components of cells from oxidative damage. In the cytosol, ascorbate function as a primary antioxidant to scavenge free radical species which are produced as by-products of cellular metabolism.[11]
However, Hegde et al., reported a reduction of salivary Vitamin C levels in potentially malignant disorders when compared to controls, with no significant reduction. The reason for insignificant reduction can be attributed to increased intake of antioxidant vitamins through diet, and also the stoppage of deleterious habits which would have otherwise increased the oxidative stress.[17]
Conclusions
These findings suggest the reduction in serum and salivary levels of Vitamin C, depicting its protective action against free radical mediated lipid peroxidation. Thus, the findings of the present study indicate that estimation of Vitamin C could assist in the early diagnosis of potentially malignant disorders and oral cancer using saliva, which is a reliable biomarker and a noninvasive diagnostic tool.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gupta A, Bhatt ML, Misra MK. Lipid peroxidation and antioxidant status in head and neck squamous cell carcinoma patients. Oxid Med Cell Longev. 2009;2:68–72. doi: 10.4161/oxim.2.2.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai B. Salivary levels Vitamin C and E in different histological grading of oral cancer. Pesqui Bras Odontopediatria Clín Integr. 2008;8:123–5. [Google Scholar]
- 3.Barikbin B, Yousefi M, Rahimi H, Hedayati M, Razavi SM, Lotfi S. Antioxidant status in patients with lichen planus. Clin Exp Dermatol. 2011;36:851–4. doi: 10.1111/j.1365-2230.2011.04152.x. [DOI] [PubMed] [Google Scholar]
- 4.Mireskandari L, Ziai SA, Salehian P, Noormohammadi I, Mahmoudian M. Serum ascorbic acid, Vitamin A and beta carotene levels in Iranian patients with cancer. Arch Iran Med. 1999;9:145–7. [Google Scholar]
- 5.Rai B, Kharb S, Jain R, Anand SC. Salivary Vitamin E and C in lichen planus. Gomal J Med Sci. 2008;6(2):91–92. [Google Scholar]
- 6.Castelli A, Martorana GE, Frasca AM, Meucci E. Colorimetric determination of plasma Vitamin C: Comparison between 2,4-dinitrophenylhydrazine and phosphotungstic acid methods (author's transl)] Acta Vitaminol Enzymol. 1981;3:103–10. [PubMed] [Google Scholar]
- 7.Kayden HJ, Chow CK, Bjornson LK. Spectrophotometric method for determination of tocopherol in red blood cells. J Lipid Res. 1973;14:533–40. [PubMed] [Google Scholar]
- 8.Borek C. Antioxidants and cancer. Sci Med (Phila) 1997;4:51–62. [Google Scholar]
- 9.Niki E, Noguchi N, Tsuchihashi H, Gotoh N. Interaction among Vitamin C, Vitamin E, and beta-carotene. Am J Clin Nutr. 1995;62(6 Suppl):1322S–6S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 10.Lawal AO, Kolude B, Adeyemi BF, Lawoyin JO, Akang EE. Serum antioxidant vitamins and the risk of oral cancer in patients seen at a tertiary institution in Nigeria. Niger J Clin Pract. 2012;15:30–3. doi: 10.4103/1119-3077.94093. [DOI] [PubMed] [Google Scholar]
- 11.Shetty SR, Babu S, Kumari S, Shetty P, Vijay R, Karikal A. Evaluation of micronutrient status in serum and saliva of oral submucous fibrosis patients: A clinicopathological study. Indian J Med Paediatr Oncol. 2012;33:224–6. doi: 10.4103/0971-5851.107087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anuradha CD, Devi CS. Serum protein, ascorbic acid & iron & tissue collagen in oral submucous fibrosis – A preliminary study. Indian J Med Res. 1993;98:147–51. [PubMed] [Google Scholar]
- 13.Tuovinen V, Väänänen M, Kullaa A, Karinpää A, Markkanen H, Kumpusalo E. Oral mucosal changes related to plasma ascorbic acid levels. Proc Finn Dent Soc. 1992;88:117–22. [PubMed] [Google Scholar]
- 14.Barikbin B, Yousefi M, Rahimi H, Hedayati M, Razavi SM, Lotfi S. Antioxidant status in patients with lichen planus. Clin Exp Dermatol. 2011;36:851–4. doi: 10.1111/j.1365-2230.2011.04152.x. [DOI] [PubMed] [Google Scholar]
- 15.Marakala V, Malathi M, Shivashankara AR. Lipid peroxidation and antioxidant vitamin status in oral cavity and oropharyngeal cancer patients. Asian Pac J Cancer Prev. 2012;13:5763–5. doi: 10.7314/apjcp.2012.13.11.5763. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Bhatt ML, Misra MK. Lipid peroxidation and antioxidant status in head and neck squamous cell carcinoma patients. Oxid Med Cell Longev. 2009;2:68–72. doi: 10.4161/oxim.2.2.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegde N, Suchetha NK, Hegde MN, Chandra MP, Nireeksha Lipid peroxidation and Vitamin C levels in saliva of oral precancerous patients – An in-vitro study. Res J Pharm Biol Chem Sci. 2011;2:13–8. [Google Scholar]


