Abstract
Bordetella pertussis (Bp) is the etiologic agent of pertussis or whooping cough, a highly contagious respiratory disease occurring primarily in infants and young children. Although vaccine preventable, pertussis cases have increased over the years leading researchers to re-evaluate vaccine control strategies. Since bacterial outer membrane proteins, comprising the surfaceome, often play roles in pathogenesis and antibody-mediated immunity, three recent Bp circulating isolates were examined using proteomics to identify any potential changes in surface protein expression. Fractions enriched for outer membrane proteins were digested with trypsin and the peptides analyzed by nano liquid chromatography-electrospray ionization-mass spectrometry (nLC-ESI-MS), followed by database analysis to elucidate the surfaceomes of our three Bp isolates. Furthermore, a less labor intensive non-gel based antibody affinity capture technology in conjunction with MS was employed to assess each Bp strains' immunogenic outer membrane proteins. This novel technique is generally applicable allowing for the identification of immunogenic surface expressed proteins on pertussis and other pathogenic bacteria.
Keywords: Pertussis, Proteomics, Membrane, Gel-free, LC-MS/MS, Antibody-affinity
1. Introduction
Bordetella pertussis (Bp) is the etiologic agent of pertussis or whooping cough, a highly contagious respiratory disease occurring primarily in infants and young children (Bordet and Gengou, 1906; Singh and Lingappan, 2006). Current vaccines used in the United States are acellular. They consist of three to five Bp proteins (Locht, 2008; Taylor and Fahm, 1999), including filamentous hemagglutinin adhesin (FHA), pertactin (Prn), pertussis toxin (Ptx), and fimbrae 2 and 3. These latter proteins are purified from Bp strains isolated from the 1940s and 1950s. Many countries throughout the world, however, continue to use whole-cell vaccines. Although pertussis is a vaccine-preventable disease, the World Health Organization (WHO) estimates that 30–50 million cases per year occur worldwide, with approximately 300,000 deaths (http://www.cdc.gov/). In fact, pertussis cases have increased over the years, leading researchers to reevaluate vaccine control strategies. This resurgence in pertussis cases has occurred globally (King et al., 2001; Das, 2002) and has occurred in populations or areas previously immunoprotected by vaccination. Although the cause for the observed increased disease incidence is not fully understood, possible contributing factors are better diagnostics and surveillance, waning vaccine-induced immunity, suboptimal vaccine formulation, and variation between circulating isolates and vaccine strains (He and Mertsola, 2008; Matoo and Cherry, 2005; Bart et al., 2010) which are all currently under investigation.
With the sequencing of many microbial pathogen genomes complete (Parkhill et al., 2003) or underway, many researchers have relied on functional genomics to translate the genetic “blueprint” of an organism and to understand biological processes. But with constantly advancing methods and technologies, the use of proteomic-based strategies has emerged as an option to study cellular function. In general, proteomics revolutionized in the mid 1970s (O'Farrell, 1975; Wilkins et al., 2007) is the analysis of an organism's proteome or, in essence, its complete array of expressed genes or proteins.
Traditional proteomic approaches, such as one- (1D) or two-dimensional (2D) gel electrophoresis (GE), are common technologies to visualize and separate proteins based on molecular weight and/or isolectric point (pI). 1D and 2D-GE, albeit fruitful, can be labor intensive and not without technical challenges. For instance, 1D-GE cannot sufficiently resolve very large proteins or complexes that generally are membrane-affiliated and hydrophobic in nature. Also, small proteins often expressed in low abundance may escape visual detection dependent upon the rate of gel migration (Kustos et al., 2007). However, to overcome these limitations subcellular compartments, such as outer membrane proteins (OMPs) or surfaceomes can be isolated by physical or chemical means, and further enriched using sodium carbonate (Thein et al., 2010) followed by differential centrifugation. Fractionation reduces sample complexity and promotes further examination by GE or mass spectrometry (MS). Nano liquid chromatography-electrospray ionization tandem MS (nLC-ESI MS/MS) is a powerful and sensitive analytical tool used to further elucidate and characterize proteins in complex mixtures (Dworzanski and Snyder, 2005; Han et al., 2008). Proteins can be proteolytically cleaved by enzymes such as trypsin, generating peptides that are first separated by differential retention on the LC column then ionized and separated based on their mass-to-charge ratio (m/z). The proteins from which they originate are identified based on the comparison between MS/MS fragmentation patterns and protein databases (Chen and Prama, 2008).
Over the years, gel-based strategies in parallel with MS have been used with great success in the characterization of bacterial surfaceomes (surface membrane fraction). For example, Somner et al. (2010) performed a comparative surfaceome analysis of pathogenic enterotoxigenic Escherichia coli (ETEC) and commensal strains using gel and MS-based proteomic approaches. In addition, nLC-ESI MS/MS analysis was used to profile the surfaceome of four genetically distinct Staphylococcus aureus strains (Dreisbach et al., 2010). Thein et al. (2010) evaluated the efficiency of multiple surfaceome isolations using nLC-ESI MS/MS, GE, and immunoblotting methodologies for OMP identification of various gram negative bacteria, including Pseudomonas aeruginosa. And Jabbour et al. (2010) performed a high-throughput proteomics study using nLC-ESI MS/MS to identify cellular proteins from pathogenic E. coli 0157:H7 and Yersinia pestis. Lastly, Bottero et al. (2007) described a procedure for the enrichment of Bp outer membrane proteins (OMPs) followed by protein identification using GE-associated mass spectrometric technologies and database search analysis as the basis for novel pertussis vaccine development.
Bp, a gram-negative organism, contains outer and cytoplasmic (or inner) membranes separated by a periplasmic space. Proteins embedded within the membrane and surface-exposed are of biological importance. These bacterial proteins act as front-line barriers to the hosts' antibody-mediated cellular environment. They contain possible virulence factors, and they play a role in the attachment to host cells as well as in the transport of nutrients into the bacteria needed for growth and survival (Poolman et al., 1990; van den Berg et al., 1999; Kustos et al., 2007; Lee et al., 2008). Thus, further examination of this subproteome (in particular for clinical pathogens) by GE or advanced technologies such as MS, would be fruitful for the development of novel diagnostics, strain comparison, or potentially for improved vaccine development.
In this study, a comparative qualitative proteomic assessment of three clinical Bp strains isolated in the United States and the well-typed acellular and whole-cell vaccine strain Tohama I was investigated. Since changes in OMP expression might affect several bacterial functions such as adherence and pathogenesis with possible implications on host cellular immunity (Kustos et al., 2007; Jabbour et al., 2010), we examined the surfaceome and immunoproteome (i.e., antigenic proteins that invoke an immune response). Protein profiles were generated using a multi-combinatorial approach of 1D-GE and/or direct nLC-ESI MS/MS tryptic peptide detection and OMP identification via database search analysis. Additionally, immunoblot-associated MS analysis and a novel approach using antibody affinity magnetic-bead-capture coupled to MS were used to identify Bp immunoreactive proteins. This antibody affinity, magnetic-bead-capture technique proved to be a labor-saving strategy that assisted in the quick assessment of Bp protein immunoreactivity. This strategy shows great promise as an expeditious approach – applicable to a broad spectrum of organisms – to identify surface-expressed antigens which, once detected and identified, could be used for strain comparisons and for improved diagnostics.
2. Materials and methods
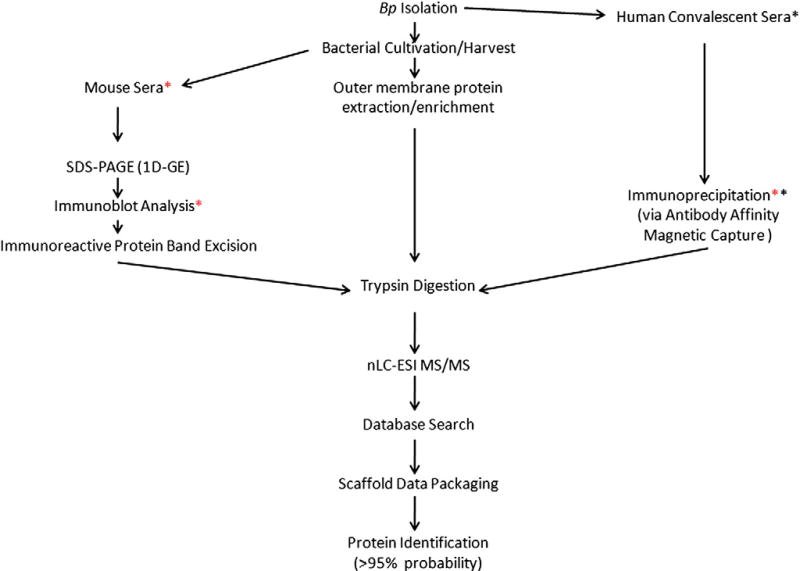
Fig. 1 is a flow diagram schematically summating the core methodologies used in this study.
Fig. 1.
Flow diagram of core methodologies used in the proteomic study. Red Asterisk (*) indicates the mouse sera was used for the immunoblot analysis and immunoprecipitation. Black asterisk (*) indicates human convalescent sera was used for the immunoprecipitation.
2.1. Reagents
All reagents and media not vendor-specified were prepared at the Centers for Disease Control and Prevention (CDC) Core Facility. In addition, chemicals used in experimentation were obtained from either Sigma-Aldrich Chemical Company (St. Louis, MO, USA) or Fisher Scientific (Pittsburg, PA, USA) as noted.
2.2. Bacterial strains
Four Bp strains, Tohama I (T) and three clinical isolates designated by CDC as C056 (C), D946 (D) and F656 (F) were used in the proteomic comparison (Table 1). T, first isolated in Japan in 1954, is a well-characterized and completely sequenced Bp strain that has been used as the basis of vaccines in many countries for several years (Advani et al., 2004). By pulse-field gel electrophoresis (PFGE) analysis, it is characterized as type II and possesses the pertactin 1 (prnA1) and pertussis toxin (ptxS1B) genotype typical of prevaccine-era isolates (Advani et al., 2004; van Loo et al., 2002; Litt et al., 2009). Strain C was isolated in Minnesota in 1998, has a PFGE CDC type 10 (Hardwick et al., 2002), and a prnA2, ptxS1A genotype common among currently circulating isolates (Litt et al., 2009). The D strain, a clinical isolate identified in Georgia in 2002, has a PFGE CDC 21, prnA1 and ptxS1A genotype. It has shown resistance to the antibiotic erythromycin. Lastly, the F strain (PFGE CDC 206, prnA2, ptxS1A) was isolated from a clinical case in 2007 in the Virgin Islands.
Table 1.
Genomic profiles of Bordetella pertussis (Bp) strains assessed in the study.
| Strain | Isolation location (Year) | PFGE | Pertactin | Pertussis toxin |
|---|---|---|---|---|
| T | Japan (1954) | Type II | prnA1 | ptxS1B |
| C | Minnesota, USA (1998) | CDC type I0 | prnA2 | ptxS1A |
| D | Georgia, USA (2002) | CDC type 21 | prnA1 | ptxS1A |
| F | Virgin Islands, USA (2007) | CDC type 206 | prnA2 | ptxS1A |
Abbreviations: T — Tohama I, C — C056, D — D946 and F — F656; USA — United States of America; CDC — Centers for Disease Control and Prevention; PFGE — pulse field gel electrophoresis; Prn — pertactin; Ptx — pertussis toxin.
2.3. Bacterial cell culture
T, C, D, and F were plated on Bordet-Gengou agar and incubated at 35 °C with 5% CO2 for 4 days (Hulbert and Cotter, 2009). The bacteria were subsequently subcultured into Modified Stainer-Schulte (MSS) media at 35 °C, with aeration at 200 rpm in a Beckman-Coulter shaker (Beckman-Coulter, Brea, CA, USA) until an optical 1.0 density was reached. The bacterial strains were then pelleted from MSS by centrifugation at 8000×g for 30 min (min) at 4 °C. The pellets were washed two times in distilled water (dH2O) and stored at −70 °C for further use.
2.4. Enriched membrane fraction collection
Enriched membrane fractions (EMFs) were collected as previously described (Molloy, 2008) with the following modifications. Briefly, cell pellets of Bp isolates were allowed to thaw gently on ice. The pellets were French-pressed at 16,000 psi in 5 ml of a 50-mM Tris–HCl (pH 8.0) buffer containing a protease inhibitor cocktail added at the manufacturer's recommendation (GE Healthcare, Piscataway, NJ, USA), and 25U of benzonase to rupture bacterial cells. The lysates were centrifuged (8000×g, 20 min, 4 °C) to remove unbroken cells, and the supernatant containing the total extracted proteome was retained. 50 ml of ice-cold sodium carbonate (pH 11.0) was added to 5 ml of each bacterial supernatant. The mixture was stirred gently at 4 °C for 2 h. The sodium carbonate infused-supernatants were subjected to ultracentrifugation (Beckman-Coulter) (115,000×g, 60 min, 4 °C) to enrich for a membrane protein fraction. The pellets containing the EMFs were washed in a 50 mM Tris–HCl buffer (pH 8.0) and ultracentrifuged twice (115,000×g, 30 min, 4 °C) to remove the enrichment buffer. The final EMFs were solubilized in 1 ml of solubilization buffer containing 7 M urea, 2 M thiourea, 2% CHAPS, 10% isopropanol, and protease inhibitor cocktail, with an optional addition of 0.5% bromophenol blue to visually assess the integrity of protein isolation. Protein concentrations of the samples were determined using a 2D-Quant Kit (GE Healthcare), and the samples were aliquoted and frozen at −20 °C until further use.
2.5. B. pertussis immune sera
Three-week-old female BALB/C mice were initially injected intraperitoneally (i.p.) with 1 × 109 colony forming units (cfu) of T, C, D, or F suspended in 10 µl of physiological saline (pH 7.2). Before injection, strains were cobalt-irradiated using 5 × 106 γ RAD to inhibit bacterial replication and infectivity, while preserving bacterial surface structures. The process was repeated 2 weeks later, every 2 weeks thereafter, with three separate i.p. immunizations of similar dosage for 6 weeks. At this time, mice were euthanized according to AALAC and IACUC standards and the Bp immune sera generated from each strain were collected from blood. The collected serum was aliquoted and stored at −70 °C until use. Additionally, a serum pool composed of sera drawn from convalescent pertussis human patients obtained from the CDC Pertussis Laboratory was used in this analysis. This pool is the Pertussis Laboratory ELISA standard reference sera acquired in accordance with CDC Institutional Review Board standards and regulations.
2.6. 1D sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis of B. pertussis EMF
Unless specified, materials, antibodies, and procedures for GE and immunoblot analysis were obtained from Bio-Rad (Hercules, CA, USA). 10 µg of total EMF proteins from each strain was suspended in Laemmli sample buffer and electrophoresed on 12.5% SDS-PAGE gels following standard protocols (Laemmli, 1970). To visualize separated proteins, gels were stained using the hot coomassie blue staining protocol. EMF proteins were electroblotted (Towbin et al., 1979) onto polyvinylidene fluoride (PVDF) membranes for 1 h and probed with either a primary Bp strain-specific immune or preimmune (normal) mouse serum. Immunoreactive bands were further probed with a secondary goat-anti-mouse horseradish peroxidase-conjugated IgG antibody and subsequently visualized with 1,4-benzenediamine dihydrochloride (Sigma-Aldrich).
2.7. PVDF on-membrane protein extraction
Protein extraction directly from blotted-PVDF membranes was performed based on Bienvenut et al. (1999), but modified accordingly. PVDF membrane bands containing EMF proteins from T and C associated with immunoreactivity were excised and destained with 50% methanol (500 µl) for 2 h at room temperature (RT). After destaining, the supernatant was removed. The membrane pieces were air dried, followed by the addition of 50 mM ammonium bicarbonate (NH4(CO3)2) digestion buffer in 30% acetonitrile (ACN) (Fisher Scientific). The protein-containing membrane pieces were then incubated overnight (ON) with trypsin (0.1 µg/µl) (Promega Corporation, Carlsbad, CA, USA) at 37 °C. After digestion, the supernatant was collected and the membranes were treated with 80% ACN to extract the peptides and sonicated at level nine (Aquasonic™ model 150-D)(VWR Scientific Products, Suwanee, GA) for 15 min. Following sonication, the extract was pooled with the previous supernatant, dried via vacuum centrifugation, and resuspended in dH2O (50 µl). Samples were prepared for nLC-ESI-MS/MS, in which peptides were suspended in equal volumes of 0.1% formic acid.
2.8. EMF protein identification
T, C, D, or F EMFs (10 µg) before direct proteolytic cleavage were treated with 0.1% rapigest (RG) (Waters Corporation, Milford, MA, USA) in NH4(CO3)2 digestion buffer at 100 °C for 5 min to denature proteins. Upon cooling at RT, the samples were incubated ON with trypsin (10 µg) (Promega) at 37 °C. After incubation, the RG was inactivated in the presence of 1 M HCl for 30 min at 37 °C, and centrifuged at 12,000×g for 15 min. The supernatant was removed and suspended in equal volumes of 0.1% formic acid and analyzed by nLC-ESI MS/MS. The data obtained represent two distinct biological preparations, each performed in triplicate.
2.9. Immunoprecipitation studies using antibody affinity magnetic bead capture technology
Dynal beads (Invitrogen Corporation, Carlsbad, CA, USA) coated with protein G for immunoglobulin (IgG) capture and subsequent immunoprecipitaton (IP) of EMFs were used as per the manufacturer's recommendation with the following changes. The Dynal beads (200 µl per sample) were initially washed three times via resuspension in 800 µl phosphate citrate buffer (PCB), pH 5.0 (Sigma-Aldrich). Next, the beads were resuspended in 800 µl PCB and incubated ON at 37 °C in the presence of immune sera (100 µg total) from mice immunized against T, C, D or F. We also prepared controls containing normal mouse immune sera (100 µg total) and beads only. After incubation, the beads were magnetically stabilized, the supernatant was removed, and the beads were washed two times with 2 M triethanolamine, pH 8.2 (Sigma-Aldrich). This was to remove unbound antibodies and to equilibrate the beads for antibody crosslinking. The immune-sera bound beads were next cross-linked with 1 ml 20 mM dimethyl pimelimidate (Sigma-Aldrich) in 2 M triethanolamine for 30 min at RT via inversion. Following crosslinking, the beads were washed two times in 800 µl phosphate buffer saline (PBS), pH 7.0 with 0.1% Tween 20 and further incubated with 800 µl TBE (Sigma-Aldrich) to reduce non-specific (NS) protein binding. The beads were resuspended in 50 µl dH2O. Then they were incubated at 37 °C ON in the presence of T, C, D or F EMFs (10 µg) that corresponded with the bead-Ab source strain (e.g., beads bound with T-specific IgG were incubated in the presence of T-EMF). After magnetic stabilization, the beads were washed via a mixer three times for 5 min with 100 µl PBS at RT to remove any unbound or NS-bound EMF proteins. The protein-bound Ab-coupled complexes were resuspended in 50 µl NH4(CO3)2 digestion buffer treated with 0.1% RG followed by ON trypsin (10 µg) digestion at 37 °C.
Upon incubation, the IP complex was magnetically stabilized, and the supernatant containing EMF tryptic peptides was transferred to a fresh tube and dried via vacuum centrifugation to concentrate samples. The RG was inactivated and the samples prepared for nLC-ESIMS/MS, in which peptides were suspended in equal volumes of 0.1% formic acid. The data represent two biological preparations, each performed in duplicate. Simultaneously, Dynal beads were conjugated with pooled human convalescent sera (100 µg total) resulting from Bp infection in addition to normal human IgG (Interstate Blood Bank, Inc., Memphis, TN, USA). The IgG bound-beads were incubated with T, C, D, or F EMF (10 µg), and the samples were processed as described above.
2.10. Nano liquid chromatography electrospray ionization mass spectrometry
Protein identification was achieved by using nanoflow liquid chromatography (nano-LC), data-dependent tandem mass spectrometry, and database searching. A pulled-needle, fused silica capillary (365 µm O.D. by 75 µm I.D.) (New Objective, Inc., Woburn, MA) was packed with 10 cm of 5 µm Symmetry 300 reverse-phase packing material (Waters Inc., Bedford, MA). Protein digests were loaded onto the analytical column and separated by gradient elution using an Eksigent 2D nanoLC system (Eksigent Technologies, Inc, Dublin, CA). The mobile phase solvents consisted of (solvent A) 0.2% formic acid (Thermo Scientific, Rockford, IL), 0.005% trifluoroacetic acid (Sigma-Aldrich) in water (Burdick and Jackson, Muskegon, MI), and (solvent B) 0.2% formic acid, 0.005% trifluoroacetic acid in acetonitrile (Burdick and Jackson). The gradient flow was set at 400 nl/min. The profile consisted of a hold at 5% B for 5 min followed by a ramp to 30% B over 100 min, then a ramp up to 90% B in 5 min and a hold at 90% for 2 min before returning to 5% B in 2 min and re-equilibration at 5% B for 20 min. After chromatography, peptides were introduced into an LTQ Orbitrap tandem mass spectrometer (Thermo Scientific, San Jose, CA). A 2.0 kV voltage was applied to the nano-LC column. The mass spectrometer was programmed to perform data-dependent acquisition by scanning the mass range from mass-to-charge (m/z) 400 to 1600 at a nominal resolution setting of 60,000 for parent ion acquisition in the Orbitrap. Most tryptic peptides fall within the stated m/z range and served as the basis for this selection. For MS/MS analysis the mass spectrometer chose the top 10 most intense ions with two or more charges. Singly charged ions were rejected for MS/MS as these ions are likely due to detergents or other sample additives. In particular for a data-dependent acquisition, time is better utilized acquiring for doubly and triply charged amino acids, which predominantly have greater sequence specificity since they are larger peptides and thus provide a higher likelihood in which to uniquely identify a protein.
All tandem mass spectra were extracted from the raw data file using Mascot Distiller (Matrix Science, London, UK; version 2.2.1.0) and searched using Mascot (version 2.2.0). Mascot was set up to search using the entire NCBInr database or a modified NCBInr database created to search “Bordetella”- or “pertussis”- recognized proteins in which trypsin is used as the digestion agent. Mascot was searched with two missed cleavages, a fragment ion tolerance mass of 0.80 Da, and a parent ion tolerance of 200 ppm, while oxidation was selected as a variable modification. Scaffold (Proteome Software, Portland, OR) was used to validate MS/MS based peptide and protein identifications.
Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm (Keller et al., 2002). Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least two identified peptides (Nesvizhskii et al., 2003). With these stringent parameters of Peptide Prophet and Protein Prophet within the Scaffold software, the probability of a wrong assignment is below 0.1%. PSORTb subcellular scores were used to predict and localize identified EMF proteins (http://www.psort.org/psortb/) (Yu et al., 2010). Lastly, KEGG identifiers using NCBI Gi accession numbers were employed to assign functions to each of the identified proteins http://www.genome.jp/kegg/kegg3.html (Tefon et al., 2011).
3. Results
3.1. 1GE and immunoblot analysis of B. pertussis species EMFs
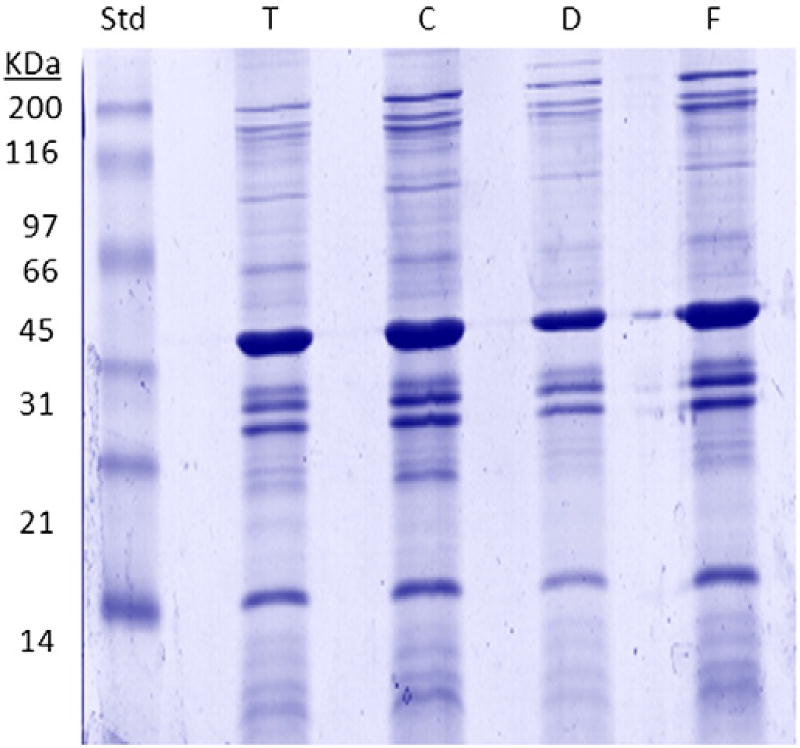
Carbonate-enriched EMF proteins were initially separated by 1D-SDS-PAGE (1D-GE). This was to observe common and differential banding patterns between the T-reference strain and clinical isolates C, D, and F. Overall, similar protein profiles among the strains were observed, with 1D-GE revealing slight differences and no unique protein banding patterns between the strains (Fig. 2). Subsequently, after observing no major protein differences, we used classical immunoblotting approaches to assess the strain's ability to invoke an immune response in vivo. EMF-transferred PVDF membranes, probed with strain-specific mouse antisera, showed comparable patterns of immunoreactivity between all four strains. Likewise, the similarity in the immunoblot corresponded with the heavy banding patterns between 30 and 70 kDa observed in the stained gel (data not shown).
Fig. 2.
SDS-PAGE of Bp T, C, D, and F enriched membrane fractions (EMF). 10 µg of total carbonate-extracted EMF proteins from each strain was suspended in Laemmli sample buffer, electrophoresed on 12.5% SDS-PAGE gels, and stained using the hot coomassie blue staining protocol. Abbreviation: T — Tohama I, C — C056, D — D946 and F — F656; SDS-PAGE — sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Collectively, the gel and immunoblot results indicate similarity in the proteins expressed among these strains. And nominal differences appeared between the immune responses seen in mice against T, C, D, and F. Nevertheless, to delineate any significant variability which was not visually observable, a few major immunoreactive protein bands from 30 to 35 and 40 to 45 kDa were analyzed for T and C. Peptides extracted from tryptically digested, excised-PVDF membranes were separated and analyzed using nLC-ESI-MS/MS. Database mining revealed a few proteins in both the T and C highly immunoreactive bands. These included a 42-kDa outer membrane porin precursor (OmpP), a 40-kDa outer membrane porin protein OmpQ (OmpQ), and a 40-kDa putative exported protein. The C-EMF-probed excised protein bands also identified a 33-kDa putative membrane protein.
3.2. Direct surfaceome analysis of Bp EMFs using nLC-ESI-MS/MS
Although Bp EMF proteins were separated and visualized using GE, and a few further identified by an on-membrane analysis in combination with MS, a more direct and less time-consuming surfaceome analysis was ultimately implemented.
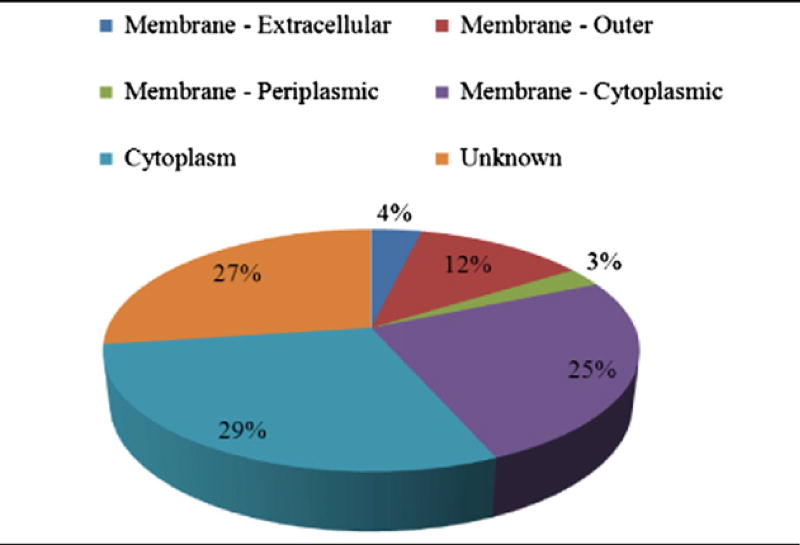
RG-treated and trypsinized-EMFs from the four strains were analyzed directly using nLC-ESI MS/MS. This was followed by database searching, in which 259, 249, 253, and 245 proteins were identified for T, C, D and F, respectively. However, based on greater than 95% Scaffold protein identification and amino acid coverage probabilities, 193 total proteins among all four strains were further selected (Table 2A). Moreover, using PSORTb subcellular localization scores, proteins considered membrane (outer, periplasmic, or cytoplasmic) or found in the cytoplasm accounted for 44% and 29%, respectively, of the total “surfaceome” identified in this study. The remaining proteins classified as “unknown” were possibly localized to the membrane or cytoplasm and comprised 27% of the total surfaceome (Table 2B).
Table 2.
| A Summary of the total number of Bp enriched membrane fraction (EMF) proteins commonly and uniquely identified among the Bp strains assessed in the study. | |
|---|---|
| Strains | Total number of proteins |
|
| |
| T | 182 |
| C | 175 |
| D | 175 |
| F | 176 |
| Strain combinations | Total number of common proteins |
|
| |
| T, C, D, and F | 163 |
| T and C only | 1 |
| T and D only | 3 |
| T and F only | 0 |
| C and D only | 0 |
| C and F only | 0 |
| D and F only | 0 |
| T, C and D only | 1 |
| T, C and F only | 5 |
| T, D and F only | 2 |
| C, D and F only | 3 |
| Strains | Total number of unique proteins |
|
| |
| T only | 7 |
| C only | 2 |
| D only | 3 |
| F only | 3 |
| D Summary of Bp EMF proteins identified among Bp strains assessed in the study (** — Table 2A). | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Protein | Accession # |
ID | Gene | Size (kDa) |
Location | T | C | D | F | Function |
| 30S ribosomal protein S2 | NP_880161 | BP1419 | rpsB | 28 | C | 28 (4) | 23 (3) | 21 (3) | 23 (3) | Translation |
| 30S ribosomal protein S3 | NP_882129 | BP3619 | rpsC | 29 | C | 27 (4) | 27 (4) | 27 (5) | 27 (4) | Translation |
| 50S ribosomal protein L16 | NP_882130 | BP3620 | rplP | 15 | C | 26 (3) | 36 (4) | 19 (2) | 29 (3) | Translation |
| 50S ribosomal protein L18 | NP_882141 | BP3632 | rplR | 13 | C | 20 (2) | 20 (2) | 30 (3) | 20 (2) | Translation |
| 50S ribosomal protein L2 | NP_882126 | BP3616 | rplB | 30 | C | 14 (3) | 11 (2) | 11 (2) | 17 (3) | Translation |
| 50S ribosomal protein L5 | NP_882137 | BP3628 | rplE | 20 | C | 17 (3) | 12 (2) | 12 (2) | 18 (3) | Translation |
| 50S ribosomal protein L6 | NP_882140 | BP3631 | rplF | 19 | C | 27 (3) | 27 (3) | 27 (3) | 19 (2) | Translation |
| 2-Isopropylmalate synthase | NP_879030 | BP0131 | leuA | 62 | U | 6 (2) | 14 (5) | 8 (3) | 11 (4) | Metabolism |
| 5-Meta^ | NP_881170 | BP2543 | metA | 84 | U | 1 (1) | 4 (2) | 4 (2) | 4 (2) | Metabolism |
| ABC transporter | NP_879529 | BP0697 | U | 29 | CM | 22 (3) | 22 (3) | 27 (4) | 28 (4) | Membrane transport |
| Acteyl-CoA carboxylase carboxyltransferase subunit alpha | NP_880596 | BP1910 | accA | 35 | C | 17 (4) | 29 (6) | 24 (5) | 17 (4) | Metabolism |
| Aconitate hydratase | NP_880684 | BP2014 | acnA | 99 | C | 5 (3) | 5 (3) | 3 (2) | 4 (2) | Metabolism |
| Acriflavine resistance protein B | NP_879779 | BP0985 | acrB | 116 | CM | 6 (4) | 3 (2) | 5 (3) | 3 (2) | Drug resistance |
| Adenylosuccinate lyase | NP_881474 | BP2890 | purB | 50 | C | 7 (3) | 5 (2) | 9 (3) | 9 (3) | Metabolism |
| Alanyl-tRNA synthetase | NP_880538 | BP1836 | alaS | 96 | C | 15 (10) | 9 (6) | 8 (6) | 8 (5) | Translation |
| ATP-dependent protease La | NP_880488 | BP1777 | Ion | 90 | C | 5 (4) | 5 (3) | 6 (4) | 5 (3) | Metabolism |
| Autotransporter | NP_880953 | BP2315 | vag8 | 101 | OM/E | 24 (15) | 37 (22) | 28 (18) | 38 (22) | Transport |
| Autotransporter subtilisin-like protease | CAC44081 | U | sphB1 | 114 | OM/E | 14 (9) | 16 (9) | 23 (15) | 17 (10) | Transport |
| Bifunctional aconitate hydratase 2/2-methylisocitrate dehydratase | NP_880691 | BP2021 | acnB | 95 | C | 8 (5) | 6 (3) | 9 (6) | 6 (3) | Metabolism |
| Bifunctional hemolysin-adenylate cyclase precursor | NP_879578 | BP0760 | cyaA | 188 | E | 8 (8) | 18 (20) | 10 (12) | 14 (15) | Metabolism |
| Capsular polysaccharide biosynthesis protein | NP_880357 | BP1629 | wbpO | 47 | C | 13 (3) | 13 (3) | 10 (2) | 10 (2) | Metabolism |
| Cell division protein | NP_879861 | BP1077 | ftsH | 69 | CM | 20 (9) | 15 (6) | 12 (6) | 15 (7) | Cell division |
| Cell division protein FtsA | NP_881594 | BP3019 | ftsA | 45 | C | 18 (5) | 18 (5) | 17 (5) | 14 (4) | Cell division |
| Chain A, structure of the membrane protein Fhac | 2QDZ_A | U | U | 61 | OM | 22 (6) | 26 (8) | 25 (8) | 27 (9) | Transport |
| Chaperonin GroEL | NP_882014 | BP3495 | groEL | 60 | C | 16 (5) | 13 (5) | 20 (8) | 12 (5) | RNA degradation |
| Competence lipoprotein | NP_879922 | BP1146 | comL | 29 | OM | 21 (4) | 12 (2) | 26 (5) | 16 (3) | Transport |
| Cytochrome C1 precursor | NP_879155 | BP0275 | petC | 31 | U | 34 (6) | 24 (4) | 13 (3) | 29 (4) | Metabolism |
| d-fructose-6-phosphate amidotransferase^ | NP_879503 | BP0666 | glmS | 67 | C | 7 (3) | 4 (1) | 10 (4) | 8 (3) | Metabolism |
| Dihydrolipoamide acetyltransferase^ | NP_879904 | BP1125 | odhB | 44 | CM | 3 (1) | 24 (4) | 19 (3) | 11 (2) | Metabolism |
| Dihydroorotate dehydrogenase 2 | NP_881968 | BP3442 | pyrD | 38 | CM | 21 (4) | 18 (3) | 18 (3) | 13 (2) | Metabolism |
| Dihydroxy-acid dehydratase | NP_879169 | BP0289 | ilvD | 68 | C | 7 (3) | 5 (2) | 5 (2) | 5 (2) | Metabolism |
| DNA gyrase subunit B | NP_879342 | BP0489 | gyrB | 90 | C | 4 (2) | 7 (4) | 3 (2) | 5 (3) | DNA synthesis |
| DNA topoisomerase III^ | NP_879317 | BP0460 | topB | 96 | C | 9 (5) | 9 (5) | 8 (4) | 2 (1) | DNA synthesis |
| DNA-directed RNA polymerase subunit beta | NP_878932 | BP0015 | rpoB | 151 | C | 3 (2) | 7 (7) | 5 (5) | 4 (4) | Metabolism |
| DNA-directed RNA polymerase subunit beta | NP_878933 | BP0016 | rpoC | 155 | C | 3 (3) | 4 (4) | 3 (4) | 2 (2) | Metabolism |
| Elongation factor G | NP_882120 | BP3610 | fusA | 77 | C | 26 (11) | 24 (10) | 13 (6) | 20 (9) | Translation |
| Elongation factor Tu | NP_878925 | BP0007 | tuf | 44 | C | 38 (9) | 31 (7) | 21 (5) | 33 (8) | Translation |
| Enoyl- (acyl carrier protein) reductase^ | NP_881766 | BP3215 | fabL | 29 | CM | 5 (1) | 9 (2) | 9 (2) | 4 (1) | Lipid metabolism |
| F0F1 ATP synthase subunit alpha^ | NP_881828 | BP3286 | atpA | 56 | C | 8 (3) | 5 (2) | 11 (4) | 2 (1) | Energy metabolism |
| F0F1 ATP synthase subunit B | NP_881826 | BP3284 | atpF | 17 | CM | 30 (4) | 30 (4) | 43 (6) | 37 (5) | Energy metabolism |
| F0F1 ATP synthase subunit beta | NP_881830 | BP3288 | atpD | 51 | CM | 17 (5) | 23 (6) | 7 (2) | 15 (3) | Energy metabolism |
| Filamentous hemagglutinin/adhesion | NP_880571 | BP1879 | fhaB | 394 | OM | 18 (43) | 16 (37) | 16 (38) | 15 (34) | Adherence |
| Glutamate synthase [NADPH] large chain precursor | NP_882256 | BP3753 | qltb | 174 | C | 7 (7) | 3 (4) | 2 (2) | 6 (6) | Amino acid metabolism |
| Guanosine-3',5'-bis (diphosphate) 3'-pyrophosphohydrolase | NP_880309 | BP1576 | spoT | 83 | U | 5 (3) | 7 (4) | 5 (3) | 10 (6) | Nucleotide metabolism |
| Histone protein | NP_881561 | BP2985 | bpH1 | 19 | C | 18 (2) | 18 (2) | 18 (2) | 18 (2) | DNA synthesis |
| HlyD family secretion protein^ | NP_882313 | BP3815 | hlyD | 46 | CM | 10 (2) | 5 (1) | 13 (3) | 10 (2) | Signal transduction |
| Homoserine dehydrogenase^ | NP_881384 | BP2784 | U | 48 | C | 15 (4) | 18 (5) | 5 (1) | 18 (5) | Amino acid metabolism |
| Hypothetical protein BP0162 | NP_879055 | BP0162 | HP | 37 | U | 14 (3) | 9 (2) | 23 (4) | 23 (4) | HP |
| Hypothetical protein BP0205 | NP_879093 | BP0205 | HP | 21 | U | 44 (7) | 47 (8) | 39 (6) | 44 (7) | HP/transport |
| Hypothetical protein BP0325^ | NP_879200 | BP0325 | HP | 43 | CM | 3 (1) | 8 (3) | 3 (1) | 5 (2) | HP/membrane transport |
| Hypothetical protein BP0387 | NP_879258 | BP0387 | HP | 27 | P | 27 (4) | 21 (3) | 32 (5) | 30 (4) | HP |
| Hypothetical protein BP0606 | NP_879449 | BP0606 | HP | 15 | U | 23 (2) | 31 (3) | 31 (3) | 23 (2) | HP |
| Hypothetical protein BP1057 | NP_879842 | BP1057 | HP | 12 | U | 51 (3) | 23 (2) | 23 (2) | 39 (2) | HP |
| Hypothetical protein BP1426 | NP_880168 | BP1426 | HP | 49 | CM | 20 (5) | 11 (3) | 7 (2) | 11 (3) | HP |
| Hypothetical protein BP1438 | NP_880180 | BP1438 | HP | 16 | U | 16 (2) | 16 (2) | 15 (2) | 16 (2) | HP |
| Hypothetical protein BP1440 | NP_880182 | BP1440 | HP | 34 | U | 42 (8) | 36 (7) | 44 (8) | 42 (9) | HP/protein stability |
| Hypothetical protein BP1485 | NP_880222 | BP1485 | HP | 58 | C | 42 (12) | 25 (8) | 31 (9) | 31 (D) | HP/protein transport |
| Hypothetical protein BP1903 | NP_880589 | BP1903 | HP | 58 | CM | 5 (2) | 7 (2) | 7 (3) | 7 (2) | HP |
| Hypothetical protein BP2141^ | NP_880795 | BP2141 | HP | 17 | U | 9 (1) | 24 (2) | 24 (2) | 31 (3) | HP |
| Hypothetical protein BP2191 | NP_880839 | BP2191 | hflK | 48 | U | 35 (8) | 22 (5) | 32 (8) | 35 (9) | HP |
| Hypothetical protein BP2197 | NP_880845 | BP2197 | HP | 23 | U | 25 (3) | 47 (6) | 32 (4) | 35 (4) | HP |
| Hypothetical protein BP2323 | NP_880961 | BP2323 | HP | 28 | U | 24 (3) | 18 (2) | 18 (2) | 18 (2) | HP |
| Hypothetical protein BP2534 | NP_881161 | BP2534 | HP | 58 | U | 25 (8) | 18 (6) | 18 (6) | 17 (7) | HP/metabolism |
| Hypothetical protein BP2535 | NP_881162 | BP2535 | HP | 43 | CM | 34 (8) | 34 (8) | 14 (4) | 29 (7) | HP/metabolism |
| Hypothetical protein BP2661^ | NP_881275 | BP2661 | HP | 32 | U | 10 (2) | 5 (1) | 10 (2) | 10 (2) | HP/transport |
| Hypothetical protein BP2717 | NP_881325 | BP2717 | HP | 40 | M/U | 29 (5) | 14 (3) | 5 (2) | 28 (5) | HP |
| Hypothetical protein BP2936 | NP_881518 | BP2936 | HP | 37 | U | 23 (6) | 17 (4) | 36 (9) | 33 (8) | HP |
| Hypothetical protein BP3467 | NP_881990 | BP3467 | HP | 92 | U | 15 (7) | 15 (7) | 10 (5) | 14 (7) | HP/membrane biogenesis |
| Hypothetical protein BP3521 | NP_882036 | BP3651 | HP | 61 | C | 7 (3) | 6 (2) | 5 (2) | 7 (3) | HP/membrane biogenesis |
| Hypothetical protein BP3559 | NP_882072 | BP3559 | HP | 39 | M/U | 30 (6) | 32 (6) | 29 (6) | 20 (4) | HP/cell division |
| Hypothetical protein BP3651 | NP_882159 | BP3651 | HP | 77 | U | 7 (3) | 7 (3) | 9 (4) | 8 (3) | HP/membrane biogenesis |
| Hypothetical protein BP3689 (LysM domain/BON SFP) | NP_882194 | BP3689 | HP | 20 | U | 24 (3) | 22 (3) | 22 (3) | 22 (3) | HP/cell wall degradation |
| Hypothetical protein BP3758^ | NP_882261 | BP3758 | HP | 29 | CM | 3 (1) | 5 (1) | 7 (2) | 7 (2) | HP/membrane transport |
| Hypothetical protein BP3819 | NP_882317 | BP3819 | HP | 27 | U | 12 (2) | 23 (3) | 19 (2) | 19 (2) | HP |
| l-lactate dehydrogenase | NP_879338 | BP0484 | ildD | 43 | C | 16 (4) | 19 (3) | 8 (2) | 28 (6) | Carbohydrate metabolism |
| Large-conductance mechanosensitive channel | NP_879158 | BP0278 | mscl | 17 | U | 25 (3) | 25 (3) | 13 (2) | 25 (3) | Transport |
| Lipoprotein^ | NP_881354 | BP2750 | HP | 24 | U | 13 (2) | 13 (2) | 11 (2) | 7 (1) | U |
| Lipoprotein | NP_879963 | BP1189 | U | 16 | U | 24 (2) | 39 (3) | 39 (3) | 25 (2) | U |
| Mce related protein | NP_882262 | BP3759 | U | 18 | U | 59 (4) | 35 (2) | 73 (5) | 45 (3) | Transport |
| Outer membrane lipoprotein | NP_881135 | BP2508 | omlA | 20 | OM | 34 (3) | 29 (3) | 40 (4) | 47 (5) | Transport |
| Outer membrane porin protein OmpQ | NP_881933 | BP3405 | ompQ | 40 | OM | 30 (8) | 27 (7) | 23 (6) | 29 (8) | Transport |
| Outer membrane porin protein precursor | NP_879650 | BP0840 | U | 42 | OM | 52 (12) | 43 (10) | 47 (10) | 43 (10) | Transport |
| Outer membrane protein A precursor | NP_879744 | BP0943 | ompA | 21 | OM | 44 (7) | 39 (5) | 39 (6) | 44 (6) | Transport |
| Outer membrane usher protein precursor | NP_880573 | BP1882 | fimC | 96 | OM | 10 (6) | 11 (6) | 13 (9) | 11 (7) | Membrane biogenesis |
| Penicillin-binding protein 1A | NP_882163 | BP3655 | U | 90 | E | 9 (3) | 11 (4) | 10 (4) | 11 (5) | Peptidoglycan metabolism |
| Putative peptidoglycan-associated lipoprotein | NP_881875 | BP3342 | U | 18 | OM | 59 (7) | 59 (6) | 67 (8) | 53 (7) | Transport |
| Peptidyl-prolyl cis-trans isomerase D | NP_880447 | BP1732 | ppiD | 7 | U | 45 (16) | 38 (13) | 38 (14) | 36 (12) | Protein folding |
| Pertactin | BAF35031 | U | prn | 101 | OM | 21 (13) | 17 (11) | 14 (8) | 15 (9) | Transport |
| Phosphoenolpyruvate synthase | NP_880178 | BP1436 | ppsA | 87 | C | 9 (6) | 3 (2) | 5 (3) | 3 (2) | Carbohydrate metabolism |
| Phosphoglucomutase/phosphomannomutase | NP_879859 | BP1075 | qlmM | 50 | C | 9 (3) | 6 (2) | 6 (2) | 6 (2) | Carbohydrate metabolism |
| Preprotein translocase subunit SecA | NP_881589 | BP3014 | secA | 100 | CM | 10 (7) | 6 (4) | 2 (2) | 2 (2) | Membrane transport |
| Preprotein translocase subunit SecD | NP_879831 | BP1046 | secD | 69 | CM | 28 (11) | 23 (10) | 29 (11) | 26 (10) | Membrane transport |
| Preprotein translocase subunit SecF | NP_879830 | BP1045 | secF | 34 | CM | 19 (3) | 19 (3) | 19 (3) | 19 (3) | Membrane transport |
| Preprotein translocase subunit SecG | NP_879617 | BP0802 | secG | 16 | U | 41 (2) | 41 (2) | 41 (2) | 41 (2) | Membrane transport |
| Putative ABC transporter | NP_880959 | BP2321 | Pu | 69 | CM | 16 (5) | 10 (3) | 15 (4) | 15 (4) | Transport |
| Putative ABC transporter ATP-binding subunit^ | NP_881029 | BP2397 | Pu | 70 | CM | 7 (2) | 8 (2) | 2 (1) | 5 (1) | Membrane transport |
| Putative ABC transporter ATP-binding subunit^ | NP_880660 | BP1986 | Pu | 70 | CM | 3 (1) | 11 (4) | 7 (2) | 7 (2) | Membrane transport |
| Putative ABC transporter ATP-binding subunit | NP_882260 | BP3757 | Pu | 31 | CM | 19 (3) | 29 (4) | 29 (4) | 23 (4) | Membrane transport |
| Putative ABC transporter ATP-binding subunit | NP_880723 | BP2057 | Pu | 46 | CM | 34 (7) | 28 (6) | 6 (2) | 24 (5) | Membrane transport |
| Putative amino acid ABC transporter ATP-binding protein | NP_882326 | BP3828 | Pu | 27 | CM | 20 (3) | 15 (2) | 20 (3) | 15 (2) | Membrane transport |
| Putative amino acid ABC transporter permease protein | NP_882328 | BP3830 | Pu | 44 | CM | 13 (3) | 8 (2) | 8 (2) | 8 (2) | Membrane transport |
| Putative bifunctional protein | NP_882247 | BP3744 | Pu | 43 | CM | 22 (6) | 17 (4) | 12 (3) | 17 (4) | Energy metabolism |
| Putative binding-protein-dependent transport permease | NP_881026 | BP2394 | Pu | 33 | CM | 5 (1) | 3 (1) | 7 (2) | 5 (1) | Membrane transport |
| Putative binding-protein-dependent transport protein | NP_881859 | BP3322 | Pu | 42 | P | 41 (7) | 27 (6) | 33 (6) | 26 (4) | Membrane transport |
| Putative cell division protein^ | NP_881100 | BP2473 | Pu | 87 | CM | 5 (2) | 2 (1) | 4 (1) | 4 (3) | Cell division |
| Putative chromosome partition protein | NP_882071 | BP3558 | Pu | 130 | C | 7 (6) | 11 (7) | 9 (6) | 8 (6) | Cell division |
| Putative dioxygenase | NP_880971 | BP2333 | Pu | 34 | CM | 16 (3) | 19 (4) | 11 (2) | 19 (4) | Amino acid metabolism |
| Putative efflux system inner membrane protein | NP_880738 | BP2075 | Pu | 49 | CM | 14 (4) | 18 (5) | 14 (4) | 14 (4) | Membrane transport |
| Putative efflux system transmembrane protein | NP_880739 | BP2076 | Pu | 118 | CM | 12 (9) | 5 (4) | 8 (7) | 10 (7) | Membrane transport |
| Putative exported solute binding protein | NP_881542 | BP2963 | Pu | 40 | U | 17 (4) | 10 (2) | 10 (2) | 13 (3) | Membrane transport |
| Putative extracellular solute-binding protein | NP_880657 | BP1983 | Pu | 82 | P | 6 (3) | 6 (3) | 6 (3) | 6 (3) | Membrane transport |
| Putative glycosyl transferase | NP_881785 | BP3238 | Pu | 34 | U | 20 (4) | 10 (2) | 12 (2) | 22 (4) | Metabolism |
| Putative inner membrane protein | NP_881862 | BP3326 | Pu | 26 | U | 27 (4) | 27 (4) | 28 (4) | 49 (6) | HP |
| Putative inner membrane protein translocase component YidC | NP_886531 | BBP4405 | Pu | 62 | CM | 9 (3) | 15 (4) | 12 (4) | 7 (3) | Membrane transport |
| Putative inner membrane-anchored protein | NP_880838 | BP2190 | Pu | 33 | U | 16 (4) | 20 (5) | 20 (4) | 15 (3) | HP |
| Putative integral membrane protein | NP_881049 | BP2420 | Pu | 41 | CM | 9 (2) | 12 (2) | 16 (4) | 7 (2) | Membrane transport |
| Putative l-lactate dehydrogenase | NP_879251 | BP0379 | Pu | 39 | C | 7 (2) | 18 (3) | 18 (3) | 21 (4) | Carbohydrate metabolism |
| Putative lipoprotein | NP_880735 | BP2072 | Pu | 22 | U | 24 (3) | 27 (3) | 24 (3) | 18 (2) | Putative transport |
| Putative lipoprotein | NP_881568 | BP2992 | Pu | 18 | OM | 59 (6) | 49 (5) | 53 (5) | 53 (5) | Putative transport |
| Putative lipoprotein | NP_882263 | BP3760 | Pu | 29 | OM | 13 (2) | 25 (4) | 16 (4) | 11 (3) | Putative transport |
| Putative lipoprotein | NP_880063 | BP1296 | Pu | 30 | U | 16 (3) | 12 (2) | 12 (2) | 12 (2) | U |
| Putative lipoprotein | NP_880303 | BP1296 | Pu | 41 | U | 26 (5) | 30 (6) | 22 (5) | 30 (7) | Putative membrane |
| Putative lipoprotein | NP_880710 | BP2043 | Pu | 24 | U | 26 (4) | 15 (2) | 23 (4) | 11 (2) | U |
| Putative membrane transport ATPase | NP_881330 | BP2722 | Pu | 86 | CM | 4 (2) | 4 (2) | 3 (2) | 5 (2) | Putative transport |
| Putative membrane transport protein | NP_881309 | BP2716 | Pu | 49 | U | 7 (2) | 7 (2) | 11 (3) | 7 (2) | Putative transport |
| Putative NADH dehydrogenase | NP_882010 | BP3491 | ndh | 48 | CM | 10 (3) | 6 (2) | 6 (2) | 6 (2) | Energy metabolism |
| Putative outer membrane (permeability) protein | NP_881865 | BP3329 | Pu | 87 | OM | 16 (7) | 9 (4) | 12 (6) | 10 (5) | Putative transport |
| Putative outer membrane ligand binding protein | NP_879893 | BP1112 | bipA | 144 | OM | 39 (28) | 15 (12) | 23 (17) | 13 (12) | Adherence |
| Putative peptidase | NP_880436 | BP1721 | Pu | 32 | OM | 22 (3) | 20 (3) | 20 (3) | 27 (4) | Protein degradation |
| Putative quinoprotein | NP_880844 | BP2196 | Pu | 42 | OM | 31 (6) | 29 (5) | 38 (7) | 20 (4) | Protein assembly |
| Putative secreted protein^ | NP_879832 | BP1047 | Pu | 13 | U | 32 (2) | 32 (2) | 32 (2) | 21 (1) | Membrane transport |
| Putative secretion system protein | NP_882292 | BP3793 | ptle | 26 | U | 14 (3) | 21 (4) | 17 (3) | 16 (3) | Putative transport |
| Putative secretion system protein | NP_882293 | BP3794 | ptlf | 30 | U | 11 (2) | 19 (3) | 19 (3) | 11 (2) | Putative transport |
| Putative secretion system protein | NP_882289 | BP3794 | ptlc | 91 | CM | 6 (3) | 9 (5) | 9 (5) | 11 (6) | Putative transport |
| Putative sugar transport protein^ | NP_881176 | BP2549 | Pu | 61 | CM | 5 (2) | 2 (1) | 5 (2) | 5 (2) | Putative transport |
| Putative sulfatase | NP_881701 | BP3136 | Pu | 72 | CM | 21 (8) | 10 (5) | 17 (8) | 17 (8) | Membrane biogenesis |
| Putative TolQ-like translocation protein | NP_881879 | BP3346 | Pu | 25 | CM | 22 (3) | 22 (3) | 23 (4) | 19 (3) | Membrane transport |
| Putative type III secretion protein | NP_880879 | BP2235 | Pu | 66 | CM | 8 (3) | 10 (3) | 12 (5) | 12 (4) | Membrane transport |
| Pyrroline-5-carboxylate reductase | NP_880048 | BP1280 | proC | 30 | C | 15 (3) | 15 (3) | 10 (2) | 11 (2) | amino acid metabolism |
| RecA | AAK85426 | U | recA | 31 | C | 36 (6) | 31 (5) | 28 (4) | 22 (4) | DNA processing |
| Ribonuclease E | NP_879331 | BP0475 | rne | 115 | C | 12 (8) | 8 (5) | 6 (4) | 8 (5) | RNA degradation |
| Ribonucleotide-diphosphate reductase subunit alpha | NP_881559 | BP2983 | nrdA | 107 | C | 10 (7) | 5 (3) | 3 (2) | 8 (5) | Nucleotide metabolism |
| RNA polymerase sigma 80 subunit | AAC45085 | BP1191 | rpoD | 81 | C | 9 (4) | 5 (2) | 6 (3) | 6 (2) | RNA processing |
| Rod shape-determining protein | NP_879246 | BP0374 | mreB | 38 | C | 16 (3) | 19 (4) | 8 (2) | 12 (3) | Cell morphology |
| SCO1/SenC family protein^ | NP_882237 | BP3734 | U | 22 | U | 17 (3) | 6 (1) | 10 (2) | 6 (1) | Transport |
| Serum resistance protein | NP_882013 | BP3494 | brkA | 111 | OM | 23 (14) | 27 (14) | 29 (17) | 38 (21) | Transport |
| Signal peptidase I | NP_881060 | BP2432 | lep | 32 | CM | 11 (2) | 19 (4) | 14 (3) | 12 (2) | Transport |
| Succinate dehydrogenase flavoprotein subunit^ | NP_880997 | BP2361 | sdhA | 65 | CM | 7 (3) | 7 (3) | 3 (1) | 11 (5) | Carbohydrate metabolism |
| Succinate dehydrogenase iron–sulfur protein^ | YP_785707 | BAV1185 | sdhB | 26 | CM | 4 (1) | 14 (4) | 9 (3) | 14 (3) | Carbohydrate metabolism |
| Succinyl-CoA synthetase subunit beta | NP_881168 | BP2541 | sucC | 42 | U | 14 (3) | 9 (2) | 9 (2) | 17 (4) | Carbohydrate metabolism |
| Surface antigen | NP_880169 | BP1427 | U | 86 | OM | 29 (15) | 29 (15) | 25 (13) | 35 (18) | Protein assembly |
| Tex | CAA64672 | BP1144 | tex | 87 | C | 5 (3) | 4 (2) | 3 (2) | 4 (2) | RNA processing |
| Thiol:disulfide interchange protein | NP_882154 | BP3646 | DsbA | 71 | CM | 15 (7) | 14 (6) | 14 (6) | 10 (4) | Membrane biogenesis |
| Threonine synthase | NP_881383 | BP2783 | thrC | 51 | C | 16 (5) | 31 (9) | 10 (3) | 17 (5) | Amino acid metabolism |
| TonB-dependent receptor for iron transport | NP_879666 | BP0856 | bfrD | 82 | OM | 34 (20) | 35 (21) | 29 (18) | 28 (15) | Membrane transport |
| Tracheal colonization factor precursor | NP_879974 | BP1201 | tcfA | 71 | OM | 12 (6) | 20 (8) | 19 (10) | 10 (6) | Membrane transport |
| Translocation protein TolB | NP_881876 | BP3343 | tolB | 48 | P | 13 (3) | 15 (4) | 21 (6) | 14 (4) | Transport |
| Trifunctional transcriptional regulator/proline DH/P-5-C DH | NP_881353 | BP2749 | putA | 140 | C | 11 (9) | 10 (8) | 14 (12) | 14 (12) | Amino acid metabolism |
| Twin argininte translocase protein A | NP_882278 | BP3777 | tatA | 8 | U | 35 (2) | 35 (2) | 35 (2) | 35 (2) | Membrane transport |
| Type II citrate synthase | NP_880994 | BP2358 | qltA | 48 | C | 11 (3) | 8 (2) | 10 (3) | 10 (3) | Carbohydrate metabolism |
| Ubiquinol oxidase polypeptide I | NP_881514 | BP2932 | cyoB | 72 | CM | 6 (3) | 6 (2) | 9 (4) | 6 (2) | Energy metabolism |
| Uridylate kinase | NP_880163 | BP1421 | pyrH | 26 | C | 13 (3) | 10 (2) | 23 (4) | 16 (3) | Nucleotide metabolism |
| Virulence factors transcription regulator | NP_880570 | BP1878 | bvgA | 23 | C | 43 (7) | 59 (9) | 52 (8) | 34 (5) | Signal transduction |
| DNA polymerase I | NP_880026 | BP1254 | polA | 99 | C | 5 (3) | 5 (3) | Nucleotide metabolism | ||
| Acetyl-CoA synthetase | NP_881040 | BP2409 | acsA | 72 | C | 6 (3) | 4 (2) | Carbohydrate metabolism | ||
| 2-Oxoglutarate dehydrogenase E1 component^ | NP_879903 | BP1124 | sucA | 106 | C | 2 (1) | 4 (2) | Metabolism | ||
| Probable Orn/Arg/Lys decarboxylase^ | NP_879079 | BP0190 | U | 83 | C | 3 (1) | 4 (2) | Amino acid metabolism | ||
| Autotransporter | NP_879378 | BP0529 | U | 246 | OM/E | 7 (2) | 5 (6) | 2 (2) | Membrane transport | |
| Cytochrome B^ | NP_879156 | BP0276 | petB | 51 | CM | 17 (5) | 3 (1) | 5 (2) | Energy metabolism | |
| DNA mismatch repair protein^ | NP_879129 | BP0244 | mutL | 69 | C | 4 (2) | 6 (2) | 5 (1) | DNA processing | |
| HP BP3084 | NP_881655 | BP3084 | HP | 41 | C | 18 (4) | 17 (4) | 10 (2) | HP | |
| Ubiquinol-cytochrome C reductase iron–sulfur subunit | NP_879157 | BP0277 | petA | 23 | CM | 24 (3) | 24 (3) | 19 (2) | Energy metabolism | |
| 30s ribosomal protein S20^ | NP_881377 | BP2773 | rspT | 90 | U | 14 (2) | 13 (1) | 14 (2) | Translation | |
| Putative membrane-bound transglycosylase^ | NP_881812 | BP3268 | Pu | 47 | OM | 4 (1) | 14 (4) | 16 (4) | Membrane biogenesis | |
| Putative transglycosylase^ | NP_881631 | BP3060 | Pu | 76 | P | 4 (2) | 4 (1) | 4 (1) | Membrane biogenesis | |
| Dermonecrotic toxin | NP_881965 | BP3439 | dnt | 161 | U | 5 (4) | 2 (2) | 4 (4) | Cell death | |
| Putative periplasmic solute-binding | NP_880224 | BP1487 | smoM | 40 | U | 8 (2) | 8 (2) | 16 (4) | Putative transport | |
| Serotype 3 fimbrial subunit | NP_880302 | BP1568 | fim3 | 22 | E | 20 (3) | 27 (4) | 15 (2) | Cell integrity | |
| CTP synthetase (synthase) | NP_881022 | BP2389 | pyrG | 61 | C | 5 (2) | Nucleotide metabolism | |||
| Cycolysin secretion protein | NP_879580 | BP0762 | cyaD | 48 | CM | 12 (3) | Membrane transport | |||
| HP BP1123 | NP_879902 | BP1123 | HP | 75 | U | 11 (5) | HP | |||
| Putative ketopantoate reductase | NP_880110 | BP1360 | Pu | 34 | C | 10 (2) | HP/metabolism | |||
| Putative transcriptional regulation protein | NP_881198 | BP2571 | Pu | 15 | U | 27 (2) | HP/RNA processing | |||
| Serotype 2 fimbrial subunit | P05788 | BP1119 | fim2 | 23 | E | 21 (2) | Cell integrity | |||
| Threonyl-tRNA-synthetase | NP_880233 | BP1497 | thrS | 71 | C | 8 (3) | Translation | |||
| ABC transport protein, ATP-binding component | NP_881414 | BP2816 | metN | 40 | CM | 10 (2) | Membrane transport | |||
| HP BP3441 | NP_881967 | BP3441 | HP | 36 | M/U | 19 (3) | HP | |||
| BpH2 (novel histone — 18323) | AAB40156 | U | bph2 | 16 | U | 19 (2) | DNA processing | |||
| Putative heme receptor | NP_879314 | BP0456 | hemC | 82 | OM | 6 (3) | Membrane transport | |||
| Trigger factor | NP_880485 | BP1774 | tig | 48 | U | 8 (2) | Cell division | |||
| Exopolyphosphatase | NP_879857 | BP1073 | ppx | 55 | C | 4 (2) | Nucleotide metabolism | |||
| HP BP2486 | NP_881113 | BP2486 | HP | 45 | CM | 8 (2) | HP | |||
| HP BP3002 | NP_881577 | BP3002 | HP | 69 | C | 6 (2) | HP | |||
Numerical values are based on a greater that 95% Scaffold protein identification probability**.
Abbreviations: T — Tohama I, C — C056, D — D946 and F — F656.
PSORTb subcellular scores were used to predict and localize the identified EMF proteins.
KEGG identifiers were employed to assign functions to each of the identified EMF proteins.
NCBI Gi accession numbers were used to further compile information such as gene identification number, subcellular localization (PSORTb) and protein function (KEGG identifiers). Values noted represent percent amino acid coverage of the identified EMF protein. (Number of unique peptides detected in parenthesis.).
indicates that identified proteins only had one unique detected tryptic peptide. No numerical value noted indicates that the protein was not identified in the strain.
Abbreviations: T — Tohama I, C — C056, D — D946 and F — F656; OM — outer membrane, CM — cytoplasmic membrane, C — cytoplasm, P — periplasm, E — extracellular, M — membrane, U — unknown, Pu — putative, HP — hypothetical protein; DH (dehydrogenase), P-5-C-DH (pyrroline-5-carboxylate DH), SFP (superfamily protein), 5-meta (5-methyltetrahydropteroyltriglutamate–homocysteine methyltransferase); BBP — B. parapertussis, BB — B. bronchiseptica, BAV – B. avium.
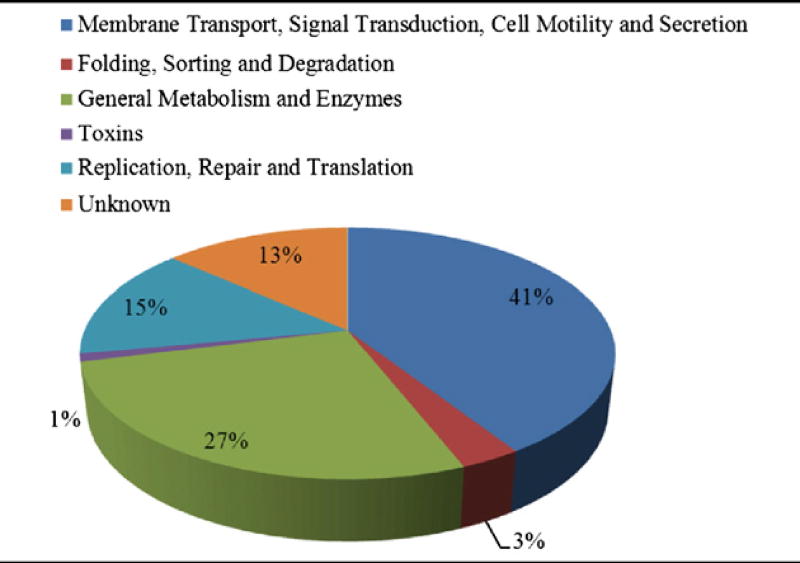
In general, the identified proteins included, but were not limited to, secreted proteins and toxins, as well as outer membrane proteins affiliated with cell membrane synthesis, cellular transport, adhesion, pathogenesis, or virulence. Additionally, the EMF proteomic profiles consisted of proteins associated with protein synthesis. These included highly abundant ribosomal proteins and elongation factors, DNA synthesis-associated proteins, metabolic enzymes, and hypothetical proteins (HP) with unknown functions (Table 2C).
In all, we discovered 163 proteins in the EMFs of all four strains (Table 2D). Among them were the expected surface proteins filamentous hemagglutinin adhesin (FHA) and pertactin (Prn), OmpQ, a serum resistance protein (BrkA), a TonB-dependent receptor for iron transport (TonB), a tracheal colonization factor precursor (TcfA), 30S ribosomal proteins S2 and S3, chaperonin GroEL (GroEL), and elongation factor Tu (EF-Tu). Common proteins were also identified in a combination of two or three strains and absent in the remaining. Other proteins were detected only in T while absent in C, D, and F, including CTP synthetase, HP Bp 1123 and, a putative ketopantoate reductase among others. Conversely, proteins were detected exclusive to C, D, and F while not present in T, such as a putative periplasmic solute-binding protein. Lastly, proteins were identified exclusively to C, D, or F. These included HP Bp 3441, a trigger factor, and an exopolyphosphatase, respectively (Table 2D).
3.3. Direct putative immunoreactive protein identification using nLC ESI-MS/MS
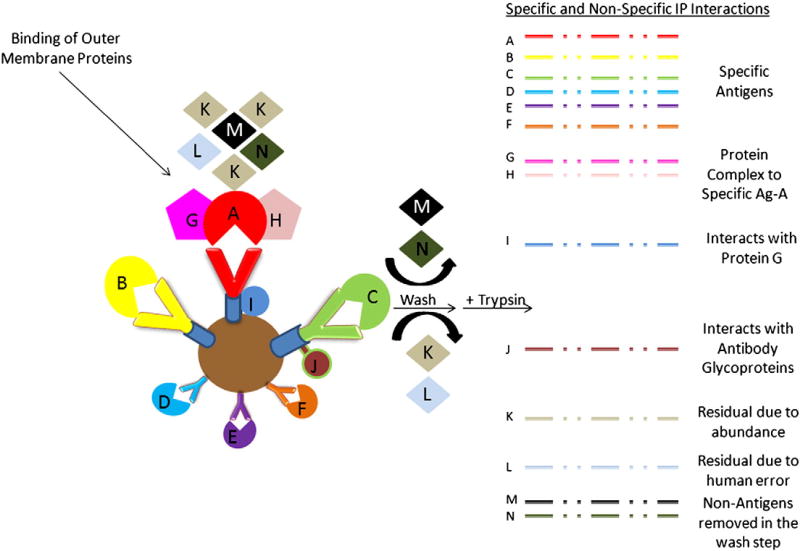
To identify putative antigenic proteins directly from the EMFs and to ascertain any differential immunoreactive proteomic profiles among the four strains, antigen-antibody (Ag-Ab) affinity capture technologies were employed. Trypsin-digested EMF proteins immunoprecipitated with coupled magnetic-bead strain-specific mouse antisera were analyzed via nLC-ESI MS/MS. Among each of the strains T, C, D, and F, 19, 31, 31, and 12 total “putative immunogenic proteins” (PIPs) were identified by database search analysis, respectively (Table 3A). Of the 48 total distinct PIPs detected between all 4 strains, 50% were membrane-associated with 60% of these proteins localized to the outer membrane and/or extracellular. The remaining 24 PIPs, accounting 23% and 27% were localized to the cytoplasm or of unknown location, respectively. For example, Prn, TonB, GroEL, EF-Tu, and a putative sulfatase were some of the proteins identified among all the strains (Table 3B). Collectively, the common PIPs (bold-black outlined box) detected in all T, C, D, and F strains were OmpQ, OmpP, putative lipoprotein, OmpA, BrkA, TcfA, and HP Bp 1440. Alternatively, a Vag8 autotransporter, preprotein translocase SecD (SecD), a putative peptidoglycan-associated protein, SCO1/SencC family and a thiol:disulfide interchange protein (DsbA) were identified in only C, D, and F (blue shaded box) and not detected in the T-EMF/Ab immunoprecipitated complex. Five, 11, and 9 strain-specific PIPs were detected in the T-, C- and D-EMF Ab-bead complexes. Included among those were HP Bp 0455, a putative inner membrane protein, and a putative bifunctional protein, respectively (Table 3B). No strain-specific PIPs were detected in the F-EMF/Ab IP complex. It is worth noting that varying degrees of cross-reactivity (CR) or nonspecific (NS) interactions were generated (denoted with an asterisk) using stable IP between the strains' EMF to the normal mouse IgG-bound bead control. And these interactions can be reasonably explained from a biological perspective as depicted in Fig. 3. TcfA, OmpP, and OmpA with evident cross reactivity were discovered in all 4 strains. Furthermore, the total PIPs correlating to mouse pertussis immunity comprised 11, 18, 18, and 7% of total T, C, D, and F surfaceome proteomic profiles, respectively (Table 3A). But taking any tentative cross-reactivity into consideration, subtracting the nonspecific interactions from the total number of PIPs resulted in a 23–39% reduction of more probable antigenic candidates.
Table 3.
| A Summary of identified Bp putative immunogenic proteins using the mouse model (** — Table 2A). | ||||
|---|---|---|---|---|
|
| ||||
| T | C | D | F | |
| Total PIPs | 19 | 31 | 31 | 12 |
| Total PIPs with CR or NS with normal mouse IgG bound-beads | 6 | 7 | 12 | 4 |
| Total PIPS without CR or NS with normal IgG bound-beads | 13 | 24 | 19 | 8 |
| % of total surfaceome (EMF) with tentative antigenic protein potential | 11% | 18% | 18% | 7% |
| B Putative Bp immunogenic proteins identified among Bp strains assessed in the study. | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Protein | Accession # |
Size (KDa) |
Localization | T | C | D | F |
| Autotransporter (Vag8) | NP_880953 | 101 | OM/E | 11(6)* | 16(10)* | 5(3) | |
|
|
|||||||
| Chaperonin GroEL | NP_882014 | 60 | C | 12(4) | 14(5) | ||
| Elongation factor Ts | CAE33099 | 34 | C | 19(5) | |||
| Elongation factor Tu | NP_878925 | 44 | C | 8(2) | 15(4) | ||
| F0F1 ATP synthase subunit B^ | NP_881826 | 17 | CM | 10(1) | |||
| Fructose-1,6-bisphosphate aldolase | NP_880254 | 39 | C | 14(4) | |||
| Glyceraldehyde-3-phosphate dehydrogenase | NP_879794 | 37 | C | 8(2) | |||
| HP BB 4955 | NP_891489 | 74 | U | 6(2) | |||
| HP BP 0205 | NP_879093 | 21 | U | 10(2) | 22(2)* | ||
| HP BP 0455 | NP_879313 | 74 | U | 3(1) | |||
| HP BP 1057 | NP_879842 | 12 | U | 23(2) | |||
|
|
|||||||
| HP BP 1440^ | NP_880182 | 34 | U | 22(4)* | 12(2)* | 34(6)* | 8(1) |
|
|
|||||||
| HP BP 1485^ | NP_880222 | 58 | C | 3(1)* | 15(6)* | ||
| HP BP 2191 | NP_880839 | 48 | U | 7(2)* | 8(2) | ||
| ketol-acid reductoisomerase | NP_879606 | 37 | C | 13(3) | |||
| N-acetyl-gamma-glutamyl-phosphate reductase | NP_881539 | 39 | C | 16(4) | |||
|
|
|||||||
| Outer membrane porin protein OmpQ | NP_881933 | 40 | OM | 8(2) | 8(2)* | 13(3)* | 8(2) |
|
|
|||||||
| Outer membrane porin protein precursor | NP_879650 | 42 | OM | 30(7)* | 50(13)* | 35(9)* | 35(9)* |
|
|
|||||||
| Outer membrane protein A precursor | NP_879744 | 21 | OM | 17(4)* | 21(5)* | 17(4)* | 35(4)* |
|
|
|||||||
| Pertactin^ | BAF35031 | 101 | OM | 6(3) | 4(2) | 2(1) | |
| Phospho-2-dehydro-3-deoxyheptonate aldolase | NP_881490 | 39 | C | 13(3) | |||
|
|
|||||||
| Preprotein translocase subunit SecD | NP_879831 | 69 | CM | 5(2) | 7(3)* | 5(2) | |
|
|
|||||||
| Putative bifunctional protein | NP_882247 | 43 | CM | 8(2) | |||
| Putative binding-protein-dependent^ | NP_881859 | 42 | P | 10(2) | 12(3) | 3(1) | |
| Putative efflux system transmembrane…^ | NP_880739 | 118 | CM | 4(2) | 2(1) | ||
| Putative exported solute binding protein | NP_881542 | 40 | U | 8(2) | |||
| Putative inner membrane protein | NP_881862 | 26 | U | 15(2) | |||
| Putative inner membrane protein | NP_886531 | 62 | CM | 6(2) | |||
| Putative inner membrane-anchored protein | NP_880838 | 33 | U | 8(2) | 8(2) | ||
| Putative l-lactate dehydrogenase | NP_879251 | 39 | C | 11(3) | |||
|
|
|||||||
| Putative lipoprotein | NP_881568 | 18 | OM | 44(4) | 14(2) | 49(5)* | 35(3)* |
|
|
|||||||
| Putative lipoprotein | NP_880303 | 41 | U | 12(3) | |||
| Putative outer membrane ligand binding protein | NP_879893 | 144 | OM | 4(3) | |||
| Putative peptidase | NP_880436 | 32 | OM | 16(2) | |||
|
|
|||||||
| Putative peptidoglycan-associated lipoprotein^ | NP_881875 | 18 | OM | 17(2) | 29(3)* | 7(1) | |
|
|
|||||||
| Putative periplasmic solute-binding protein | NP_880224 | 40 | U | 15(4) | |||
| Putative secreted protein^ | NP_879832 | 13 | U | 21(1) | 32(2) | ||
| Putative sulfatase | NP_881701 | 72 | CM | 4(2) | |||
| Probable inner-membrane protein | CAE35357 | 61 | CM | 6(2) | |||
| Rod-shape determining protein | NP_879246 | 38 | C | 16(4) | |||
|
|
|||||||
| SCO1/SenC family protein^ | NP_882237 | 22 | U | 6(1) | 6(1) | 6(1) | |
|
|
|||||||
| Serotype 3 fimbrial subunit | NP_880302 | 22 | E | 17(2) | |||
|
|
|||||||
| Serum resistance protein (BrkA) | NP_882013 | 111 | OM | 4(3)* | 3(2) | 4(3) | 4(3) |
|
|
|||||||
| Signal peptidase I | NP_881060 | 32 | CM | 7(2) | |||
| Surface antigen | NP_880169 | 86 | OM | 6(3) | |||
|
|
|||||||
| Thiol:disulfide interchange protein | NP_882154 | 71 | CM | 3(2) | 3(2)* | 3(2) | |
|
|
|||||||
| TonB-dependent receptor for iron transport | NP_879666 | 82 | OM | 4(2) | 5(3) | 13(7) | |
|
|
|||||||
| Tracheal colonization factor precursor | NP_879974 | 71 | OM | 6(3)* | 7(3)* | 7(3)* | 9(4)* |
Abbreviations: T — Tohama I, C — C056, D — D946 and F — F656; CR — cross-reactivity, NS — non-specificity, IgG — immunoglobulin G, EMF — enriched membrane fraction, PIPs — putative immunogenic proteins.
Values noted represent percent amino acid coverage of the identified IP protein using the mouse model. (Number of unique peptides detected in parenthesis.). No numerical value noted indicates that the protein was not identified in the mouse IP.
indicates that identified proteins only had one unique detected tryptic peptide.
Asterisk (*) indicates that the protein identified in the mouse IP was cross-reactive with normal mouse IgG-bound beads. Blue-shaded box: common in all 3 clinical isolates; black-bordered box: common in all 4 strains.
Abbreviations: T — Tohama I, C — C056, D — D946 and F — F656; OM — outermembrane, CM — cytoplasmic membrane, C — cytoplasm, P — periplasm, E — extracellular, M — membrane, U — unknown, HP — hypothetical protein, BB — B. bronchiseptica.
Fig. 3.
Illustration of potential specific and non-specific interactions during an immunoprecipitation (IP) from a biological and technical perspective.
Finally, we performed a MS-based immunoproteomic study using coupled magnetic bead-pooled human convalescent serum in combination with EMFs. This was to identify novel antigenic proteins that potentially correlate with human response to pertussis infection. The study, as summated in Tables 4A and 4B, revealed that human antibodies contained in the pooled serum immunoreacted with 4, 12, 8, and 10 proteins present in T-, C-, D-, and F-EMFs, respectively. Moreover, of the 15 total distinct PIPs detected by nLC-ESI MS/MS among all 4 strains, more than half were extracellular or localized to the outer membrane, including Vag8, BrkA, and TonB. Five proteins: HP Bp 0205, HP Bp 1485, HP Bp 3689, a probable inner membrane, and TonB revealed strain-specific detection in the human IP. Only two – a probable inner membrane protein and HP Bp 3689 – were unique to C and F, respectively. Once again, accounting for any non-specific interactions between normal human IgG antibodies and the EMFs, the pool of PIPs identified in this foundational assessment is diminished to 1, 3, 0, and 5 for T, C, D, and F, respectively. Lastly, in both the mouse and human immunoproteome examinations, 14 proteins were commonly identified, of which OmpQ and OmpA were the only proteins detected in all four immune complexes.
Table 4.
| A Summary of identified Bp putative immunogenic proteins using the human model (** — Table 2A). | ||||
|---|---|---|---|---|
|
| ||||
| T | C | D | F | |
| Total PIPs | 4 | 12 | 8 | 10 |
| Total PIPs with CR or NS with normal mouse IgG bound-beads | 3 | 9 | 8 | 7 |
| Total PIPS without CR or NS with normal IgG bound-beads | 1 | 3 | 0 | 3 |
| % of total surfaceome (EMF) with tentative antigenic protein potential | 1% | 2% | 0% | 2% |
| B Putative Bp immunogenic proteins identified among Bp strains assessed in the study. | |||||
|---|---|---|---|---|---|
|
| |||||
| Protein | Accession # | T | C | D | F |
| Autotransporter (Vag8) | NP_880953 | 12(8)* | 17(12)* | 7(4)* | |
|
|
|||||
| HP BP 0205^ | NP_879093 | 6(1)* | 21(4) | ||
| HP BP 1440 | NP_880182 | 10(3) | 27(5)* | 11(3) | |
| HP BP 1485 | NP_880222 | 9(3)* | 16(7)* | 6(2) | |
| HP BP 3689 | NP_882194 | 18(2) | |||
|
|
|||||
| Outer membrane porin protein OmpQ^ | NP_881933 | 3(1)* | 7(2)* | 11(3)* | 4(1)* |
|
|
|||||
| Outer membrane porin protein precursor | NP_879650 | 23(6)* | 21(7)* | ||
|
|
|||||
| Outer membrane protein A precursor | NP_879744 | 10(3)* | 21(5)* | 21(5)* | 17(3)* |
|
|
|||||
| Preprotein translocase subunit SecD | NP_879831 | 12(5)* | |||
| Probable inner membrane protein | CAE35357 | 4(2)* | |||
| Putative lipoprotein | NP_881568 | 37(3)* | 22(2)* | ||
| Serum resistance protein (BrkA) | NP_882013 | 2(2)* | |||
| Thiol:disulfide interchange protein | NP_882154 | 3(2) | |||
| TonB-dependent receptor for iron transport | NP_879666 | 7(4) | 19(11)* | 3(2) | |
| Tracheal colonization factor protein | NP_879974 | 9(4)* | 10(5)* | 7(3)* | |
Abbreviations: T — Tohama I, C — C056, D — D946 and F — F656; CR — cross-reactivity; NS — non-specificity; IgG — immunoglobulin G; EMF — enriched membrane fraction; PIPs — putative immunogenic protein.
Values noted represent percent amino acid coverage of the identified IP protein using the human model. (Number of unique peptides detected in parenthesis.). No numerical value noted indicates that the protein was not identified in the human IP.
indicates that identified proteins only had one unique detected tryptic peptide.
Asterisk (*) indicates that the protein identified in the human IP was cross-reactive with normal human IgG-bound beads. Pink-shaded box — protein identified in human and mouse IP; yellow-shaded box — strain-specific protein identification only in human IP; black-bordered box: common in all 4 strains.
Abbreviation: T — Tohama I, C — C056, D — D946 and F — F656.
4. Discussion
In spite of widespread vaccination, disease caused by Bp is rapidly increasing in the United States. In this pilot study we compared the proteomes of one past Bp strain with three current circulating strains in which any subtle changes in their proteome profile could have pathogenic and immunological implications. Additionally, we employed a non gel-based technique to compare surface proteins and immunoproteins from the four Bp strains. Previous studies of bacterial surfaceomes for the identification of clinical diagnostic biomarkers (and more so, novel vaccine candidates) have all incorporated approaches that involve to a certain extent subproteome fractionation and gel-based separation followed by MS and protein identification (Thein et al., 2010). Our initial path of study in characterizing the surfaceomes of three recent Bp circulating isolates and one older isolate from 1954 began with a 1D-GE EMF protein assessment. This revealed no major protein banding differences or unique patterns. In an effort to maximize protein discovery, however, we implemented a gel-free surfaceome profiling approach. This proved advantageous and fruitful in identifying total proteins and was in partial concurrence with previous Bp surfaceome analysis.
Bottero et al. (2007) described a comparative surfaceome analysis of 3 Bp vaccine producing strains. The methodology included T and an Argentinean clinical isolate 106, in which 54 total proteins from enriched Bp surface extracts were identified using 2D-GE in parallel with matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) MS analysis.
In our direct nLC-ESI MS/MS Bp surface assessment, 139 more proteins were identified than in the gel-based Bottero study (Bottero et al., 2007). Bottero similarly identified 21 of the total 193 proteins, of which approximately 40% of this subset localized to the outer membrane. These included a competence lipoprotein, FHA, OmpQ, OmpP, OmpA, Prn, BrkA, and a putative quino protein. Among the cytoplasmic proteins, both studies discovered a capsular polysaccharide biosynthesis protein, putative l-lactate dehydrogenase, GroEL, and EF-Tu. And as summated in Table 2D, the remaining 172 proteins identified in our study were localized to the membrane or cytoplasm. They either “moonlight” (perform multiple cellular roles), serve in a cellular housekeeping capacity such as energy production, or engage in membrane-associated activities such as biogenesis, adhesion, or transport.
Our surfaceome profile resulted in a higher number of proteins identified. Given that our preparation is enriched and not exclusive for the surface membrane, more nonsorted cytoplasmic proteins may have been retained in the fraction compared with Bottero's enrichment method, thus resulting in more proteins in the starting material. Additionally, the profile generated is a direct analysis of a conformationally native in-solution protein pool versus a protein-embedded, gel excised spot or band. Consequently, this state would ideally allow proteolytic enzymes greater accessibility to the protein itself and result in a potentially higher yield of peptides generated by tryptic digestion. MALDI-TOF is a fruitful MS technology, in particular for its speed in analyzing a sample, but generally in terms of accuracy, peptide capacity, peptide resolution, and detection sensitivity, the nLC-ESI MS/MS used in our study is more advantageous and lends itself to higher numbers of proteins identified.
Other factors such as database annotation, peptide ionization potential, subproteome extraction, and protein abundance can also affect the success of protein identification. First, the Bp clinical isolates used in these studies have yet to be sequenced and thus are not present in the database. Therefore, some MS/MS data may not be matched to proteins in the existing database, resulting in lower protein discovery. Also, as in the case for both studies, due to amino acid composition (i.e., hydrophobic peptides) some peptides do not ionize well. Thus, their abundance may not be enough to trigger the mass spectrometer to conduct an MS/MS experiment. And some peptides may not fragment efficiently, leading to complete inability for the searching algorithm to match the data to the protein, irrespective of the MS instrumentation used. Again, due to chemical composition of the proteins and the extraction buffers utilized, some proteins may even be lost in the preparation (Bottero et al., 2007; Altindis et al., 2009). Note too that proteins commonly identified between both studies are cellularly abundant (i.e. EF-Tu) and large in molecular weight (i.e., FHA). Consequently, when these proteins are enzymatically digested they will likely generate more peptides that would have a greater propensity of detection. This could hinder the detection of smaller proteins, which obviously would have fewer tryptic peptides. Though our direct, gel-free, EMF nLC-ESI MS/MS analysis may require optimization of extraction steps to ensure a greater retention of outer membrane proteins and reduce “contamination” by abundant cytoplasmic proteins that may overshadow less abundant proteins, the approach can be used to examine other Bp circulating isolate surfaceomes for novel surface-expressed protein discovery.
The next phase of our study moved from elucidating what proteins comprised the enriched surfaceome to what proteins actually have the ability to induce an Ab-mediated response. We deviated from classical immunoblotting techniques, such as those used by Altindis et al. (2009), for a less-labor intensive, more rapid Ag–Ab affinity approach. The Ag–Ab method has an added benefit of identifying surface proteins with both continuous and noncontinuous epitopes while immunoblotting techniques generally can only probe continuous epitopes. Today, conjugated Ab-magnetic bead capture technologies are widely used for protein IP. Generally, protein G-coated magnetic Dynal™ beads capture from antisera IgG populations traditionally associated with direct Ab-mediated immune responses. This Ab-bead complex is immunoprecipitated or “pulled down” with proteins, if immunostimulatory would uniquely “match” and interact with its specific Ab. Once the protein-Ab-bead complex is pulled-down and washed to remove nonspecific binding, the beads are subjected to enzymatic digestion by trypsin and the peptides are analyzed by nLC-MS/MS. The respective proteins are identified by database searching.
Kudva et al. (2005) used this Ab-bead capture technology to identify anthrax spore surface proteins in response to human anthrax vaccine adsorbed-induced immunity. Here we similarly describe the use of Ag–Ab affinity for stable IP of Bp EMFs using strain-specific mouse-antisera, and couple this technique to MS to identify novel putative antigenic proteins. Of the 48 total proteins detected among our strains, 10 well-known immunogens were identified, including Vag8, GroEL, Prn, BipA, serotype 3 fimbrial subunit (Fim3), BrkA, OmpQ, OmpP, OmpA and TcfA (van den Berg et al., 1999; Oliver and Fernandez, 2001; Fuchslocher et al., 2003; Elder and Harvill, 2004; Matoo and Cherry, 2005; Zhu et al., 2010). The latter five are commonly detected in all four strains. Other known Bp immunogens such as Ptx, FHA, Dnt, Fim2, or CyaA, the latter four identified in our total surfaceome profile, may have been immunoprecipitated and, due to peptide composition, simply not detected by MS. EF-Tu, EF-Ts, glyceraldehyde-3-phosphate dehydrogenase (Gdh), and a putative l-lactate dehydrogenase (Ldh) are immunogens commonly conserved in other pathogens (Zhu et al., 2010; Chitlaru et al., 2007; Ling et al., 2004) were also identified in our Bp affinity capture assessment. Additionally, Altindis et al. (2009) used strain-specific mouse antisera to perform a 2D-GE immunoproteomic study aimed at identifying novel immunogens in T and in the Bp Saadet strain, a 1948 Turkish isolate. The Altindis study discovered 25 total proteins, of which EF-Tu, Prn, BrkA, and ketol-acid reductoisomerase were comparably identified in all or some of the isolates in our examination. Even more, Tefon et al. (2011) described an extension of the 2009 Altindis study using 2D-GE in parallel with nLC-MS/MS to identify 11 more immunogens of Bp T and the Saadet strain, of which three – Prn, GroEL and BrkA – were, as stated, discovered in our study.
Identification of more similar proteins from the Altindis and Tefon studies is likely dependent on differential method design. Nevertheless, these proteins, along with the OmpP and OmpQ comparably identified in both our pilot immunoblot and IP study, in addition to the other known antigen identifications are confirmation that the capture technology is a fruitful alternate approach available for Bp immunogen discovery.
Of the remaining PIPs determined as common among T, C, D, and F, HP Bp 1440 and a putative lipoprotein are both possibly associated with transport and adhesion and could be considered as novel putative Bp antigens. Moreover, of the 5 PIPs only detected in the recent circulating C, D, and F isolates, only Vag8 was previously identified as immunogenic. The putative peptidoglycan associated-lipoprotein, SCO1/SenC family protein, DsbA, and SecD are proteins involved in outer membrane lipid attachment; membrane biogenesis and cytochrome c assembly, protein turnover, protein folding and energy production; and membrane protein secretion and export, respectively (Parkhill et al., 2003). Note too that their unique identification to the clinical strains could provide insight into the overall immunopathogenesis of past and recent pertussis cases.
Even more, immunogen screening via IP also identified PIPs unique to each strain. First, the T-EMF study detected five specific PIPs, one of which is BipA, a known immunogen. HP Bp 0455 and its Bordetella bronchioseptica (Bb) BB4955 homolog, both putatively associated with organic anion transport, also were identified (Parkhill et al., 2003), as were a putative inner membrane protein (Bp 3326) and a surface antigen, similar to the outer membrane protein assembly complex YaeT.
Second, the C-EMF study detected 11 specific PIPs, which included previously identified immunogens EF-Ts, GDH, LDH, and ketolacid reductoisomerase. Fructose 1-6-bisphosphate aldolase (Fba), N-acetyl-gamma-glutamyl-phosphate reductase (ArgC), and phosphor-2-dehydro-3-deoxyheptonate aldolase (AroG) are all metabolic enzymes involved in glycolysis, gluconeogenesis, or amino acid biosynthesis (Parkhill et al., 2003; Matoo and Cherry, 2005). Two proteins, similar to the bacterial, extracellular solute-binding protein family 7, exported solute-binding and periplasmic solute-binding (SmoM) were also found to be immunostimulatory (Parkhill et al., 2003; Matoo and Cherry, 2005). A rod-shape determining protein associated with the MreBCD complex was also identified.
Third, the D-EMF study detected nine specific PIPs, which included HP BP1057—a protein similar to E. coli Elab. A putative bifunctional protein, a metabolic protein similar to P. aeruginosa cytochrome c oxidase, and a F0F1 ATP synthase subunit B (an enzyme part of the membrane proton pump involved in ATP synthesis) were discovered. A probable Bp inner membrane protein and its homologous Bb translocase YidC, a membrane insertase, were identified as immunoreactive. A putative peptidase, associated with bacterial wall degradation and a signal peptidase I – an enzyme similar to the essential membrane bound serine protease leader peptidase B – were uniquely immunoprecipitated in the D antibody/EMF complex. A putative sulfatase containing phosphoglycerol transferase activity-like domains possibly associated with cell envelope biogenesis, was also discovered. Protective antibodies, for instance generated against this sulfatase, if not expressed in humans, could potentially impair bacterial cell growth and ultimately reduce infectivity and transmission of pertussis. Lastly, of the 14 PIPs identified in F, none were unique to this isolate.
Moreover, with differential and lower immunoreactivity among the strains, as in the case with strain F, one may wonder why variation exists and the usefulness of strain-specific PIPs as potential candidates for clinical diagnostics and improved vaccine development. First, an important reminder is that Bp T is a lab-adapted strain in which virulence observed over the years may have weakened, reducing its immunopotency and contributing to fewer PIPs identified.
Second, C, D, and F strains all were isolated in different U.S. geographical regions between 1998 and 2007 and presumably from different individuals, thus, the identification of unique, strain-specific PIPs is not unlikely. Also, although Bp T, C, D, and F OMP extraction and enrichments were performed under the same conditions and time, some proteins exhibiting antigenic properties might have been lost during the preparation process. Nevertheless, regardless of strain differences, all the aforementioned PIP biological roles could prove insightful into the overall immunopathogenesis of Bp and provide a greater epidemiological understanding of pertussis incidence and how better to diagnose, treat, and – most importantly – prevent the disease.
As alluded, though specific Ag–Ab interactions were formed and measures taken in the method to reduce nonspecific binding, cross-reactivity of strain-specific Ags from the EMFs to normal mouse IgG-bound bead controls was observed. Proteins commonly immunoprecipitated in all strains, such as OmpQ and OmpA, exhibit cross-reactive or nonspecific tendencies and are reasonably explained from a biological perspective (Fig. 3). First, the EMF protein interactive with the Ab-bead in its natural state exists as a complex. Second, because not all of the available sites on the protein G-beads will be occupied by IgG-specific antibodies, Bp proteins present in the EMF with biochemical properties that allow for interaction directly to the protein G could exist. Third, Bp proteins could nonspecifically bind to glycoprotein-antibody moieties. Also, cross-reactivity may simply persist due to protein abundance in which residual amounts of detectable protein remain even after several washes upon IP and before protein digestion. Under all of these scenarios, upon tryptic digestion not only the bound specific protein is digested, but also those making up complex formations are digested as well, all of which could be simply considered as nonspecific. With all that said, regardless of the origin of cross-reactivity, identified immunogens are merely putative. To be considered true candidates as biomarkers for clinical diagnostics and improved vaccine development, they have to be validated extensively on a proteomic level and, most importantly, in vivo.
Information gained from immunoproteomic studies that use Bp-generated mouse antisera as the basis for novel immunogen discovery are quite valuable to identify possible immunoreactive proteins. But individual, Bp-stimulated human antibody or pooled serum from actively infected or even convalescent pertussis patients is more ideal and provides a more relevant immunological perspective of human pertussis infection. In our study, 15 total PIPs among T, C, D, and F were recognized by pooled human antibodies from patients recovering from pertussis infection—a more than 3-fold reduction in total PIPs identified using the mouse model. Moreover, strain-specific antisera generated from whole inactivated Bp T, C, D, and F from immunized animals versus a pooled serum from convalescent pertussis-infected human patients are a clear factor that may play a role in differential immunoreactivity. Although not comparable with methodologies performed in our study, Zhu et al. (2010) recently described a 2D-GE proteomic assessment of total Bp membrane enriched and extracellular protein preparations. The purpose was to investigate the complete set of Bp antigenic proteins using antisera generated in response to Chinese whole-cell, vaccine-induced immunity.
In the Zhu study, twice as many total immunogens were identified for human sera compared with our human sera pool. The disparity was possibly due to Bp strain differences and lower post-pertussis infection specific circulating Ab titer levels of the pooled human antisera (caused by prior serum dilution) used in our study. Interestingly, irrespective of the subproteome extraction, protein enrichment, and immunodetection methods used, OmpQ was the only protein pulled down using both mouse and human antisera from all four Bp EMFs in our study, as well as in Zhu's immunoproteomic examinations.
In conclusion, we used Ag–Ab affinity capture technologies in conjunction with MS to identify immunoreactive proteins in three recent circulating Bp strains. The overall goals were to identify biomarkers that could be evaluated for use in pertussis strain differentiation and in clinical diagnostics, and to detect novel targets for design of prevention and therapeutic strategies.
As a first step in this qualitative proteomics study, we examined the applicability of a gel-free direct enzymatic-treated Bp EMF surfaceome analysis. Common and strain-specific surface expressed protein profiles compared to the current vaccine-producing T strain were generated, suggesting that there may be subtle differences of surface protein expression among Bp strains. Further characterization of these differentially expressed proteins at the molecular level is warranted; which may reveal minor changes that could have implications on virulence and pathogenesis.
Second, antibodies generated from pertussis interperitoneal mouse inoculation were used to assess protein immunogenicity and revealed the identification of known and unknown Bp antigens using a stable IP approach. A comprehensive proteomic examination and an in vivo immunopotency assessment are further needed to validate these PIPs as true biomarker and vaccine candidates. Also, reassessing the immunoproteome of our unique Bp strains by stable IP using several human sera from active pertussis infected human patients will provide a more consistent depiction of the pertussis immunoproteome. Additionally, efforts to enhance novel immunogen discovery may require immunodepletion of known and highly abundant antigenic-specific antibody populations, such as OmpQ and BrkA. Highly abundant antigenic proteins would in essence generate a larger specific IgG antibody pool. This larger antibody subset would have a greater chance of binding to protein G-beads, which invariably could overshadow lower expressed immunostimulatory proteins.
Nonetheless, the gel-free approach described here for immunoproteome identification remains a fruitful alternative for antigen discovery. And because this is a pilot study using a small number of temporally limited and genetically diverse strains, future studies will entail using the described methods to examine a larger set of epidemiologically significant strains with these putative, novel candidates laying the foundation for clinical diagnostic design and improved vaccine development, leading to the ultimate prevention of this deadly infection.
4.1. Disclaimer
References in this article to any specific commercial products, process, service, manufacturer, or company do not constitute an endorsement or a recommendation by the U.S. Government or the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of CDC.
References
- Advani A, Donnelly D, Hallander H. Reference system for characterization of Bordetella pertussis pulsed-field gel electrophoresis profiles. J. Clin. Microbiol. 2004;42(7):2890–2897. doi: 10.1128/JCM.42.7.2890-2897.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altindis E, Tefon BE, Özcengiz Y, Becher D, Hecker M, Özcengiz G. Immunoproteomic analysis of Bordetella pertussis and identification of new immunogenic proteins. Vaccine. 2009;27:542–548. doi: 10.1016/j.vaccine.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Bart MJ, van Gent M, van der Heide HG, Boekhorst J, Hermans P, Parkhill J, Mooi FR. Comparative genomics of prevaccination and modern Bordetella pertussis strains. BMC Genomics. 2010;11(627):1–10. doi: 10.1186/1471-2164-11-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenut WV, Sanchez JC, Karmime A, Rouge V, Rose K, Binz PA, Hochstrasser DF. Toward a clinical molecular scanner for proteome research: parallel protein chemical processing before and during western blot. Anal. Chem. 1999;17(21):4800–4807. doi: 10.1021/ac990448m. [DOI] [PubMed] [Google Scholar]
- Bordet J, Gengou O. Le microbe de la coqueluche. Ann. Inst. Pasteur. 1906;20:48–68. [Google Scholar]
- Bottero D, Gaillard ME, Fingermann M, Weltman G, Fernandez J, Sisti F, Graieb A, Roberts R, Rico O, Rios G, Regueira M, Binsztein N, Hozbor D. Pulsed-field gel electrophoresis, pertactin, pertussis toxin S1 subunit polymorphisms, and surfaceome analysis of vaccine and clinical Bordetella pertussis strains. Clin. Vaccine Immunol. 2007;14:1490–1498. doi: 10.1128/CVI.00177-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Prama BN. LC-MS for protein characterization: current capabilities and future trends. Expert Rev. Proteomics. 2008;5:435–444. doi: 10.1586/14789450.5.3.435. [DOI] [PubMed] [Google Scholar]
- Chitlaru T, Gat O, Grosfeld H, Inbar I, Gozlan Y, et al. Identification of in vivo-expressed immunogenic proteins by serological proteome analysis of the Bacillus anthracis secretome. Infect. Immun. 2007;75:2841–2852. doi: 10.1128/IAI.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P. Whooping cough makes global comeback. Lancet Infect. Dis. 2002;2:322. doi: 10.1016/s1473-3099(02)00308-0. [DOI] [PubMed] [Google Scholar]
- Dreisbach A, Hempel K, Buist G, Hecker M, Cecher D, van Dijl JM. Profiling the surfaceome of Staphylococcus aureus. Proteomics. 2010;10:1–15. doi: 10.1002/pmic.201000062. [DOI] [PubMed] [Google Scholar]
- Dworzanski JP, Snyder AP. Classification and identification of bacteria using mass spectrometry-based proteomics. Exp. Rev. Proteomics. 2005;2(6):863–878. doi: 10.1586/14789450.2.6.863. [DOI] [PubMed] [Google Scholar]
- Elder KD, Harvill ET. Strain-dependent role of BrkA during Bordetella pertussis infection of the murine respiratory tract. Infect. Immun. 2004;72:5919–5924. doi: 10.1128/IAI.72.10.5919-5924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchslocher B, Millar LL, Cotter PA. Comparison of bipA alleles within and across Bordetella species. Infect. Immun. 2003;71:3043–3052. doi: 10.1128/IAI.71.6.3043-3052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Aslanian A, Yates JR., III Mass spectrometry for proteomics. Curr. Opin. Chem. Biol. 2008;12:483–490. doi: 10.1016/j.cbpa.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick TH, Cassiday P, Weyant RS, Bisgard KM, Sanden GN. Changes in predominance and diversity of genomic subtypes of Bordetella pertussis isolated in the United States, 1935 to 1999. Emerg. Infect. Dis. 2002;8(1):44–49. doi: 10.3201/eid0801.010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Mertsola J. Factors contributing to pertussis resurgence. Future Microbiol. 2008;3:329–339. doi: 10.2217/17460913.3.3.329. [DOI] [PubMed] [Google Scholar]
- http://www.cdc.gov/.
- Hulbert RR, Cotter PA. Laboratory maintenance of Bordetella pertussis. Curr. Protoc. Microbiol. 2009;15(4B):1.1–1.9. doi: 10.1002/9780471729259.mc04b01s15. [DOI] [PubMed] [Google Scholar]
- Jabbour RE, Wade MM, Desphande SV, Stanford MF, Wick CH, Zullich AW, Snyder AP. Identification of Yersinia pestis and Escherichia coli strains by whole cell and membrane protein extracts with mass spectrometry-based proteomics. J. Proteome Res. 2010;9(7):3647–3655. doi: 10.1021/pr100402y. [DOI] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- King AJ, Berbers G, van Oirschot HF, Hoogerhout P, Knipping K, Mooi FR. Role of the polymorphic region 1 of the Bordetella pertussis protein pertactin in immunity. Microbiology. 2001;147:2885–2895. doi: 10.1099/00221287-147-11-2885. [DOI] [PubMed] [Google Scholar]
- Kudva IT, Griffin RW, Garren JM, Calderwood SB, John M. Identification of a protein subset of the anthrax spore immunome in humans immunized with the anthrax vaccine adsorbed preparation. Infect. Immun. 2005;73(9):5685–5696. doi: 10.1128/IAI.73.9.5685-5696.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustos I, Kocsis B, Kilár F. Bacterial outer membrane protein analysis by electrophoresis and microchip technology. Expert Rev. Proteomics. 2007;4(1):91–106. doi: 10.1586/14789450.4.1.91. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spec. Rev. 2008;27:535–555. doi: 10.1002/mas.20175. [DOI] [PubMed] [Google Scholar]
- Ling E, Feldman G, Portnoi M, Dagan R, Overweg K, Mulholland F, Chalifa-Caspi V, Wells J, Mizrachi-Nebenzahl Y. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin. Exp. Immunol. 2004;138:290–298. doi: 10.1111/j.1365-2249.2004.02628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt DJ, Neal SE, Fry NK. Changes in genetic diversity of the Bordetella pertussis population in the United Kingdom between 1920 and 2006 reflect vaccination coverage and emergence of a single dominant clonal type. J. Clin. Microbiol. 2009;47:680–688. doi: 10.1128/JCM.01838-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C. A common vaccination strategy to solve unsolved problems of tuberculosis and pertussis? Microbes Infect. 2008;10:1051–1056. doi: 10.1016/j.micinf.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Matoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 2005;18(2):326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MD. Isolation of bacterial cell membrane proteins using carbonate extraction. Methods Mol. Biol. 2008;424:397–401. doi: 10.1007/978-1-60327-064-9_30. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of protein. J. Biol. Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oliver DC, Fernandez RC. Antibodies to BrkA augment killing of Bordetella pertussis. Vaccine. 2001;20:235–241. doi: 10.1016/s0264-410x(01)00269-9. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchioseptica. Nat. Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Poolman J, Hamstra HJ, Barlow A, Kuipers B, Loggen H, Nagel J. Outer membrane vesicles of Bordetella pertussis are protective antigens in the mouse intracerebral challenge model. FDA. 1990:202–206. [Google Scholar]
- Singh M, Lingappan K. Whooping cough—the current scene. Chest. 2006;130:1547–1553. doi: 10.1378/chest.130.5.1547. [DOI] [PubMed] [Google Scholar]
- Somner U, Peterson J, Pfeiffer M, Schrotz-King P, Morsczeck C. Comparison of surface proteomes of enterotoxigenic (ETEC) and commensal Escherichia coli strains. J Microbiol Methods. 2010;83(2010):13–19. doi: 10.1016/j.mimet.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Taylor B, Fahm R. Pasteur Merieux Connaught five-component acellular pertussis vaccine. Biologicals. 1999;27:103–104. doi: 10.1006/biol.1999.0190. [DOI] [PubMed] [Google Scholar]
- Tefon B, MaaB S, Ozcengiz E, Becher D, Hecker M, Ozcengiz G. A comprehensive analysis of Bordetella pertussis surface proteome and identification of new immunogenic proteins. Vaccine. 2011;29(19):3583–3595. doi: 10.1016/j.vaccine.2011.02.086. [DOI] [PubMed] [Google Scholar]
- Thein M, Sauer G, Paramasivam N, Grin I, Linke D. Efficient subfractionation of gram-negative bacteria for proteomics studies. J. Proteome Res. 2010;9(12):6135–6147. doi: 10.1021/pr1002438. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gets to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg BM, Beekhuizen H, Mooi FR, van Furth R. Role of antibodies against Bordetella pertussis virulence factors in adherence of Bordetella pertussis and Bordetella parapertussis to human bronchial epithelial cells. Infect. Immun. 1999;67:1050–1055. doi: 10.1128/iai.67.3.1050-1055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo IH, Heuevelman KJ, King AJ, Mooi FR. Multilocus sequence typing of Bordetella pertussis based on surface protein genes. J. Clin. Micro. 2002;40:1994–2001. doi: 10.1128/JCM.40.6.1994-2001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MR, Appel RD, Williams KL, Hochstrasser DF, editors. Proteome Research: Concepts, Technology and Application. 2. Springer; Berlin: 2007. [Google Scholar]
- Yu NY, Wagner JR, Laird MR, Mellj G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, Brinkman FS. Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26(13):1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YZ, Cai CS, Zhang W, Guo HX, Zhang JP, Ji YY, Ma GY, Wu JL, Li QT, Lu CP, Guo XK. Immunoproteomic analysis of human serological antibody responses to vaccination and whole-cell pertussis vaccine (WCV) PLoS One. 2010;5(11):1–11. doi: 10.1371/journal.pone.0013915. [DOI] [PMC free article] [PubMed] [Google Scholar]