Summary
Transformation in most bacteria is dependent on orthologues of Type 2 secretion and Type 4 pilus system proteins. In each system, pilin proteins (major and minor) are required to make the pilus structure and are essential to the process, although the precise roles of the minor pilins remain unclear. We have explored protein–protein interactions among the competence minor pilins of Bacillus subtilis through in vitro binding studies, immunopurification and mass spectrometry. We demonstrate that the minor pilins directly interact, and the minor pilin ComGG interacts with most of the known proteins required for transformation. We find that ComGG requires other ComG proteins for its stabilization and for processing by the prepilin peptidase. These observations, C-terminal mutations in ComGG that prevent processing and the inaccessibility of pre-ComGG to externally added protease suggest a model in which pre-ComGG must be associated with other minor pilins for processing to take place. We propose that ComGG does not become a transmembrane protein until after processing. These behaviours contrast with that o fpre-ComGC, themajor pilin, which is accessible to externally added protease and requires only the peptidase to be processed. The roles of the pilins and of the pilus in transformation are discussed.
Introduction
Many species of bacteria have acquired the ability to take up environmental DNA (Lorenz and Wackernagel, 1994; Johnsborg et al., 2007), presumably to incorporate potentially beneficial genes into their genomes. This process is referred to as natural transformation, and those cells capable of transformation are called competent. Transformation begins with the binding of double-stranded DNA to the cell. In Bacillus subtilis, optimal binding requires the seven proteins of the comG operon, which encodes proteins orthologous to those needed for the formation of Type 4 pili (T4P) and for functional Type 2 secretion (T2S) systems of Gram-negative bacteria (Albano and Dubnau, 1989; Hobbs and Mattick, 1993; Dubnau and Provvedi, 2000). After binding, DNA is internalized through an aqueous channel formed by the multipass membrane protein, ComEC (Draskovic and Dubnau, 2005). Also required for internalization are the cytoplasmic membrane-associated ComFA,anATPase that may provide energy for transport (Londono-Vallejo and Dubnau, 1994b; Hahn et al., 2005; Takeno et al., 2012), and the bitopic membrane protein ComEA, which possessesa helix–loop–helix DNA-binding motif in its extracellular domain (Inamine and Dubnau, 1995; Provvedi and Dubnau, 1999). During uptake, one DNA strand is degraded and the other is internalized (Piechowska and Fox, 1971; Dubnau and Cirigliano, 1972; Lacks et al., 1975). Once in the cytoplasm, the single-stranded DNA interacts with a number of soluble proteins that mediate homologous recombination, including DprA, SsbB and RecA (Fernandez et al., 2000; Berge et al., 2003; Grove et al., 2005; Mortier-Barriere et al., 2007; Yadav et al., 2013).
The competence, T4P and T2S systems (Chen and Dubnau, 2004; Craig and Li, 2008; Douzi et al., 2012) encode one or two peripheral membrane ATPases (ComGA, in B. subtilis), a polytopic membrane protein (ComGB) and several pilin proteins (ComGC, ComGD, ComGE and ComGG). ComGF is a single-pass transmem-brane protein that has some similarities in its organization to pilin-like proteins, but little if any sequence similarity. In all three systems, a dedicated protease (ComC) is encoded elsewhere in the genome and is required to process the pilin proteins, removing short N-terminal leader peptides. In B. subtilis, each of the ComG proteins is required for DNA-uptake as well as for optimal DNA-binding to occur, presumably because they are needed for the formation of the competence pseudopilus (Chen et al., 2006; Briley et al., 2011), analogous to structures of the T4P and T2S systems.
Prior to processing, pilin proteins are anchored in the membrane by an N-terminal α-helix (Strom and Lory, 1993). A short, positively charged N-terminal leader upstream of the helix is then removed by the processing protease (Strom and Lory, 1991). The most abundant of the mature pilins, referred to as the major pilin, is translocated out of the membrane for assembly of the Type 4 pilus; this structure extends outside the cell (Craig et al., 2006; Hansen and Forest, 2006). Evidence for a T2S system ‘pseudopilus’ was obtained when PulG, the major pseudopilin from Klebsiella oxytoca, and XcpT, the major pseudopilin from Pseudomonas aeruginosa, were overproduced (Sauvonnet et al., 2000; Durand et al., 2003; 2005; Vignon et al., 2003). As noted above, ComGC, the major pseudopilin of B. subtilis, oligomerizes to form a competence pseudopilus (Chen et al., 2006). All seven of the ComG proteins and the ComC peptidase are needed for this structure to form. Recently, the transformation pseudopilus of Streptococcus pneumoniae has been shown to form a fibre that extends several microns from the cell surface and binds DNA (Laurenceau et al., 2013). Thus, in all three systems the major pilin forms a fibre-like structure at the cell surface, and does so with the help of a largely conserved set of proteins. In all three systems, three to four less abundant minor pilins are also processed by the pre-pilin peptidase and are essential for the systems to function. The roles of the minor pilins (ComGD, ComGE and ComGG in B. subtilis) are not well defined and may well differ in the various systems. Although the terms pseudopilin and pseudopilus have been used widely for the T2S and competence systems, we will instead refer to pilins and pili for simplicity and because what appear to be pili have been identified in S. pneumoniae (Laurenceau et al., 2013).
Here we provide the first evidence that ComGD, ComGE and ComGG, the B. subtilis minor pilins interact directly with one another and that the minor pilin complex is part of a larger transformation machine, containing all the ComG proteins, as well as other competence proteins. Unexpectedly, we have found that ComGG processing requires other ComG proteins in addition to the peptidase, and that pre-ComGG adopts an unusual membrane topology in which neither of its termini are exposed outside the membrane. Cell fractionation reveals that the bulk of ComGG remains in the membrane even after processing, unlike the major pilin ComGC, which is translocated from the membrane into the periplasm. The implications of these findings for the roles of the pilins, and of the transformation pilus during transformation are discussed.
Results
ComGG stability and processing depends on other ComG proteins
To investigate possible interactions among the ComG proteins we determined the relative abundance of ComGG in the absence of other proteins, reasoning that ComGG may be destabilized by the lack of a binding partner. For this, the ComGG coding sequence was placed under the control of an IPTG-inducible (Phyperspank) promoter at the ectopic amyE locus in a series of strains carrying deletions. The amount of protein produced from the Phyperspank promoter was higher than that from the native promoter (data not shown), but overproduction had no discernible impact on transform-ability and the ectopically expressed gene was able to fully complement a comGG deletion at its native locus (Table 1, compare strains BD2528 with BD5575). To increase the ComGG signal in Western blots and to minimize signal contribution from the expression of comGG in non-competent cells, most experiments were performed in a strain that overproduces the regulatory protein ComS (Hahn et al., 1996), which results in competence expression in 80–90% of the cells. ComGG was chosen for this study because of the availability of a good antiserum for Western blotting, and because it is encoded at the end of the comG operon, facilitating genetic manipulations without the introduction of polar effects on downstream genes.
Table 1.
Transformability of ComGG point mutants.
| Straina | Relevant genotype | Transformability (%)b | Fold deficiencyc |
|---|---|---|---|
| BD2528 | Wild type | 0.53 ± 0.067 | n.a. |
| BD5310 | comGGΔKan | 1.85E-6 ± 2.32E-6* | 2.9E5 |
| BD5575 | comGG wild type | 0.54 ± 0.16 | n.a. |
| BD5802 | comGG I104N | 0.0045 ± 0.0018* | 120 |
| BD5803 | comGG E87G | 0.39 ± 0.07 | wt |
| BD5804 | comGG Y75N | 0.00046 ± 0.0028* | 1100 |
| BD5805 | comGG L114R | 0.41 ± 0.09 | wt |
| BD5806 | comGG F8L | 0.41 ± 0.028 | wt |
| BD5821 | comGG G(−1)D | 0.0058 ± 0.0018* | 100 |
| BD5822 | comGG V20A | 0.40 ± 0.25 | wt |
| BD5823 | comGG V6E | 0.18 ± 0.07* | 3 |
| BD5825 | comGG T80I | 0.38 ± 0.19 | wt |
| BD5831 | comGG N42D | 0.036 ± 0.015* | 15 |
| BD5834 | comGG I88N | 5.2E-4 ± 2.0E-5* | 1000 |
| BD5835 | comGG G(−1)V | 0.0075 ± 0.0029* | 75 |
| BD6782 | comGG A5E | 0.15 ± 0.085* | 4 |
| BD6835 | comGG C28S | 0.56 ± 0.20 | wt |
All strains carried mcComS, and as a result, 80–90% of the cells in the culture expressed competence.
Standard transformation assay measuring the conversion to leucine prototrophy. Each experiment was performed in triplicate. A two-tailed t-test was performed to compare the transformation frequencies with that of the controls. The asterisk (*) indicates a P < 0.05 as compared to wild type (BD5575).
The fold transformation deficiency was determined by dividing the average wild-type transformability by that of the specified mutant. wt denotes a transformability not statistically significant from wild type.
When comGG expression was induced in the absence of the pre-pilin peptidase ComC, the protein was completely unprocessed, as expected (Fig. 1A, lane 1). Furthermore, the amount of unprocessed ComGG was slightly reduced compared to that in the comC+ control strain (lane 2), suggesting that the unprocessed form of the protein may be somewhat unstable. In a ΔcomK background (lane 3), in which competence proteins are not expressed (van Sinderen et al., 1995), the IPTG-induced ComGG was unprocessed, as predicted from the absence of ComC, and the level of ComGG was reduced compared to that in the presence of ComK (Fig. 1A, compare lanes 1 and 3). This low abundance suggests that one or more additional competence proteins stabilize the unprocessed form of ComGG. To determine if the ComG proteins were responsible for this effect, ComGG stability was determined in a comK+ background with a deletion of the entire comG operon (Fig. 1A, lane 4). In this case, the amount of ComGG was comparable to that in the comK deletion strain (compare lanes 3 and 4). Unexpectedly, ComGG was mostly unprocessed, although upon prolonged exposure a small amount of processed protein was evident (Fig. S1). These results suggested that pre-ComGG is destabilized in the absence of other ComG proteins and that either ComGG cannot be processed or ComC is less active in the absence of these proteins. To test the second possibility, we determined if the major pilin ComGC could be processed in the absence of other ComG proteins. comGC was expressed from Phyperspank at the amyE locus and analysed in an analogous fashion to ComGG. ComGC was unstable in the absence of ComC, as evident from the greatly reduced levels of protein (Fig. 1B, compare lanes 1 and 2). More importantly, ComGC was stable and processed in the absence of all the other ComG proteins (Fig. 1B, compare lanes 1 and 3). It had been shown previously that when only comGC and comC were coex-pressed in Escherichia coli, ComGC was processed (Chung and Dubnau, 1995), consistent with the present results. We conclude that in contrast to ComGC, both the abundance and processing of ComGG are dependent on the presence of other ComG proteins. Moreover, the low abundance of ComGG in the absence of the other ComG proteins is not purely because it remains unprocessed, as pre-ComGG levels in the comC deletion background are significantly higher than in the ΔcomG strain (Fig. 1A, compare lanes 1 and 4).
Fig. 1.
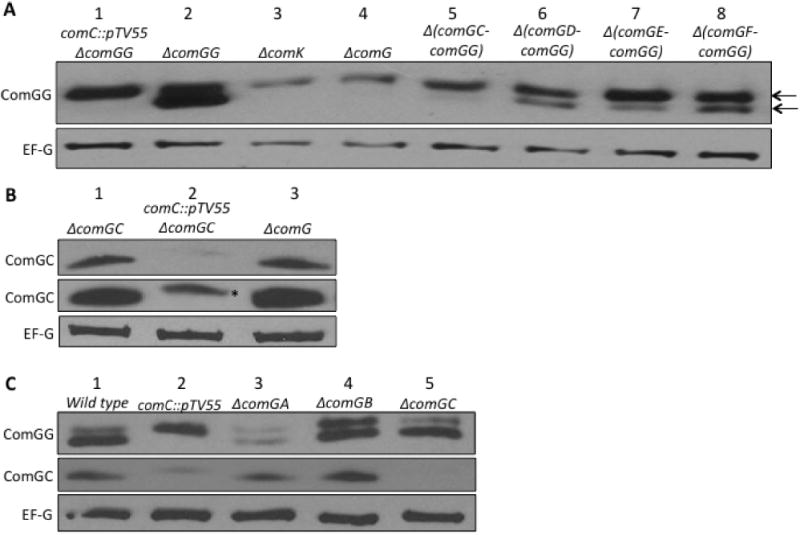
Dependence of ComGG stability and processing on ComG proteins.
A. All the strains were induced with IPTG to express comGG from the amyE∷Phyperspank-comGG construct. The top and bottom arrows indicate the unprocessed (pre-ComGG) and processed ComGG protein signals respectively. The strains used were BD6060, lane 1; BD5965, lane 2; BD6003, lane 3; BD6026, lane 4; BD6059, lane 5; BD6053, lane 6; BD6051, lane 7; and BD6052, lane 8. The EF-G signal is shown as a loading control. Although the loading control signal was slightly lower in lanes 3 and 4, the ComGG signal was proportionally even lower and the results depicted here have been confirmed in other experiments (not shown).
B. Similarly, strains BD6732, lane 1; BD6739, lane 2; and BD6742, lane 2 were induced with 1 mM IPTG to turn on comGC expression from the amyE∷Phyperspank-comGC construct. The asterisk represents the unprocessed ComGC protein signal. The lower ComGC blot was intentionally overexposed to reveal the ComGC signal from the ΔcomGC comC∷pTV55 (BD6739) lysate.
C. Wild type BD2528, lane 1; BD3844, lane 2; BD2786, lane 3; BD4139, lane 4 and BD2788, lane 5.
To identify which ComG proteins are required for the stabilization and processing of ComGG, we tested a series of comG operon deletions. The results suggested that the proteins encoded by comGC thru comGF contribute to both the abundance and processing of pre-ComGG. The levels of ComGG decreased with the removal of ComG proteins and it remained predominantly in the unprocessed form (Fig. 1A, lanes 5–8). We interpret the effects on protein abundance exhibited in Fig. 1A as reflecting relative stabilities, because in each case comGG was expressed from the identical construct at the amyE locus and polar effects of the various deletions were absent.
Because the Δ(comGC–comGG) strain did not display as drastic a decrease in ComGG levels as the ΔcomG mutant (Fig. 1A, lanes 4 and 5), we determined the impact of individually eliminating the first three proteins in the operon, ComGA, ComGB and ComGC, using in-frame non-polar deletion mutants. In these strains, comGG was expressed from its native promoter. In the absence of ComGB or ComGC, ComGG was present at wild-type levels and was apparently processed (Fig. 1C, compare lane 1 with lanes 4 or 5), although perhaps with a minor processing defect in the absence of ComGB. In contrast, ComGG was much less abundant when ComGA was absent, although processing clearly took place (Fig. 1C, lane 3). If the in-frame deletions had polar effects on the expression, stability or processing of downstream proteins, we would expect to see changes in the amount of ComGC, but this was not the case (Fig. 1C, middle panel). To further confirm that these upstream deletions were non-polar, we verified that the decreased ComGG levels in the ΔcomGA strain reflect its instability. The ComGG protein was indeed degraded faster in the absence of ComGA (Fig. S2). ComGA is a peripherally bound membrane protein, and it presumably binds and hydrolyses ATP through Walker A and Walker B sequence motifs (Albano et al., 1989; Briley et al., 2011). A ComGA Walker A mutation, which is known to drastically reduce transformability and competence pilus formation (Haijema et al., 2001; Chen et al., 2006; Briley et al., 2011), did not affect ComGG stability (Fig. S3A). This suggests that the stabilizing effect of ComGA on ComGG does not depend on ATP hydrolysis and most likely reflects the existence of protein–protein interactions, perhaps directly between ComGG and ComGA. Although the stability of ComGG depends on ComGA, the reverse is not true, as determined using a polar Tn917 transposon insertion in comGB (Fig. S3B).
N- and C-terminal residues of ComGG are important for processing
The conserved glycine residue at the −1 position relative to the cleavage site is required for processing of the major pilin of P. aeruginosa (Strom and Lory, 1991). To characterize the sequence requirements for processing of ComGG, we introduced mutations in the conserved N-terminal leader sequence (Fig. 2A) and then assayed for transformation defects, as well as ComGG processing and stability. When the conserved −1 glycine, relative to the mature protein, was mutated to either aspartate or valine, processing was reduced (Fig. 2B) and there was a 75- to 100-fold reduction in transformability (Table 1).
Fig. 2.
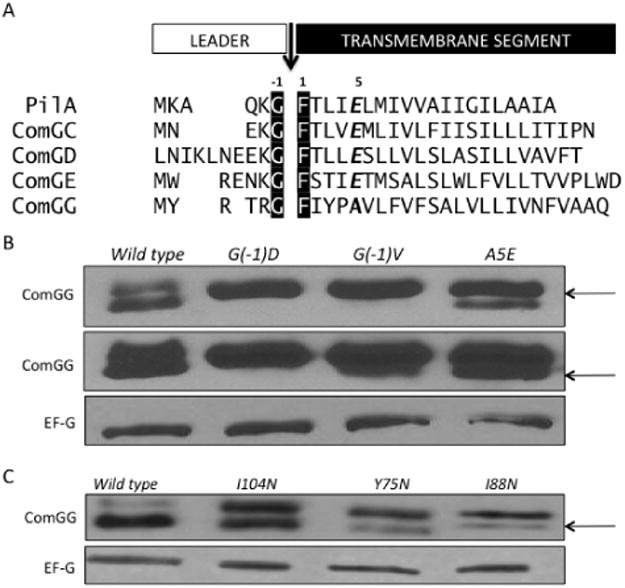
ComGG processing depends on both N- and C-terminal residues.
A. The N-terminal segments of B. subtilis pilin proteins, ComGC, ComGD, ComGE and ComGG, are aligned with PilA of P. aeruginosa. The black arrow denotes the end of the leader sequence with the canonical pilin cleavage motif G/F, highlighted in black. ComGG lacks the conserved glutamate residue at the +5 position of the mature pilin, indicated in bold italics in the other pilins. The residues immediately following the cleavage sites are predicted to be part of the transmembrane segment (Cserzo et al., 1997; Krogh et al., 2001).
B. Representative Western blot of ComGG N-terminal point mutants: G(−1)D (BD5821), G(−1)V (BD5835) and A5E (BD6782). A more exposed ComGG blot is also shown.
C. Representative Western blot for ComGG C-terminal mutants I104N (BD5802), Y75N (BD5804) and I88N (BD5834) identified in a ComGG loss of function screen. EF-G is included as a loading control. The arrows indicate processed ComGG protein.
Clearly processing of ComGG is necessary for function and the conserved G(−1) residue is essential for processing, as observed in other systems. Although the fifth residue in pilins is most often a glutamate, the T4P and T2S systems generally include one minor pilin in which residue E5 is replaced by an aliphatic amino acid. In the competence system, ComGG is that unique pilin in which E5 is replaced by an alanine (Fig. 2A). When this residue was changed to glutamate to produce the A5E mutant protein, pre-ComGG processing was impeded (Fig. 2B), resulting in a fourfold reduction in transformability (Table 1). A V6E mutation (Fig. 2A) also reduced transformation threefold (Table 1) and decreased processing (data not shown). The relatively small defect exhibited by the E5 mutant may reflect a relaxed selection for glutamate at this position, rather than strong selection for an alanine residue.
Since not only ComC but also the pilin proteins are needed for pre-ComGG processing, it seemed likely that protein–protein interactions are important for the processing of ComGG and that point mutations that disrupt these interactions might be detected by screening for comGG loss-of-function mutations (Fig. S5). We screened a library of approximately 1700 clones carrying comGG that had been replicated using an error-prone polymerase, for loss of transformability. We selected 10 mutations to analyse further, as well as the mutation C28S, because it had been proposed that ComGG might form an intermolecular disulphide bond (Chung et al., 1998). Some mutants had defects in transformation frequency (Table 1) and some exhibited reduced levels of ComGG protein (Table S3). The C28S mutation had no effect, suggesting that the proposed disulphide bond either does not exist or has no relevance.
Several mutations located within the C-terminus of ComGG, distant from the processing site, conferred significant processing and transformation deficiencies (Fig. 2C and Table 1). These mutations, Y75N, I88N and I104N all reside within predicted β-strands inthe C-terminal domain (Fig. S4). These data suggest that both the N- and C-terminal moieties of ComGG are important for processing and that the residues surrounding the processing site may not be sufficient for cleavage to occur. These data were unexpected because the pre-pilins are usually envisaged as bitopic proteins with their cleavage sites accessible to the peptidase on the cytoplasmic side of the membrane and with their C-terminal domain distant from the processing site and separated from it by the cell membrane. We return to this point below.
The comG minor pilins interact directly
Because ComGD and ComGE contribute to ComGG stability and processing, we investigated the possible interaction of ComGG and ComGD by co-immunoprecipitation. ComGG was isolated from solubilized membranes of wild-type (BD2528),ΔcomGG∷Tet(BD5404) andcomC∷pTV55 (BD3844) strains. Although ComGD was present in the extracts (Load) obtained from all three strains, it was only present in the eluate when ComGG was present. ComGD also co-immunoprecipitated with ComGG in the comC deletion strain, suggesting that the ComGG–ComGD interaction in the membrane is independent of processing (Fig. 3C). ComGE interaction was not tested in this way because we lack an adequate antiserum for this protein.
Fig. 3.
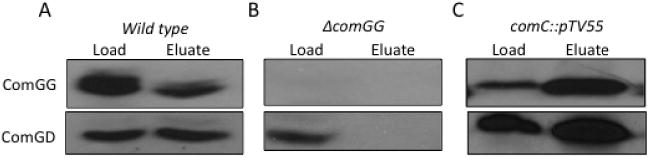
ComGD and ComGG interact in vivo. Cell membranes were isolated from (A) wild type (BD2528), (B) ΔcomGG∷Tet (BD5404) and (C) comC∷pTV55 (BD3844) strains and treated with Triton X-100. The solubilized membrane proteins (Load) were incubated with ComGG antiserum and the immune complexes were isolated using Protein A agarose. The co-immunoprecipitated proteins (Eluates) and the Load samples were analysed by immunoblotting with ComGG and ComGD antisera.
To extend these results and to determine whether the pilin interactions are direct, we used affinity-tagged pro-teins expressed in E. coli. The C-terminal portions of ComGC, ComGD, ComGE and ComGG were cloned into pQlinkG2, to express them as N-terminally GST-tagged recombinant proteins, and the same moieties of ComGD and ComGG were also cloned into pQlinkN (Scheich et al., 2007), to express them without tags. The proteins were expressed individually in E. coli and centrifugal pellets containing the untagged C-terminal domain of a pilin, either ComGD or ComGG, were mixed and lysed with pellets of GST-tagged pilins. As controls, pellets expressing individual untagged pilins were treated similarly without mixing or were combined with pellets expressing GST alone. All of the expected GST-tagged proteins were detected in the eluates (Fig. 4A and C). The GST–ComGE construct was somewhat unstable and multiple degradation products were consistently observed. ComGD was pulled down in the presence of GST–ComGD, GST–ComGE and GST–ComGG, suggesting that it interacts directly with itself and with the other minor pilins (Fig. 4B). ComGD failed to interact detectably with GST–ComGC. Similarly, ComGG was found to interact with ComGD, ComGE and ComGG, but not with ComGC (Fig. 4D). These data suggest that the ComG minor pilins interact directly with one another and also individually with themselves through their C-terminal segments.
Fig. 4.
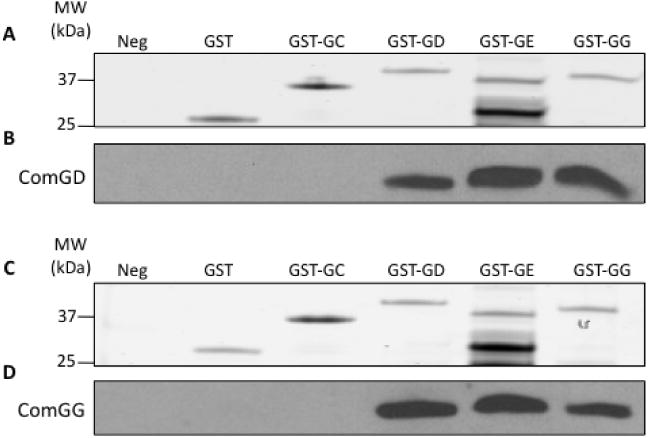
C-terminal domains of ComGD, ComGE and ComGG interact directly. Pilin proteins lacking their N-terminal transmembrane domains were expressed individually in E. coli, with or without N-terminal GST-tags. Pellets from cells expressing either untagged ComGD (ED1484, A and B) or untagged ComGG (ED1491, C and D) were lysed alone (Neg) or mixed before lysis with pellets expressing GST (ED1604), GST–ComGC (ED1650), GST–ComGD (ED1502), GST–ComGE (ED1503) or GST–ComGG (ED1504). The mixed suspensions were lysed by sonication, centrifuged and the supernatants were applied to GSH-superflow resin. The elution fractions were separated by SDS-PAGE. The top of each gel (A or C) was stained with Coomassie blue and the bottom was immunoblotted with ComGD (B) or ComGG (D) antisera.
Analysis of the ComGG interactome
To gain further insight into protein–protein interactions involving ComGG, we analysed its in vivo binding partners by immunoaffinity purification and mass spectrometry. ComGG-containing complexes were immunopurified from wild-type (BD2528), ΔcomGG∷Tet (BD5404) and ComGG overproducing (BD5575) strains. The ΔcomGG strain was included as a control for specificity, and the over-producing strain was included to aid in the identification of lower abundance interacting proteins. Cells were subjected to cryogenic lysis, and ComGG-containing protein complexes were isolated using affinity-purified ComGG antibodies conjugated to magnetic beads as described (Cristea et al., 2005; Carabetta et al., 2013). The co-precipitated proteins were identified by MS/MS with an LTQ-Orbitrap Velos ETD mass spectrometer (Cristea et al., 2005; Carabetta et al., 2013). It should be noted that the likelihood of detecting peptides from interacting proteins is impacted by the abundance of these proteins as well as their affinities for ComGG, and the number of peptides detected is limited by the size of the proteins, particularly for the low-molecular-mass pilins. As expected from our prior studies, ComGD and ComGE were co-isolated with ComGG, as was the pre-pilin peptidase ComC (Table 2). In addition, all of the remaining ComG proteins, the competence membrane proteins ComFA, NucA and ComEC, and the competence cytoplasmic proteins RecA, SsbB, DprA and Maf were identified as well. Our data suggest that all the ComG proteins are, at one time or another, in direct or indirect contact with ComGG, and most likely part of a larger transformation complex.
Table 2.
Proteins that were co-isolated with ComGG.
| Peptide count | Per cent sequence coverage | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Proteina | Wild typeb | ΔcomGG | O/E comGG | Wild type | ΔcomGG | O/E comGG |
| ComGG | 3 | 0 | 11 | 23% | n.a. | 57% |
| ComGA | 11 | 3 | 15 | 33% | 9.8% | 42% |
| ComGB | 2 | 0 | 4 | 7.1% | n.a. | 13% |
| ComGC | 5 | 0 | 6 | 38% | n.a. | 39% |
| ComGD | 0 | 0 | 4 | n.a. | n.a. | 25% |
| ComGE | 0 | 0 | 3 | n.a. | n.a. | 28% |
| ComGF | 4 | 0 | 7 | 43% | n.a. | 39% |
| ComFA | 9 | 2 | 12 | 20% | 2.6% | 25% |
| ComEC | 1 | 2 | 7 | 2.6% | 5.9% | 10% |
| ComC | 0 | 0 | 3 | n.a. | n.a. | 15% |
| NucA | 0 | 0 | 2 | n.a. | n.a. | 18% |
| RecA | 6 | 1 | 15 | 21% | 4.6% | 54% |
| SsbB | 6 | 0 | 8 | 64% | n.a. | 74% |
| DprA | 7 | 0 | 10 | 28% | n.a. | 28% |
| Maf | 0 | 0 | 3 | n.a. | n.a. | 22% |
| YqfA (FloA) | 8 | 1 | 21 | 31% | 4.5% | 74% |
| YuaG (FloT) | 1 | 0 | 14 | 2.2% | n.a. | 33% |
| FtsH | 12 | 5 | 21 | 21% | 11% | 36% |
| MntB | 7 | 2 | 13 | 36% | 6.4% | 62% |
Proteins indicated in boldface were reproducibly isolated in at least two out of three independent experiments. Representative data are displayed.
Wild type (BD2528), ΔcomGG∷Tet (BD5404), O/E comGG (BD5575). O/E: overexpressing. n.a. indicates ‘not applicable’.
Because MntB, YqfA (FloA), FtsH and YuaG (FloT), which were co-immunopurified with ComGG (Table 2), were not known to be transformation proteins, we tested deletion strains for transformability (Table S4). Only the ftsH deletion had a statistically significant transformation deficiency. Inactivation of ftsH depresses comK expression (data not shown), providing a sufficient explanation for the transformation phenotype of this mutant.
ComGG is an integral membrane protein, while ComGD associates with the membrane by protein–protein interactions
It has been well documented that the major pilin is translocated out of the membrane after processing to assemble the competence pilus. Although the minor pilins are processed, it is unclear whether they too are translocated or remain within the membrane. Therefore, we analysed periplasm, cytoplasm and membrane fractions to determine ComGG localization (Fig. 5A). The cell wall fraction consists of the supernatant recovered after sedimenting protoplasts, and includes material that is derived from the outer surface of the cell membrane including the cell wall and the periplasmic space. EF-G, ComEA and ComGA were used as controls for cytoplasmic, integral membrane and peripheral membrane protein localizations respectively, and ComGC was included as an example of a protein that resides in both the membrane and the cell wall fraction (Chung et al., 1998; Chen et al., 2006). Figure 5A shows that each control protein was detected in its expected fraction. Although ComGG was detected in the membrane fraction, it was not found in the cytoplasmic or cell wall fractions.
Fig. 5.
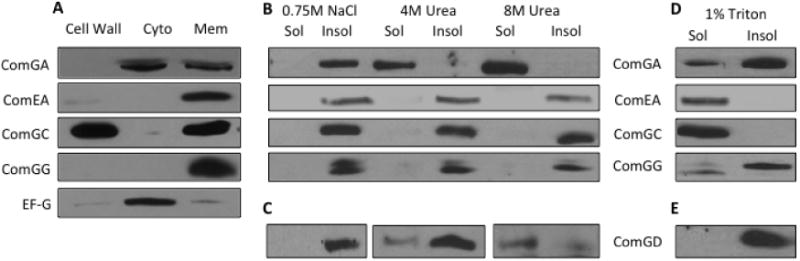
Characterization of pilin protein membrane associations.
A. The cell wall, cytoplasmic and membrane fractions from wild-type (BD2528) cells were analysed by Western blotting with EF-G, ComEA, ComGA, ComGC and ComGG antisera.
B–E. Membranes isolated from cultures of (B, D) wild-type (BD2528) or (C, E) ComGD over-producing (BD5727) strains were incubated with buffers containing 0.75 M NaCl, 4 M urea, 8 M urea or 1% Triton X-100 as described in Experimental procedures. The soluble and insoluble fractions were separated by ultracentrifugation. Samples were immunoblotted with (B, D) ComGA, ComEA, ComGC, ComGG or (C, E) ComGD antisera.
To further investigate the membrane association of ComGG, we tested its solubility by incubating isolated membranes in solutions containing 0.75 M NaCl, 4 M urea or 8 M urea (Fig. 5B, Pailler et al., 2010). As expected, ComGA was completely solubilized by treatment with 4 M and 8 M urea but not by NaCl, suggesting that it is associated with the membrane via non-ionic protein–protein interactions. ComEA and the membrane-associated ComGC were insoluble in all three buffers, as expected for integral membrane proteins (Inamine and Dubnau, 1995; Chung et al., 1998). ComGG also displayed the solubilization pattern expected of an integral membrane protein.
Because ComGD is difficult to detect, we utilized an overproducing strain to examine its membrane association. Like ComGC and ComGG it was not released by NaCl treatment (Fig. 5C). Unlike these proteins, it was partially solubilized by 4 M urea and nearly completely by 8 M urea, suggesting that it is anchored by protein–protein interactions, unlike a typical transmembrane protein.
Like ComGG from wild-type membranes (Fig. 5B), unprocessed ComGG in membranes from a comC deletion strain is insoluble in NaCl and urea (data not shown). We conclude that both pre-ComGG and mature ComGG are integral membrane proteins. In contrast, ComGD is anchored to the membrane largely through protein–protein interactions although it may be partially embedded in the membrane. Membrane samples were also treated with Triton X-100, which can be used to solubilize integral membrane proteins (Fig. 5D and E). Only about half of the total ComGG was solubilized by Triton and the unprocessed form (pre-ComGG) appeared to be less soluble than the mature form (Fig. 5D). Likewise, only a portion of the ComGA protein was Triton soluble (Fig. 5D) and all of ComGD was insoluble (Fig. 5E). Partialor incomplete solubility in Triton X-100 implies either that these proteins precipitate when removed from the membrane, and thus pellet with the insoluble material, or that they are associated with a detergent-resistant component of the B. subtilis membrane (Donovan and Bramkamp, 2009; Lopez and Kolter, 2010; Yepes et al., 2012; Bach and Bramkamp, 2013). This issue will be discussed below.
Pre-ComGG and mature ComGG are oriented differently with respect to the membrane
It was previously suggested that unprocessed pre-ComGG in intact protoplasts was not susceptible to externally added protease (Chung et al., 1998). This was unexpected because it is generally believed that unprocessed pilins are oriented with their C-terminal domains exposed to the outside of the cell membrane. Although ComGC and processed ComGG are pronase accessible in intact protoplasts, pre-ComGG was indeed resistant to pronase degradation (Fig. 6A), in agreement with previous results. The cytoplasmic EF-G was also resistant, reflecting the integrity of the protoplasts. After detergent solubilization all three proteins were degraded, showing that they were not intrinsically resistant to proteolysis. To determine if pre-ComGG resistance is a characteristic of only this protein when associated with the membrane or is due to its association with other competence proteins, we examined the susceptibility of pre-ComGG in the absence of other competence proteins. comGG was expressed from the Phyperspank promoter at the amyE locus in a ΔcomGG∷Tet (BD5965) or ΔcomK∷Tet (BD6003) background. Again, pre-ComGG was not susceptible to pronase degradation in either strain, whereas mature ComGG was produced and degraded in the comGG knockout, as expected (Fig. 6B). It is important to note that pre-ComGG is pronase resistant in the wild-type background, when ComC is present. This excludes the possibility that the resistance results from a failure of pre-ComGG to interact with the membrane or to fold properly when ComC is absent. In Fig. 6, lanes 2 and 6, the unprocessed ComGG was resistant to pronase even in the wild-type strain when ComC was present, excluding the possibility that pronase resistance was due to artefactual insolubility of pre-ComGG in the cytoplasm when ComC is absent. These results suggest that prior to processing by ComC, ComGG is not exposed on the outer surface of the membrane, contrary to expectations. Additionally, this is a characteristic of pre-ComGG that is independent of other competence proteins. It remained possible that all unprocessed competence pilins exhibit this property, and to test this we analysed pre-ComGC in a comC∷pTV55 (BD6739) background to prevent it from being processed (Fig. 6C). Clearly pre-ComGC, unlike pre-ComGG, is susceptible to pronase degradation in protoplasts.
Fig. 6.

Pre-ComGG is not susceptible to pronase degradation in intact protoplasts, unlike Pre-ComGC. Intact protoplasts were isolated from the following strains: (A) wild type (BD2528), (B) (wild type) ΔcomGG∷Tet amyE∷Phyperspank-comGG (BD5965) and ΔcomK∷Tet amyE∷Phyperspank-comGG (BD6003) and (C) (wild type) ΔcomGC amyE∷Phyperspank-comGC (BD6732) and comC∷pTV55 amyE∷Phyperspank-comGC (BD6739). The protoplasts were then incubated in protoplast-supernatant buffer with or without pronase and with or without 1% Triton X-100. Samples were analysed by Western blotting for EF-G, ComGG and ComGC, and representative blots are shown. The upper and lower arrows indicate the unprocessed and processed forms of ComGG or ComGC respectively.
Discussion
The minor pilins of the T2S systems form complexes that are believed to initiate assembly of the pilin fibres, perhaps also forming the tip of the fibre where they may mediate contacts with other components, possibly even with substrates for secretion (Korotkov and Hol, 2008). Although progress has been made in understanding protein–protein interactions among proteins in the T2S system (Sauvonnet et al., 2000; Durand et al., 2003; 2005; Hansen and Forest, 2006; Johnson et al., 2006; Forest, 2008; Korotkov and Hol, 2008; Yanez et al., 2008; Douzi et al., 2009; 2012; Giltner et al., 2012; McLaughlin et al., 2012; Burrows, 2012a,b; Cisneros et al., 2012b), little was known in this regard about the competence pilus system.
Several important findings emerge from the present work. First, we have shown that the minor pilins ComGD, ComGE and ComGG interact directly with one another (Figs 1, 3 and 4). We have extended the interacting partners of ComGG to include nearly all the known proteins needed for transformation (Table 2), although many of these interactions may be indirect. Although some of these proteins were previously shown to be in close proximity using FRET analysis (Kramer et al., 2007), no other ComG proteins other than ComGA were found. Common sense would suggest that proteins that function at different sequential phases of the transformation process must associate at various times. The colocalization of competence proteins predominantly at the cell poles (Hahn et al., 2005), the aforementioned FRET analysis and the data presented here, all substantiate this expectation. Naturally our data do not demonstrate the existence of a super-complex in which all of these proteins are together at the same time.
It has been generally considered that pilins of the T4P, T2S and competence systems were organized prior to processing as bitopic integral membrane proteins with their C-termini facing outward (Douzi et al., 2012). In the T2S system this simple picture has been modified based on evidence that the minor pilins associate with one another in a staggered conformation, with one or more proteins partially withdrawn from the membrane (Cisneros et al., 2012a). This staggered complex has a rise between complex subunits similar to that of the T2S pilus, and for this reason it has been proposed that the minor pilin complex primes pilus assembly (Burrows, 2012a; Cisneros et al., 2012a). For two reasons, our data imply that the simple picture must be modified for the competence system as well. First, ComGD is partially solubilized by urea (Fig. 5C), implying that its association with the membrane is mediated in part by protein–protein interactions. Perhaps ComGD is partially withdrawn from the membrane like its orthologues in the T2S system.
Second, and more striking, although pre-ComGG is susceptible to pronase, it is not degraded when this protease is added to the outside of intact protoplasts. In contrast, mature ComGG, pre-ComGC and processed ComGC are susceptible to extracellular pronase (Fig. 6). This departure of pre-ComGG from the behaviour expected of a bitopic transmembrane protein does not depend on interaction with other ComK-dependent competence proteins (Fig. 6B). We interpret these findings as suggesting an unusual arrangement of pre-ComGG with respect to the membrane (Fig. 7). Because pre-ComGG is accessible for processing by ComC while its C-terminus is protected from proteolysis, we propose that both the N-and C-termini of pre-ComGG reside in the cytoplasm. The resistance of pre- and post-ComGG to solubilization by high salt or urea suggests that the protein is strongly associated with the membrane. In fact the addition of 100 mM Na2CO3 together with Triton X-100, completely solubilized both processed and unprocessed ComGG (data not shown), consistent with interactions with both membrane lipids and protein. However, pre-ComGG is mostly insoluble in Triton X-100, contrary to post-ComGG. We therefore propose that pre-ComGG resides largely on the cytoplasmic face of the membrane, closely associated with the membrane in complex with other ComG proteins, and that this association is required for processing by ComC (Fig. 7). This unusual topology is consistent with both the demonstrated requirement for other ComG proteins for pre-ComGG processing and the existence of comGG mutations in the C-terminal domain that prevent processing. These mutations may prevent proper folding of pre-ComGG and/or direct interaction with these other proteins, thus preventing processing. In any case these data pose interesting questions, because T4P and T2S substrates are thought to be inserted in the membrane via the Sec pathway (Pugsley et al., 1991; Lee and Schneewind, 2001), which is generally dependent only on signal sequence interactions. In contrast, pre-ComGC adopts a more classical bitopic membrane topology so that only a few residues are exposed in the cytoplasm and its correct interaction with the processing peptidase presumably requires only a conserved N-terminal pilin sequence. We do not know if the other minor competence pre-pilins are arranged similarly or if this unexpected topologyis a unique characteristic of pre-ComGG. We have not been able to detect ComGG in the cell wall fraction (Fig. 5A) or in the culture medium (data not shown) and there is no evidence for the presence of this minor pilin in the competence pilus. The resistance of pre-ComGG to complete solubilization by Triton X-100 is similar to what has been described for other B. subtilis proteins (Donovan and Bramkamp, 2009; Lopez and Kolter, 2010) and to a defining characteristic of lipid rafts in eukaryotic membranes (Brown, 2006; Pike, 2009). Lipid rafts are membrane micro-domains that are considered to contain highly ordered lipids, responsible for the detergent-resistance of associated proteins. Although ComGG exhibits detergent-resistance and is also associated with the flotillin-like proteins YqfA (FloA) and YuaG (FloT) (Table 2), we remain cautious in concluding that this implies association with an ordered lipid domain. It is very possible that the apparent partial detergent-resistance of ComGG (and ComGA) is due to its insolubility when removed from the membrane. In other words these proteins may not be detergent-resistant but may be inherently insoluble when away from the membrane and may therefore pellet when centrifuged. The ability of Na2CO3 with Triton X-100 to completely solubilize ComGG is consistent with this hypothesis, because high pH can assist in the solubilization of proteins (D. Brown, personal correspondence). It will be necessary to test this idea by carrying out flotation experiments with the ‘detergent-resistant’ material to demonstrate that it remains in association with lipids. Despite our caution, we are intrigued by these observations and by the finding that inactivation of either flotillin orthologue [yqfA (floA) or yuaG (floT)] resulted in elevated rates of transformation, because this may imply a regulatory role for the flotillin-dependent organization of the competence proteins in the membrane.
Fig. 7.
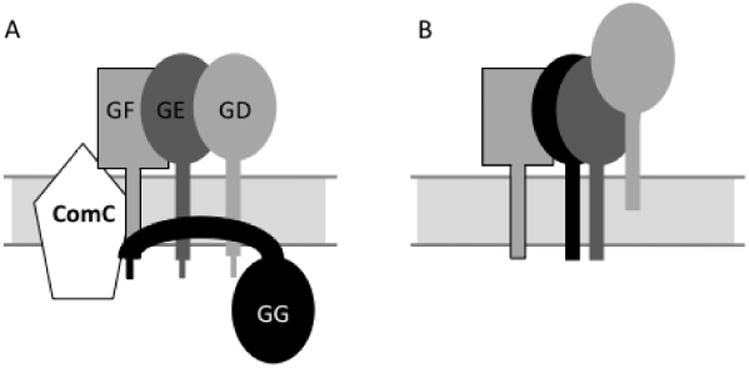
Model for assembly of the Bacillus minor pilin complex.
A. The N- and C-termini of pre-ComGG are in the cytoplasm. The protein interacts with ComGD (GD), as well as with ComGE (GE) and ComGF (GF). Some of these transmembrane segment interactions are speculative. The interactions are essential for efficient processing of ComGG.
B. After processing, ComGG is embedded in the membrane with the typical pilin orientation. The pilin complex is stabilized through contacts between their C-terminal domains. ComGD is less tightly associated with the membrane than the other minor pilins and protein–protein interactions play an important role in this association.
Because each of the minor pilin proteins, as well as ComGF, is required for assembly of the competence pilus, we propose that the complex formed by these proteins is required for pilus initiation, elongation or both. The ATPase ComGA is also required to assemble the pilus and our studies show that association with ComGA stabilizes ComGG and that ComGA and ComGC can be co-immunoprecipitated with ComGG. We have shown elsewhere that ComGA is required to translocate ComGC from the membrane and that the Walker A motif of ComGAplays a role in translocation (Briley et al., 2011). It is tempting to suggest that ComGA uses direct or indirect contacts with the minor pilin complex to mobilize processed ComGC for pilus assembly.
ComEA is a bitopic DNA-binding protein, which is needed, together with ComFA and ComEC, for transport of DNA into the cell. In its absence, DNA binding to the cell surface is reduced but still present (Briley et al., 2011). ComEA may conceivably associate with ComGC, positioning the membrane transport apparatus near the base of the pilus. The pilus may serve to gather DNA from the cell environment and deliver the DNA to ComEA. ComEA may then hand the DNA to the ComEC channel. This general idea is supported by the recent important observation that the ComG proteins of S. pneumoniae elabo-rate a long DNA binding pilus fibre that extends into the environs of the cell (Laurenceau etal., 2013). This structure may retract, bringing the DNA near the cell surface where it can bind to ComEA, much as bacteriophage are transported to the cell surface by retraction of T4P (Burrows, 2012b). Indeed such a role for pilus retraction is consistent with our data suggesting a role for this structure in DNA uptake in addition to its role in DNA binding to the cell (Briley etal., 2011). The T4P systems require two ATPases, one for pilus extension and another for retraction. In our case, ComGA is clearly needed for construction of the pilus (Chen etal., 2006; Briley etal., 2011). ComFA, an unrelated membrane-associated ATPase, is required for DNA transport across the membrane (Londono-Vallejo and Dubnau, 1994a). ComFA has the sequence motifs of a helicase (Londono-Vallejo and Dubnau, 1993) and is more likely to be involved in moving the DNA; it is probably not needed for pilus retraction, although this has not been ruled out. An alternative hypothesis, which avoids the problems posed by the absence of two ComGA-related ATPases, is that the transformation pilus may not retract at all, but may serve to trap DNA near the cell where the extended DNA fibre attached to a flexible pilus would have an increased probability of encountering cell surface receptors. The reduced binding observed in the absence of pili (Briley etal., 2011) is consistent with this suggestion and would imply that DNA can bind directly to one or more cell surface receptors, bypassing the pilus, albeit with reduced efficiency. Further work to distinguish between trapping and retraction models is needed urgently.
Experimental procedures
Bacterial strains, growth conditions, transformation and genetic constructions
The B. subtilis strains used for this work are listed in Table S1. Unless otherwise noted, experiments were performed in a background that overproduces the regulatory protein ComS (pUB110∷comS, Hahn etal., 1993). Strain construction was carried out by transformation, with selection for appropriate antibiotic resistance (Albano etal., 1987) or by transduction using bacteriophage PBS1 (Takahashi, 1963). Antibiotic concentrations used with B. subtilis were as follows: 20 μg ml−1 chloramphenicol (Cat), 10 μg ml −1 kanamycin (Kan), 100 μg ml −1 spectinomycin (Spc), 5 μg ml −1 phleomycin (Phle) and 20 μg ml −1 tetracycline (Tet). For most experiments B. subtilis was grown in liquid competence medium (Albano et al, 1987). Phyperspank constructs were induced by the addition of 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) to the medium, following a 1 h growth period at 37°C. E. coli DH5α (Invitrogen) was utilized for plasmid storage. E. coli BL21 (DE3) was used for protein expression. E. coli was grown in LB or 2× yeast tryptone, supplemented with the appropriate antibiotics. Antibiotic concentrations used for plasmid selection in E. coli were as follows: 100 μg ml −1 ampicillin (Amp) and 50 μg ml −1 Kan. Genetic constructions are described in Supporting information and all constructs were confirmed by DNA sequencing analysis (Genewiz). Primers are listed in Table S2.
Transformability was assayed as described previously (Briley et al., 2011) using genomic DNA from a leu prototroph. Appropriate dilutions were plated on minimal medium agar supplemented with histidine and methionine for enumeration of transformants and on LB agar to determine viable counts.
Production of antibodies against ComGG and ComGD
The C-terminal domain of ComGG, residues 30–124, or the C-terminal domain of ComGD, residues 43–143, were cloned into the expression vector pQE30 (Qiagen) by Roberta Prov-veddi in our laboratory, generating N-terminally His6-tagged constructs. The His6-tagged proteins were partially purified under denaturing conditions using standard protocols and antibodies were raised in rabbits by Lampire Biological Laboratories (Pipersville, PA).
SDS-PAGE and immunoblotting
Cell pellets were resuspended in STM buffer (50 mM Tris pH 8.0, 25% sucrose, 50 mM NaCl, 5 mM MgCl2) containing 350 μg ml−1 lysozyme and incubated at 37°C for 5 min. The volume of STM was normalized to the turbidity measurement of the culture when the sample was collected. Sample buffer [final concentration of 20 mM Tris HCl pH 6.8, 10% glycerol, 1% SDS, 0.01% bromophenol blue, 2% 2-mercaptoethanol (Sigma-Aldrich) ] was added to the samples, which were then incubated at 100°C for 5 min. Samples were separated by electrophoresis on either 12% or 15% Tris-tricine SDS poly-acrylamide gels (Schagger and von Jagow, 1987). The proteins were transferred to a nitrocellulose membrane (Millipore) using a Bio-Rad semidry transfer apparatus (Bio-Rad) at 12 V for 1 h. Primary antisera was used at the following dilutions: 1:1000 guinea pig anti-ComEA (Inamine and Dubnau, 1995), 1:5000 rabbit anti-ComGC (Breitling and Dubnau, 1990), 1:5000 rabbit anti-ComGA (Briley et al., 2011), 1:3000 rabbit anti-ComGG, 1:2000 rabbit anti-ComGD and 1:3000 rabbit anti-elongation factor G(EF-G) (agenerous gift from Jonathon Dworkin). Most signals were detected using a secondary dilution of 1:10 000 goat anti-rabbit conjugated to horseradish-peroxidase (HRP) (Calbiochem) followed by Enhanced Chemiluminescence (ECL) Prime Western Blot Detection Reagent (Amersham) as per the manufacturers’ instructions. The bands were visualized by exposing the blot to Hyblot film (Denville Scientific). The ComEA signal was detected using a 1:30 000 IRDye 800 Donkey anti-guinea pig IgG (LI-COR Biosciences) and then analysed using the Odyssey Clx infrared imaging system (LI-COR Biosciences).
Subcellular fractionation
Cell wall and periplasm fractions were isolated from protoplasts in an osmotically protective buffer containing MgCl2 and sucrose with lysozyme. Protoplasts were lysed for separation of cytoplasm and membrane as previously described (Breitling and Dubnau, 1990), with slight modifications. The cytoplasmic (supernatant) and membrane (pellet) fractions were separated by centrifugation in a SW55Ti rotor (Beckman) at 35 000 r.p.m. for 25 min (4°C). The membrane pellet was resuspended in 0.5 ml 1× sample buffer. Equal volumes of cell wall and cytoplasmic fractions were loaded, and the volume of the membrane fraction loaded was adjusted to ensure that comparable amounts of cell-equivalents were loaded on the gel. Samples were analysed by SDS-PAGE followed by immunoblotting with appropriate antisera.
For the separation of peripheral and integral membrane proteins, four membrane fractions prepared in parallel were incubated for 1 h at 4°C in one of the following solutions: 0.75 M NaCl, 8 M urea, 4 M urea, all in TED buffer (20 mM Tris-HCl, pH 8.0, 1.25 mM EDTA, 2 mM DTT, Pailler etal, 2010). For further classification of detergent insolubility, membranes were incubated in 1% Triton X-100 in TED buffer for 1 h at 37°C or 1% Triton X-100 and 0.1 M Na2CO3 in TED buffer for 1 h at 4°C (Brown, 2002). The insoluble and soluble fractions were separated by centrifugation in a SW55Ti rotor (Beckman) at 50 000 r.p.m. for 1 h at either 4°C or 25°C, based on the solubilization temperature. The insoluble fractions were resuspended in 1 ml of 1% Triton X-100 in TED buffer. Samples from each fraction where analysed by SDS-PAGE followed by immunoblotting.
ComGG co-immunoprecipitation
Membranes were prepared as described above from 60 ml cultures of wild-type (BD2528), comC∷pTV55 (BD3844) and AcomGG∷Tet (BD5404) strains and solubilized in 1.5 ml of MS Buffer [50 mM Tris HCl pH 8.0, 150 mM NaCl, 0.5% Triton X-100, one tablet of complete protease inhibitor cocktail (Roche) ] by incubation overnight at 4°C. The insoluble (pellet) and soluble (supernatant) fractions were separated by centrifugation. The soluble fraction was mixed with ComGG antiserum at a dilution of 1:200 for 3 h at 4°C followed by the addition of 0.1 ml of Protein A resin slurry (Pierce Thermo Scientific) and incubation at 25°C for 3 h. Resin was collected by centrifugation for 2 min at 2500 g and then washed several times with 1 ml of MS Buffer. The immune complexes were eluted by incubating the resin with 0.1 ml of 0.1 M glycine (pH 2.5) at 25°C for 15 min then neutralized with 5 μl 1 M Tris HCl pH 8.0 and 2 μl of 1 M NaOH. Samples were analysed by SDS-PAGE and immunoblotting for ComGD and ComGG.
Mass spectrometry
The sample preparation, conjugation of magnetic beads, immunopurification of ComGG-containing protein complexes, and mass spectrometric analyses were carried out essentially as described previously (Cristea etal., 2005; Carabetta etal., 2010; 2013; Greco etal., 2012; Joshi etal, 2013). A strain producing wild-type levels of ComGG (BD2528), overproducing ComGG (BD5575) and a comGG knockout (BD5404) were grown to competence, then harvested and frozen as described (Cristea etal, 2005; Carabetta etal, 2013). Lysis buffer conditions were optimized, and a suitable buffer that allowed for efficient ComGG solubilization was used: 20 mM HEPES pH 7.4, 100 mM CH3CO2K, 2 mM MgCl2, 0.1% tween-20 (v/v), 1 mM ZnCl2, 1 mM CaCl2, 1% Triton-X, 200 mM NaCl, 0.5% deoxycholate, 1:100 protease inhibitor cocktail (Sigma) and 2 mg ml−1 phenylmethylsulphonyl fluoride (PMSF). Affinity-purified ComGG antiserum was conjugated to magnetic M270 Epoxy Dynabeads (Dynal, Invitrogen) at a concentration of 10 μg of antibody per mg of beads. Immunopurified complexes were eluted into TEL buffer [26 mM Tris-HCl, 35 mM Tris-base, 127 mM EDTA, 0.5% lithium dodecyl sulphate (LDS, Invitrogen) ], and prepared for in-solution digestion. All samples were analysed by nLC-MS/MS on a Dionex Ultimate 3000 RSLC coupled to an LTQ-Orbitrap Velos ETD mass spectrometer (Thermo Fisher Scientific, San Jose, CA), as described (Joshi etal, 2013).
Pull-down of GST-tagged proteins
BL21 (DE3) E. coli strains, harbouring either a pQLinkN or pQlinkG2 (Scheich etal, 2007), were grown at 37°C to OD600 = 0.6, induced with 1 mM IPTG and grown at 25°C for 6 h. Cell pellets were harvested by centrifugation and stored at −20°C. The amount of each cell pellet used was adjusted based on the efficiency of protein expression, determined from prior experiments, so that approximately equal amounts of expressed proteins were combined. Pellets from cultures ranging in volume from 2 to 35 ml were used. Each cell pellet was resuspended in 1.75 ml of GST Buffer [20 mM Tris pH 7.5, 150 mM NaCl with complete protease inhibitor cocktail (Roche) ]. Each resuspended pellet from a culture with an untagged protein was mixed with one expressing a GST-tagged protein. The suspensions were immediately lysed by sonication and samples were centrifuged in a SW55Ti rotor (Beckman) at 25 000 r.p.m. for 30 min at 4°C to remove cell debris. One and half millilitres of the supernatants were combined with 0.1 ml of glutathione (GSH) superflow agarose beads (Clontech Laboratories), previously equilibrated with GST Buffer, for 1 h at 4°C. The samples were centrifuged at 2000 g for 2 min and the supernatants were discarded. The recovered resin was washed several times with 1 ml of GST Buffer and proteins were eluted with 0.1 ml of 45 μM L-glutathione (Sigma-Aldrich) in 180 mM Tris-HCl pH 8.0. Samples were analysed by SDS-PAGE followed by Coomassie blue staining and immunoblotting for ComGD or ComGG.
Pronase treatment
The experiment was performed essentially as previously described (Chung etal, 1998). Eighty millilitres of cultures of wild type (BD2528), mcComS AcomGG∷Tet amyE∷Phyperspank-comGG (BD5965) and mcComS AcomK∷TetamyE∷Phyperspank-comGG (BD6003) for analysis of ComGG, as well as mcComS AcomGC amyE∷Phyperspank-comGC (BD6732) and mcComS comC∷pTV55 amyE∷Phyperspank-comGC (BD6739) for ComGC analysis, were grown to competence in the presence of IPTG, to induce comGG or comGC expression from the ectopic locus respectively. Pellets from 20 ml aliquots of each culture were collected by centrifugation. The pellets were resuspended in protoplast-supernatant buffer (25% sucrose, 50 mM Tris HCl pH 8.0, 50 mM NaCl, 5 mM MgCl2, 350 μg ml−1 lysozyme) and incubated for 30 minat37°C. Protoplasts were separated from the cell wall fraction by centrifugation at 3300 g for 2 min. The supernatants were further clarified by centrifugation at 13 500 r.p.m. for 2 min. Each protoplast pellet was resuspended in protoplast-supernatant buffer or protoplast-supernatant buffer plus 1% Triton X-100, with or without 2.5 mg ml−1 pronase (Sigma Aldrich). Samples were incubated at 37°C for 30 min, after which 4 mM PMSF, 1 mM EDTA and 50 μl of complete protease inhibitor cocktail (One tablet was dissolved into 0.5 ml protoplast-supernatant buffer, Roche) were added. The samples with Triton X-100 were immediately heated at 100°C for 5 min. The samples without Triton X-100 were washed three times with protoplast-supernatant buffer containing 4 mM PMSF, 1 mM EDTA and complete protease inhibitor cocktail. After washing, protoplasts were lysed with protoplastlysing buffer (50 mM Tris HCl pH 8.0, 50 mM NaCl, 5 mM MgCl2, 10 μg ml−1 DNase, 10 μg ml−1 RNase) and incubated for 5 min at 4°C. The samples were then heated at 100°C for 5 min. All samples were analysed by SDS-PAGE and immu-noblotted for EF-G, ComGC and ComGG.
Supplementary Material
Acknowledgments
We thank all members of the Dubnau lab and Todd Greco from the Cristea lab for discussion and advice. We appreciate the gift of EF-G antiserum from Jonathan Dworkin, as well as strains provided by John Helmann and Daniel Lopez. This work was supported by 1F31AI084542-01 awarded to J.M.M., GM043756 awarded to D.D. and DP1DA026192 and 1R21HD073044 awarded to I.M.C.
Footnotes
Supporting information: Additional supporting information may be found in the online version of this article at the publisher's web-site.
References
- Albano M, Dubnau DA. Cloning and characterization of a cluster of linked Bacillus subtilis late competence mutations. J Bacteriol. 1989;171:5376–5385. doi: 10.1128/jb.171.10.5376-5385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano M, Hahn J, Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987;169:3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano M, Breitling R, Dubnau DA. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J Bacteriol. 1989;171:5386–5404. doi: 10.1128/jb.171.10.5386-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JN, Bramkamp M. Flotillins functionally organize the bacterial membrane. Mol Microbiol. 2013;88:1205–1217. doi: 10.1111/mmi.12252. [DOI] [PubMed] [Google Scholar]
- Berge M, Mortier-Barriere I, Martin B, Claverys JP. Transformation of Streptococcus pneumoniae relies on DprA- and RecA-dependent protection of incoming DNA single strands. Mol Microbiol. 2003;50:527–536. doi: 10.1046/j.1365-2958.2003.03702.x. [DOI] [PubMed] [Google Scholar]
- Breitling R, Dubnau D. Amembrane protein with similarity to N-methylphenylalanine pilins is essential for DNA binding by competent Bacillus subtilis. J Bacteriol. 1990;172:1499–1508. doi: 10.1128/jb.172.3.1499-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley K, Jr, Dorsey-Oresto A, Prepiak P, Dias MJ, Mann JM, Dubnau D. The secretion ATPase ComGA is required for the binding and transport of transforming DNA. Mol Microbiol. 2011;81:818–830. doi: 10.1111/j.1365-2958.2011.07730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA. Isolation and use of rafts. In: Coligan JE, editor. Current Protocols in Immunology. New York: John Wiley & Sons; 2002. pp. 11.10.01–11.10.23. [DOI] [PubMed] [Google Scholar]
- Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- Burrows LL. Prime time for minor subunits of the type II secretion and type IV pilus systems. Mol Microbiol. 2012a;86:765–769. doi: 10.1111/mmi.12034. [DOI] [PubMed] [Google Scholar]
- Burrows LL. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol. 2012b;66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- Carabetta VJ, Silhavy TJ, Cristea IM. The response regulator SprE (RssB) is required for maintaining poly(A) polymerase I-degradosome association during stationary phase. J Bacteriol. 2010;192:3713–3721. doi: 10.1128/JB.00300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabetta VJ, Tanner AW, Greco TM, Defrancesco M, Cristea IM, Dubnau D. A complex of YlbF, YmcA and YaaT regulates sporulation, competence and biofilm formation by accelerating the phosphorylation of Spo0A. Mol Microbiol. 2013;88:283–300. doi: 10.1111/mmi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Dubnau D. DNA uptake during bacterial transformation. Nat Rev Microbiol. 2004;2:241–249. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- Chen I, Provvedi R, Dubnau D. A macromolecular complex formed by a pilin-like protein in competent Bacillus subtilis. J Biol Chem. 2006;281:21720–21727. doi: 10.1074/jbc.M604071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YS, Dubnau D. ComC is required for the processing and translocation of comGC, a pilin-like competence protein of Bacillus subtilis. Mol Microbiol. 1995;15:543–551. doi: 10.1111/j.1365-2958.1995.tb02267.x. [DOI] [PubMed] [Google Scholar]
- Chung YS, Breidt F, Dubnau D. Cell surface localization and processing of the ComG proteins, required for DNA binding during transformation of Bacillus subtilis. Mol Microbiol. 1998;29:905–913. doi: 10.1046/j.1365-2958.1998.00989.x. [DOI] [PubMed] [Google Scholar]
- Cisneros DA, Bond PJ, Pugsley A, Campos M, Francetic O. Minor pseudopilin self-assembly primes type II secretion pseudopilus elongation. EMBO J. 2012a;31:1041–1053. doi: 10.1038/emboj.2011.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros DA, Pehau-Arnaudet G, Francetic O. Heterologous assembly of type IV pili by a type II secretion system reveals the role of minor pilins in assembly initiation. Mol Microbiol. 2012b;86:805–818. doi: 10.1111/mmi.12033. [DOI] [PubMed] [Google Scholar]
- Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M, Egelman EH, Tainer JA. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol Cell. 2006;23:651–662. doi: 10.1016/j.molcel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Cristea IM, Williams R, Chait BT, Rout MP. Fluorescent proteins as proteomic probes. Mol Cell Proteomics. 2005;4:1933–1941. doi: 10.1074/mcp.M500227-MCP200. [DOI] [PubMed] [Google Scholar]
- Cserzo M, Wallin E, Simon I, von Heijne G, Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997;10:673–676. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- Donovan C, Bramkamp M. Characterization and subcellular localization of a bacterial flotillin homologue. Microbiology. 2009;155:1786–1799. doi: 10.1099/mic.0.025312-0. [DOI] [PubMed] [Google Scholar]
- Douzi B, Durand E, Bernard C, Alphonse S, Cambillau C, Filloux A, et al. The XcpV/GspI pseudopilin has a central role in the assemblyofa quaternary complex within the T2SS pseudopilus. J Biol Chem. 2009;284:34580–34589. doi: 10.1074/jbc.M109.042366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douzi B, Filloux A, Voulhoux R. On the path to uncover the bacterial type II secretion system. Philos Trans R Soc Lond B Biol Sci. 2012;367:1059–1072. doi: 10.1098/rstb.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draskovic I, Dubnau D. Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulphide bonds. Mol Microbiol. 2005;55:881–896. doi: 10.1111/j.1365-2958.2004.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D, Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972;64:9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D, Provvedi R. Internalizing DNA. Res Microbiol. 2000;151:475–480. doi: 10.1016/s0923-2508(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Durand E, Bernadac A, Ball G, Lazdunski A, Sturgis JN, Filloux A. Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure. J Bacteriol. 2003;185:2749–2758. doi: 10.1128/JB.185.9.2749-2758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E, Michel G, Voulhoux R, Kurner J, Bernadac A, Filloux A. XcpX controls biogenesis of the Pseudomonas aeruginosa XcpT-containing pseudopilus. J Biol Chem. 2005;280:31378–31389. doi: 10.1074/jbc.M505812200. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Ayora S, Alonso JC. Bacillus subtilis homologous recombination: genes and products. Res Microbiol. 2000;151:481–486. doi: 10.1016/s0923-2508(00)00165-0. [DOI] [PubMed] [Google Scholar]
- Forest KT. The type II secretion arrowhead: the structure of GspI–GspJ–GspK. Nat Struct Mol Biol. 2008;15:428–430. doi: 10.1038/nsmb0508-428. [DOI] [PubMed] [Google Scholar]
- Giltner CL, Nguyen Y, Burrows LL. Type IV pilin proteins: versatile molecular modules. Microbiol Mol Biol Rev. 2012;76:740–772. doi: 10.1128/MMBR.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco TM, Miteva Y, Conlon FL, Cristea IM. Complementary proteomic analysis of protein complexes. Methods Mol Biol. 2012;917:391–407. doi: 10.1007/978-1-61779-992-1_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove DE, Willcox S, Griffith JD, Bryant FR. Differential single-stranded DNA binding properties of the paralogous SsbA and SsbB proteins from Streptococcus pneumoniae. J Biol Chem. 2005;280:11067–11073. doi: 10.1074/jbc.M414057200. [DOI] [PubMed] [Google Scholar]
- Hahn J, Inamine G, Kozlov Y, Dubnau D. Characterization of comE, a late competence operon of Bacillus subtilis required for the binding and uptake of transforming DNA. Mol Microbiol. 1993;10:99–111. doi: 10.1111/j.1365-2958.1993.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Hahn J, Luttinger A, Dubnau D. Regulatory inputs for the synthesis of ComK, the competence transcription factor of Bacillus subtilis. Mol Microbiol. 1996;21:763–775. doi: 10.1046/j.1365-2958.1996.371407.x. [DOI] [PubMed] [Google Scholar]
- Hahn J, Maier B, Haijema BJ, Sheetz M, Dubnau D. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell. 2005;122:59–71. doi: 10.1016/j.cell.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijema BJ, Hahn J, Haynes J, Dubnau D. A ComGA-dependent checkpoint limits growth during the escape from competence. Mol Microbiol. 2001;40:52–64. doi: 10.1046/j.1365-2958.2001.02363.x. [DOI] [PubMed] [Google Scholar]
- Hansen JK, Forest KT. Type IV pilin structures: insights on shared architecture, fiber assembly, receptor binding and type II secretion. J Mol Microbiol Biotechnol. 2006;11:192–207. doi: 10.1159/000094054. [DOI] [PubMed] [Google Scholar]
- Hobbs M, Mattick JS. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- Inamine GS, Dubnau D. ComEA, a Bacillus subtilis integral membrane protein required for genetic transformation, is needed for both DNA binding and transport. J Bacteriol. 1995;177:3045–3051. doi: 10.1128/jb.177.11.3045-3051.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsborg O, Eldholm V, Havarstein LS. Natural genetic transformation: prevalence, mechanisms and function. Res Microbiol. 2007;158:767–778. doi: 10.1016/j.resmic.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Johnson TL, Abendroth J, Hol WG, Sandkvist M. Type II secretion: from structure to function. FEMS Microbiol Lett. 2006;255:175–186. doi: 10.1111/j.1574-6968.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- Joshi P, Greco TM, Guise AJ, Luo Y, Yu F, Nesvizhskii AI, Cristea IM. The functional interactome landscape of the human histone deacetylase family. Mol Syst Biol. 2013;9:672. doi: 10.1038/msb.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkov KV, Hol WG. Structure of the GspK– GspI–GspJ complex from the enterotoxigenic Escherichia coli type 2 secretion system. Nat Struct Mol Biol. 2008;15:462–468. doi: 10.1038/nsmb.1426. [DOI] [PubMed] [Google Scholar]
- Kramer N, Hahn J, Dubnau D. Multiple interactions among the competence proteins of Bacillus subtilis. Mol Microbiol. 2007;65:454–464. doi: 10.1111/j.1365-2958.2007.05799.x. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Lacks S, Greenberg B, Neuberger M. Identification of a deoxyribonuclease implicated in genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975;123:222–232. doi: 10.1128/jb.123.1.222-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenceau R, Pehau-Arnaudet G, Baconnais S, Gault J, Malosse C, Dujeancourt A, et al. A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS Pathog. 2013;9:e1003473. doi: 10.1371/journal.ppat.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VT, Schneewind O. Protein secretion and the pathogenesis of bacterial infections. Genes Dev. 2001;15:1725–1752. doi: 10.1101/gad.896801. [DOI] [PubMed] [Google Scholar]
- Londono-Vallejo JA, Dubnau D. comF, a Bacillus subtilis late competence locus, encodes a protein similar to ATP-dependent RNA/DNAhelicases. Mol Microbiol. 1993;9:119–131. doi: 10.1111/j.1365-2958.1993.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Londono-Vallejo JA, Dubnau D. Membrane association and role in DNA uptake of the Bacillus subtilis PriA analogue ComF1. Mol Microbiol. 1994a;13:197–205. doi: 10.1111/j.1365-2958.1994.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Londono-Vallejo JA, Dubnau D. Mutation of the putative nucleotide binding site of the Bacillus subtilis membrane protein ComFA abolishes the uptake of DNA during transformation. J Bacteriol. 1994b;176:4642–4645. doi: 10.1128/jb.176.15.4642-4645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D, Kolter R. Functional microdomains in bacterial membranes. Genes Dev. 2010;24:1893–1902. doi: 10.1101/gad.1945010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MG, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin LS, Haft RJ, Forest KT. Structural insights into the Type II secretion nanomachine. Curr Opin Struct Biol. 2012;22:208–216. doi: 10.1016/j.sbi.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier-Barriere I, Velten M, Dupaigne P, Mirouze N, Pietrement O, McGovern S, et al. A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell. 2007;130:824–836. doi: 10.1016/j.cell.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Pailler J, Aucher W, Pires M, Buddelmeijer N. Phosphatidylglycerol:prolipoprotein diacylglyceryl transferase (Lgt) of Escherichia coli has seven transmembrane segments, and its essential residues are embedded in the membrane. J Bacteriol. 2010;194:2142–2151. doi: 10.1128/JB.06641-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechowska M, Fox MS. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971;108:680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl.):S323–S328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provvedi R, Dubnau D. ComEA is a DNA receptor for transformation of competent Bacillus subtilis. Mol Microbiol. 1999;31:271–280. doi: 10.1046/j.1365-2958.1999.01170.x. [DOI] [PubMed] [Google Scholar]
- Pugsley AP, Poquet I, Kornacker MG. Two distinct steps in pullulanase secretion by Escherichia coli K12. Mol Microbiol. 1991;5:865–873. doi: 10.1111/j.1365-2958.1991.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Sauvonnet N, Vignon G, Pugsley AP, Gounon P. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 2000;19:2221–2228. doi: 10.1093/emboj/19.10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Scheich C, Kummel D, Soumailakakis D, Heinemann U, Bussow K. Vectors for co-expression of an unrestricted number of proteins. Nucleic Acids Res. 2007;35:e43. doi: 10.1093/nar/gkm067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sinderen D, Luttinger A, Kong L, Dubnau D, Venema G, Hamoen L. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol Microbiol. 1995;15:455–462. doi: 10.1111/j.1365-2958.1995.tb02259.x. [DOI] [PubMed] [Google Scholar]
- Strom MS, Lory S. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J Biol Chem. 1991;266:1656–1664. [PubMed] [Google Scholar]
- Strom MS, Lory S. Structure–function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- Takahashi I. Transducing phages for Bacillus subtilis. J Gen Microbiol. 1963;31:211–217. doi: 10.1099/00221287-31-2-211. [DOI] [PubMed] [Google Scholar]
- Takeno M, Taguchi H, Akamatsu T. Role of ComEA in DNA uptake during transformation of competent Bacillus subtilis. J Biosci Bioeng. 2012;113:689–693. doi: 10.1016/j.jbiosc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Vignon G, Kohler R, Larquet E, Giroux S, Prevost MC, Roux P, Pugsley AP. Type IV-like pili formed by the type II secreton: specificity, composition, bundling, polar localization, and surface presentation of peptides. J Bacteriol. 2003;185:3416–3428. doi: 10.1128/JB.185.11.3416-3428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav T, Carrasco B, Hejna J, Suzuki Y, Takeyasu K, Alonso JC. Bacillus subtilis DprA recruits RecA onto ssDNA and mediates annealing of complementary ssDNA strands coated by SsbB and SsbA. J Biol Chem. 2013;288:22437–22450. doi: 10.1074/jbc.M113.478347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez ME, Korotkov KV, Abendroth J, Hol WG. The crystal structure of a binary complex of two pseudopilins: EpsI and EpsJ from the type 2 secretion system of Vibrio vulnificus. J Mol Biol. 2008;375:471–486. doi: 10.1016/j.jmb.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes A, Schneider J, Mielich B, Koch G, Garcia-Betancur JC, Ramamurthi KS, et al. The biofilm formation defect of a Bacillus subtilis flotillin-defective mutant involves the protease FtsH. Mol Microbiol. 2012;86:457–471. doi: 10.1111/j.1365-2958.2012.08205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


