Abstract
Primary myoblast culture is a valuable tool in research of muscle disease, pathophysiology, and pharmacology. This protocol describes techniques for dissociation of cells from human skeletal muscle biopsies and enrichment for a highly myogenic population by fluorescence-activated cell sorting (FACS). We also describe methods for assessing myogenicity and population expansion for subsequent in vitro study.
Keywords: Skeletal muscle, Myoblast isolation, Tissue dissociation, Fluorescence-activated cell sorting (FACS)
Background
Primary human myoblasts from muscle biopsies are a valuable resource for modeling human muscle disease in vitro. Alterations in myoblast proliferation, differentiation, and fusion are features shared by many neuromuscular disorders, and can be used to assay cell-based and pharmacological therapies. Human skeletal muscle biopsies, especially those affected by disease, often contain extensive populations of non-myogenic cells such as adipocytes and fibroblasts. Thus, it is important to purify a myogenic population for in vitro study of skeletal muscle development and disease. Early studies of muscle disease involved use of tissue explants or unpurified dissociated cells (Geiger and Garvin, 1957; Herrmann et al., 1960 ; Goyle et al., 1967 ; Bishop et al., 1971 ), and later, Blau and Webster introduced a pre-plating technique to remove fibroblasts (Blau and Webster, 1981). Here, we describe an effective technique for dissociation of mononuclear cells from human muscle biopsies, and purification of a highly myogenic population utilizing FACS with the cell surface markers CD56 and CD82 (see Note 1). We recently demonstrated that CD82 is an excellent myogenic marker in both human fetal and adult skeletal muscle that is also retained on activated and differentiating myogenic progenitors ( Alexander et al., 2016 ). This protocol also describes methods to culture these myoblasts and confirm a myogenic population by in vitro fusion assay. Isolation and expansion of these cells from normal individuals and from individuals with muscle disorders will help accelerate the development of therapies for human disorders such as muscular dystrophies.
Materials and Reagents
-
Dissociation of primary human skeletal muscle tissue (see Note 2)
Protected sterile disposable scalpels with stainless steel blade size #10 (Aspen Surgical, Bard-ParkerTM, catalog number: 372610)
Sterile 10 cm tissue culture-treated plastic dishes (Corning, Falcon®, catalog number: 353003)
Assorted sterile 5, 10, and 25 ml pipettes (Olympus Plastics, catalog numbers: 12-102, 12-104, 12-106)
Sterile 0.22 µm PES (low protein binding) filters (250 and 500 ml volumes) (such as Olympus Plastics, catalog number: 25-227)
Sterile 15 and 50 ml conical centrifuge tubes (Olympus Plastics, catalog numbers: 21-101 and 21-106)
BD Falcon sterile nylon cell strainers (100 µm and 40 µm pore sizes) (Corning, catalog numbers: 352360 and 352340)
Sterile 1.8 ml CryoTubeTM vials (Thermo Fisher Scientific, Thermo Scientific TM, catalog number: 377267)
-
10x Hank’s balanced saline solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14185052) or 10x Dulbecco’s phosphate buffer saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14200075), free of calcium chloride, magnesium chloride and magnesium sulfate, diluted to 1x with double distilled water and filter sterilized with a 0.22 µm PES filter
Note: This solution can be stored at 4 °C or room temperature (RT).
Sterile red blood cell lysis solution (QIAGEN, catalog number: 158904) (stored at RT)
Sterile HEPES buffered saline solution, without phenol red (Lonza, catalog number: 12-747F)
Dulbecco’s modified Eagle’s medium (DMEM 4.5 g glucose) (Thermo Fisher Scientific, GibcoTM, catalog number: 10564011)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437)
100x penicillin-streptomycin-glutamine (PSG) (Thermo Fisher Scientific, GibcoTM, catalog number: 10378016)
Calcium chloride dihydrate (Sigma-Aldrich, catalog number: C7902-500G)
Dispase II (Roche Diagnostics, catalog number: 04942078001)
Collagenase D (Roche Diagnostics, catalog number: 11088882001 2.5g)
Complete growth medium (500 ml) (see Recipes)
1 M calcium chloride solution (CaCl2·2H2O, FW 147) (see Recipes)
Dispase stock solution (see Recipes)
Collagenase D stock solution (see Recipes)
Sterile freezing medium (see Recipes)
-
Purification of myoblasts from dissociated human skeletal muscle mononuclear cells
-
Thawing of cryopreserved sample prior to FACS
Sterile 0.22 µm PES filter (500 ml volume) (Thermo Fisher Scientific, catalog number: 569-0020)
Sterile 50 ml conical centrifuge tubes (Olympus Plastics, catalog number: 21-106)
Sterile tissue culture-treated plastic dishes (10 or 15 cm size) (Corning, Falcon®, catalog numbers: 353003 and 353025)
CryoTubeTM vial containing dissociated unpurified primary cells (Simport, catalog number: T309-2A)
Dulbecco’s modified Eagle’s medium (DMEM) (DMEM 4.5 g glucose) (Thermo Fisher Scientific, GibcoTM, catalog number: 10564011)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437)
100x penicillin-streptomycin-glutamine (PSG) (Thermo Fisher Scientific, GibcoTM, catalog number: 10378016)
Complete growth medium (see Recipes)
-
Preparation of sample for FACS
Sterile 0.22 µm PES filter (50 ml volume) Cole Palmer Steriflip-GP Filter, 0.22 µm PES Item # UX-29969-20 (EMD Millipore, catalog number: SCGP00525)
Sterile 15 and 50 ml conical centrifuge tubes (Olympus Plastics, catalog numbers: 21-101 and 21-106)
Sterile 5 ml round bottom test tubes with cell strainer caps (Corning, Falcon®, catalog number: 352054)
Dissociated unpurified primary cells, thawed one day prior to FACS
Sterile 1x HBSS (diluted from 10x stock) (Thermo Fisher Scientific, GibcoTM, catalog number: 14185052)
Sterile 1x DPBS (diluted from 10x stock) (Thermo Fisher Scientific, GibcoTM, catalog number: 14200075)
TrypLE ExpressTM dissociation enzyme with phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 12605010)
-
Antibodies
APC anti-CD56 antibody, Clone HCD56 (BioLegend, catalog number: 318310)
PE anti-CD82 antibody, Clone ASL-24 (BioLegend, catalog number: 342103)
-
Calcein blue (1 mg vial) (Thermo Fisher Scientific, catalog number: C1430)
Note: Resuspend in 200 µl dimethyl sulfoxide (DMSO, AmericanBio, catalog number: AB03091-00050) aliquot in 25 µl aliquots and store at -20 °C (stock). Use 0.5 µl stock calcein/106 cells.
Sterile 5% FBS solution (50 ml) (see Recipes)
-
Fluorescence-activated cell sorting (FACS)
FACS or 5 ml round-bottom tubes with cell strainer caps (Corning, Falcon®, catalog number: 352235)
-
-
In vitro culture and analysis of human skeletal myoblasts
Sterile 50 ml conical centrifuge tubes (Olympus Plastics, catalog number: 21-106)
Sterile 10 cm tissue culture-treated plastic dishes (Corning, Falcon®, catalog number: 353003)
Sterile 0.22 µm PES filter (50 ml volume) Cole Palmer Steriflip-GP Filter, 0.22 µm PES Item # UX-29969-20 (EMD Millipore, catalog number: SCGP00525)
Sterile 1x HBSS (diluted from 10x stock) (Thermo Fisher Scientific, GibcoTM, catalog number: 14185052)
TrypLETM Express Dissociation Enzyme with Phenol Red (Thermo Fisher Scientific, GibcoTM, catalog number: 12605010)
Dulbecco’s modified Eagle’s medium (DMEM) 4.5 g glucose for proliferation medium (Thermo Fisher Scientific, GibcoTM, catalog number: 10564011) and DMEM 1 g glucose for differentiation medium (Thermo Fisher Scientific, GibcoTM, catalog number: 10567014)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437)
100x penicillin-streptomycin-glutamine (PSG) (Thermo Fisher Scientific, GibcoTM, catalog number: 10378016)
Gelatin Type A from porcine skin (Sigma-Aldrich, catalog number: G1890)
Horse serum (Thermo Fisher Scientific, GibcoTM, catalog number: 16050122)
Complete growth medium (see Recipes)
Differentiation medium (50 ml) (see Recipes)
0.1% gelatin (see Recipes)
-
Immunofluorescence for in vitro fusion assay
Aluminum foil
4-well chamber slides, Nunc Lab-Tek II Permanox (Thermo Fisher Scientific, catalog number: 177437)
-
10x Dulbecco’s phosphate buffered saline (PBS) (diluted from 10x stock) (Thermo Fisher Scientific, GibcoTM, catalog number: 14200075)
Note: Diluted to 1x with double distilled water. Store at RT.
-
Antibodies (stored at 4 °C)
Anti-Myosin Heavy Chain (Developmental Studies Hybridoma Bank, MF-20) or anti human desmin (clone D33) (Abcam, catalog number: ab8470)
AffiniPure F(Ab’)2 Alexa Fluor 594 Donkey anti-Mouse IgG (H+L) (Jackson ImmunoResearch, catalog number: 715-586-150) (Protect from light)
Vectashield HardSet mounting medium with DAPI (Vector Laboratories, catalog number: H-1500)
Triton X-100 (Sigma-Aldrich, catalog number: T8787-250ml)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437)
16% paraformaldehyde (PFA) (Electron Microscopy Sciences, catalog number: 15710-S), diluted to 4%
4% paraformaldehyde (PFA) (see Recipes)
Permeabilization solution (see Recipes)
Blocking solution (see Recipes)
Equipment
-
Dissociation of primary human skeletal muscle tissue
Bench top centrifuge (Beckman Coulter, model: Allegra® 6R, catalog number: 366816)
Bright-LineTM hemocytometer (0.1 mm) (Hausser Scientific, catalog number: 1492)
Sterile laminar flow biosafety cabinet (SterilGard® Class II Type A/B3) (The Baker Company, model: SG400)
-150 °C freezer, with liquid nitrogen storage backup tank (VIP® PLUS) (Panasonic, model: MDF-C2156VANC)
Humidified 5% CO2 incubator set to 37 °C (Thermo Fisher Scientific, Thermo ScientificTM, model: FormaTM Series II 3110, catalog number: 3110)
FACS: Becton Dickinson Aria II equipped with 4 lasers and a biosafety cabinet
-
Purification of myoblasts from dissociated human skeletal muscle mononuclear cells
Sterile laminar flow biosafety cabinet (SterilGard® Class II Type A/B3) (The Baker Company, model: SG400)
Water bath set to 37 °C (Sheldon Manufacturing, SHEL LAB®, model: SWB15)
Bench top centrifuge (Beckman Coulter, model: Allegra® 6R, catalog number: 366816)
Inverted microscope (Nikon, model: TMS-F, catalog number: 210775)
Bright-LineTM hemocytometer (0.1 mm) (Hausser Scientific, catalog number: 1492)
Cell sorter, such as Becton Dickinson Aria II equipped with 4 lasers and a biosafety cabinet
-
In vitro culture and analysis of human skeletal myoblasts
1,000 µl pipette
Rotating shaker
Sterile laminar flow biosafety cabinet (SterilGard® Class II Type A/B3) (The Baker Company, model: SG400)
Water bath set to 37 °C (Sheldon Manufacturing, SHEL LAB®, model: SWB15)
Humidified 5% CO2 incubator set to 37 °C (Thermo Fisher Scientific, Thermo ScientificTM, model: FormaTM Series II 3110, catalog number: 3110)
Bench top centrifuge (Beckman Coulter, model: Allegra® 6R, catalog number: 366816)
Inverted microscope (Nikon, model: TMS-F, catalog number: 210775)
Bright-LineTM hemocytometer (0.1 mm) (Hausser Scientific, catalog number: 1492)
Inverted microscope with epi-fluorescence capabilities including ultraviolet/DAPI and FITC/GFP filter sets (such as Nikon, model: Eclipse E1000)
Software
Cell sorter analysis software (FlowJo: https://www.flowjo.com/solutions/flowjo)
Procedure
-
Dissociation of primary human skeletal muscle tissue
Note: All steps in this protocol should be performed in a sterile laminar flow biosafety cabinet using sterile tissue culture technique. Human skeletal muscle can only be obtained following approval from the Institutional IRB. We obtain de-identified, discarded skeletal muscle tissue under a protocol approved by Boston Children’s Hospital IRB.
Pre-weigh one 10 cm tissue culture plate with lid, and place the tissue sample to be dissociated in a second (non pre-weighed) 10 cm tissue culture plate.
Using sterile scalpels, remove any connective tissue from the muscle biopsy. Tissue should be kept moist. Add a few drops of sterile 1x HBSS as necessary to prevent it from drying out. Place muscle tissue in the pre-weighed 10 cm tissue culture plate, replace lid, and weigh the plate again. Subtract from this number the tare of the empty plate to calculate the amount of muscle tissue to be dissociated.
Thaw frozen aliquots of dispase II and collagenase D in a 37 °C water bath. Thawed collagenase D stock solution (see Recipes) and dispase II stock (see Recipes) will be added at a volume of 3.5 ml each per gram of muscle tissue to be dissociated. Thaw only the amounts of collagenase D and dispase II necessary for dissociation. If an excess of enzymes is thawed, it can be refrozen once and re-used.
Using sterile scalpels, mince muscle tissue until it resembles a fine paste. During mincing, add a few drops of sterile 1x HBSS to prevent exposed tissue from drying out. Tissue should always appear moist, but with no excess of liquid.
After tissue is finely minced, add equal amounts of the thawed dispase II and collagenase D solutions. The final concentration will be 5 mg/ml for collagenase D and 1.2 U/ml for dispase II in this solution. Pipette minced tissue and enzyme solution up and down through a sterile wide-bore 25 ml pipette a few times.
Incubate plate in a humidified 5% CO2 incubator set to 37 °C for 15 min.
Pipette the digestion solution up and down through a sterile 25 ml pipette a few times and incubate again for 15 min. Repeat this step additional 1-2 times, until the slurry easily passes through a sterile 5 ml pipette and all tissue chunks are dissolved. The total digestion time will range between 45 min and 1 h 15 min.
Add 2 volumes of complete growth medium (see Recipes) (based on the total volume of dispase II and collagenase D) to the digested slurry and filter the digestion solution through a 100 µm cell strainer over a 50 ml conical tube. Change cell strainer if it appears clogged.
Pellet cells for 10 min at 1,100 × g, RT.
Resuspend the pellet in 1 volume of complete growth medium (i.e., 3 ml) and add 7 volumes (i.e., 21 ml) of red blood cell lysis solution. Invert the tube a few times and then filter the solution through a 40 µm cell strainer over a 50 ml conical tube.
Count cells using a hemocytometer, then pellet the cells for 10 min at 1,100 × g, RT. Expect approximately 107 cells/gram tissue from postnatal skeletal muscle and 108 cells/gram tissue from fetal skeletal muscle. Cell numbers vary among individuals.
Freeze cells at a concentration of 1 x 107 cells/ml in ice-cold freezing medium (see Recipes). Store cryovials at -80 °C overnight, then transfer them to -150 °C where they can be permanently stored until necessary. Cell freezing is not required if all reagents and FACS equipment are immediately available.
-
Purification of myoblasts from dissociated human skeletal muscle mononuclear cells
Note: All steps in this protocol should be performed in a sterile laminar flow biosafety cabinet using sterile tissue culture technique. Cell sorting should be performed in as clean an environment as possible, including sorting human cells under a BSL-2 biosafety cabinet, if possible.
-
Thawing of cryopreserved sample prior to FACS
Cryopreserved cells should be carefully thawed and plated 1 day prior to cell sorting. This allows the cells to recover from the freezing process before undergoing FACS.
Pre-warm complete growth medium in a 37 °C water bath. Then, pipette 10 ml pre-warmed medium into a sterile 50 ml conical tube.
Coat sterile tissue culture-treated plates (10 cm plate) with 10 ml 0.1% gelatin (see Recipes) for 1 h at 37 °C, then remove the gelatin solution by aspiration. Let plates dry briefly in the biosafety cabinets and replace lid.
Carefully and quickly thaw a vial of cryopreserved, dissociated cells in a 37 °C water bath and transfer the cells into the 50 ml conical tube with 15 ml pre-warmed proliferation medium using a 1 ml pipette. Rinse the inside of the cryovial with fresh complete growth medium to remove as many cells as possible. This step should be performed very quickly as the DMSO used during the cryopreservation process is toxic to the cells at RT.
-
Plate the cells in the pre-warmed medium onto sterile, tissue-culture treated plates at approximately 0.5-1 x 107 cells/10 cm plate or 1.5-3 x 107 cells/15 cm plate. If using a 15 cm plate, add 15 ml pre-warmed medium to bring the total medium volume to 25 ml.
Note: No plate coating is required for this step.
Incubate the cells in a humidified 5% CO2 incubator set to 37 °C overnight. If plating cells several days in advance, change the growth medium every other day and do not allow confluence to exceed 80%.
-
Preparation of sample for FACS
Pre-warm complete growth medium and 1x DPBS in a 37 °C water bath. Place the 5% FBS/HBSS (see Recipes) on ice.
-
Check the cells under a phase contrast microscope with 10x magnification. Ensure that there is no contamination and that the cells look healthy.
Note: There will be many floating, live cells in your culture, which is normal for dissociated human skeletal muscle. It is also likely that there will be small clumps of cells in the culture, and the number of clumps will vary. These clumps will be filtered out prior to cell sorting. Additionally, the dissociation process results in a large amount of debris in addition to cells. This will make the culture appear ‘dirty’ (i.e., little black specks, etc.), but again, this is normal and should not be considered contamination. This debris will be removed during the FACS sample preparation process.
Wash the cells with 5 ml (10 cm plate) or 10 ml (15 cm plate) of 1x DPBS at least twice or ideally three times.
Pipette 2 ml (10 cm plate) or 5 ml (15 cm plate) of TrypLE ExpressTM dissociation enzyme onto the plate and incubate in a humidified 5% CO2 incubator set to 37 °C for 2 min.
Check under the microscope if cells have lifted and are now floating freely in the medium. If cells still adhere to the plate, gently tap the bottom of the plate to loosen the cells and return the plate to the incubator for another minute.
-
After incubation, remove the cells by gently swirling the medium, pipetting the cells a few times and pooling the cells to one side of the plate by tilting it at an angle (~45°), then carefully pipette the medium into the 50 ml conical tube.
Note: When the plate is tilted at an angle, the cells can be seen on the surface of the plate as a light opaque coating. Repeatedly rinse gently the cells off the plate using the trypsin solution (TrypLE ExpressTM Dissociation Enzyme with Phenol Red) until this coating is no longer visible.
-
Repeat step B2f with growth medium (5 ml for 10 cm plate and 10 ml for 15 cm plate) to collect any remaining cells and quench the TrypLETM.
Note: Prolonged exposure of the cells to undiluted TrypLE ExpressTM may negatively affect the health of the cells. Addition of growth medium to the trypsinized cells will quench this effect.
Check the plate under a phase contrast microscope at 10x magnification for the presence of cells. There should be very few cells on the surface of the plate after this process.
Centrifuge the 50 ml conical tubes containing the cells and washes at 1,100 × g at 4 °C for 10 min to pellet the cells.
Remove the supernatant, and resuspend the cells in 10 ml 5% FBS/HBSS.
Determine the cell concentration using a hemocytometer or other cell counting device.
-
For FACS controls, use 5 ml round-bottom test tubes and set aside 2.5 x 105 cells in 500 µl 5% FBS/HBSS for each of the following controls:
‘Unstained’ control
Calcein blue single color control
CD56 single color control
CD82 single color control
-
Pipette the ‘unstained’ control sample through the strainer cap of a 5 ml round-bottom test tube. Keep on ice.
Note: Some FACS machines may require tubes that are different in diameter/size from the tube specified in this protocol. Check in advance that the tubes fit in the FACS machine.
Centrifuge the remaining cells (to be labeled with both CD56 and CD82 antibodies or single color controls) for 10 min at 1,100 × g at 4 °C.
Resuspend cells at a concentration of 1 x 107/ml in 5% FBS/HBSS.
Primary antibody incubation: add CD56 and CD82 antibodies to the appropriate cell solutions at a concentration of 5 µl per 1 x 106 cells.
-
To gate for live cells, add calcein blue at a concentration of 0.5 µl per 1 x 106 cells to the appropriate cell solutions. Gently mix and place on ice protected from the light for 30 min.
Note: Calcein blue is a cell viability dye and is used in this protocol to discriminate live from dead cells during the FACS.
After this incubation, wash the cells 1 x in 2ml of 5% FBS/HBSS.
Centrifuge the cells for 10 min at 1,100 × g at 4 °C.
Resuspend the CD56 and CD82 single color controls in 500 µl of 5% FBS/HBSS, and pipette through the strainer cap of a 5 ml round-bottom test tube. Store on ice in the dark.
Resuspend the CD56/CD82/calcein blue stained cells in 1 ml of 5% FBS/HBSS, and pipette through the strainer cap of a 5 ml round-bottom test tube. Store on ice in the dark.
Prepare collection tube for CD56+CD82+ sorted cells by pipetting 500 µl of growth medium into a new tube. Store on ice.
-
-
Fluorescence-activated cell sorting
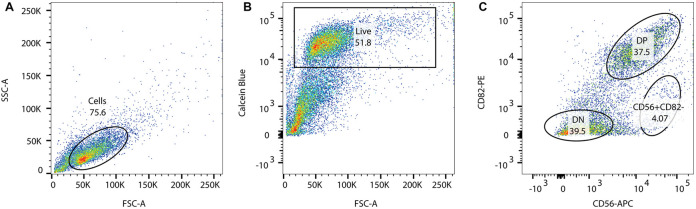
It is beyond the scope of this chapter to review FACS or flow cytometry in detail. Gating specifications are briefly indicated (Figure 1).
Determine optimal excitation voltages and compensation values using the ‘no stain’ and single color controls.
Determine the live cell population gating for calcein blue positive cells.
Determine the double positive (DP) CD56+/CD82+ and double negative (DN) populations. Gate and sort for the DP cell population.
-
In vitro culture and analysis of human fetal skeletal myoblasts
-
In vitro cell culture
Note: All steps in this protocol should be performed in a sterile laminar flow biosafety cabinet using sterile tissue culture technique.
Coat sterile tissue culture-treated plates (10 cm plate) with 10 ml 0.1% gelatin (see Recipes) for 1 h in a humidified 5% CO2 incubator set to 37 °C, then remove the gelatin solution by aspiration. Let plates dry briefly in the biosafety cabinets and replace lid.
Pre-warm complete growth medium in a water bath set to 37 °C.
Resuspend sorted CD56/CD82 double positive cells at 0.5-1 x 106 cells/10 ml complete growth medium and plate on coated plates. Gently rock plate(s) to evenly distribute cells, and then incubate in a CO2 incubator. Sorted cells will be small and have a bright, rounded appearance and should attach within 1 day post-sorting (Figure 2).
Propagate the cells to 65-75% confluency. This should take approximately 2-3 days; however, if necessary, replace the medium with fresh growth medium every 2 days until the plate is at 65-75% confluency.
-
To passage cells
Coat sterile tissue culture-treated plates with 0.1% gelatin as above (as in step D1a).
Pre-warm the following in a water bath set to 37 °C: 1x DPBS, TrypLETM Express dissociation enzyme, and complete growth medium.
Remove medium from plate by aspiration and wash the cells twice with 10 ml (10 cm plate) 1x DPBS. Remove DPBS by aspiration.
Pipette 2 ml TrypLETM Express onto the plate and incubate in a humidified 5% CO2 incubator set to 37 °C for 2-3 min. Gently remove the cells from the plate by pipetting up and down a few times before transferring cells into a sterile conical tube. Wash any remaining cells from the surface of the plate with additional complete growth medium.
Centrifuge the cells at 1,100 × g at RT for 10 min.
Resuspend the cells in 10 ml fresh complete growth medium.
Determine the cell concentration using a hemocytometer and plate the cells at 0.5-1 x 106 cells in 10 ml complete growth medium/10 cm plate.
-
Cells should be passaged every 2-3 days and should not be grown past 70% confluency.
Note: Cells should never reach 100% confluency when proliferating, as they will begin to differentiate and fuse on contact. The high serum growth medium will lower in serum concentration over time and will not be able to prevent fusion.
-
To freeze cells
Trypsinize and centrifuge cells as in steps D2c-D2e.
Resuspend cells in ice-cold freezing medium (10% DMSO in complete growth medium) at desired cell concentration (106-107/ml).
Store cryovials at -80 °C overnight then transfer to -150 °C where they can be permanently stored until necessary.
-
To perform an in vitro fusion assay
Coat 4-well chamber slides with 0.1% gelatin.
Trypsinize the cells (with TrypLE ExpressTM Dissociation Enzyme with Phenol Red) and determine the cell concentration as described above, then plate 20,000 cells in 500 µl complete growth medium/well.
Incubate the cells in a humidified 5% CO2 incubator set to 37 °C until cells are ~80% confluent.
-
When cells are ~80% confluent, remove the growth medium from each well and replace with 500 µl pre-warmed differentiation medium (see Recipes). Incubate the cells in a humidified 5% CO2 incubator set to 37 °C overnight.
Note: Low serum medium induces differentiation and fusion of myoblasts in culture (Yaffe and Saxel, 1977).
Replace the differentiation medium in each well daily during the course of the fusion assay.
Monitor the differentiation of the cells using a phase contrast microscope at 10x or 20x magnification. Fusion should occur within 1 week of exposure to differentiation medium (Figure 2). When mature myotubes of > 10 nuclei/myotube are present, perform the following immunocytochemistry protocol to visualize the cells by fluorescence microscopy.
-
Immunofluorescence for in vitro fusion assay
Note: This immunocytochemical protocol may also be utilized for the detection of other myogenic markers of proliferating or differentiating cells.
Thaw 4% PFA (see Recipes) at RT.
Carefully wash the cells with 200 µl 1x PBS/well. Remove PBS using a 1,000 µl pipette.
Fix the cells with 200 µl 4% PFA for 20 min at RT; remove fixation liquid using a pipette, then permeabilize the cells with 200 µl permeabilization solution (see Recipes) for 3 min at RT.
Remove permeabilization solution using a pipette, and then block the cells for 30 min at RT with 200 µl blocking solution (see Recipes).
Prepare the primary antibody solution by diluting the anti-human desmin antibody 1:100 in fresh blocking solution. Incubate the cells with primary antibody solution overnight at 4 °C.
Wash the cells 3 times with 1x PBS for 5 min at RT. The plate may be gently agitated on a rotating shaker.
Prepare the secondary antibody solution by diluting the anti-mouse antibody 1:1,000 in blocking solution. Incubate the cells in the dark with secondary antibody solution for 1 h at RT.
Wash the cells 3 times with 1x PBS for 5 min at RT in the dark. The plate may be gently agitated on a rotating shaker.
Mount the cells with Vectashield with DAPI and store at 4 °C protected from light with aluminum foil.
Visualize the cells by fluorescent microscopy using ultraviolet/DAPI and FITC/GFP filter sets for DAPI and desmin, respectively.
-
Figure 1. Example of FACS gating technique for human myogenic cells immunostained with anti-CD56 and anti-CD82 antibodies using calcein AM blue as vital dye marker.
A. Unstained control sample, gate on single cells; B. Calcein blue signal (calcein AM blue) to gate on live cells; C. Gated live cells stained for CD82-PE and CD56-APC. DN = double negative cells; DP = double positive cells.
Figure 2. In vitro culture of FACS sorted CD56 and CD82 double negative (DN) and double positive (DP) human cells.
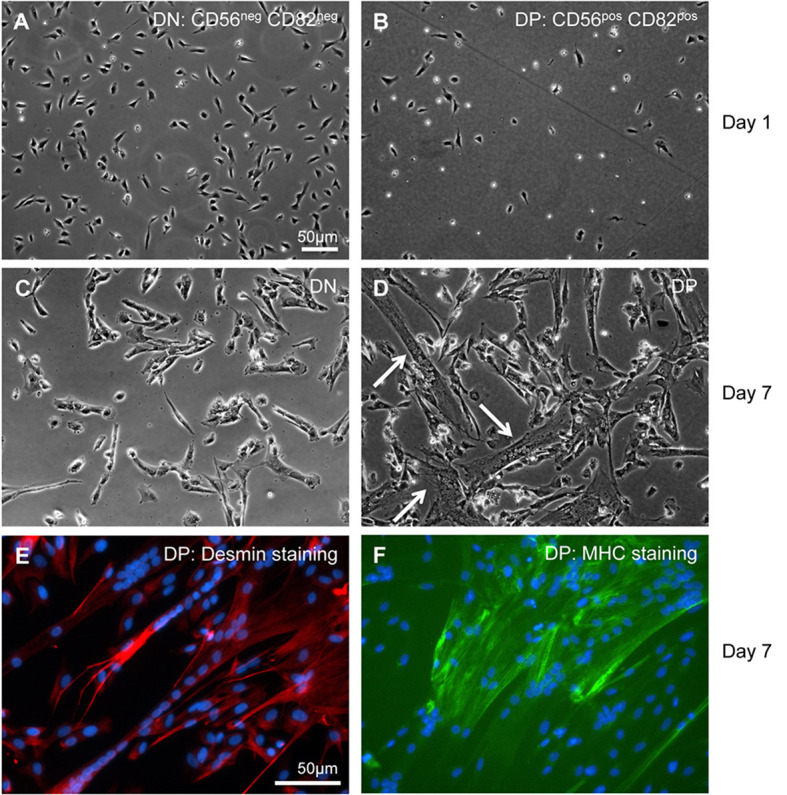
(A and B) On day 1 after sorting, both DN and DP cells appear bright and rounded, while some cells have flattened and firmly adhered to the plate. (C and D) On day 7 after sorting (4 days in growth medium and 3 days in differentiation medium [see Recipes]), DN cells remain mononuclear, whereas DP cells have fused and formed multinucleated myotubes (arrows). (E and F) Examples of immunostaining for myosin heavy chain (MHC) and desmin on double-positive cells are shown.
Data analysis
In vitro fusion assay–fusion index calculation
-
After immunofluorescent staining of myotubes, count the following in each of five random fields per well (x 4 wells/sample):
# of total nuclei
# of nuclei within myotubes
Calculate fusion index (%) as ((# nuclei within myotubes/# total nuclei) x 100).
Average the fusion index of the five fields.
Compare the fusion index of sorted myoblasts versus unsorted.
Notes
We would like to note that FACS with either CD56 or MCAM in conjunction with CD82 as enriching markers is a highly effective method for isolating human fetal myogenic progenitors. We refer the readers to the following protocol describing use of MCAM as a positive selection marker in cells sorted from human fetal tissue (Lapan and Gussoni, 2012). It is not appropriate to use CD82 and MCAM for adult human skeletal muscle, since endothelial cells express MCAM in adult muscle.
Institutional review and protocol approval are required prior to collection and processing of human tissue. All personnel handling human tissue must receive appropriate safety and human subject education training.
Recipes
-
Growth medium (500 ml)
395 ml of high glucose (4.5 g) Dulbecco’s modified Eagle’s medium (DMEM)
100 ml of fetal bovine serum (FBS)
5 ml of 100x penicillin-streptomycin-glutamine (PSG)
Sterilize by filtering the solution through a 500 ml 0.22 µm PES filter unit
Store at 4 °C and use within 1 month
Note: FBS varies considerably between companies and between lots. Therefore, we recommend testing several different FBS samples with the in vitro fusion assay described here.
-
Dispase II stock solution
Dissolve 1 g powder dispase II in 100 ml HEPES buffered saline solution
Add 316 ml of high glucose (4.5 g) DMEM to generate a stock solution of 2.4 U/ml
Filter-sterilize the solution through a PES 500 ml filter; aliquot into 15 ml conical tubes (10 ml/tube)
Store aliquots at -20 °C
-
1 M calcium chloride solution (CaCl2·2H2O, FW 147)
Dissolve 1.47 g powder in 10 ml of double distilled water
Store at 4 °C
-
Collagenase D stock solution
Dissolve 2.5 g powder collagenase D in 250 ml solution of 1x HBSS supplemented with 1.25 ml of 1 M calcium chloride solution
Sterilize by filtering through a 250 ml PES filter unit
Aliquot in 15 ml conical tubes (10 ml/tube) and stored at -20 °C
-
Sterile freezing medium
Combine 90% FBS and 10% dimethyl sulfoxide (DMSO)
Prepare freezing medium and immediately store on ice
Unused sterile freezing medium can be stored at 4 °C for up to 4 weeks
-
Differentiation medium (50 ml)
48.5 ml of low glucose (1 g) Dulbecco’s modified Eagle’s medium (DMEM)
1 ml of horse serum (HS)
0.5 ml of 100x penicillin-streptomycin-glutamine (PSG)
Sterilize by filtering the solution through a 150 ml 0.22 µm PES filter unit
Store at 4 °C and use within 1 month
-
Sterile 5% FBS/HBSS solution (50 ml)
Add 2.5 ml of FBS to 1x HBSS
Sterilize by filtering the solution through a 50 ml 0.22 µm PES filter unit
Store at 4 °C
-
0.1% gelatin
Add 0.5 g gelatin to 500 ml of double distilled water. DO NOT SHAKE
Sterilize the solution by autoclaving for 20 min and store at 4 °C
-
4% paraformaldehyde (4% PFA)
Dilute 16% paraformaldehyde with 1x PBS
USE CAUTION as paraformaldehyde is extremely toxic; it is recommended that paraformaldehyde be used in a fume hood for safety. Aliquot and store at -20 °C
Aliquots should not be repeatedly frozen and thawed; discard unused PFA after initial use
-
Permeabilization solution
Mix 50 µl of Triton X-100 with 10 ml of 1x PBS
-
Blocking solution
Mix together 1 ml of fetal bovine serum (FBS), 10 µl of Triton X-100, and 9 ml of 1x PBS
Acknowledgments
This work is supported by a grant from the Muscular Dystrophy Association #479606 (EG) and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 1R01AR069582-01 (EG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This protocol was modified from previous work, specifically from the listed references 1 and 7.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Alexander M. S., Rozkalne A., Colletta A., Spinazzola J. M., Johnson S., Rahimov F., Meng H., Lawlor M. W., Estrella E., Kunkel L. M. and Gussoni E.(2016). CD82 is a marker for prospective isolation of human muscle satellite cells and is linked to muscular dystrophies. Cell Stem Cell 19(6): 800-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop A., Gallup B., Skeate Y. and Dubowitz V.(1971). Morphological studies on normal and diseased human muscle in culture. J Neurol Sci 13(3): 333-350. [DOI] [PubMed] [Google Scholar]
- 3.Blau H. M. and Webster C.(1981). Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A 78(9): 5623-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geiger R. S. and Garvin J. S.(1957). Pattern of regeneration of muscle from progressive muscular dystrophy patients cultivated in vitro as compared to normal human skeletal muscle . J Neuropathol Exp Neurol 16(4): 523-543. [PubMed] [Google Scholar]
- 5.Goyle S., Kalra S. L. and Singh B.(1967). The growth of normal& dystrophic human skeletal muscle in tissue culture. Neurol India 15(4): 149-151. [PubMed] [Google Scholar]
- 6.Herrmann H., Konigsberg U. R. and Robinson G.(1960). Observations on culture in vitro of normal and dystrophic muscle tissue . Proc Soc Exp Biol Med 105: 217-221. [DOI] [PubMed] [Google Scholar]
- 7.Lapan A. D. and Gussoni E.(2012). Isolation and characterization of human fetal myoblasts. Methods Mol Biol 798: 3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaffe D. and Saxel O.(1977). A myogenic cell line with altered serum requirements for differentiation. Differentiation 7(3): 159-166. [DOI] [PubMed] [Google Scholar]