Abstract
IN BRIEF Several new endoscopic bariatric therapies have been approved by the U.S. Food and Drug Administration for the treatment of obesity, with many more devices and procedures undergoing investigational studies. This article describes these devices and procedures and special considerations for their use in patients with diabetes.
Endoscopic bariatric and metabolic therapies (EBMTs) are a new category of therapies that expand the options for effective obesity treatment. These therapies require flexible endoscopy for either placement, removal, or procedure performance. EBMTs for primary obesity treatment can be divided into two categories: gastric therapies and small-bowel therapies.
Gastric EBMTs are placed or performed in the stomach, whereas small-bowel EBMTs are placed or performed in the small intestine. In general, gastric therapies result in weight loss, which is associated with improvements in metabolic outcomes, but do not have weight loss–independent effects on metabolic outcomes. Small-bowel therapies likely have both weight loss–dependent and weight loss–independent effects on metabolic outcomes, including blood glucose control.
Currently, the only EBMT devices or procedures that are approved for use by the U.S. Food and Drug Administration (FDA) are gastric therapies. These include intragastric balloons (IGBs), aspiration therapy, and a suturing device that has been approved for the general indication of tissue approximation in the gastrointestinal (GI) tract and is used for a procedure called endoscopic sleeve gastroplasty (ESG). Multiple small-bowel therapies are being developed but are still in the research phase.
FDA-Approved EBMTs
IGBs
IGBs are space-occupying devices that are placed endoscopically or swallowed by patients. Although the IGBs currently available in the United States were approved in the past 2 years, they are the not the first balloons to have received approval. The Garren-Edwards Gastric Bubble was the first IGB approved for use in the United States in 1985. Complications from this balloon, including gastric mucosa injury; deflation with migration into the small bowel, causing obstruction; and lack of weight loss (1–4) resulted in its removal from the market in 1988.
All of the currently approved IGBs were designed to address the issues that plagued the Garren-Edwards device (Table 1). Multicenter trials for FDA approval of both the ReShape and Obalon balloons were randomized, sham-controlled trials, whereas the multicenter trial for Orbera was an open-label randomized, controlled trial (RCT). All three trials demonstrated a significant percentage of total body weight loss (TBWL) compared to control conditions in intention-to-treat (ITT) analyses that included all subjects who underwent device or sham device placement (ReShape active 6.8% vs. control 3.3% TBWL; Obalon active 6.6 ± 5.1% vs. control 3.4 ± 5.0% TBWL; and Orbera active 10.2 ± 6.6% vs. control 3.3 ± 5.0% TBWL) (5–7). As expected, subjects in the open-label Orbera trial achieved higher weight loss than in the sham trials. Clinical case series from outside of the United States have demonstrated greater weight loss than in the U.S. RCTs (8,9). This discrepancy is likely multifactorial and may include increased patient baseline motivation to change, increased personalized lifestyle therapy, and patient self-payment, resulting in increased adherence to lifestyle changes. Clinical case series from the United States are not yet available, but it is possible that clinical experience within the United States will be similar to that from elsewhere. Moreover, in the U.S. multicenter trials, patients maintained 70–89.5% of their weight loss 6 months after balloon removal, and clinical case series demonstrate weight loss maintenance in up to 23% of patients at 5 years (10).
TABLE 1.
FDA-Approved Intragastric Balloons
| Images | Characteristics | FDA Status | |
|---|---|---|---|
| ReShape dual balloon system (ReShape Medical, San Clemente, Calif.) |
|
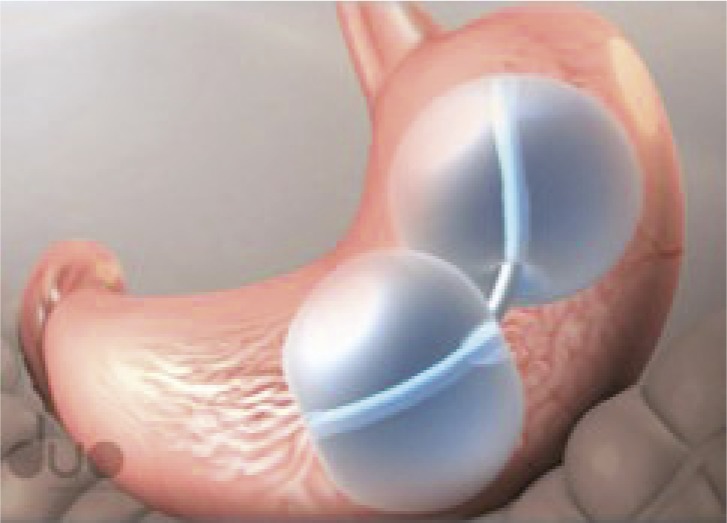
• Two medical-grade silicone spheres joined by a flexible shaft | • Approved 28 July 2015 |
| • Each balloon is filled with 375–450 mL saline dyed with methylene blue | • For patients with a BMI of 30–40 kg/m2 and one obesity-related comorbidity | ||
| • Endoscopically placed and removed | • Remains in place for 6 months | ||
| Orbera intragastric balloon system (Apollo Endosurgery, Austin, Tex.) |  |
• Medical-grade silicone sphere | • Approved 5 August 2015 |
| • Filled with 400–700 mL saline | • For patients with a BMI of 30–40 kg/m2 | ||
| • Endoscopically placed and removed | • Remains in place for 6 months | ||
| Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) |

|
• Thin polymer ellipse shape | • Approved 8 September 2016 |
| • Filled with 250 mL nitrogen mix gas | • For patients with a BMI of 30–40 kg/m2 | ||
| • Three balloons administered in an 8- to 12-week period | • Remains in place for 6 months from first administration | ||
| • Swallowed and endoscopically removed |
Cardiometabolic changes were evaluated in the U.S. pivotal trials of the ReShape, Orbera, and Obalon balloon systems. It is important to note that baseline metabolic parameters (i.e., fasting blood glucose, lipids, A1C, and blood pressure) were normal on average, and very few participants had a diagnosis of diabetes at study entry. Compared to subjects receiving the control condition of lifestyle therapy only, those treated with the Obalon balloon system had small but significant improvements in systolic blood pressure, fasting glucose, LDL cholesterol, and triglycerides at 24 weeks (11). Metabolic outcomes for the ReShape dual balloon were not reported compared to the control condition (12). No differences were seen between subjects in the control group and those treated with the Orbera balloon (13).
A clinic case series of 143 consecutive patients with a metabolic syndrome rate of 34.8% and a type 2 diabetes rate of 32.6% found a reduction in the incidence of metabolic syndrome to 14.5% and of diabetes to 20.9% at IGB removal, with 14.1 ± 5.7% TBWL (14). Moreover, 12 months after IGB removal, the TBWL remained high at 11.2 ± 4.6%, and incidence of metabolic syndrome and diabetes remained low at 11.6 and 21.3%, respectively (14).
Serious adverse events (AEs) were higher in the ReShape and Orbera trials than in the Obalon trial (10.6, 10, and 0.5%, respectively). Rates of serious AEs for both ReShape and Orbera were driven by dehydration requiring intravenous fluids due to nausea and vomiting after IGB placement or intolerability resulting in early device removal. The Obalon trial had only one serious AE due to a bleeding ulcer in a patient on protocol-prohibited nonsteroidal anti-inflammatory drugs. All serious AEs resolved without sequela.
Nonserious AEs were common but varied slightly among the different IGBs. Vomiting was a more common AE in the ReShape and Orbera trials (86.7 and 86.8%, respectively) than in the Obalon trial (17.3%). Gastric ulceration occurred in 35% of ReShape subjects compared to 0 and 0.9% of Orbera and Obalon subjects, respectively; however, a design change during the ReShape trial reduced the ulceration rate to 10%. Incidence of gastroesophageal reflex disease was highest in the Orbera trial, at 30% (5–7).
There are several management considerations for using IGB therapy for weight loss in patients with diabetes. First, IGBs are indicated for patients with a BMI of 30–40 kg/m2, with the ReShape dual balloon indications also requiring at least one obesity-related comorbidity. Use outside of this BMI range is considered off-label use, but evidence suggests that the percentage of TBWL in such instances is consistent with that in subjects with higher BMIs (15). Second, all patients initiating therapy with IGB and starting a low-calorie diet should have reductions in medications that can cause hypoglycemia. Moreover, the fluid-filled IGBs require a liquid and pureed diet for at least 2 weeks after IGB placement. Third, the fluid-filled IGBs can induce vomiting after placement, while a patient’s stomach is adjusting to accommodate the IGB. This can be managed with antiemetic medication, but it is important to adjust antidiabetic medications in anticipation of significant vomiting. Fourth, data suggest that the fluid-filled IGBs alter gut physiology, resulting in delayed gastric emptying that may correlate with weight loss (16), and baseline gastroparesis is considered a contraindication for IGB placement.
Aspiration Therapy
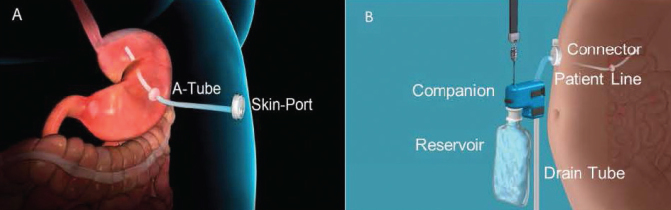
Aspiration therapy allows patients to remove a portion of their gastric contents after eating a meal with the use of the AspireAssist system (Aspire Bariatrics, King of Prussia, Pa.). The AspireAssist system contains both implanted components similar to a percutaneous endoscopic gastrostomy tube and components that are only used during aspiration (Figure 1). The implanted components include the A-tube, which is a silicone tube with a fenestrated 15-cm intragastric and extragastric portion separated by an intragastric bumper, and the skin-port, which is a <1-cm-high disc attached to the A-tube that can be opened to allow the flow of gastric contents. The components that are only used during aspiration include the connector, which attaches to and opens the skin-port and has a counter that locks and prevents further skin-port opening after 115 openings; the patient line, which connects the connector to the companion; the companion, which is a siphon with a one-way valve allowing for passive flow of gastric contents out or water flush into the stomach; the reservoir, which is a 600-mL soft water bottle filled with tap water used for flushing the system; and the drain tube, which is a silicone tube attached to the companion that directs the flow of gastric contents into the toilet or sink.
FIGURE 1.
AspireAssist system. A) Implanted components include the A-tube and skin-port. B) Components used only during aspiration include the connector, patient line, companion, reservoir, and drain tube.
In addition to reducing the amount of food entering the small bowel, aspiration therapy also results in a decrease in food consumed at meals. This is caused by multiple factors, including the need to increase chewing to reduce food particle size to <5 mm to reliably fit through the A-tube, the requirement for increased water consumption during a meal, and an increased awareness of negative food choices due to the ability to see food contents exit through the clear drain tube. Analyses of the gastric aspirate in a U.S. pilot study revealed that only 80% of the weight loss was explained by aspiration of calories if the patient aspirated all meals and snacks optimally. Because subjects in that study only aspirated two meals on average and did not aspirate snacks, weight loss due to aspiration of gastric contents was likely significantly less than 80%, with reduced food intake responsible for significantly more than 20% of the weight loss (17).
Weight loss in trials for aspiration therapy have resulted in 14.2–19.8% TBWL in subjects with a BMI of 35–55 kg/m2 who completed 12 months of therapy (17–19) and 21.4% TBWL in 11 subjects with a BMI of >55 kg/m2 who completed 12 months of therapy (20). In the U.S. multicenter RCT (the PATHWAY study), the TBWL in the AspireAssist group was 12.1 ± 9.6% in the modified ITT analysis (n = 111) and 14.2 ± 9.8% in the completer analysis (n = 82) at 12 months compared to 3.5 ± 6.0% in the control group modified ITT analysis (n = 60) and 4.9 ± 7.0% in the control group completer analysis (n = 31) at 12 months (19). Two-year weight loss was reported in the U.S. pilot trial (n = 7) and a Swedish single-arm prospective study (n = 15), with 20.1 ± 3.5% TBWL and 61.5 ± 28.5% excess body weight loss (EBWL), respectively (17,18).
As with the IGB trials, few subjects had abnormal cardiometabolic risk factors at baseline in the PATHWAY study, and only nine subjects had a diagnosis of diabetes. The study was not powered to detect changes in cardiometabolic risk factors; however, despite these limitations, A1C was decreased significantly more in the AspireAssist group than in the control group. Significant improvements were also seen in triglycerides and HDL cholesterol in the AspireAssist group compared to baseline (19).
Five serious AEs occurred in four subjects in the PATHWAY study. One subject had severe abdominal pain after A-tube placement and required two separate overnight hospitalizations for pain control; one had mild peritonitis 2 days after A-tube placement requiring a 2-day hospitalization with intravenous antibiotics; one had gastric ulceration from the A-tube that was treated with removal of the A-tube; and one experienced a product malfunction that required replacement of an A-tube. All serious AEs resolved without sequela.
The most common nonserious AEs were similar to those of percutaneous endoscopic gastrostomy tubes, including peristomal granulation tissue, abdominal pain after A-tube placement, and peristomal irritation. Only four subjects in the AspireAssist group developed hypokalemia (potassium 3.2–3.7 mEq/L), which was treated with oral potassium supplementation (19).
Aspiration therapy management considerations for patients with diabetes are similar in principle to those for patients with a percutaneous endoscopic gastrostomy tube with regard to A-tube care. It is important to keep the A-tube site clean and dry. Patients can also use skin barriers that contain zinc oxide to prevent skin irritation. FDA indications for treatment with the AspireAssist system include patients with a BMI of 35–55 kg/m2. As with IGBs, use outside of this BMI range is considered off-label use. Patients will also have an altered diet after initiation of aspiration, with pureed food and a reduction in food intake. As with IGBs, medications that cause hypoglycemia should be reduced with the initiation of therapy. Patients also need to be instructed to take their oral medications after aspiration to ensure consistent absorption of the medications. Finally, as with all patients who initiate aspiration therapy, patients need to be instructed about how to adequately chew their food to ensure successful aspiration.
Suturing and Plicating
Two devices have received FDA clearance for the generic indication of tissue approximation in the GI tract. The Overstitch endoscopic suturing system (Overstitch, Apollo Endosurgery, Austin, Tex.) is a suturing device that attaches to the end of a double-channel upper endoscope. The Incisionless Operating Platform (IOP; USGI Medical, San Clemente, Calif.) is a 54 French flexible tube with a control handle like an endoscope and four channels with specialized instruments for placing plications in the GI tract.
The Overstitch has been used to perform ESG, a new technique initially described in 2013 that uses the Overstitch to reduce gastric volume by suturing along the greater curvature of the stomach to create a tube shape along the lesser curve of the stomach (21). Although the Overstitch device does have FDA approval, it does not have a specific indication for ESG, and no RCTs have been performed with this therapy. A study on mechanisms of action suggests that this procedure may cause a delay in gastric emptying that correlates with increased satiety; however, only four participants were studied (22).
The largest case series of ESG was reported in 2017 and included 248 patients at three centers. TBWL 6 months after ESG was 15.2% (95% CI 14.2–16.25%), with 13% of patients lost to follow-up. Fifty-seven of the 92 patients who were eligible for the 24-month follow-up visit completed it, yielding a follow-up rate of 62%. TBWL in the patients who completed the 24-month visit was 18.6% (95% CI 15.7–21.5%) (23).
Cardiometabolic outcomes were not reported in this case series but were reported in a case series of a subset of the patients (n = 91) included in the original 248-case series. Follow-up at 12 months was conducted in 53 of the 91 patients with significant reductions in A1C, systolic blood pressure, fasting serum triglycerides, and serum alanine aminotransferase (24).
Five serious AEs occurred in the 248-patient case series. These included two perigastric fluid collections requiring drainage and intravenous antibiotics, one case of extra-gastric bleeding requiring blood transfusion, one pulmonary embolism, and one pneumoperitoneum with pneumothorax requiring chest tube placement. It is important to note that the number of patients who required hospitalization for dehydration, nausea, or pain was not recorded. Nonserious AEs also were not recorded (23).
The IOP has been used for the primary obesity surgery endoluminal (POSE) procedure. Case series of the POSE procedure in Europe in 147 patients (BMI 38.0 ± 4.8 kg/m2) demonstrated a TBWL of 15.1 ± 7.8% with 21% lost to follow-up at 12 months (25). The POSE procedure was also studied in a multicenter, open-label RCT in Europe that demonstrated significantly more weight loss in the active group than in the control group (TBWL of 13.0 ± 1.4% [SE] vs. 5.3 ± 2.5%, respectively, P = 0.01) (26).
The POSE procedure was also studied in a U.S. multicenter, randomized, sham-controlled, double-blind study with 332 subjects (active group n = 221, BMI 36.0 ± 2.4 kg/m2; sham control group n = 111, BMI 36.2 ± 2.2 kg/m2) (27). Serious AEs occurred in 4.7% of subjects, with 10 of the 12 serious AEs related to post-procedure symptoms that resolved with medical therapy. Although subjects in the active group achieved significantly more weight loss than sham control subjects at 12 months (4.94 ± 7.04 vs. 1.38 ± 5.58%, respectively, P <0.0001), the predefined study endpoints were not met, and the FDA did not approve the IOP for the specific indication of the POSE procedure.
Of note, the trial design only used a low-intensity lifestyle therapy program (6 visits in the first 12 months of therapy), which led to lower-than-expected weight loss in both the active and sham control groups. Only 26 patients who completed the trial had diabetes; however, resolution of diabetes, defined as cessation of antidiabetic medications, was seen in 9 of 16 patients in the active group and 1 of 10 in the sham group (P = 0.0367) (27). It is likely that, with more intensive lifestyle therapy, both the active and control groups’ weight loss would have been closer to the weight loss seen in the European multicenter trial. It is possible that future studies may lead to FDA approval, but currently the IOP is not available in the United States for the POSE procedure.
Considerations for management of patients with diabetes after ESG or POSE are similar to those for IGB, with the exception of BMI indications, since ESG in particular has not been specifically approved by the FDA. Patients will have altered diet, including a liquid diet for 2–3 weeks, a pureed diet for 2 weeks, and then transition to a regular diet. Patients may also have significant nausea and vomiting after the procedure. These features require adjustments to antihyperglycemic medications. This procedure may also result in delayed gastric emptying and may not be appropriate for patients who have delayed gastric emptying from diabetic gastroparesis.
Investigational EBMTs
Two gastric EBMTs are currently being studied in U.S. multicenter RCTs, including one adjustable intragastric balloon (Spatz3 adjustable balloon system; Spatz FGIA, Great Neck, N.Y.) and the Transplyoric Shuttle (TPS; BAROnova, Inc., Goleta, Calif.). The Spatz3 system is a fluid-filled intragastric balloon with a catheter that allows for fill volume adjustment during an endoscopy after the initial placement. The TPS is a 56-mm outer silicone skin filled with a coiled cord of silicone tethered by silicone to a small weight. Unlike the intragastric balloons, this device causes intermittent gastric outlet obstruction by intermittently blocking the pylorus.
In addition, a U.S. multicenter RCT is being initiated on a completely procedureless intragastric balloon (Ellipse, Allurion Technologies, Natick, Mass.). This balloon is made of a thin film that is filled with 550 mL saline. It is swallowed by the patient and inflated after confirmation of the capsule in the stomach. The balloon has a release valve that catastrophically opens and deflates the balloon at 4 months. The balloon is then passed naturally through the GI tract.
Pilot studies of these devices have been performed outside of the United States, but whether these devices will become available in the United States will depend on the outcomes of the current trials.
Investigational Small-Bowel EBMTs
Multiple small-bowel EBMTs are being studied for the management of obesity and diabetes. These technologies were developed to mimic the effects on diabetes of proximal small-bowel exclusion seen with bariatric surgery.
Both animal and human data support the role of the proximal small bowel in glucose absorption, incretin secretion, and insulin resistance. Rat studies suggest that glucose sensing is decreased in the setting of diabetes (28), and high-fat feeding may stimulate duodenal proliferation of enteroendocrine cells that differentiate into K cells, producing glucose-dependent insulinotropic polypeptide (29). Moreover, proteins extracted from the duodenum and jejunum of diabetic mice and insulin-resistant humans caused insulin resistance in cultured muscle cells, suggesting that the duodenum and jejunum may also secrete a protein that causes skeletal muscle insulin resistance (30). Humans with diabetes have also been shown to have mucosal hypertrophy and increased enteroendocrine cells in the proximal small bowel (31), and nutrient infusion into the jejunum with a feeding tube in patients with diabetes led to increases in the glucose absorption rate and insulin sensitivity (32).
Endoluminal Bypass Liners
The most well studied of these devices is the duodenal-jejunal bypass liner (EndoBarrier, GI Dynamics, Lexington, Mass.). This bypass liner is made from a 60-cm-long, ultra-thin film with a self-expanding nitinol ring with 10 barbs to anchor the device in the duodenal bulb. The ultra-thin film blocks nutrient interaction with the small-bowel mucosa until the film ends at 60 cm. A meta-analysis found a percentage of EBWL of 35.4% (95% CI 24.7–46.1%) at 12 months in three studies, with a decrease in A1C of –1.5% (95% CI –2.2 to –0.8) (9). However, the device had a pooled early removal rate of 18.4%, a migration rate of 4.9%, bleeding in 3.9%, and liver abscess in 0.13% (9).
A U.S. multicenter, randomized, sham-controlled trial was stopped early after enrolling 325 of a planned 500 subjects because of a 3.5% incidence of hepatic abscess. All patients recovered after intravenous antibiotics and percutaneous drainage (33). Despite the reduced number of subjects, the study still demonstrated a significant decrease in A1C in the active group compared to the sham control group (–1.0 and –0.3%, respectively). It is important to note that the average A1C of the study population was 8.8%, and they were already on oral antihyperglycemic agents (33). It is unclear why the rate of hepatic abscess was higher in this trial than in studies performed outside of the United States. The device is not available with the United States but is still available elsewhere.
Another endoluminal bypass liner, a gastro-duodeno-jejunal bypass liner (ValenTx, Maple Grove, Minn.), has been developed to bypass both the stomach and proximal small bowel. The device is a 120-cm-long fluoropolymer that is anchored to the gastroesophageal junction and deployed through the pylorus into the small bowel. Endoscopic placement initially required laparoscopic assistance (34) and was successfully performed in 22 subjects, with 17 subjects completing 12 weeks of implantation. Baseline BMI was 42 kg/m2 (range 35.4–50.8 kg/m2), and weight loss was 16.8 kg (range 8.6–30.8 kg) (34). The study reported on seven subjects with diabetes on oral antihyperglycemic medications who did not need the medications at the end of the study, but no further detail was provided about this. An additional single-arm trial was performed with planned implantation duration of 12 months. Twelve patients underwent successful implantation, 10 completed 12 months of testing, and 6 patients had attached functional devices at 12 months (35). Weight loss was 14.9 kg at 12 months. Of four subjects with diabetes, three had a 1% decrease in A1C, and one stopped oral antihyperglycemic medications (35).
Duodenal Mucosal Resurfacing
Duodenal mucosal resurfacing (DMR) employs hydrothermal ablation to destroy the superficial mucosal layer and stimulate regrowth of normal mucosal tissue (Revita DMR system, Fractyl Laboratories, Lexington, Mass.). The device is a catheter capable of circumferential saline lift to protect the submucosa, followed by inflation of a 2-cm-long balloon filled with fluid heated to 90° C. Duodenal mucosa distal to the papilla and up to the ligament of Treitz are ablated under direct visualization in 2-cm increments. Similar principles are used in clinical practice for the ablation of Barrett’s esophagus mucosal tissue (36), with regrowth of normal squamous epithelium.
One human, open-label trial in 39 subjects (BMI 30.8 ± 3.5 kg/m2, weight 84.4 ± 11.9 kg, and A1C 9.6 ± 1.4%) has been published (37). Subjects had 3–15 cm of ablation in the duodenum, with a decrease in A1C of 1.2 ± 0.3% at 6 months with only a 3% TBWL. In a subanalysis of subjects who had at least 9 cm of ablation and did not stop antihyperglycemic medications, the decrease in A1C was 1.8 ± 0.5% (37).
Dual-Path Enteral Bypass
The Incisionless Magnet Anastomotic System (GI Windows, Bridgewater, Mass.) is a new endoscopic therapy that requires the simultaneous deployment of self-assembling magnets in the proximal jejunum and ileum using pediatric colonoscopes under fluoroscopic guidance. The magnets attract each other and cause necrosis of the tissue compressed between the magnets, which leads to the creation of an anastomosis. The new dual-path enteral bypass allows for nutrients to flow through both the native and newly created anastomosis. The first human study was performed in 10 subjects (BMI 41 kg/m2), with data reported for 6-month outcomes. TBWL was 10.6%, and in four subjects who had diabetes (baseline A1C 7.8%), A1C decreased by 1.8%.
Conclusion
EBMTs are a new class of treatment for obesity and include gastric and small-bowel therapies. In contrast to gastric therapies, which have metabolic effects related to weight loss, small-bowel therapies have both weight loss–dependent and weight loss–independent effects on glucose homeostasis. Several gastric EBMTs are approved or available in the United States. The FDA-approved devices, including three intragastric balloons and aspiration therapy, have demonstrated safety and efficacy in RCTs. A large case series is available in support of the ESG procedure, which does not have specific FDA approval. Additional gastric and small-bowel devices and procedures are being studied for the treatment of obesity and diabetes and may become available in the United States soon. EBMTs offer needed options to improve obesity and diabetes treatment in the United States and abroad.
Duality of Interest
S.S. is a member of the American Gastroenterological Association Institute’s council section for Obesity, Metabolism, and Nutrition, an advisory board member for the Association for Bariatric Endoscopy, and vice president of The Obesity Society’s Bariatric Section. She is also currently a consultant for Obalon Therapeutics, Aspire Bariatrics, USGI Medical, and Spatz FGIA. In the past 12 months, she has performed contracted research through her institution for Aspire Bariatrics, Obalon Therapeutics, and Allurion Technologies. No other potential conflicts of interest relevant to this article were reported
References
- 1.Benjamin SB. Small bowel obstruction and the Garren-Edwards gastric bubble: an iatrogenic bezoar. Gastrointest Endosc 1988;34:463–467 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin SB, Maher KA, Cattau EL Jr, et al. . Double-blind controlled trial of the Garren-Edwards gastric bubble: an adjunctive treatment for exogenous obesity. Gastroenterology 1988;95:581–588 [DOI] [PubMed] [Google Scholar]
- 3.Kirby DF, Wade JB, Mills PR, et al. . A prospective assessment of the Garren-Edwards Gastric Bubble and bariatric surgery in the treatment of morbid obesity. Am Surg 1990;56:575–580 [PubMed] [Google Scholar]
- 4.Zeman RK, Benjamin SB, Cunningham MB, et al. . Small bowel obstruction due to Garren gastric bubble: radiographic diagnosis. AJR Am J Roentgenol 1988;150:581–582 [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration Summary of safety and effectiveness data (SSED) ORBERA intragastric balloon system. Available from https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008B.pdf. Accessed 22 September 2016
- 6.U.S. Food and Drug Administration Summary of safety and effectiveness data (SSED) ReShape integrated dual balloon system. Available from https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140012b.pdf. Accessed 22 September 2016
- 7.U.S. Food and Drug Administration Summary of safety and effectiveness data (SSED) Obalon balloon system. Available from https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160001b.pdf. Accessed 22 September 2016
- 8.Lopez-Nava G, Bautista-Castaño I, Jimenez-Baños A, Fernandez-Corbelle JP. Dual intragastric balloon: single ambulatory center Spanish experience with 60 patients in endoscopic weight loss management. Obes Surg 2015;25:2263–2267 [DOI] [PubMed] [Google Scholar]
- 9.Abu Dayyeh BK, Kumar N, Edmundowicz SA, et al. . ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 2015;82:425–438.e5 [DOI] [PubMed] [Google Scholar]
- 10.Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg 2012;22:896–903 [DOI] [PubMed] [Google Scholar]
- 11.Sullivan S, Swain JM, Woodman G, et al. . The Obalon swallowable 6-month balloon system is more effective than moderate intensity lifestyle therapy alone: results from a 6-month randomized sham controlled trial [Abstract 812d]. Gastroenterology 2016;150(Suppl. 1):S1267 [Google Scholar]
- 12.Ponce J, Woodman G, Swain J, et al. . The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis 2015;11:874–881 [DOI] [PubMed] [Google Scholar]
- 13.Courcoulas A, Abu Dayyeh BK, Eaton L, et al. . Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. Int J Obes 2017;41:427–433 [DOI] [PubMed] [Google Scholar]
- 14.Crea N, Pata G, Della Casa D, et al. . Improvement of metabolic syndrome following intragastric balloon: 1 year follow-up analysis. Obes Surg 2009;19:1084–1088 [DOI] [PubMed] [Google Scholar]
- 15.ASGE Technical Committee; Abu Dayyeh BK, Thosani N, Konda V, et al. . ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2015;81:502.e1–502.e16 [DOI] [PubMed] [Google Scholar]
- 16.Gómez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: results of a prospective study. Obesity (Silver Spring) 2016;24:1849–1853 [DOI] [PubMed] [Google Scholar]
- 17.Sullivan S, Stein R, Jonnalagadda S, Mullady D, Edmundowicz S. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology 2013;145:1245–1252.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norén E, Forssell H. Aspiration therapy for obesity; a safe and effective treatment. BMC Obes 2016;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson CC, Abu Dayyeh BK, Kushner R, et al. . Percutaneous gastrostomy device for the treatment of class II and class III obesity: results of a randomized controlled trial. Am J Gastroenterol 2017;112:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machytka E, Turro R, Huberty V, et al. . Aspiration therapy in super obese patients: pilot trial [Abstract No. 1944]. Gastroenterology 2016;150(Suppl. 1):S822–S823 [Google Scholar]
- 21.Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc 2013;78:530–535 [DOI] [PubMed] [Google Scholar]
- 22.Abu Dayyeh BK, Acosta A, Camilleri M, et al. . Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol 2017;15:37–43.e1 [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. . Endoscopic sleeve gastroplasty for obesity: a multicenter study of 248 patients with 24 months follow-up. Obes Surg. Epub ahead of print on 27 April 2017 (DOI: 10.1007/s11695-017-2693-7) [DOI] [PubMed] [Google Scholar]
- 24.Sharaiha RZ, Kumta NA, Saumoy M, et al. . Endoscopic sleeve gastroplasty significantly reduces body mass index and metabolic complications in obese patients. Clin Gastroenterol Hepatol 2017;15:504–510 [DOI] [PubMed] [Google Scholar]
- 25.López-Nava G, Bautista-Castaño I, Jimenez A, de Grado T, Fernandez-Corbelle JP. The primary obesity surgery endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis 2015;11:861–865 [DOI] [PubMed] [Google Scholar]
- 26.Miller K, Turró R, Greve JW, Bakker CM, Buchwald JN, Espinós JC. MILEPOST multicenter randomized controlled trial: 12-month weight loss and satiety outcomes after POSESM vs. medical therapy. Obes Surg 2017;27:310–322 [DOI] [PubMed] [Google Scholar]
- 27.Sullivan S, Swain JM, Woodman G, et al. . Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: the ESSENTIAL trial. Obesity (Silver Spring) 2017;25:294–301 [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Cummings BP, Martin E, et al. . Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 2012;302:R657–R666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gniuli D, Calcagno A, Dalla Libera L, et al. . High-fat feeding stimulates endocrine, glucose-dependent insulinotropic polypeptide (GIP)-expressing cell hyperplasia in the duodenum of Wistar rats. Diabetologia 2010;53:2233–2240 [DOI] [PubMed] [Google Scholar]
- 30.Salinari S, Debard C, Bertuzzi A, et al. . Jejunal proteins secreted by db/db mice or insulin-resistant humans impair the insulin signaling and determine insulin resistance. PLoS One 2013;8:e56258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theodorakis MJ, Carlson O, Michopoulos S, et al. . Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 2006;290:E550–E559 [DOI] [PubMed] [Google Scholar]
- 32.Salinari S, Carr RD, Guidone C, et al. . Nutrient infusion bypassing duodenum-jejunum improves insulin sensitivity in glucose-tolerant and diabetic obese subjects. Am J Physiol Endocrinol Metab 2013;305:E59–E66 [DOI] [PubMed] [Google Scholar]
- 33.Kaplan LM, Buse JB, Mullin C, et al. . EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral antihyperglycemic agents. Presented at the American Diabetes Association 76th Scientific Sessions, New Orleans, La., 2016 [Google Scholar]
- 34.Sandler BJ, Rumbaut R, Paul Swain C, et al. . Human experience with an endoluminal, endoscopic, gastrojejunal bypass sleeve. Surg Endosc 2011;25:3028–3033 [DOI] [PubMed] [Google Scholar]
- 35.Sandler BJ, Rumbaut R, Swain CP, et al. . One-year human experience with a novel endoluminal, endoscopic gastric bypass sleeve for morbid obesity. Surg Endosc 2015;29:3298–3303 [DOI] [PubMed] [Google Scholar]
- 36.Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low-grade dysplasia in Barrett’s esophagus: expert review from the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835 [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan H, Cherrington AD, Thompson CC, et al. . Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-month interim analysis from the first-in-human proof-of-concept study. Diabetes Care 2016;39:2254–2261 [DOI] [PubMed] [Google Scholar]