Abstract
Background
Anthracyclines-induced cardiotoxicity has become one of the major restrictions of their clinical applications. Klotho showed cardioprotective effects. This study aimed to investigate the effects and possible mechanisms of klotho on doxorubicin (DOX)-induced cardiotoxicity.
Material/Methods
Rats and isolated myocytes were exposed to DOX and treated with exogenous klotho. Specific inhibitors and siRNAs silencing MAPKs were also used to treat the animals and/or myocytes. An invasive hemodynamic method was used to determine cardiac functions. Intracellular ROS generation was evaluated by DHE staining. Western blotting was used to determine the phosphorylation levels of JNK, ERK, and p38 MAPKs in plasma extracts and Nrf2 in nuclear extracts. Nuclear translocation of Nrf2 in myocytes was evaluated by immunohistochemistry. Cell apoptosis was evaluated by TUNEL assay and flow cytometry.
Results
Klotho treatment improved DOX-induced cardiac dysfunction in rats. The DOX-induced ROS accumulation and cardiac apoptosis were attenuated by klotho. Impaired phosphorylations of MAPKs, Nrf2 translocation and expression levels of HO1 and Prx1 were also attenuated by klotho treatment. However, the anti-oxidant and anti-apoptotic effects of klotho on DOX-exposed myocardium and myocytes were impaired by both specific inhibitors and siRNAs against MAPKs. Moreover, the recovery effects of klotho on phosphorylations of MAPKs, Nrf2 translocation and expression levels of HO1 and Prx1 were also impaired by specific inhibitors and siRNAs against MAPKs.
Conclusions
By recovering the activation of MAPKs signaling, klotho improved cardiac function loss which was triggered by DOX-induced ROS mediated cardiac apoptosis.
MeSH Keywords: Apoptosis, Doxorubicin, Oxidative Stress
Background
Chemotherapy is still one of the most frequent primary treatments for patients with various malignant tumors including liver cancer, lung cancer, and ovarian cancer. Many chemotheraputic agents, such as doxorubicin (DOX), have been proven potent in suppressing cancer growth and increasing survival rates in patients with cancer [1]. However, the side effects of these chemotherapeutic agents have drawn the attention of oncologists. Heart failure has been found correlated with administration of chemotherapy, which is considered as cardiotoxicity [2]. It was been reported that reduction in left ventricular ejection fraction (LVEF) was impaired in 12.5% of cancer patients receiving anthracycline/trastuzumab administration [3]. Though several mechanisms have been proposed, such as endoplasmic reticulum stress, mitochondria damage, and calcium overload [4–6], the exact molecular mechanisms of cardiotoxicity are still unclear.
DOX is broadly used in cancer patient treatment due to its potent anti-cancer activity and wide spectrum. Nevertheless, the irreversible cardiotoxicity limits its clinical applications. The administration of DOX is thought to induce excessive generation of reactive oxygen species (ROS) which triggers cell apoptosis via multiple signaling pathways [7]. Cardiac apoptosis leads to continuous loss of contractile units, contributing to heart failure. Nuclear factor erythroid 2-related factor (Nrf2) is a key modulator maintaining intracellular anti-oxidative/oxidative balance. By binding with antioxidant response element (ARE), the transcription of its targeted genes were initiated to produce a set of antioxidant enzymes such as peroxiredoxin1 (Prx1) and heme oxygenase (HO1) [8,9]. The activation of Nrf2/ARE signaling pathway is believed to be regulated by its upstream kinases mitogen-activated protein kinases (MAPKs) including p38, c-Jun N-terminal kinase (JNK), and extracellular signal regulated kinase 1/2 (ERK 1/2) [10–12].
Klotho was originally reported as a single-pass transmembrane protein highly expressed in renal tubular epithelium, regulating ageing-related processes [13]. There are two forms of klotho: the full-length form and the secreted form which is generated by proteolytic shedding or splicing of the extracellular part of the full-length klotho. The secretive klotho is soluble and could be considered a hormone that affects remote organs via circulation [14]. Anti-oxidative and anti-apoptotic effects have been suggested. According to previous studies, klotho was associated with the regulation of activation of MAPKs [15,16]. In the current study, 109-KDa recombinant secretive klotho protein was administrated to DOX-exposed animals and myocytes. The cardiac protective role of klotho in DOX-induced cardiotoxicity was investigated. Furthermore, by using specific MAPKs inhibitors and small interference RNAs (siRNAs), the possible role of klotho in regulating MAPKs/Nrf2/ARE signaling in DOX-treated heart and myocytes was also studied. We believe that results from this study could not only deepen our current knowledge concerning chemotherapy-related cardiotoxicity and cardiac protective effects of klotho, but also provide novel theoretical basis for application of klotho in alleviating chemotherapy-related cardiotoxicity in clinical practice in the future.
Material and Methods
Animals and treatments
Sixty male Sprague-Dawley (SD) rats (eight-weeks old, weighting 220 to 250 g) were used in this study. Rats were maintained in separated polypropylene cages within an artificial environment providing 12-hour light/dark circle, 25±2°C room temperature and 50% humidity. Rats were free to eat standard chow and drink clean tap water.
Several rats were exposed to DOX (3 mg/kg, Sigma-Aldrich) by intraperitoneal injections (single dose of DOX every another day for total three injections) [17]. Recombinant klotho protein (0.01 mg/kg, R&D) was administrated to rats by intraperitoneal injections every another day for total two injections [16]. The JNK inhibitor SP600125 (15 mg/kg, Tocris Bioscience), ERK1/2 inhibitor U0126 (0.22 μg/kg, Sigma-Aldrich) and p38 inhibitor SB203580 (1 mg/kg, Selleck Chemicals,) were administrated to rats by intraperitoneal injections daily for consecutive four days [18–20]. Control animals received treatments of equal volume of physiological saline. According to the different treatments, equal amount rats (n=10) were assigned into six groups: Control group (control animals), DOX (animals exposed to DOX); DOX+KL (DOX-exposed animal treated with klotho); DOX+KL+JNKi (DOX-exposed animal cotreated with klotho and SP600125); DOX+KL+ERKi (DOX-exposed animal cotreated with klotho and U0126); DOX+KL+p38i (DOX-exposed animal cotreated with klotho and SB203580). The animal experiment protocol was carried out according to Recommended Guideline for the Care and Use of Laboratory Animals issued by Chinese Council on Animal Research. The protocol was reviewed and approved Animal Ethics Committee of Shaanxi Provincial People’s Hospital.
Myocytes isolation and treatment
Primary myocytes were isolated from neonate male SD rats in accordance to previous studies [21]. The harvested hearts were perfused by Liberase (4.5 mg/mL, Roche) and the myocytes were isolated and purified in accordance with previous descriptions [22]. Cells were cultured in minimum essential medium (MEM, Gibco) containing bovine calf serum (Gibco), Hank’s buffered salt solution (HyClone), 2,3-butanedione monoxime (10 mmol/L, Sigma-Aldrich), L-glutamine (2 mmol/L, Gibco) and antibiotics mix (Sigma-Aldrich) for one hour. Then the cells were maintained in MEM containing bovine serum albumin (0.1 mg/mL, Gibco), L-glutamine (2 mmol/L, Gibco) and antibiotics mix (Sigma-Aldrich) in a cell incubator providing humidified environment, constant temperature at 37°C and fresh air (5% CO2). DOX (1 μmol/L for 24 hours) [17], klotho (400 pmol/L, four hours prior to DOX exposure) [23], specific MAPKs inhibitors and specific small interference RNAs (siRNA) against genes MAPKs were used to incubate the isolated myocytes. Control cells were incubated with MEM. Final concentrations of the MAPKs inhibitors were 10 μmol/L. The grouping details were listed in Table 1.
Table 1.
Grouping and treatments of cultured primary myocytes.
| Groups | Treatment1 | Treatment2 | Treatment3 | |||
|---|---|---|---|---|---|---|
| Agent | Dosage | Agent | Dosage | Agent | Dosage | |
| Control | MEM | Equal volume | MEM | Equal volume | Scrambled siRNA | 100 nmol/l |
| DOX | Doxorubicin | 1 μmol/l | MEM | Equal volume | – | – |
| DOX+KL | Doxorubicin | 1 μmol/l | Klotho | 400 pmol/l | – | – |
| DOX+KL+JNKi | Doxorubicin | 1 μmol/l | Klotho | 400 pmol/l | SP600125 | 10 μmol/l |
| DOX+KL+ERKi | Doxorubicin | 1 μmol/l | Klotho | 400 pmol/l | U0126 | 10 μmol/l |
| DOX+KL+p38i | Doxorubicin | 1 μmol/l | Klotho | 400 pmol/l | SB203580 | 10 μmol/l |
| DOX+KL+JNK-siRNA | Doxorubicin | 1 μmol/l | Klotho | 400 pmol/l | jnk-shRNA | 100 nmol/l |
| DOX+KL+ERK-siRNA | Doxorubicin | 1 μmol/l | Klotho | 400 pmol/l | erk-shRNA | 100 nmol/l |
| DOX+KL+p38-siRNA | Doxorubicin | 1 μmol/l | Klotho | 400 pmol/l | p38-shRNA | 100 nmol/l |
siRNAs transfections
The expressions of MAPKs were silenced by small RNAs interference technique in this study. SignalSilence p38 MAPK siRNA (#6564, Cell Signaling Tech), SignalSilence p44/42 (Erk1/2) siRNA (#6560, Cell Signaling Tech) and SignalSilence JNK siRNA (#6232, Cell Signaling Tech) were used to transfect the cells. The scrambled duplex control siRNA (#6568, Cell Signaling Tech) was used as a negative control. The siRNAs were introduced to cultured myocytes by transfection with Mirus TransIT-TKO reagent (Mirus) in six-well culturing dishes according to the instructions provided by the manufacturer. The final concentrations of siRNAs were 100 nmol/L.
Cardiac function evaluation
The cardiac function was evaluated by invasive hemodynamic determination in this study. Briefly, the animals were anesthetized by isoflurane inhalation. Through the right carotid artery, a catheter was intubated into the left ventricle and connected to a pressure transducer. The pressure curve was plotted with Powerlab biological analysis system (AD instruments). Hemodynamic parameters including left ventricular diastolic pressure (LVDP) and left ventricular systolic pressure (LVSP) were determined.
ROS detection
ROS was detected in vivo by using dihydroethidium (DHE, Molecular Probes). Ventricular tissue was embedded with optimal cutting temperature compound (OCT, Sakura) and frozen on dry ice. Then the tissues were made into 6 μm-thick slides. DHE (10 μmol/L) was used to incubate the slides in a dark humidified chamber for 45 minutes at 37°C. The cultured myocytes were washed by PBS and then incubated with DHE in a dark humidified chamber for 30 minutes at 37°C. Fluorescent images were captured by a fluorescence microscope and further analyzed by software ImageJ.
Fluorescence staining
Fluorescence staining was used to evaluate the nuclear translocation of Nrf2 in vitro. After fixing, the cultured myocytes were incubated with antibody against Nrf2 (Cell Signaling Tech, 1: 500) for 10 hours at 4°C. Then cells were incubated with secondary antibodies conjugated with Alexa 488 (Sigma-Aldrich) and DAPI. SlowFade Light Antifade kit (Molecular Probes) was used to alleviate the quenching. After exited at 519 nm, the cells were observed at 442 nm and 495 nm for Alexa 488 and DAPI by using a fluorescence microscope respectively. Captured images were merged and analyzed by software ImageJ.
Apoptosis assessment
The apoptosis was assessed by terminal transferase UTP nick end labeling (TUNEL) assays. The cardiac slides or cultured myocytes were treated with proteinase K (20 μmol/L, Sigma-Aldrich). A TUNEL assay kit (Roche) was used according to the protocol provided by the manufacturer. Then the fluorescence images were captured by a fluorescence microscope. The TUNEL-positive cells were considered as apoptotic cells (tagged green). The images were analyzed by software ImageJ.
Western blotting
Whole cell extracts from cardiac tissue or cultured myocytes were prepared by using cell lysis buffer system (Santa Cruz). Nuclear extraction reagents (Pierce) and cytoplasmic extraction reagents (Pierce) were used to extract nuclear proteins and cytoplasmic proteins respectively according to the protocol provided by the manufacturer. Concentrations of protein samples were determined by BCA protein assay kit (Pierce). 20 μg of protein samples were loaded and then separated by SDS-PAGE. The protein samples were transferred electrically to PVDF or NC membranes which were then incubated with primary antibodies against p38 MAPK (Cell Signaling Tech, 1: 1,000), phospho-p38 MAPK (p-p38 MAPK, Cell Signaling Tech, 1: 1,000), JNK (Abcam, 1: 500), phospho-JNK (p-JNK, Abcam, 1: 500), ERK1/2 (Cell Signaling Tech, 1: 1,000), phospho-ERK1/2 (p-ERK1/2, Cell Signaling Tech, 1: 1,000), Nrf2 (Invitrogen, 1: 500), HO1 (Santa Cruz, 1: 1,000), Prx1 (Santa Cruz, 1: 1,000), GAPDH (Santa Cruz, 1: 2,000) and Lamin A (Santa Cruz, 1: 1,000) at 4°C for 10 hours. After washing with TBST, membranes were incubated with horseradish peroxidase conjugated secondary antibodies (1: 5,000) at room temperature for one hour. ECL kit (Pierce) was used to incubate the membranes which were then exposed with Gene Genius (Syngene); then the intensities of the immunoblots were analyzed by software ImageJ.
Statistics
Data acquired in this study was presented as mean ±SEM. Differences between groups were analyzed by Student’s t-tests or one-way ANOVA with software SPSS (version 16.0, SPSS). Differences were considered significant when p<0.05.
Results
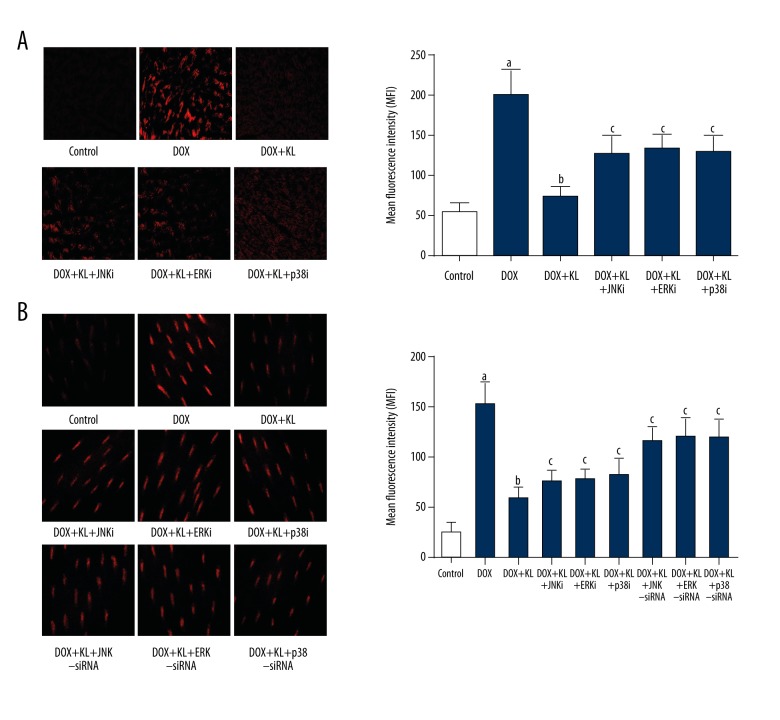
Klotho treatment alleviated DOX exposure-induced ROS production in myocardium and myocytes which was impaired by MAPKs inhibitors or siRNAs.
The results are shown in Figure 1. The ROS was tagged by DHE fluorescence staining. The production of ROS increased significantly in both DOX-exposed cardiac tissue and cultured myocytes. The administration of klotho dramatically suppressed the ROS production in myocardium and myocytes. However, the treatment of inhibitors of p38 MAPK, JNK, and ERK1/2 significantly impaired klotho’s inhibitory effects on ROS production in myocardium and myocytes. Moreover, treatments of siRNAs silencing p38 MAPK, JNK, and ERK1/2 were also found to impair the anti-oxidant effects of klotho in cultured myocytes.
Figure 1.
Klotho reduced DOX-induced ROS in myocardium and myocytes which was reversed by MAPKs inhibitors or siRNAs. (A) Left panel demonstrates the captured fluorescent images of DHE stained myocardium. Intracellular ROS is tagged red. Columns on the right side indicate the mean fluorescence intensities of ROS in myocardium harvested from control, DOX, DOX+KL, DOX+KL+JNKi, DOX+KL+ERKi and DOX+KL+p38i respectively. (B) Images on the left side are DHE staining of cultured myocytes. Columns on the right side indicate the mean fluorescence intensities of ROS in myocardium harvested from control, DOX, DOX+KL, DOX+KL+JNKi, DOX+KL+ERKi, DOX+KL+p38i, DOX+KL+JNK-siRNA, DOX+KL+ERK-siRNA, and DOX+KL+p38-siRNA respectively. a Differences were significant when compared with control (p<0.05); b differences were significant when compared with DOX (p<0.05); c differences were significant when compared with DOX+KL (p<0.05).
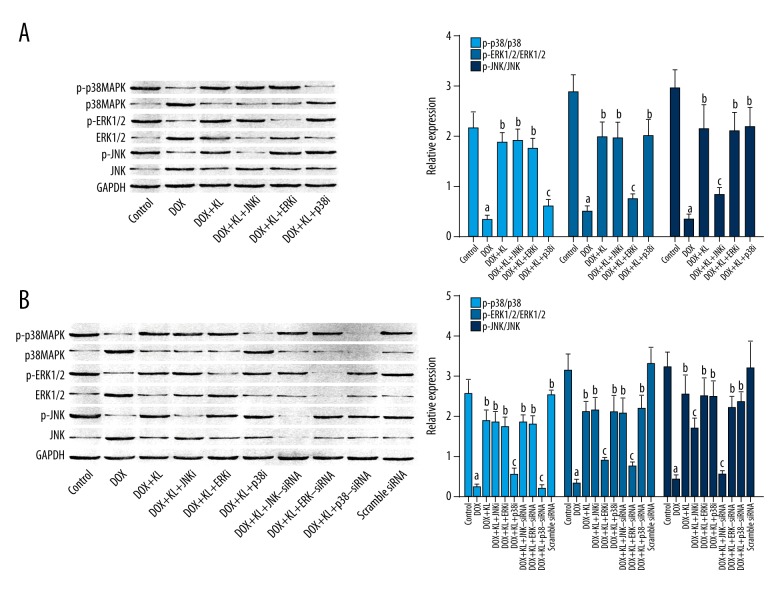
Klotho treatment recovered activation of MAPKs in DOX-exposed myocardium and myocytes which was impaired by MAPKs inhibitors or siRNAs.
The immunoblots of p-p38 MAPK, p38 MAPK, p-JNK, JNK, p-ERK1/2, ERK1/2, and GAPDH of myocardium and myocytes are shown in Figure 2. The phosphorylation levels of p38 MAPK, JNK, and ERK1/2 were dramatically decreased in DOX-exposed myocardium and cultured myocytes. The administration of klotho, however, increased the phosphorylation levels of these MAPKs. Nevertheless, in both myocardium and myocytes, treatment of inhibitors of MAPK, JNK, and ERK1/2 significantly impaired the recovering effects of klotho on phosphorylation levels of corresponding MAPKs. Furthermore, siRNAs silencing p38 MAPK, JNK, and ERK1/2 also reversed the recovering effects of klotho on phosphorylation levels of corresponding MAPKs.
Figure 2.
Klotho activated MAPKs in DOX-treated myocardium and myocytes which was reversed by MAPKs inhibitors or siRNAs. (A, B) The left panels show the immunoblots of p-p38 MAPK, p38 MAPK, p-ERK1/2, ERK1/2, p-JNK, JNK, and GAPDH in cytoplasmic protein samples extracted from myocardium and myocytes. Columns on the right panels indicate the ratio of p-p38MAPK/p38MAPK (light blue), p-ERK2/1/ERK1/2 (blue), and p-JNK/JNK (dark blue) in myocardium and cultured myocytes respectively. a Differences were significant when compared with control (p<0.05); b differences were significant when compared with DOX (p<0.05); c differences were significant when compared with DOX+KL (p<0.05).
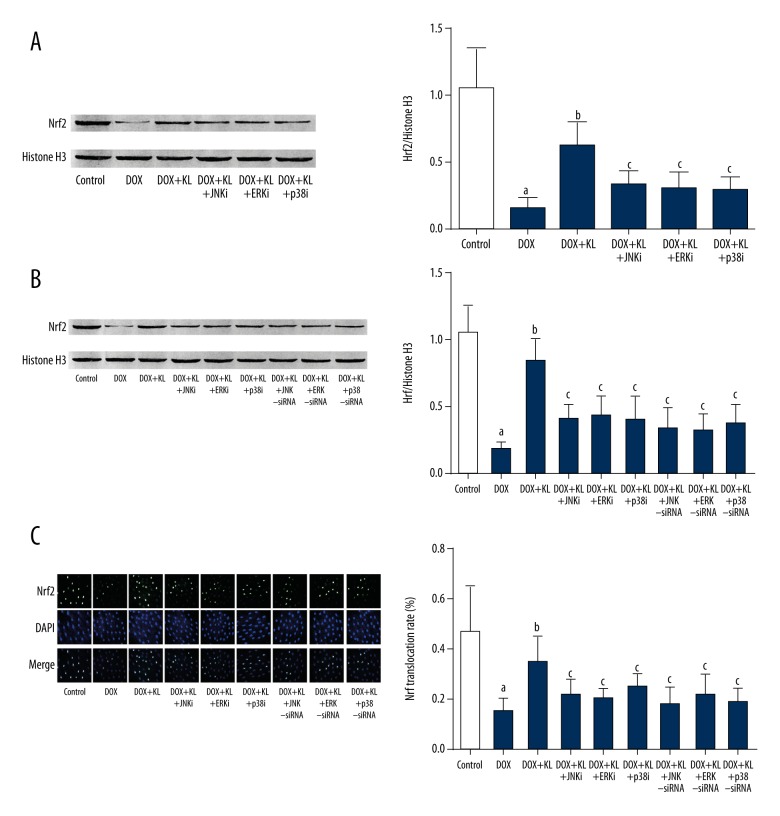
Klotho treatment recovered Nrf2 nuclear translocations in DOX-exposed myocardium and myocytes which was impaired by MAPKs inhibitors or siRNAs.
The results are shown in Figure 3. DOX exposure significantly suppressed the nuclear translocation of Nrf2 in both myocardium and myocytes. The administrations of Klotho dramatically improved the nuclear translocation of Nrf2 in both myocardium and cultured myocytes. However, the treatment of inhibitors of MAPKs impaired the recovery effects of klotho on Nrf2 nuclear translocation in vivo and in vitro. Similarly, siRNAs silencing MAPKs impaired the recovery effects of Klotho on Nrf2 nuclear translocation in cultured myocytes.
Figure 3.
Klotho improved nuclear translocation of Nrf2 in DOX-exposed myocardium and myocytes which was reversed by MAPKs inhibitors or siRNAs. (A, B) The left panels show the immunoblots of Nrf2 and Histone H3 in nuclear protein samples extracted from myocardium and myocytes. Columns on the right panels indicate the ratio of Nrf2/histone H3 in myocardium and cultured myocytes respectively. (C) The left panel demonstrates the captured fluorescent images of Nrf2, DAPI, and their merged images in cultured myocytes. Columns on the right part indicate the Nrf2 nuclear translocation rate in different groups. a Differences were significant when compared with control (p<0.05); b differences were significant when compared with DOX (p<0.05); c differences were significant when compared with DOX+KL (p<0.05).
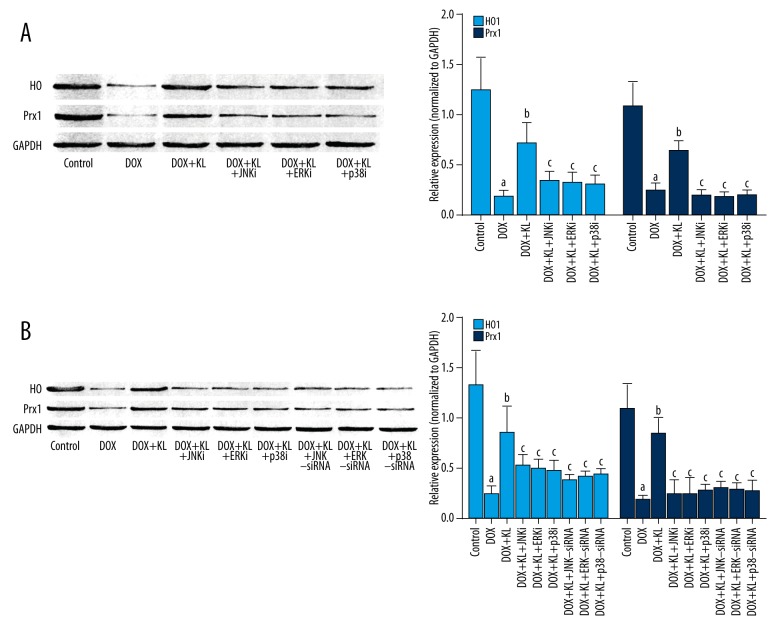
Klotho treatment improved expression of anti-oxidant enzymes in DOX-exposed myocardium and myocytes which was impaired by MAPKs inhibitors or siRNAs.
The results are shown in Figure 4. The DOX-exposure significantly decreased the expression levels of HO1 and Prx1 in myocardium and cultured myocytes. The administration of klotho dramatically recovered the expression levels of HO1 and Prx1 both in vivo and in vitro. The treatment of inhibitors of MAPKs impaired the recovery effect of klotho on HO1 and Prx1 in myocardium and myocytes. In addition, specific siRNAs silencing MAPKs also impaired the recovery effect of klotho on expression levels of HO1 and Prx in cultured myocytes.
Figure 4.
Klotho increased expression levels of anti-oxidant enzymes in DOX-treated myocardium and myocytes which was reversed by MAPKs inhibitors or siRNAs. (A, B) The immunoblots of HO1, Prx1, and GAPDH in myocardium and cultured myocytes in different groups are shown on the left panels. Columns on the right part indicate the ratio of HO/GAPDH and Prx1/GAPDH in myocardium and cultured myocytes respectively. a Differences were significant when compared with control (p<0.05); b differences were significant when compared with DOX (p<0.05); c differences were significant when compared with DOX+KL (p<0.05).
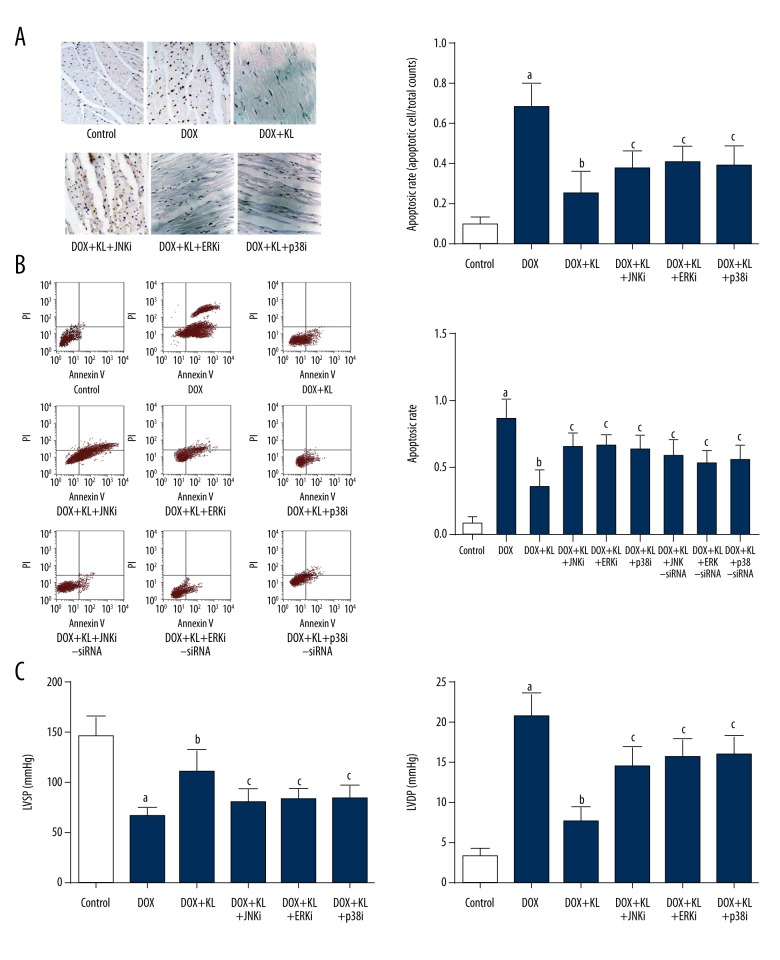
Klotho administration attenuated DOX exposure-induced cardiac apoptosis and dysfunctions which were impaired by MAPKs inhibitors.
The results are shown in Figure 5. The DOX- exposure induced cell apoptosis in both myocardium and cultured myocytes significantly. The treatment of klotho, however, repressed the apoptosis. Treatments of specific MAPKs inhibitors impaired the anti-apoptotic effects of klotho in vivo and in vitro. Treatments of specific siRNAs silencing MAPKs also impaired the anti-apoptotic effects of klotho in cultured myocytes. Additionally, the treatment of klotho dramatically improved the cardiac functions in DOX-exposed rats. However, the cotreatments of MAPKs inhibitors impaired the improvements.
Figure 5.
Klotho attenuated DOX-induced apoptosis and cardiac dysfunction which were reversed by treatment of MAPKs and siRNAs. (A) Left panel demonstrates the captured images of TUNEL assay in myocardium. Apoptotic cells are stained brown. Columns on the right part indicate the cell apoptotic rate in different groups. (B) Charts of flow cytometry of apoptosis in cultured myocytes in different groups are shown on the left side. Columns on the right part indicate the cell apoptotic rate in different groups. (C) Columns in this panel indicate the detected LVSP (left side) and LVDP (right side) by hemodynamic method in rats from different groups. a Differences were significant when compared with control (p<0.05); b differences were significant when compared with DOX (p<0.05); c differences were significant when compared with DOX+KL (p<0.05).
Discussion
In the current study, we investigated the protective effects of exogenous klotho protein on DOX-induced cardiotoxicity. The possible underlining molecular mechanisms were also investigated. Our results suggested that the administration of klotho attenuated DOX-induced oxidative stress and cardiac apoptosis, and improved cardiac dysfunctions. We made further investigations to explore the possible molecular mechanisms in this study. Using the specific MAPKs inhibitors and siRNAs silencing MAPKs, we found that klotho exerted its cardiac protective role by recovering the activation of MAPKs/Nrf2 pathway.
Chemotherapy is one of the fundamental therapies for human malignant tumors. Anthracyclines are potent ant-cancer chemotherapeutic agents, which were applied alone or in combination with other kinds of chemotherapeutic agents. DOX is one of the most frequently used anthracyclines which is effective against many human malignant cancers. However, during the treatment, the DOX-induced cardiotoxicity requires our attention because of the limited clinical application of DOX [1]. Though exact mechanisms of DOX-induced cardiotoxicity is still not completely elucidated, cardiac apoptosis has been believed to be an important feature of the cardiotoxicity, which could cause loss of contractile units, leading to heart failure [24]. In the present study, we found that DOX treatment induced cell apoptosis in both the myocardium and cultured myocytes. The in vivo hemodynamic assessments showed that DOX administration impaired both systolic and diastolic cardiac functions. Thus, agents exert anti-apoptotic effects would be helpful in improve DOX induced cardiac dysfunctions.
It has been recognized that DOX-induced cardiotoxicity is also characterized by excessive intracellular ROS production [25]. ROS mediates cell apoptosis via multiple mechanisms such as impairing membrane integrity and causing mitochondrial damage. In this study, we found that compared with normal control, DOX treatment dramatically resulted in excessive ROS accumulation in both the myocardium and cultured primary myocytes. The heart is more sensitive to ROS induced injury because the heart possesses a lower content of anti-oxidative enzymes such as HO and Prx1 compared to other mammalian organs [26]. That may explain why DOX is most toxic to the heart. Results from this study indicated that DOX exposure dramatically downregulated the expression levels of HO1 and Prx1 in both the myocardium and primary myocytes.
Nrf2 is an important member of the cap ‘n’ collar family of basic leucine zipper transcription factors, playing a part in the anti-oxidative defense system by upregulating expressions of anti-oxidant enzymes [27]. After activation, Nrf2 translocates to the nuclear area to bind with ARE which is located in the promoter region of genes encoding the phase II anti-oxidant enzymes [8]. In this study, results from both in vivo and in vitro investigations showed that DOX exposure suppressed the nuclear translocation of Nrf2. A set of protein kinases referred as MAPKs were believed to regulate the activation of Nrf2/ARE signaling [28]. MAPKs including JNK, ERK1/2, and p38MAPK were reported involved in cellular defense responses to stressful factors such as ROS [29]. The activation of MAPKs would further facilitate the activation of Nrf2/ARE signaling [30]. Evidence gathered in this study indicated that DOX exposure deactivated MAPKs by reducing their phosphorylation in vivo and in vitro.
The Klotho gene was initially identified as a putative aging-suppressor gene. The mutation of Klotho was reported to shorten life span [31]. The product klotho protein was believed to be a humoral factor attenuating oxidative stress, inflammation, and apoptosis [32,33]. Though several mechanisms were proposed, deeper understanding is still needed. Unlike the ROS scavengers, such as NAC, klotho may exert its anti-oxidative and anti-apoptotic effects by taking part in the anti-oxidative defense system. Several previous studies suggested that klotho could regulate the activation of JNK [16], ERK1/2, and p38MAPK [23]. A very recent communication indicated that the Nrf2 activation was also regulated by klotho [34]. Thus, our hypothesis was: klotho activates MAPKs/Nrf2/ARE signaling. This pathway could facilitate anti-oxidant enzymes synthesis which reduces ROS mediated apoptosis in myocytes exposed to DOX.
In this study, klotho was administrated to DOX-exposed rats and primary myocytes. The results showed that klotho treatment reduced intracellular ROS accumulation. Meanwhile, the cardiac apoptosis was also dramatically attenuated. As a result, the cardiac systolic and diastolic functions were improved. Further investigations showed that klotho treatment recovered the phosphorylation of MAPKs which activated Nrf2/ARE signaling pathways. As a result, the expressions of the anti-oxidant enzymes HO1 and Prx1 increased significantly. MAPKs and siRNAs against MAPKs were used to further testify our hypothesis. We found that the application of specific inhibitors of JNK, ERK1/2, and p38MAPK impaired klotho’s anti-oxidative and anti-apoptotic effects in vivo and in vitro. As a result, cardiac function improvements in rats receiving klotho treatment were also impaired by MAPKs inhibitors administration. Moreover, the recovery of MAPKs/Nrf2 signaling and expression levels of anti-oxidant enzymes were also impaired by MAPKs inhibitors and siRNAs silencing MAPKs in klotho-treated myocytes. These results indicated that MAPKs were the targets for klotho administration.
Conclusions
The deactivation of MAPKs/Nrf2 signaling was involved in DOX-induced cardiotoxicity, which was characterized by cardiac apoptosis, and which lead to cardiac dysfunctions. Klotho recovered activation of MAPKs/Nrf2 signaling which reduced ROS-mediated cardiac apoptosis and eventually improved cardiac dysfunctions. Results from this study not only deepen our knowledge concerning chemotherapy-induced cardiotoxicity, but also show the theoretical basis of the application of klotho as a cardiac protective agent for patients undergoing chemotherapies.
Limitations
However, there are limitations of this study. 1) Specific siRNAs against MAPKs were used in the in vitro study rather than in vivo studies because the knock-down efficacy of siRNA in vivo is hard to guarantee. Nevertheless, these siRNAs should be utilized in vivo to make the evidence more solid. 2) More functional indicators could be employed to make the study more persuasive. Take echocardiography for instance, both cardiac index and LVEF could be evaluated. 3) Evidence proving Nrf2/ARE signaling activation was not complete. The binding activity of Nrf2/ARE could be assessed by ARE-luciferase activity assay which could provide more direct evidences.
Footnotes
Source of support: This work received internal financial support from Department of Cardiology, Shaanxi Provincial People’s Hospital
References
- 1.Xu F, Li X, Liu L, et al. Attenuation of doxorubicin-induced cardiotoxicity by esculetin through modulation of Bmi-1 expression. Exp Ther Med. 2017;14(3):2216–20. doi: 10.3892/etm.2017.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson R, Shabalala S, Louw J, et al. Aspalathin reverts doxorubicin-induced cardiotoxicity through increased autophagy and decreased expression of p53/mTOR/p62 signaling. Molecules. 2017;22(10) doi: 10.3390/molecules22101589. pii: E1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamirani Y, Fanous I, Kramer CM, et al. Anthracycline- and trastuzumab-induced cardiotoxicity: A retrospective study. Med Oncol. 2016;33(7):82. doi: 10.1007/s12032-016-0797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lou Y, Wang Z, Xu Y, et al. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int J Mol Med. 2015;36(3):873–80. doi: 10.3892/ijmm.2015.2291. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira BL, Niederer S. A biophysical systems approach to identifying the pathways of acute and chronic doxorubicin mitochondrial cardiotoxicity. PLoS Comput Biol. 2016;12(11):e1005214. doi: 10.1371/journal.pcbi.1005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarmohmmadi F, Rahimi N, Faghir-Ghanesefat H, et al. Protective effects of agmatine on doxorubicin-induced chronic cardiotoxicity in rat. Eur J Pharmacol. 2017;796:39–44. doi: 10.1016/j.ejphar.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Priya LB, Baskaran R, Huang CY, Padma VV. Neferine ameliorates cardiomyoblast apoptosis induced by doxorubicin: Possible role in modulating NADPH oxidase/ROS-mediated NFkappaB redox signaling cascade. Sci Rep. 2017;7(1):12283. doi: 10.1038/s41598-017-12060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RE, Tran K, Smith CC, et al. The role of the Nrf2/ARE antioxidant system in preventing cardiovascular diseases. Diseases. 2016;4(4) doi: 10.3390/diseases4040034. pii: E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SK, Yang JW, Kim MR, et al. Increased expression of Nrf2/ARE-dependent anti-oxidant proteins in tamoxifen-resistant breast cancer cells. Free Radic Biol Med. 2008;45(4):537–46. doi: 10.1016/j.freeradbiomed.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Fan Z, Han Y, Ye Y, et al. l-carnitine preserves cardiac function by activating p38 MAPK/Nrf2 signalling in hearts exposed to irradiation. Eur J Pharmacol. 2017;804:7–12. doi: 10.1016/j.ejphar.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Dai LH, Fei A, et al. Isoquercetin activates the ERK1/2-Nrf2 pathway and protects against cerebral ischemia-reperfusion injury in vivo and in vitro. Exp Ther Med. 2017;13(4):1353–59. doi: 10.3892/etm.2017.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng CK, Hsu SP, Lin CK, et al. Celastrol inhibits hepatitis C virus replication by upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in human hepatoma cells. Antiviral Res. 2017;146:191–200. doi: 10.1016/j.antiviral.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Zhuang X, Huang Z, et al. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-kappaB-mediated inflammation both in vitro and in vivo. Biochim Biophys Acta. 2017 doi: 10.1016/j.bbadis.2017.09.029. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Dalton G, An SW, Al-Juboori SI, et al. Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc Natl Acad Sci USA. 2017;114(4):752–57. doi: 10.1073/pnas.1620301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brobey RK, German D, Sonsalla PK, et al. Klotho protects dopaminergic neuron oxidant-induced degeneration by modulating ASK1 and p38 MAPK signaling pathways. PLoS One. 2015;10(10):e0139914. doi: 10.1371/journal.pone.0139914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song S, Gao P, Xiao H, et al. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One. 2013;8(12):e82968. doi: 10.1371/journal.pone.0082968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YY, Yi M, Huang YP. Oxymatrine ameliorates doxorubicin-induced cardiotoxicity in rats. Cell Physiol Biochem. 2017;43(2):626–35. doi: 10.1159/000480471. [DOI] [PubMed] [Google Scholar]
- 18.Bulut G, Kurdoglu Z, Donmez YB, et al. Effects of jnk inhibitor on inflammation and fibrosis in the ovary tissue of a rat model of polycystic ovary syndrome. Int J Clin Exp Pathol. 2015;8(8):8774–85. [PMC free article] [PubMed] [Google Scholar]
- 19.Muller AH, Edwards AVG, Larsen MR, et al. Proteomic expression changes in large cerebral arteries after experimental subarachnoid hemorrhage in rat are regulated by the MEK-ERK1/2 pathway. J Mol Neurosci. 2017;62(3–4):380–94. doi: 10.1007/s12031-017-0944-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan W, Xiaoli L, Guoliang A, et al. SB203580 inhibits epithelial-mesenchymal transition and pulmonary fibrosis in a rat silicosis model. Toxicol Lett. 2016;259:28–34. doi: 10.1016/j.toxlet.2016.07.591. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Zhao N, Zhu H, et al. Circulating interleukin-1beta promotes endoplasmic reticulum stress-induced myocytes apoptosis in diabetic cardiomyopathy via interleukin-1 receptor-associated kinase-2. Cardiovasc Diabetol. 2015;14:125. doi: 10.1186/s12933-015-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo L, Youtz DJ, Wold LE. Particulate matter exposure exacerbates high glucose-induced cardiomyocyte dysfunction through ROS generation. PLoS One. 2011;6(8):e23116. doi: 10.1371/journal.pone.0023116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang JS, Chuang CT, Liu MH, et al. Klotho attenuates high glucose-induced fibronectin and cell hypertrophy via the ERK1/2-p38 kinase signaling pathway in renal interstitial fibroblasts. Mol Cell Endocrinol. 2014;390(1–2):45–53. doi: 10.1016/j.mce.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Khan AA, Ashraf A, Singh R, et al. Incidence, time of occurrence and response to heart failure therapy in patients with anthracycline cardiotoxicity. Intern Med J. 2017;47(1):104–9. doi: 10.1111/imj.13305. [DOI] [PubMed] [Google Scholar]
- 25.Lagoa R, Ganan C, Lopez-Sanchez C, et al. The decrease of NAD(P)H: Quinone oxidoreductase 1 activity and increase of ROS production by NADPH oxidases are early biomarkers in doxorubicin cardiotoxicity. Biomarkers. 2014;19(2):142–53. doi: 10.3109/1354750X.2014.885084. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp. 2009;57(6):435–45. doi: 10.1007/s00005-009-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JY, Zhu GY, Su XH, et al. 7-deacetylgedunin suppresses inflammatory responses through activation of Keap1/Nrf2/HO-1 signaling. Oncotarget. 2017;8(33):55051–55063. doi: 10.18632/oncotarget.19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu L, Zhang B, Liu L, et al. The Role of p38 MAPK, JNK, and ERK in antibacterial responses of chilo suppressalis (Lepidoptera: Crambidae) J Econ Entomol. 2017;110(4):1460–64. doi: 10.1093/jee/tox126. [DOI] [PubMed] [Google Scholar]
- 29.Ye J, Piao H, Jiang J, et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-kappaB and Nrf2/HO-1 pathways. Sci Rep. 2017;7(1):11895. doi: 10.1038/s41598-017-12252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong YH, Park JS, Kim DH, Kim HS. Lonchocarpine increases Nrf2/ARE-mediated antioxidant enzyme expression by modulating AMPK and MAPK signaling in brain astrocytes. Biomol Ther. 2016;24(6):581–88. doi: 10.4062/biomolther.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiraki-Iida T, Iida A, Nabeshima Y, et al. Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J Gene Med. 2000;2(4):233–42. doi: 10.1002/1521-2254(200007/08)2:4<233::AID-JGM110>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Sun Z. Antiaging gene klotho attenuates pancreatic beta-cell apoptosis in type 1 diabetes. Diabetes. 2015;64(12):4298–311. doi: 10.2337/db15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin-Nunez E, Lopez-Castillo A, Delgado-Molinos A, et al. Soluble levels and endogenous vascular gene expression of KLOTHO are related to inflammation in human atherosclerotic disease. Clin Sci. :2017. doi: 10.1042/CS20171242. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Maltese G, Psefteli PM, Rizzo B, et al. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med. 2017;21(3):621–27. doi: 10.1111/jcmm.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]