Abstract
Case series
Patient: Female, 60 • Male, 45 • Male, 56 • Male, 65 • Female, 57 • Male, 35
Final Diagnosis: Klebsiella pneumoniae liver abscess
Symptoms: Fever
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Rare co-existance of disease or pathology
Background:
Liver abscesses represent a serious infection of hepatic parenchyma and are associated with significant morbidity and mortality. The emergence of a new hypervirulent variant of Klebsiella pneumoniae, which can cause serious infections in the Asian population, is under investigation. We report a case series of six Asian patients hospitalized at our institution from January 2013 to November 2015 for liver abscess due to Klebsiella pneumoniae.
Case Report:
Charts of six Asian patients were retrospectively reviewed. Four patients were male and two were female. The mean age was 53 years (range: 35–64 years). All patients had no known past medical history of immunodeficiency. Three patients had multiple liver abscesses at the time of initial presentation. In five patients, the source of entry of the pathogenic microorganism was unknown and in one patient the suspected source of entry was the gastrointestinal tract. In three patients there was also concomitant Klebsiella pneumoniae bacteremia. The mean duration of antibiotic treatment was seven weeks and the mean duration of hospital stay was 13.5 days.
Conclusions:
Liver abscess should always be included in the differential diagnosis in cases of sepsis without obvious source and/or in the clinical scenarios of fever, abdominal pain, and liver lesions.
MeSH Keywords: Klebsiella Pneumoniae, Liver Abscess; Pyogenic, Sepsis
Background
Liver abscesses can develop as a complication of abdominal and biliary infections, or following hematogenous spread, or by contiguity. The etiology of liver abscesses can be bacterial, parasitic and, rarely, fungal [1,2]. Streptococci and Escherichia coli are the most commonly reported pathogens [3–6]. Empiric antibiotic regimen upon diagnosis of a liver abscess should include coverage against Gram-negative bacilli, Gram-positive cocci, and anaerobes. Antibiotic regimens should be tailored based on available culture results and sensitivities. The recommended duration of antibiotic therapy is usually between two and six weeks [1,7–9]. Mortality rates for patients with liver abscesses remain high, and appropriate antimicrobial treatments combined with percutaneous or surgical drainage for adequate source control have already increased survival rates and improved clinical outcomes [10,11].
Klebsiella species have emerged in recent years as important causes of liver abscesses in the Asian population. In 1986, a new syndrome of pyogenic liver abscess caused by hypervirulent community-acquired Klebsiella pneumoniae was first described in Taiwan [12,13]. These community-acquired primary infections were observed in healthy individuals with some specific predisposing risk factors such as diabetes mellitus [14]. The source of entry for the bacteria remains unclear, and the abscesses are usually cryptogenic [15]. Intestinal colonization and colonization of portal venous flow, genetic predisposition, and geographic strain acquisition appear to play a role [12,15]. Characteristics of the hypervirulent strain include the increase in capsule production, resulting to the ability of the bacteria to cause serious, life threatening infections with metastatic potential. The most virulent stains are K1 and K2. In Asia, isolated strains are mainly serotype K1, followed by serotype K2 [15,16–18]. The hypermucoviscous phenotype correlates with “stickiness” of colonies on culture media [19,20].
The emergence of Klebsiella pneumoniae as an important causative agent for liver abscesses has been reported by multiple studies worldwide. This syndrome has been reported not only in Asian countries, but is also now is increasingly recognized in America, Europe, Africa, Australia, and the Caribbean [12,21,22].
We report on a case series of six Asian patients hospitalized at our institution from January 2013 to November 2015 for liver abscess due to Klebsiella pneumoniae.
Case Reports
The most important information on patients’ clinical presentation, diagnosis, and management are summarized in Table 1. In our case series, four of the six patients were male and two were female. The mean age was 53 years (range: 35–64 years). One out of six patients had a past medical history of diabetes mellitus; and one out of six patients had a remote past medical history of malignancy but that patient was not on recent chemotherapy. One out of six patients had a past medical history of liver disease (chronic hepatitis B infection). All patients had no known past medical history of immunodeficiency. The initial clinical manifestations included fever-chills, vomiting, and abdominal pain. Three out of six patients met the sepsis criteria upon initial presentation. Three out of six patients were initially admitted to the intensive care unit, and one out of six patients was admitted to the medical intensive care unit for septic shock requiring vasopressors for hemodynamic support.
Table 1.
Basic characteristics of cases.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Gender | Female | Male | Male | Male | Female | Male |
| Age (years) | 60 | 45 | 56 | 65 | 57 | 35 |
| Origin | Chinese | Malaysian | Chinese | Chinese | Chinese | Chinese |
| History of diabetes mellitus | Yes | No | No | No | No | No |
| History of malignancy | Yes | No | No | No | No | No |
| History of immunodeficiency | No | No | No | No | No | No |
| Sepsis – septic shock | No | Yes | No | Yes | Yes | No |
| Intensive care unit stay | No | Yes | No | Yes | Yes | No |
| Abnormal liver function tests | Yes | Yes | Yes | Yes | Yes | Yes |
| Leukocytosis | Yes | Yes | Yes | Yes | No | No |
| Multiple abscesses | Yes | No | No | No | Yes | Yes |
| Location | Liver Dome | Right lobe | Liver dome | Right lobe | Right lobe | Left lobe |
| Source of entry | Gastrointestinal tract | Unknown | Unknown | Unknown | Unknown | Unknown |
| Bacteremia | No | Yes | Yes | No | Yes | No |
| Drainage | Yes | Yes | No | Yes | Yes | Yes |
| Duration of antibiotics (weeks) | 12 | 8 | 8 | 6 | 4 | 5 |
| Length of stay (days) | 18 | 25 | 7 | 8 | 13 | 10 |
| Mortality | No | No | No | No | No | No |
| Gastrointestinal work-up | No | No | No | Yes | No | No |
| Anaerobic coverage | Yes | Yes | Yes | Yes | No | Yes |
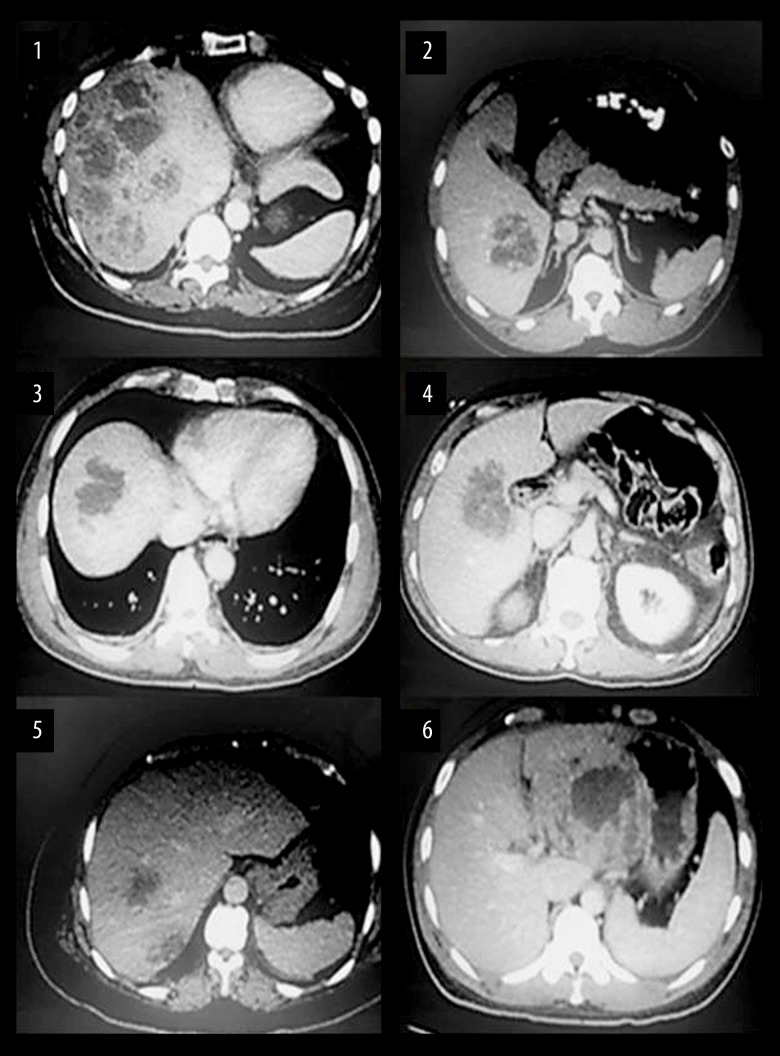
Three out of six patients also had concomitant bacteremia, but none of the patients had signs and symptoms suggestive of metastatic infection. Upon initial presentation, all patients had abnormal liver function tests and four out of six patients had leukocytosis. Three out of six patients had multiple liver abscesses at the time of initial presentation. In three out of six patients, the abscesses were located on the right liver lobe (Figure 1). In five out of six patients the source of entry of the pathogenic microorganism was unknown, and in one out of six patients the suspected source of entry was the gastrointestinal tract (Table 1). Further work-up with upper endoscopy and colonoscopy for evaluation of possible underlying malignancy was performed for one out of six patients. Five out of six patients underwent drainage of abscesses by Interventional Radiology. The antibiotic choices in all cases included beta-lactams, quinolones, and additionally in five out of six patients the antibiotic regimen also included anaerobic coverage. The mean duration of antibiotic treatment was seven weeks and the mean duration of hospital stay was 13.5 days (range 7–25 days). All patients were discharged in stable condition. All patients had favorable outcomes and satisfactory clinical progress with 0% mortality rate for this case series.
Figure 1.
Initial CT abdomen images at the time of liver abscess diagnosis.
Discussion
Klebsiella pneumoniae liver abscess is a rare and emerging infectious disease in patients in Asia, the United States, and Europe, and it tends to spread globally [23]. In the majority of these liver abscesses, the etiology cannot be established, thus these abscesses are characterized as cryptogenic [24]. Since Klebsiella pneumoniae strains may colonize the human gastrointestinal tract, translocation from the gastrointestinal tract is the most likely route by which Klebsiella pneumoniae causes formation of liver abscesses [15,23].
The optimal management of pyogenic liver abscess requires appropriate potent broad-spectrum antibiotic regimen tailored to the culture results, in conjunction with source control and abscess drainage and/or surgical intervention. Percutaneous intervention remains the preferred first-line therapeutic choice for liver abscess drainage, especially after the development of advances in imaging technology, the advantages of the simplicity of treatment, and avoidance of general anesthesia and laparotomy [23]. Additionally, needle aspiration can help to identify the responsible etiologic microorganisms and its susceptibilities to antimicrobial agents [1]. In our case series, five out of six patients underwent percutaneous drainage, while in one out of six patients the abscess was not drained due to abscess location just below the diaphragm and the high risk for pneumothorax.
The duration of antibiotic therapy must be determined by clinical response, including the resolution of fever and leukocytosis, and the resolution of the liver abscess as demonstrated by repeat imaging [4,15]. In our case series, all patients underwent repeat imaging during the follow-up period, which confirmed resolution of their abscesses. The mean duration of antibiotic treatment was 7.16 weeks.
It has been shown that diabetes mellitus (DM) can be an important predisposing factor that correlates with a high incidence of K1 serotype liver abscesses due to neutrophil dysfunction and chemotaxis failure [25,26], and that the incidence rate of metastatic infections is higher in patients with DM [23,24]. Metastatic infections are a clinical, diagnostic, and therapeutic challenge and are only diagnosed in one third of the cases on admission [15]. In our case series, no patient developed metastatic infection, despite concomitant bacteremia in three out of six patients.
It has been previously suggested that liver abscesses can be related to gastrointestinal malignancy [27,28]; therefore, further work-up with endoscopy and biliary imaging is indicated.
In our case series, only one out of six patients underwent upper endoscopy and colonoscopy, which were negative for gastrointestinal malignancy.
Pyogenic liver abscesses are considered polymicrobial with estimates ranging from 20–50%, and with frequent presence of anaerobes; thus, anaerobic coverage is warranted since cultures often can be of low yield [25]. In clinical practice, continuation of anaerobic coverage is common even in the absence of anaerobic growth in cultures [29–31]. In a 2015 study by Kim et al., it was suggested that in pyogenic liver abscess cases, anaerobic coverage can be discontinued if Klebsiella is isolated, and that early discontinuation of anaerobic coverage did not affect the clinical outcome in these patients [25]. This observation has important implications for avoiding unnecessarily prolonged use of anti-anaerobic agents, which are associated with suppression of normal gut flora and selective pressure for antibiotic-resistant microorganisms. Moreover, the length of hospital stay was shorter in the discontinuation group compared to the continuation group, and it was assumed that early discontinuation of intravenous anti-anaerobic agents could reduce the need for continuing hospitalization [25]. In our case series, five out of six patients received treatment with anaerobic coverage, and in six out of six patients, no anaerobes grew in fluid cultures from abscesses.
The cornerstones of diagnosis of liver abscess are imaging studies, such as ultrasonography and CT abdomen. In our case series, the initial imaging study of choice for diagnosis of liver abscess was CT abdomen and pelvis, which is consistent with existing literature data. It has been reported that CT was more sensitive than ultrasonography in the detection of liver abscesses, with 98% being clearly identified by CT [3].
The absence of mortality in our case series can be attributed to the increased suspicion for the disease, the early diagnosis, and the prompt initiation of antibiotics combined with source control and drainage of abscess; similar observations have occurred in studies in the past [10,11]. Overall, the prognosis is better for patients with Klebsiella liver abscesses (KLAs) than for those with other bacterial liver abscesses; however, the prognosis for patients with metastatic infections is grim [33].
As mentioned previously, Klebsiella pneumoniae isolates from liver abscesses are often hypermucoviscous, frequently belong to serotype K1 or K2, demonstrate unique characteristics such as metastatic potential, and represent a new increasingly recognized health problem in the Asian population [32–34].
In our case series, Klebsiella serotype and virulence factor testing was not performed because these tests were not part of routine care at our institution at the time these patients were hospitalized.
Conclusions
Liver abscesses due to Klebsiella pneumoniae is an emerging entity with increased morbidity and mortality if undiagnosed and untreated. Since the clinical presentation is often atypical and insidious and physical findings may be subtle, increasing awareness and high clinical suspicion are essential and play a vital role in the early diagnosis and effective treatment in cases of fever and abdominal pain and/or sepsis without an obvious source of infection.
References:
- 1.Lardière-Deguelte S, Ragot E, Amroun K, et al. Hepatic abscess: Diagnosis and management. J Visc Surg. 2015;152(4):231–33. doi: 10.1016/j.jviscsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Nakanishi Y, Kayahara T, Yamashita Y, et al. A case of ruptured giant liver cyst complicated by Candida infection. Nihon Shokakibyo Gakkai Zasshi. 2009;106(7):1056–52. [PubMed] [Google Scholar]
- 3.Ali AH, Smalligan RD, Ahmed M, Khasawneh FA. Pyogenic liver abscess and the emergence of Klebsiella as an etiology: A retrospective study. Int J Gen Med. 2013;7:37–32. doi: 10.2147/IJGM.S54448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meddings L, Myers RP, Hubbard J, et al. A population-based study of pyogenic liver abscesses in the United States: Incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105(1):117–24. doi: 10.1038/ajg.2009.614. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez Pérez JA, González JJ, Baldonedo RF, et al. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. 2001;181(2):177–86. doi: 10.1016/s0002-9610(00)00564-x. [DOI] [PubMed] [Google Scholar]
- 6.Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14(10):1592–600. doi: 10.3201/eid1410.071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and man-agement of complicated intra-abdominal infection in adults and children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–34. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 8.Hope WW, Vrochides DV, Newcomb WL, et al. Optimal treatment of hepatic abscess. Am Surg. 2008;74(2):178–82. [PubMed] [Google Scholar]
- 9.Bamberger DM. Outcome of medical treatment of bacterial abscesses without therapeutic drainage: Review of cases reported in the literature. Clin Infect Dis. 1996;23(3):592–93. doi: 10.1093/clind/23.1.592. [DOI] [PubMed] [Google Scholar]
- 10.Siu LK, Yeh KM, Lin JC, et al. Klebsiella pneumoniae liver abscess: A new invasive syndrome. Lancet Infect Dis. 2012;12:881–87. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Wu SS, Chang HC, Chang YJ. Initial presentations and final outcomes of primary pyogenic liver abscess: A cross-sectional study. BMC Gastroenterol. 2014;14:133. doi: 10.1186/1471-230X-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence. 2013;4(2):107–8. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JC, Koh TH, Lee N, et al. Genotypes and virulence in serotype K2 Klebsiella pneumoniae from liver abscess and non-infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathog. 2014;6:21. doi: 10.1186/1757-4749-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcántar-Curiel MD, Girón JA. Klebsiella pneumoniae and the pyogenic liver abscess: implications and association of the presence of rpmA genes and expression of hypermucoviscosity. Virulence. 2015;6(5):407–9. doi: 10.1080/21505594.2015.1030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lübbert C, Wiegand J, Karlas T. Therapy of liver abscesses. Viszeralmedizin. 2014;30(5):334–41. doi: 10.1159/000366579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilal S, Volz MS, Fiedler T, et al. Klebsiella pneumoniae – induced liver asbscesses, Germany. Emerg Infect Dis. 2014;20(11):1939–40. doi: 10.3201/eid2011.140149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung DR, Lee SS, Lee HR, et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect. 2007;54:578–73. doi: 10.1016/j.jinf.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Wang Y, Ye L, Yang J. Molecular epidemiology and virulence factors of pyogenic liver abscess causing Klebsiella pneumoniae in China. Clin Microbiol Infect. 2014;20(11):O818–24. doi: 10.1111/1469-0691.12664. [DOI] [PubMed] [Google Scholar]
- 19.Nadasy KA, Domiati-Saad R, Tribble MA. Invasive Klebsiella pneumoniae syndrome in North America. Clin Infect Dis. 2007;45(3):25–28. doi: 10.1086/519424. [DOI] [PubMed] [Google Scholar]
- 20.Lee HC, Chuang YC, Yu WL, et al. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: Association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med. 2006;259:606–14. doi: 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 21.Chuang YP, Fang CT, Lai SY, et al. Genetic determinants of capsular sero-type K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis. 2006;193:645–54. doi: 10.1086/499968. [DOI] [PubMed] [Google Scholar]
- 22.Vila A, Cassata A, Pagella H, et al. Appearance of Klebsiella pneumoniae liver abscess syndrome in Argentina: Case report and review of molecular mechanisms of pathogenesis. Open Microbiol J. 2011;5:107–13. doi: 10.2174/1874285801105010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Wang JY, Jiang W. An increasing prominent disease of Klebsiella pneumoniae liver abscess: Etiology, diagnosis, and treatment. Gastroenterol Res Pract. 2013;2013:258514. doi: 10.1155/2013/258514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dulku G, Mohan G, Samuelson S, et al. Percutaneous aspiration versus catheter drainage of liver abscess: A retrospective review. Australas Med J. 2015;8(1):7–18. doi: 10.4066/AMJ.2015.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HA, Chung DR, Yeom JS, et al. Anti-anaerobic coverage is not necessary for Klebsiella pneumoniae liver abscess: A propensity score-matched cohort study. Diagn Microbiol Infect Dis. 2015;81(1):60–65. doi: 10.1016/j.diagmicrobio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Eltawansy SA, Merchant C, Atluri P, Dwivedi S. Multi-organ failure secondary to a Clostridium perfringens gaseous liver abscess following a self-limited episode of acute gastroenteritis. Am J Case Rep. 2015;16:182–86. doi: 10.12659/AJCR.893046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong SW, Jang JY, Lee TH, et al. Cryptogenic pyogenic liver abscess as the herald of colon cancer. J Gastroenterol Hepatol. 2012;27:248–55. doi: 10.1111/j.1440-1746.2011.06851.x. [DOI] [PubMed] [Google Scholar]
- 28.Koo HC, Kim YS, Kim SG, et al. Should colonoscopy be performed in patients with cryptogenic liver abscess? Clin Res Hepatol Gastroenterol. 2013;37:86–92. doi: 10.1016/j.clinre.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 29.Baron MJ, Kasper DL. Intraabdominal infections and abscesses. In: Longo DL, Fauci AS, Kasper DL, editors. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill Companies, Inc.; 2012. [Google Scholar]
- 30.Frey CF, Zhu Y, Suzuki M, Isaji S. Liver abscesses. Surg Clin North Am. 1989;69:259–61. doi: 10.1016/s0039-6109(16)44784-5. [DOI] [PubMed] [Google Scholar]
- 31.Sifri CD, Madoff LC. Infections of the liver and biliary system. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; Elsevier: 2010. [Google Scholar]
- 32.Melot B, Colot J, Guerrier G. Bacteremic community-acquired infections due to Klebsiella pneumoniae: Clinical and microbiological presentation in New Caledonia, 2008–2013. Int J Infect Dis. 2015;41:29–31. doi: 10.1016/j.ijid.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Yu VL, Hansen DS, Ko WC, et al. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;13:986–93. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh KM, Kurup A, Siu LK, et al. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol. 2007;45:466–71. doi: 10.1128/JCM.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]