Abstract
Bladder cancer (BC) is one of the leading causes of cancer-related deaths worldwide. Despite, the majority of the cases were diagnosed as non-muscle invasive bladder cancer (NMIBC) with favorable prognosis, it has tendency to recur or progress to a higher grade or stage. The first line treatment of patients with NMIBC is transurethral resection with adjuvant therapies primarily intravesical Bacillus Calmette-Guérin (BCG) immunotherapy. However, in a portion of patients whose BCG treatment failed, alternative treatments may be required. Furthermore, intravesical BCG may be contraindicated in or untolerated by a group of patients. For these patients, some treatment options are readily available and a variety of them are currently under clinical investigation. In this review, these alternative therapies have been summarized.
Keywords: BCG, bladder cancer, instillation, survival
Introduction
Bladder cancer (BC) is a serious health problem with an estimated 430.000 new cases and 165.000 deaths in 2012 worldwide.[1] The age-standardized incidence rates were found to be 9.0 per 100.000 men and 2.2 per 100.000 women (male/female ratio: 4.1). While BC has a strong association with gender and age, rates are also associated with the human development index (HDI). The HDI is a summary measure of average achievement in key dimensions of human development ie. a long and healthy life, being knowledgeable and have a decent standard of living. The highest rates of BC were reported to be in men from countries with very HDIs (16.7 and 3.7 per 100.000 for men and women, respectively); whereas the lowest rates were from countries with low HDIs (3.1 and 1.4 per 100.000 for men and women, respectively). Despite these high rates in countries with high HDIs, the mortality rates were higher (0.48 vs. 0.26) in less developed countries compared with the developed ones.[2]
Bladder cancer develops from the epithelium (urothelium) covering the inner surface of the bladder and specifically named as an “urothelial” carcinoma. Non-muscle invasive bladder cancer (NMIBC) corresponds to disease confined to mucosa and submucosa (stages Ta, T1 or carcinoma in situ [CIS]); which accounts for 75% of the newly diagnosed cases. During the course of NMIBC, as many as 50–70% of the cases will recur and roughly 10–20% of them will invade muscularis propria (muscle-invasive disease).[3]
Treatment of NMIBC includes transurethral bladder tumor resection (TUR-BT) with risky use of adjuvant intravesical treatments. Adjuvant treatment after TUR-BT is generally categorized by risk groups that combine pathologic and clinical features.[4] Summarizing the tumors with risk groups as “low”, “intermediate” and “high” risk for recurrence and progression generally helps the physician to counsel patients regarding intensity of surveillance and treatment. Recently, a review of the four major organizational guidelines on NMIBC released by the American Urological Association (AUA)/Society of Urologic Oncology (SUO), European Association of Urology (EAU), National Comprehensive Cancer Network (NCCN), and National Institute for Health and Care Excellence (NICE) was published largely based on risk stratification of patients (Table 1).[5]
Table 1.
Risk categories of NMIBC created by 3 major urological organizations
| EAU | AUA | NICE | |
|---|---|---|---|
| Low |
|
|
|
| Intermediate |
|
|
|
| High |
|
|
|
Subgroup of highest risk tumors
|
CIS: carcinoma in situ; LG: low grade; HG: high grade; LVI: lymphovascular invasion; PUNLMP: papillary neoplasm of low malignant potential; AUA: American Urological Association; EUA: European Urological Association; NICE: National Institute for Health and Care Excellence; NMIBC: non-muscle invasive bladder cancer
Standard adjuvant therapy for NMIBC is delivered as intravesical therapies and is classified as perioperative, induction and maintenance therapies. The main mechanism of action of these therapies is to achieve immunotherapy and chemotherapy.[6] Bacillus Calmette-Guérin (BCG) vaccine is an immunotherapeutic agent that was developed from Mycobacterium Bovis and its mechanism of action is not completely elucidated. However, it is known that BCG activates the immune system by adhering to urothelium and tumor cells through the action of fibronectin.[7] After being internalized, MHC class II molecules are upregulated and cytokine production is increased leading to immune mediated cytotoxicity.[7] A recent systematic review suggested that 6 weeks of induction therapy with BCG is associated with a decreased recurrence (RR 0.56 95% CI 0.43–0.1) and progression (RR 0.39, 95% CI 0.24–0.64) risk compared to TUR-BT alone.[8]
Some patients may have contraindications to BCG and some patients and physicians may prefer to use intravesical chemotherapy in recurrent low grade tumors. Several agents are being used for intravesical chemotherapy; primarily exert their effects by disrupting DNA synthesis, such as mitomycin C (MMC) which is the most commonly used chemotherapeutic agent. However, maintenance therapy with BCG seems to be superior to intravesical MMC chemotherapy in terms of recurrence accounting for 32% reduction in recurrence rates with BCG compared to MMC.[9] Meanwhile, no statistical significant difference between BCG and MMC in terms of disease-progression and mortality rates was found.
Despite being an effective drug, 20–40% of the patients undergoing BCG treatment experience recurrence and every course of additional treatment increases this risk around 7 percent.[10] More importantly, around 20% of the patients progress to muscle invasive state.[3,6] Generally, radical cystectomy (RC) is encouraged in case of failure of BCG; as categorized in Table 2 and schematized in Figure 1, but it is not always a viable option due to multiple comorbidities or reluctance of the patients to undergo such a morbid surgery.[6] Consequently, the aim of this review is to discuss alternative treatment regimens depicted in Table 3 in patients with NMIBC.
Table 2.
Categories of BCG failure and their definition (Adopted from reference 8)
| BCG intolerance |
|
| BCG relapsing |
|
| BCG resistance |
|
| BCG refractory |
|
| BCG unresponsive |
|
BCG: Bacillus Calmette-Guérin
Figure 1.
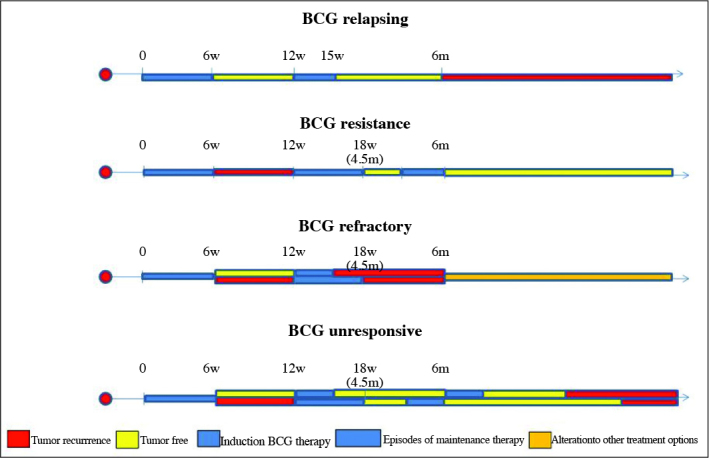
Schematic demonstration of Categories of BCG failure. BCG unresponsive patients also includes BCG refractory patients
Table 3.
Outlined alternative treatment options in patients with non-muscle invasive bladder cancer
| Valrubicin |
| Gemcitabine |
| IFN-2α |
| Taxanes |
| Combination chemotherapeutics (e.g: doxorubicin-BCG, MMC-doxorubicin, cisplatin) |
| Keyhole limpet hyocyanin (KLH) |
| Mycobacterium phlei cell wall-nucleic acid complex (MCNA) |
| Mistletoe lectin (ML) |
| Apaziquone |
| Chemothermotherapy |
| Electromotive drug administration (EMDA) |
| Oncolytic viruses |
| Interferon α2b (IFN-α2b) producing adenoviruses |
| Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) |
| Checkpoint inhibitors |
| Vaccines |
Clinical research consequences
Valrubicin
Valrubicin is the only FDA approved agent for salvage therapy of CIS in BCG-unresponsive patients. It is a semisynthetic analogue of anthracycline doxorubicin that was launched in 1999 but withdrawn because of manufacturing issues and consequently relaunched in 2009. Different from doxorubicin, valrubicin passes the cystoplasmic membrane rapidly and accumulates in the cytoplasm leading to cytolytic cell death. This potential advantage was demonstrated in a marker lesion study.[11] Steinberg et al.[12] evaluated intravesical valrubicin in 90 patients with recurrent CIS after at least 2 prior courses of intravesical therapy. The authors reported complete response in 19 patients (21%) at 3–6 months of follow-up and 7 of these were disease free at a mean follow-up 30 months. The median time of failure in complete responders was 18 months or more. In a further study, Dinney et al.[13] estimated the chances of undergoing cystectomy ın patients with failed BCG treatment, intolerance to BCG or for BCG- naïve cases as 12, 24, and 30% at 6 months, 1, and 2 years, respectively.
Gemcitabine
Gemcitabine (GEM) is a deoxycytidine analogue that inhibits DNA synthesis and is currently being used as a component of systemic chemotherapy for muscle- invasive BC (MIBC). It is relatively safe at standard intravesical concentrations (2000 mg/day) with minimal absorption to systemic circulation. Ablative efficacy of GEM was investigated in a marker lesion study, and complete response rate was found to be 44% in a weekly single dose group; whereas 56% of the patient cohort was found to have new tumor occurrences.[14] A similar study observed complete response in 22 out of 39 (56%) intermediate-risk NMIBC patients and no progression was observed among non-responding patients.[15] In a phase II study, Bartoletti et al. used GEM once a week for 6 weeks with an intravesical dosage of 2000 mg in 116 patients with intermediate-high risk BC.[16] In the intermediate risk group, 21 of 81 patients (25.9%) had recurrence (2 cases with disease-progression) whereas, in the high risk group 27 of 35 patients (77.1%) experienced recurrences (5 cases with disease-progression) after 1 year. Tumor recurrence was observed in 35.2% (13/40) and 21% (16/76) of the BCG-relapsing, and BCG naïve patients, respectively. The treatment was well tolerated and no greater risk of toxicity was observed. Meanwhile, the study by Porena et al.[17] demonstrated a significant advantage for BCG in terms of recurrence rates (28.1% vs. 53.1%, p=0.037) after a mean follow-up of 44 months. No patients in this study developed disease progression. Additionally, Addeo et al.[18], compared 6-week course of GEM with 4-week course of MMC in an RCT. Responders of the induction therapy further received maintenance therapy consisting of 10- monthly MMC treatments. After a median follow-up of 36 months, the relative risk of recurrence (0.72 vs. 0.94, p=0.29) and the rate of disease progression by stage was not statistically significantly different (11% vs. 18%, p=0.14) for intravesical GEM and MMC therapies, respectively. However, GEM demonstrated a significant disease- free survival (DFS) advantage (p=0.0021) over MMC and a better safety profile. On the other hand, Di Lorenzo et al.[19] reported a better 2-year recurrence- free survival (3% vs. 19%, p<0.008) with BCG compared to GEM in cases with BCG failure. However this trend was not verified for disease-progression (37.5% vs. 33%, p=0.12) and both groups had similar rates of patients undergoing RC. Consequently, despite better toxicity profile GEM alone is not generally used as primary therapy but may have a role in patients with BCG failure; who do not wish to undergo RC.
Interferon 2α
Interferon (IFN) is a local immune booster that may enhance the local antitumoral activity of immunotherapeutics. With this concept, Joudi et al.[20] published the outcomes of phase II trial of BCG plus IFNα combination therapy in BCG- naïve and BCG- failure (recurrence any time in the past but not deemed as BCG intolerant) patients. The authors used standard dose BCG with 50 IU IFNα for BCG naïve patients whereas; one third of the standard dose with IFNα in patients with recurrent disease. At 24 months, 59% vs. 45% (p<0.0001) of these patients were disease free. Multivariant analysis revealed that T1 disease, tumor size >5 cm, multifocality and prior BCG therapy were significant risk factors for recurrence. In a further analysis, Gallagher et al.[21] evaluated the effect of the interval to recurrence after BCG therapy on the subsequent response to intravesical BCG plus IFN-α therapy. The authors reported a comparable recurrence rate for patients with BCG failure after 12 months of remission relative to BCG naïve patients. Similarly, Nepple et al.[22] found no difference in terms of recurrence-free survival after a 24-month median follow-up; when IFNα and daily allowance vitamins were added to maintenance treatment with BCG (63% vs. 55%, p>0.05) in a prospectively randomized trial. Accordingly, adding IFNα to BCG does not improve the oncological outcomes of the patients.
Taxanes
Taxanes such as docetaxel and paclitaxel are cytotoxic agents that stabilize microtubules against depolymerization leading to cell cycle arrest and death. Laudano et al.[23] reported the clinical outcomes of a phase I trial of intravesical use of docetaxel as 6 weeks induction therapy in patients with at least one course of failed BCG therapy. Of 18 patients, 4 patients had demonstrated complete, and 3 patients partial responses; whereas 11 patients had failed treatment without significant toxicity after a median follow-up of 48.3 months. The updated version of the study with 33 patients revealed a complete response rate of 61% at post-induction cystoscopy (67% who received the dose of 75 mg/100 mL) and 2 year recurrence-free survival rate was given as 32% in patients with and without monthly maintenance therapy.[24] However, among 10 patients who maintained disease-free status, 7 had received maintenance therapy.
One of the problems faced in taxanes and other high- molecular weight chemotherapeutics is their low solubility in water leading to poor drug uptake into the bladder tissue. For this reason, researchers synthesize substances to make taxanes highly soluble in water. For instance, PMB30W increases the solubility of paclitaxel 1000-fold higher than in water. Tamura et al.[25] investigated the impact of PMB30W coupled with paclitaxel in comparison with cremophor; the conventional solubilizer of paclitaxel, on BC using an orthotopic BC model. The authors reported that the paclitaxel concentration in the bladder tumors was significantly higher in the paclitaxel-PMB30W group in comparison with paclitaxel-cremophor group. Meanwhile, more reduction in bladder wet volume was also achieved with paclitaxel-PMB30W compared with paclitaxel-cremophor. More research is needed to deliver the drugs to target tissue in the unique conditions of bladder within a limited amount of time.
Combination chemotherapeutics
Intravesical delivery of chemotherapeutic combinations is an attractive idea for the prevention of disease-progression to MIBC since similar to the systemic therapy for MIBC, single agents in NMIBC do not provide enough efficacy to control disease. For this purpose, researchers have introduced a genetically engineered mouse (GEM) model. Briefly in this model p53 and Pten; the major tumor suppressor genes were deleted by delivery of adenovirus expressing Cre recombinase (Adeno-cre) into the bladder lumen. These tumor suppressor gene-deficient mice develop CIS in 8 weeks and CIS progresses to invasive disease in 5–6 months of age.[26] With this model, Delto et al.[27] compared cisplatin, GEM and/or docetaxel, alone or by combining maximum 2 agents for a biweekly instillation to determine whether these treatments inhibit tumor progression. The authors reported that bladder weights of mice treated with GEM reduced 5.4-fold (p=0.02); whereas those threated with docetaxel and cisplatin reduced 1.8 and 3.73-fold respectively. Moreover, the reduction in bladder weight was 7.6-fold, 6.0- fold and 4.53- fold for GEM-docetaxel, GEM-cisplatin and docetaxel-ciplatin combinations. Thus the authors concluded that GEM was the most effective chemotherapeutics for prevention of disease-progression into muscle invasive state. Other in vitro studies revealed that paclitaxel plus cisplatin and doxorubicin plus mitomycin might be good combinations.[28,29]
In a clinical study, Chen et al.[30] retrospectively compared an intravesical cocktail including MMC, doxorubicin and cisplatin with single agent doxorubicin and BCG. Both doxorubicin and BCG groups received 6 weeks of induction therapy followed by a 3 year maintenance therapy; whereas the cocktail group had 3 weekly instillations of sequential MMC, doxorubicin and cisplatin and also received maintenance therapy with the same schedule. After a mean follow-up of 50 months, the cocktail group had a lower disease- progression rate (4.4% vs. 17.2% for doxorubicin and 12.7% for BCG) in spite of having more unfavorable features. Recurrence rate was higher in doxorubicin group while it was almost the same in BCG and cocktail groups. Furthermore, discontinuation rate was far better for the cocktail group (16.5%) compared with the BCG group (22.5%). Meanwhile, some other multi-agent combination studies such as cabazitaxel+GEM+cisplatin (NCT02202772) are under investigation. Accordingly, despite considerable effort with the idea of overcoming drug resistance with combinations of drugs, currently no intravesical drug combination is widely utilized.[31]
Others
Keyhole limpet hyocyanin (KLH) is a strong humoral and cellular immune-stimulant in both experimental animal models and humans. It is filtered from hemolymph of Megathura cranulata, also called the giant keyhole limpet, a native sea creature in Southern Carolina and Mexico. After some phase I and phase II studies showing limited side effect profile, Lammers et al.[32] evaluated the intravesical use of KHL in a prospective randomized phase III trial in comparison with MMC. In the KLH arm, patients were started on intracutaneous injections of 1 mg KLH (up to 4 times) before immunization until delayed-type hypersensitivity response was obtained. Patients in KLH arm received a total of 16 intravesical instillations in 9 months; whereas patients in the MMC group received 11 instillations in 12 months. The authors showed that receiving KLH is associated with more recurrences in patients with intermediate-high BC without CIS. There was also no reduction in stage-adjusted disease-progression.
Mycobacterium phlei cell wall-nucleic acid complex (MCNA) contains mycobacterial cell wall fragments complexed with nucleic acid oligomers derived from Mycobacterium Phlei or other mycobacteria species.[33] It is a rational idea to use BCG structural extracts instead of mycobacterium itself to avoid the potential side effects of live mycobacterium such as BCG cystitis, reactive arthritis and even interstitial pneumonitis.[34] MCNA exerts its antitumoral activity with both direct cytotoxic and immune-mediated mechanisms. It has been shown that MCNA has negative direct effect on cell proliferation and viability leading to the death of tumor cells.[35] Furthermore, it stimulates production of IL-12 which also has an anti-cancer activity.[35] In a multi-institutional study, Morales et al. evaluated the efficacy and safety of intravesical MCNA in patients who failed BCG treatment.[36] The study comprised a 6 week induction phase and a maintenance phase; in which 129 patients were to receive 3-or 6- (re-induction) weekly instillations at month 3 followed by 3 weekly instillations at months 6, 12, 18 and 24. The authors reported DFS rates of 25, and 19% at 1, and 2 years, respectively. DFS at 1 and 2 years were 35.1% and 32.2% for papillary only lesions, 25% and 16.1% for CIS only lesions and 13.3% and 8.9% for CIS + papillary lesions, respectively. Patients with BCG relapse had better RFS than those with BCG-refractory disease (22.1% vs. 15.8% at 1 year). Thus, the authors concluded that MCNA is comparable with valrubicin, the only FDA approved agent for CIS. This study completed with 99% compliance with the number of planned instillations. As an advantage, MCNA might be used during the early postoperative period of TURBT similar to other cytotoxic agents such as MMC or epirubicin used for chemoresection of the tumors. Despite these promising outcomes, this drug failed to have FDA approval because of a lack of control arm, small size of the study and ill-defined patient population of the related studies.
Mistletoe lectin (ML) is a semi-parasitic plant used for decades in Europe for different diseases. In vitro studies have shown that it has cytotoxic effects on cancer cells with its antiproliferative, antimetabolic, cytotoxic and immunomodulatory effects.[37] In a prospective Phase II trial, Goebel et al. evaluated the influence of subcutaneously applied ML on pTa G1–2 tumor recurrence in 45 patients. ML was applied twice a week for 3 months, which was followed by a therapy-free period and then a second cycle was initiated.[38] After a follow-up of 18 months, the number of recurrences and recurrence free intervals were not different in ML group in comparison with control group. Elsasser-Beile et al.[39] assessed the intravesical use of ML in group of patients with pTa and PT1, grades 1 or 2. The authors used ML weekly for 6 weeks with increasing doses up to 5.000 ng/mL. The study had no control group but revealed a similar recurrence rate (30%) when compared with a historical group.
Apaziquone is a synthetic alkylating agent that is converted to its cytotoxic metabolites after enzymatic activation by deoxythymidine-diaphorase. After an extensive preclinical evaluation and marker lesion study, two phase III trials evaluated the immediate use of apaziquone following TURBT for Ta/T1 disease.[40] These studies were designed to detect a 12% absolute difference between apaziquone and placebo in the primary endpoint (2-year recurrence rate) at 5% level of significance and powered at 80%. However, both trials with 6.6% (SPI-611) and 6.2% (SPI-612) reductions in recurrence rates when compared with the placebo groups failed to achieve this outcome. Thus, recently FDA voted against approving apaziquone for the immediate use after TURBT. Currently another phase III trial (NCT03224182) using 8 mg of apaziquone for immediate intravesical installation is ongoing. Subsequently, Hendricksen et al.[41] evaluated the efficacy of 4 mg in 40 mL apaziquone as 6 week induction therapy in patients with intermediate-high risk BC in a phase 2 study. Of 49 patients with intent- to- treat analysis, 34.7% and 44.9% of them experienced tumor recurrence at 12 and 18 months, respectively. The authors compared this outcome with EORTC risk tables and found it within the normal range (24%–61%) for 1 year. While 92% of the patients had some type of side effects which was thought to be comparable with recurrence rates reported for other chemotherapeutic drugs.
Device assisted therapies
Chemothermotherapy
Despite, MMC being considered as a sufficient drug in the first line setting, only 19% of the patients with one course of failed BCG treatment remained free of recurrences.[42] Since better longer term success rate (averages 35%) with second course of BCG treatment was reported, intravesical MMC is generally not considered for BCG-relapsing patients.[43] However, combination of MMC with thermotherapy is a promising treatment alternative. The idea of using heat for treating cancer is not new but in recent years combined use of chemotherapy and heat as “chemothermotherapy” (CHT) has been evaluated for patients with high risk disease and failed BCG treatments. It is known that the distance over which MMC concentration decreases by one-half was 500 microns and the MMC concentrations of 5.6 mcg, 2.7 mcg and 0.9 mcg have been found for urothelium, lamina propria and detrusor muscle, respectively.[44] The rationale of CHT generally depends on the improvement of efficacy of MMC which is based on tumor cell toxicity, altered blood flow and localized immune response.[45] The most commonly used device is Synergo HT system (Medical Enterprises Europe B.V., Amstelven, The Netherlands); which uses 915-Mhz intravesical microwave antenna integrated in a 20F treatment catheter for increasing the heat of bladder epithelium up to 41–44°. Meanwhile, MMC is circulated through a closed circuit and subsequently cooled to prevent overheating of the urethra. This concept was evaluated for the first time in a randomized trial in 2003; which was updated in 2011. Briefly, Colombo et al.[46] reported the outcomes of 65 patients with Ta-Ta and G1–3 BC who were randomized to receive intravesical chemohyperthermia (CHT) with MMC or intravesical MMC alone. All patients received 8 weekly, 60 min treatment sessions, followed by 4-monthly sessions. The 10 year DFS and bladder preservation rates for the CHT and chemotherapy arms were 53% and 15% (p<0.001); 86.1% and 78.9% (p=0.129), respectively. Subsequently, a review of CHT using 15 original non-randomized articles published until 2011 compared CHT with intravesical MMC alone.[47] The authors reported 59% lower recurrence rates after CHT in comparison with MMC alone and bladder preservation rate was 87.6%. However, the authors could not make any definitive statement on the role of CHT in patients with failed intravesical treatments because of the heterogeneous intravesical regimens. Despite this deficit, they mentioned that a significant number of these patients were salvaged with CHT. Side effects of CHT were generally found to be mild and reversible. The most common side effects were bladder spasms and bladder pain encountered in 21.6% and 17.5% of the patients, respectively. Of note, serious burn injuries were not reported but as a thermal reaction a small, superficial, darkly, discolored patch was seen on the posterior wall in 40.2% of the patients.
Despite being widely accepted in Europe for years, a multi-institutional RCT comparing CHT with BCG has been recently reported. Arends et al.[48] randomized 190 patients to CHT including 6 weeks induction (30 min treatment schedules with 20 mg MMC) and 6 maintenance treatments and 1 year classical BCG immunotherapy. The authors reported 2 year RFS as 81.1% vs. 64.8% in favor of CHT in per protocol analysis. Regarding CIS patients, complete response rates were not significantly different (88.9% vs. 85.7%). Progression to muscle invasive state was seen in 1 patient in the BCG group whereas no patients experienced this outcome in the CHT group. Nine (1 contracted bladder) and 5 serious adverse reactions were encountered in the study groups, respectively.
The most rationale use of this technology was seen in patients with failed BCG treatment. A study involving 111 patients revealed DFS rates of 85% and 56% at 1 and 2 years, respectively.[49] This outcome is probably the best of the treatment alternative used for BCG failures up to date. Despite these promising outcomes, some questions such as the number and duration of sessions and the need for maintenance therapy have remained to be answered. Moreover, high cost of the disposable catheters is another challenging issue.
On the other hand, a recent mathematical model challenged the internal application of heat since it was speculated that heating the solution to 42° outside the bladder and then circulating it within the bladder has the same effect with using the antenna and heating the fluid inside the body.[50] By using a device (Unithermia, Elmedical Ltd, Hod-Hasharon, Israel) for the application of this principle, Soria et al.[51] aimed to evaluate the efficacy of this device in patients with TA-T1, G1–2 NMIBC relapsing after BCG treatment. The authors used 45 min treatments with 40 mg of MMC in 34 patients with minimum follow-up of 24 months. Overall, initial treatment response rate was found to be 59% and 44.1% in patients who remained disease free after a median follow-up of 41 months. As an advantage the cost of disposables of Unitermia is less than Synergo. Consequently, CHT in either form has potential to be widely used especially for patients whose disease recurred despite application of BCG.
Electromotive drug administration
Electromotive drug administration (EMDA) relies on the enhancement of drug penetrance through the bladder wall by using electrical current.[45] In vitro studies have shown that EMDA increases MMC delivery by 4–7 times in comparison with passive diffusion.[52] Briefly, intravesical EMDA is given by a battery operated generator delivering a controlled electric current that passes from the active (integrated to the urethral catheter) to the ground electrodes pasted on the skin of the patient. Using this technique, Di Stasi et al. compared 6 weeks of induction therapy plus monthly BCG treatment for 9 months with sequential use of BCG and EMDA with MMC (EMDA-MMC) with an RCT including 212 patients.[53] In the latter protocol, patients received BCG for 2 weeks followed by 40 mg EMDA-MMC once a week as one cycle for 3 cycles. Furthermore, the latter group also received EMDA-MMC once a month for 2 months and followed by BCG once a month as one cycle for 3 months. After a median follow-up of 88 months, 41.9% and 57.9% (p=0.0012) of the patients were disease free in the BCG only and EMDA-MMC groups, respectively. In another RCT, the same group compared immediate pre-TURBT instillation of 40 mg EMDA-MMC with TURBT alone and immediate post-TURBT instillation.[54] After a mean follow-up of 86 months, 124 patients in the first group had lower rate of recurrence (38%) than the 124 (64%) and 126 (59%) patients in the second and third groups, respectively. Meanwhile, disease-free intervals for the study groups were 52, 12, and 16 months, respectively. The authors noted improvement in intermediate and high risk groups and patients with multifocal disease and no significant concerns were raised regarding safety of the drug and used technique.
Future concepts
In the management of BC, clinical parameters such as current grading and staging systems are insufficient for foreseeing the patients’ future. Additional tools are needed for optimizing the treatment and human genome is probably the most valuable source for this purpose. Van Allen et al.[55] showed that ERCC2 (a nucleotide excision repair gene) mutation detected by whole-exome sequencing is associated with cisplatin response. Similarly, ERCC1, BRCA1, P53 were found to be useful for the prediction of sensitivity to cisplatin; whereas hENT1 and RRM1 are reported as beneficial for GEM.[56,57] Thus, comparable with the other cancers such as breast and lung cancers, the revolutionary “genome medicine” will allow prediction of drug sensitivity and tailor the individualized approaches. Moreover, since BC is one of the easiest cancers in the body to obtain tumor tissue, we might use in vitro assays of BC for predicting drug resistance for effective intravesical chemotherapies to be applied in the future.
Targeting cancer cells with genetically engineered oncolytic viruses is another promising concept. Briefly, the replication ability of oncolytic viruses has been modified to be replicated in cancer cells without giving harm to normal cells.[58] This concept is different from gene therapy where a virus is used as a carrier for transgene delivery. For BC, an oncolytic virus (CG0070) destroying retinoblastoma (RB) pathway defective cells armed with GM-CSF was used for the treatment of recurrent NMIBC.[59] Of 35 patients, 13 had received single dose (up to 3× 103 virus particles); whereas the remaining 22 (28 days × 3 or weekly ×6) patients had multiple doses. The authors reported that urine GM-CSF peaked in the second day of the treatment in 94.3% of the patients; which is correlated with the dose administered. The overall response rate was 48.6%; whereas it was found to be 63.6% for patients with multidose treatment. The minor side effects of mainly lower urinary symptoms were noted except one patient who experienced transient grade 3 lymphopenia. A phase III single arm study is ongoing by using this treatment in patients who were unresponsive to BCG therapy and refused cystectomy (NCT02365818). Meanwhile, a recent in vitro study has shown that BC tissue-specific adenovirus improved the antitumor efficacy of radiotherapy.[60] Thus, it is rational to consider that efficiency of anti-tumor treatments such as immune checkpoint inhibitors or chemotherapy might be increased with the addition of oncolytic virus therapy to these treatment alternatives.
Another application is use of a genetically engineered adenovirus for producing interferon α2b (IFN-α2b) for stimulating the local immune system.[61] In a phase I study, Dinney et al.[62] used intravesical recombinant adenovirus mediated IFN-α2b gene therapy in 17 BCG unresponsive patients. Of 14 patients treated with adequate dosage, 6 (43%) patients experienced complete response with an average duration of 31 months, whereas 2 patients were disease free at the last follow-up. They also aimed to augment the clinical response by giving an additional dosage on day 4.[63] Overall, 5 of 7 patients were disease free with a minimum follow-up of 23.9 months; including 2 patients receiving an additional course of treatment at 3 months. Furthermore, a recent study including 40 patients with BCG refractory or relapsing disease with a primary end point as recurrence- free survival revealed that 35% (14/40) of the patients remained free of HG recurrence at 12 months after the initiation of the treatment.[64] Of 11 patients with long-term (3 years) data, 8 remained free of disease during a period ranging between 15 and ≥36 months. Ninety-seven percent of the patients experienced adverse events, for majority (78%) of them adverse events were transient and classified as either grade 1 or 2. Meanwhile, a phase III multicenter study is also ongoing (NCT02773849).
Nanotechnology is already being used for the new drug delivery systems that increase the efficiency of the drug with controlled release of the substances leading to minimized side effects. As an example, nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is widely used in metastatic breast and pancreas cancers with its facilitated drug delivery mechanism into tumor cells with albumin receptor mediated transport. In a phase I trial McKiernan et al.[65] used nab-paclitaxel in 18 patients with high risk NMIBC ineligible for cystectomy in patients who failed at least one (median 2) course of intravesical regimen as an induction therapy. The authors reported a complete response in 5 (28%) patients and no patient experienced significant side effects. At the phase II trial the same group reported the outcomes of 28 patients who experienced at least one course of failed BCG treatment.[66] Of these patients, 10 (37.5%) exhibited a complete response that was durable after 6 months of maintenance therapy. Overall, 19 of 28 patients remained with intact bladder after a mean follow-up of 21 months and RC free survival was calculated as 74%, 74% and 55% for 12, 24 and 36 months, respectively. Meanwhile, other products of nanotechnology such as paclitaxel and docetaxel loaded into hydrophobically derivatized hyperbranched polyglycerols or mucoadhesive polymers for delivering the drugs in a more efficient way to endothelium is currently being under investigation.[67,68]
Checkpoint inhibitors are one of the breakthroughs in BC and currently atezolizumab and nivolumab, monoclonal antibodies targeting PD-L1 on both the tumor and T cells have become almost the standard of care for patients with advanced and metastatic BC which progressed after platinum-based therapy.[69,70] Currently some studies are recruiting patients using different regimens such as atezolizumab (NCT02792192, NCT02844816) and pembrolizumab (NCT02625961) and their outcomes are awaited.
Vaccines are systemic immunomodulators that aim the recognition of specific tumor- associated proteins. Currently three systemic immunomodulators are under investigation (Vesigenurtacel-L [HS-410], ALT-801 and PANVAC) and no clinical data is yet available. Among these, vesigenurtacel-L is made of an allogeneic cell line, has been selected for high expression of bladder tumor antigens to induce immune response over CD+ cytotoxic t-lymphocytes.[71] Despite lack of any clinical data, a phase 1 study has revealed an increased rate of tumor infiltrating lymphocytes (60% vs. 16%) after intradermal vaccine injections combined with BCG treatment.[72] Meanwhile, ALT-801 is a fusion molecule between IL-2 and t-cell receptor that enhances immune response by displaying target peptide/HLA complexes.[73] The ongoing phase Ib/II study (NCT01625260) basically evaluates systemic administration of ALT-801 with GEM. The primary end point is clinical efficacy which will be evaluated at 13 weeks; whereas duration of response, progression- free and event-free survival rates are to be evaluated in up to 3 years. Lastly, PANVAC is a vector based vaccine that contains tumor associated antigens (carcinoembryonic antigen and mucin-1) and costimulatory proteins (B7-1, ICAM-1, lymphocyte function- associated antigen 3 [LFA-3] and aim to boost CD4 and CD8 dependent antigenic response. The ongoing phase II RCT (NCT02015104) aims to compare PANVAC in combination with BCG versus BCG alone. The primary outcome measure is DFS and the study is estimated to be completed soon.
Radiotherapy combined with chemotherapy is being used increasingly in muscle-invasive bladder cancer with certain indications. This is particularly attractive for patients with NMIBC for avoiding RC. However, a multi-intuitional RCT in the UK revealed no advantage of adding radiotherapy in terms of progression- free survival over conservative treatments defined as observation for single tumors and intravesical therapy (BCG or MMC) for multiple tumors or CIS.[74] Meanwhile, a non-randomized trial has shown comparable outcomes for RC as 89%, 19%, and 80% complete response, 5-year progression and bladder preservation rates, respectively.[75] The ongoing RTOG-0926 trial (NCT00981656) comparing 61.2 Gy radiation plus cisplatin or 5-FU/MMC in patients with recurrent disease after BCG treatment in a randomized-controlled study will provide conclusive evidence to this debate.
Conclusion
In this review, we have overviewed some alternative treatment options in NMIBC. Up to date, numerous molecules have been tested as an alternative to BCG. However, none of them has been found to be superior compared to BCG. Nevertheless, they have a role in patients in whom BCG is contraindicated and patients with BCG failure, those who have comorbidities or unwilling to undergo RC. Extensive research is being done to provide more effective therapies and many of them are under clinical assessment. Hopefully, less toxic options with better efficacy will be available in the future. Until this time, RC seems to be the best treatment alternative to achieve long-term disease-free survival.
Footnotes
Peer-review: This manuscript was prepared by the invitation of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept – Ö.Ş., Y.L.; Design – Ö.Ş., Y.L.; Supervision – Y.L.; Data Collection and/or Processing – Ö.Ş.; Analysis and/or Interpretation – Ö.Ş., Y.L.; Literature Search – Ö.Ş.; Writing Manuscript – Ö.Ş., Y.L.; Critical Review – Ö.Ş., Y.L.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. https://doi.org/10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Greiman AK, Rosoff JS, Prasad SM. Association of Human Development Index with global bladder, kidney, prostate and testis cancer incidence and mortality. BJU Int. 2017 doi: 10.1111/bju.13875. https://doi.org/10.1111/bju.13875. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. https://doi.org/10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 4.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. https://doi.org/10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int. 2017;119:371–80. doi: 10.1111/bju.13760. https://doi.org/10.1111/bju.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanli O, Dobruch J, Knowles MA, Burger M, Alemozaffar M, Nielsen ME, et al. Bladder cancer. Nat Rev Dis Primers. 2017;3:17022. doi: 10.1038/nrdp.2017.22. https://doi.org/10.1038/nrdp.2017.22. [DOI] [PubMed] [Google Scholar]
- 7.Donin NM, Lenis AT, Holden S, Drakaki A, Pantuck A, Belldegrun A, et al. Immunotherapy for the Treatment of Urothelial Carcinoma. J Urol. 2017;197:14–22. doi: 10.1016/j.juro.2016.02.3005. https://doi.org/10.1016/j.juro.2016.02.3005. [DOI] [PubMed] [Google Scholar]
- 8.Kamat AM, Colombel M, Sundi D, Lamm D, Boehle A, Brausi M, et al. BCG-unresponsive non-muscle-invasive bladder cancer: recommendations from the IBCG. Nat Rev Urol. 2017;14:244–55. doi: 10.1038/nrurol.2017.16. https://doi.org/10.1038/nrurol.2017.16. [DOI] [PubMed] [Google Scholar]
- 9.Malmstrom PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–56. doi: 10.1016/j.eururo.2009.04.038. https://doi.org/10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Catalona WJ, Hudson MA, Gillen DP, Andriole GL, Ratliff TL. Risks and benefits of repeated courses of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol. 1987;137:220–4. doi: 10.1016/s0022-5347(17)43959-0. https://doi.org/10.1016/S0022-5347(17)43959-0. [DOI] [PubMed] [Google Scholar]
- 11.Newling DW, Hetherington J, Sundaram SK, Robinson MR, Kisbenedek L. The use of valrubicin for the chemoresection of superficial bladder cancer -- a marker lesion study. Eur Urol. 2001;39:643–7. doi: 10.1159/000052521. https://doi.org/10.1159/000052521. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000;163:761–7. https://doi.org/10.1097/00005392-200003000-00014. [PubMed] [Google Scholar]
- 13.Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urol Oncol. 2013;31:1635–42. doi: 10.1016/j.urolonc.2012.04.010. https://doi.org/10.1016/j.urolonc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Gardmark T, Carringer M, Beckman E, Malmstrom PU. Randomized phase II marker lesion study evaluating effect of scheduling on response to intravesical gemcitabine in recurrent Stage Ta urothelial cell carcinoma of the bladder. Urology. 2005;66:527–30. doi: 10.1016/j.urology.2005.03.084. https://doi.org/10.1016/j.eururo.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Gontero P, Casetta G, Maso G, Sogni F, Pretti G, Zitella A, et al. Phase II study to investigate the ablative efficacy of intravesical administration of gemcitabine in intermediate-risk superficial bladder cancer (SBC) Eur Urol. 2004;46:339–43. doi: 10.1016/j.eururo.2004.05.001. https://doi.org/10.1016/j.urology.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 16.Bartoletti R, Cai T, Gacci M, Giubilei G, Viggiani F, Santelli G, et al. Intravesical gemcitabine therapy for superficial transitional cell carcinoma: results of a Phase II prospective multicenter study. Urology. 2005;66:726–31. doi: 10.1016/j.urology.2005.04.062. https://doi.org/10.1159/000273461. [DOI] [PubMed] [Google Scholar]
- 17.Porena M, Del Zingaro M, Lazzeri M, Mearini L, Giannantoni A, Bini V, et al. Bacillus Calmette-Guerin versus gemcitabine for intravesical therapy in high-risk superficial bladder cancer: a randomised prospective study. Urol Int. 2010;84:23–7. doi: 10.1159/000273461. https://doi.org/10.1200/JCO.2008.20.8199. [DOI] [PubMed] [Google Scholar]
- 18.Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol. 2010;28:543–8. doi: 10.1200/JCO.2008.20.8199. https://doi.org/10.1002/cncr.24914. [DOI] [PubMed] [Google Scholar]
- 19.Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116:1893–900. doi: 10.1002/cncr.24914. https://doi.org/10.1016/j.urolonc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Joudi FN, Smith BJ, O’Donnell MA. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 2006;24:344–8. doi: 10.1016/j.urolonc.2005.11.026. https://doi.org/10.1016/j.urology.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher BL, Joudi FN, Maymi JL, O’Donnell MA. Impact of previous bacille Calmette-Guerin failure pattern on subsequent response to bacille Calmette-Guerin plus interferon intravesical therapy. Urology. 2008;71:297–301. doi: 10.1016/j.urology.2007.09.050. https://doi.org/10.1016/j.juro.2010.06.147. [DOI] [PubMed] [Google Scholar]
- 22.Nepple KG, Lightfoot AJ, Rosevear HM, O’Donnell MA, Lamm DL. Bacillus Calmette-Guerin with or without interferon alpha-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol. 2010;184:1915–9. doi: 10.1016/j.juro.2010.06.147. https://doi.org/10.1016/j.urology.2009.06.112. [DOI] [PubMed] [Google Scholar]
- 23.Laudano MA, Barlow LJ, Murphy AM, Petrylak DP, Desai M, Benson MC, et al. Long-term clinical outcomes of a phase I trial of intravesical docetaxel in the management of non-muscle-invasive bladder cancer refractory to standard intravesical therapy. Urology. 2010;75:134–7. doi: 10.1016/j.urology.2009.06.112. https://doi.org/10.1007/s00345-009-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlow LJ, McKiernan JM, Benson MC. The novel use of intravesical docetaxel for the treatment of non-muscle invasive bladder cancer refractory to BCG therapy: a single institution experience. World J Urol. 2009;27:331–5. doi: 10.1007/s00345-009-0377-1. https://doi.org/10.1186/s12885-015-1338-2. [DOI] [PubMed] [Google Scholar]
- 25.Tamura K, Kikuchi E, Konno T, Ishihara K, Matsumoto K, Miyajima A, et al. Therapeutic effect of intravesical administration of paclitaxel solubilized with poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate) in an orthotopic bladder cancer model. BMC Cancer. 2015;15:317. doi: 10.1186/s12885-015-1338-2. https://doi.org/10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–80. doi: 10.1101/gad.1772909. https://doi.org/10.18632/oncotarget.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delto JC, Kobayashi T, Benson M, McKiernan J, Abate-Shen C. Preclinical analyses of intravesical chemotherapy for prevention of bladder cancer progression. Oncotarget. 2013;4:269–76. doi: 10.18632/oncotarget.852. https://doi.org/10.1016/S0022-5347(17)38406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seraphim LA, Perrapato SD, Slocum HK, Rustum YM, Huben RP. In vitro study of the interaction of doxorubicin, thiotepa, and mitomycin-C, agents used for intravesical chemotherapy of superficial bladder cancer. J Urol. 1991;145:613–7. doi: 10.1016/s0022-5347(17)38406-9. https://doi.org/10.1111/j.1464-410X.2008.07571.x. [DOI] [PubMed] [Google Scholar]
- 29.Hadaschik BA, ter Borg MG, Jackson J, Sowery RD, So AI, Burt HM, et al. Paclitaxel and cisplatin as intravesical agents against non-muscle-invasive bladder cancer. BJU Int. 2008;101:1347–55. doi: 10.1111/j.1464-410X.2008.07571.x. https://doi.org/10.1016/j.urolonc.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Chen CH, Yang HJ, Shun CT, Huang CY, Huang KH, Yu HJ, et al. A cocktail regimen of intravesical mitomycin-C, doxorubicin, and cisplatin (MDP) for non-muscle-invasive bladder cancer. Urol Oncol. 2012;30:421–7. doi: 10.1016/j.urolonc.2010.06.012. https://doi.org/10.1016/j.urolonc.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui MR, Grant C, Sanford T, Agarwal PK. Current clinical trials in non-muscle invasive bladder cancer. Urol Oncol. 2017;35:516–27. doi: 10.1016/j.urolonc.2017.06.043. https://doi.org/10.1200/JCO.2011.39.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lammers RJ, Witjes WP, Janzing-Pastors MH, Caris CT, Witjes JA. Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: results from a prospective randomized phase III trial. J Clin Oncol. 2012;30:2273–9. doi: 10.1200/JCO.2011.39.2936. https://doi.org/10.1159/000324331. [DOI] [PubMed] [Google Scholar]
- 33.Yuksel ZS, Buber E, Kocagoz T, Alp A, Saribas Z, Acan NL. Mycobacterial strains that stimulate the immune system most efficiently as candidates for the treatment of bladder cancer. J Mol Microbiol Biotechnol. 2011;20:24–8. doi: 10.1159/000324331. https://doi.org/10.3109/23744235.2015.1055794. [DOI] [PubMed] [Google Scholar]
- 34.Pommier JD, Ben Lasfar N, Van Grunderbeeck N, Burdet C, Laouenan C, Rioux C, et al. Complications following intravesical bacillus Calmette-Guerin treatment for bladder cancer: a case series of 22 patients. Infect Dis. 2015;47:725–31. doi: 10.3109/23744235.2015.1055794. https://doi.org/10.1038/sj.bjc.6690038. [DOI] [PubMed] [Google Scholar]
- 35.Filion MC, Lepicier P, Morales A, Phillips NC. Mycobacterium phlei cell wall complex directly induces apoptosis in human bladder cancer cells. Br J Cancer. 1999;79:229–35. doi: 10.1038/sj.bjc.6690038. https://doi.org/10.1016/j.juro.2014.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morales A, Herr H, Steinberg G, Given R, Cohen Z, Amrhein J, et al. Efficacy and safety of MCNA in patients with nonmuscle invasive bladder cancer at high risk for recurrence and progression after failed treatment with bacillus Calmette-Guerin. J Urol. 2015;193:1135–43. doi: 10.1016/j.juro.2014.09.109. https://doi.org/10.1016/j.juro.2011.11.101. [DOI] [PubMed] [Google Scholar]
- 37.Urech K, Buessing A, Thalmann G, Schaefermeyer H, Heusser P. Antiproliferative effects of mistletoe (Viscum album L.) extract in urinary bladder carcinoma cell lines. Anticancer Res. 2006;26:3049–55. [PubMed] [Google Scholar]
- 38.Elsasser-Beile U, Leiber C, Wetterauer U, Buhler P, Wolf P, Lucht M, et al. Adjuvant intravesical treatment with a standardized mistletoe extract to prevent recurrence of superficial urinary bladder cancer. Anticancer Res. 2005;25:4733–6. [PubMed] [Google Scholar]
- 39.Hendricksen K, Cornel EB, de Reijke TM, Arentsen HC, Chawla S, Witjes JA. Phase 2 study of adjuvant intravesical instillations of apaziquone for high risk nonmuscle invasive bladder cancer. J Urol. 2012;187:1195–9. doi: 10.1016/j.juro.2011.11.101. https://doi.org/10.1016/S0022-5347(01)61607-0. [DOI] [PubMed] [Google Scholar]
- 40.Malmstrom PU, Wijkstrom H, Lundholm C, Wester K, Busch C, Norlen BJ. 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group. J Urol. 1999;161:1124–7. https://doi.org/10.1007/s00345-006-0112-0. [PubMed] [Google Scholar]
- 41.O’Donnell MA, Boehle A. Treatment options for BCG failures. World J Urol. 2006;24:481–7. doi: 10.1007/s00345-006-0112-0. https://doi.org/10.1007/s00345-006-0112-0. [DOI] [PubMed] [Google Scholar]
- 42.Wientjes MG, Badalament RA, Wang RC, Hassan F, Au JL. Penetration of mitomycin C in human bladder. Cancer Res. 1993;53:3314–20. [PubMed] [Google Scholar]
- 43.Slater SE, Patel P, Viney R, Foster M, Porfiri E, James ND, et al. The effects and effectiveness of electromotive drug administration and chemohyperthermia for treating non-muscle invasive bladder cancer. Ann R Coll Surg Engl. 2014;96:415–9. doi: 10.1308/003588414X13946184901001. https://doi.org/10.1308/003588414X13946184901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21:4270–6. doi: 10.1200/JCO.2003.01.089. https://doi.org/10.1200/JCO.2003.01.089. [DOI] [PubMed] [Google Scholar]
- 45.Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol. 2011;60:81–93. doi: 10.1016/j.eururo.2011.04.023. https://doi.org/10.1016/j.eururo.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Arends TJ, Nativ O, Maffezzini M, de Cobelli O, Canepa G, Verweij F, et al. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guerin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. Eur Urol. 2016;69:1046–52. doi: 10.1016/j.eururo.2016.01.006. https://doi.org/10.1016/j.eururo.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Nativ O, Witjes JA, Hendricksen K, Cohen M, Kedar D, Sidi A, et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette-Guerin. J Urol. 2009;182:1313–7. doi: 10.1016/j.juro.2009.06.017. https://doi.org/10.1016/j.juro.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Sadee C, Kashdan E. A model of thermotherapy treatment for bladder cancer. Math Biosci Eng. 2016;13:1169–83. doi: 10.3934/mbe.2016037. https://doi.org/10.3934/mbe.2016037. [DOI] [PubMed] [Google Scholar]
- 49.Soria F, Milla P, Fiorito C, Pisano F, Sogni F, Di Marco M, et al. Efficacy and safety of a new device for intravesical thermochemotherapy in non-grade 3 BCG recurrent NMIBC: a phase I–II study. World J Urol. 2016;34:189–95. doi: 10.1007/s00345-015-1595-3. https://doi.org/10.1007/s00345-015-1595-3. [DOI] [PubMed] [Google Scholar]
- 50.Di Stasi SM, Giannantoni A, Massoud R, Dolci S, Navarra P, Vespasiani G, et al. Electromotive versus passive diffusion of mitomycin C into human bladder wall: concentration-depth profiles studies. Cancer Res. 1999;59:4912–8. [PubMed] [Google Scholar]
- 51.Di Stasi SM, Giannantoni A, Giurioli A, Valenti M, Zampa G, Storti L, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncol. 2006;7:43–51. doi: 10.1016/S1470-2045(05)70472-1. https://doi.org/10.1016/S1470-2045(05)70472-1. [DOI] [PubMed] [Google Scholar]
- 52.Di Stasi SM, Valenti M, Verri C, Liberati E, Giurioli A, Leprini G, et al. Electromotive instillation of mitomycin immediately before transurethral resection for patients with primary urothelial non-muscle invasive bladder cancer: a randomised controlled trial. Lancet Oncol. 2011;12:871–9. doi: 10.1016/S1470-2045(11)70190-5. https://doi.org/10.1016/S1470-2045(11)70190-5. [DOI] [PubMed] [Google Scholar]
- 53.Van Allen EM, Mouw KW, Kim P, Iyer G, Wagle N, Al-Ahmadie H, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4:1140–53. doi: 10.1158/2159-8290.CD-14-0623. https://doi.org/10.1158/2159-8290.CD-14-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan K, Cunningham D, Peckitt C, Barton S, Tait D, Hawkins M, et al. miR-21 expression and clinical outcome in locally advanced pancreatic cancer: exploratory analysis of the pancreatic cancer Erbitux, radiotherapy and UFT (PERU) trial. Oncotarget. 2016;7:12672–81. doi: 10.18632/oncotarget.7208. https://doi.org/10.18632/oncotarget.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cimino GD, Pan CX, Henderson PT. Personalized medicine for targeted and platinum-based chemotherapy of lung and bladder cancer. Bioanalysis. 2013;5:369–91. doi: 10.4155/bio.12.325. https://doi.org/10.4155/bio.12.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–9. doi: 10.1111/cas.13027. https://doi.org/10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burke JM, Lamm DL, Meng MV, Nemunaitis JJ, Stephenson JJ, Arseneau JC, et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012;188:2391–7. doi: 10.1016/j.juro.2012.07.097. https://doi.org/10.1016/j.juro.2012.07.097. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, Wang F, Mao C, Zhang Z, Fu S, Lu J, et al. Effect of combined treatment of radiation and tissue-specific recombinant oncolytic adenovirus on bladder cancer cells. Int J Radiat Biol. 2017;93:174–83. doi: 10.1080/09553002.2017.1231942. https://doi.org/10.1080/09553002.2017.1231942. [DOI] [PubMed] [Google Scholar]
- 59.Adam L, Black PC, Kassouf W, Eve B, McConkey D, Munsell MF, et al. Adenoviral mediated interferon-alpha 2b gene therapy suppresses the pro-angiogenic effect of vascular endothelial growth factor in superficial bladder cancer. J Urol. 2007;177:1900–6. doi: 10.1016/j.juro.2007.01.003. https://doi.org/10.1016/j.juro.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Dinney CP, Fisher MB, Navai N, O’Donnell MA, Cutler D, Abraham A, et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-alpha2b formulated in Syn3 for Bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. J Urol. 2013;190:850–6. doi: 10.1016/j.juro.2013.03.030. https://doi.org/10.1016/j.juro.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Navai N, Benedict WF, Zhang G, Abraham A, Ainslie N, Shah JB, et al. Phase 1b Trial to Evaluate Tissue Response to a Second Dose of Intravesical Recombinant Adenoviral Interferon alpha2b Formulated in Syn3 for Failures of Bacillus Calmette-Guerin (BCG) Therapy in Nonmuscle Invasive Bladder Cancer. Ann Surg Oncol. 2016;23:4110–4. doi: 10.1245/s10434-016-5300-6. https://doi.org/10.1245/s10434-016-5300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, et al. Intravesical rAd-IFNalpha/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol. 2017;35:3410–6. doi: 10.1200/JCO.2017.72.3064. https://doi.org/10.1200/JCO.2017.72.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKiernan JM, Barlow LJ, Laudano MA, Mann MJ, Petrylak DP, Benson MC. A phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette-Guerin refractory nonmuscle invasive bladder cancer. J Urol. 2011;186:448–51. doi: 10.1016/j.juro.2011.03.129. https://doi.org/10.1016/j.juro.2011.03.129. [DOI] [PubMed] [Google Scholar]
- 64.McKiernan JM, Holder DD, Ghandour RA, Barlow LJ, Ahn JJ, Kates M, et al. Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guerin treatment failure. J Urol. 2014;192:1633–8. doi: 10.1016/j.juro.2014.06.084. https://doi.org/10.1016/j.juro.2014.06.084. [DOI] [PubMed] [Google Scholar]
- 65.Mugabe C, Liggins RT, Guan D, Manisali I, Chafeeva I, Brooks DE, et al. Development and in vitro characterization of paclitaxel and docetaxel loaded into hydrophobically derivatized hyperbranched polyglycerols. Int J Pharm. 2011;404:238–49. doi: 10.1016/j.ijpharm.2010.11.010. https://doi.org/10.1016/j.ijpharm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 66.Mugabe C, Hadaschik BA, Kainthan RK, Brooks DE, So AI, Gleave ME, et al. Paclitaxel incorporated in hydrophobically derivatized hyperbranched polyglycerols for intravesical bladder cancer therapy. BJU Int. 2009;103:978–86. doi: 10.1111/j.1464-410X.2008.08132.x. https://doi.org/10.1111/j.1464-410X.2008.08132.x. [DOI] [PubMed] [Google Scholar]
- 67.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. https://doi.org/10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–22. doi: 10.1016/S1470-2045(17)30065-7. https://doi.org/10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 69.Keehn A, Gartrell B, Schoenberg MP. Vesigenurtacel-L (HS-410) in the management of high-grade nonmuscle invasive bladder cancer. Future Oncol. 2016;12:2673–82. doi: 10.2217/fon-2016-0284. https://doi.org/10.2217/fon-2016-0284. [DOI] [PubMed] [Google Scholar]
- 70.Steinberg GD, Shore ND, Karsch LI, Bailen JL, Bivalacqua TJ, Charmie K, et al., editors. Immune response results from vesigenurtacel-l (HS-410) in combination with BCG from a randomized phase 2 trial in patients with non-muscle invasive bladder cancer (NMIBC). ASCO Annual Meeting; 2017 June 02–06, 2017; Chicago, Ilinois. [Google Scholar]
- 71.Wen J, Zhu X, Liu B, You L, Kong L, Lee HI, et al. Targeting activity of a TCR/IL-2 fusion protein against established tumors. Cancer Immunol Immunother. 2008;57:1781–94. doi: 10.1007/s00262-008-0504-7. https://doi.org/10.1007/s00262-008-0504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harland SJ, Kynaston H, Grigor K, Wallace DM, Beacock C, Kockelbergh R, et al. A randomized trial of radical radiotherapy for the management of pT1G3 NXM0 transitional cell carcinoma of the bladder. J Urol. 2007;178:807–13. doi: 10.1016/j.juro.2007.05.024. https://doi.org/10.1016/j.juro.2007.05.024. [DOI] [PubMed] [Google Scholar]


