Abstract
Objective
We investigated the effect of the use of multiparametric prostate magnetic resonance imaging (mp-MRI) on the dissection plan of the neurovascular bundle and the oncological results of our patients who underwent robot-assisted radical prostatectomy.
Material and methods
We prospectively evaluated 60 consecutive patients, including 30 patients who had (Group 1), and 30 patients who had not (Group 2) mp-MRI before robot-assisted radical prostatectomy. Based on the findings of mp-MRI, the dissection plan was changed as intrafascial, interfascial, and extrafascial in the mp-MRI group. Two groups were compared in terms of age, prostate-specific antigen (PSA), Gleason sum scores and surgical margin positivity.
Results
There was no statistically significant difference between the two groups in terms of age, PSA, biopsy Gleason score, final pathological Gleason score and surgical margin positivity. mp-MRI changed the initial surgical plan in 18 of 30 patients (60%) in Group 1. In seventeen of these patients (56%) surgical plan was changed from non-nerve sparing to interfascial nerve sparing plan. In one patient dissection plan was changed to non-nerve sparing technique which had extraprostatic extension on final pathology. Surgical margin positivity was similar in Groups 1, and 2 (16% and 13%, respectively) although, Group 1 had higher number of high- risk patients. mp-MRI confirmed the primary tumour localisation in the final pathology in 27 of of 30 patients (90%).
Conclusion
Preoperative mp-MRI effected the decision to perform a nerve-sparing technique in 56% of the patients in our study; moreover, changing the dissection plan from non-nerve-sparing technique to a nerve sparing technique did not increase the rate of surgical margin positivity.
Keywords: Dissection, magnetic resonance, prostate, prostatectomy
Introduction
The use of robot-assisted radical prostatectomy (RARP) is increasing worldwide, and RARP is being performed for advanced diseases more frequently. Accurate clinical staging before a RARP operation is crucial. The main aim of surgeries today is to achieve a negative surgical margin using a nerve-sparing approach with both open and laparoscopic techniques.[1] Compared to prostate-confined disease, prostate cancer (PCa) with extracapsular extension (ECE) is associated with decreased overall and cancer-specific survival after radical prostatectomy.[2] The extent of ECE predicts disease recurrence and cancer progression, which in turn affects cancer-specific survival.[3] RARP is advantageous due to its improved visualization and instrument controls, but the lack of tactile feedback is a serious drawback when dissecting the neurovascular bundle (NVB) and adjacent tissues around the tumor. Extensive resection of the NVB or extrafascial dissection are associated with high risk for erectile dysfunction and compromised continence. On the other hand, preservation of the NVB without preliminary evaluation of the extent and localization of the tumor may lead to residual tumor tissue at the bundle and fascial sites. For this reason, it is a common practice to peroperatively resect the cavernous nerves when ECE is suspected to achieve negative margins.[4]
The basic methods of clinical staging of prostate cancer are digital rectal examination and transrectal ultrasound (TRUS), which detect ECE with low accuracy. Preliminary multiparametric prostate magnetic resonance imaging (mp-MRI) could be a better method of imaging and planning for the dissection of NVB. mp-MRI localizes high-volume and high-grade PCa tumor areas more accurately than conventional magnetic resonance imaging (MRI).[5–9] It is estimated that mp-MRI detects ECE with 48–76% accuracy.[4,10–14] Walz et al.[15] detailed the anatomy of the prostate and adjacent tissues, especially the fascia and NVB. In light of these studies, preoperative mp-MRI of the prostate may guide surgeons’ dissection plans for RARP. We aimed to report the oncologic results of patients who underwent RARP with or without preoperative mp-MRI.
Material and methods
Between January 2014 and December 2015, we prospectively evaluated 60 consecutive patients who had biopsy-proven PCa and were candidates for RARP. Of these, 30 underwent preoperative mp-MRI (Group 1) and 30 did not (Group 2). The approval of the Institutional Review Board was obtained and an informed consent was obtained from each patient. mp-MRI for each patient in Group I was performed with the use of a phased array body coil at 1.5 Tesla (14 patients) or 3 Tesla (16 patients). Prostate-specific antigen (PSA) levels, clinical stage (determined by digital rectal examination), Gleason score (determined by TRUS-guided prostate biopsy), and identified pathologic specimens were recorded for each patient. Diagnosis of a metastatic disease, previous usage of anti-androgens or androgen blockers, clinical T3–T4 disease and pre-existing erectile dysfunction were exclusion criteria. All images were evaluated for ECE by a single radiologist with 15 years of experience in abdominal MRI. Three techniques (T2W imaging, diffusion-weighted imaging, and dynamic contrast-enhanced MRI) were used for localization of the tumor and its possible ECE.
Eleven patients in Group I underwent mp-MRI before the prostate biopsy, and 19 underwent mp-MRI six weeks after the biopsy to reduce inaccurate interpretation of the images due to hemorrhage. The radiologist evaluated the images obtained from mp-MRI to identify lesions and the presence of ECE. Based on the findings, intrafascial (nerve sparing), interfascial (partial nerve sparing), or extrafascial (non-nerve sparing) dissection plans were chosen for the patients in Group 1. If the tumor was not near the prostate capsule or in the half part of the prostate, intrafascial dissection was chosen. If the tumor was near the prostate capsule or had invaded the capsule but did not extend to the NVB, interfascial dissection was chosen. In Group 2, dissection was planned according to digital rectal examinations and preoperative risk was determined according to the D’Amico risk classification. A single surgeon performed all the operations, and RARP was performed in accordance with standard surgical procedures based on widely known publications as previously described.[16] Surgical margin status, ECE, seminal vesicle invasion and localization of the tumor were also determined for the final surgical specimen and subsequently mapped on macroscopic photographs by two dedicated pathologists according to the standards of the International Prostate Consensus Group to demonstrate the extent and multifocality of the tumors.[17]
Statistical analysis
The two groups were compared in terms of mean age, PSA levels, surgical marginal status, and Gleason sum scores for the biopsy and final pathology. A univariate analysis of patients’ age, PSA, Gleason sum scores of biopsy, and prostatectomy specimens was performed using the Mann-Whitney U test and T test. Statistical Package for the Social Sciences (SPSS Inc.; Chicago, IL, USA) version 15 was used for statistical analysis with the two-tailed significance level set at p<0.05.
Results
In total, 60 patients were enrolled in the study, 30 of whom underwent preoperative mp-MRI evaluation (Group 1) and 30 of them who did not (Group 2). The mean age was 62.1 years in Group 1 and 61.9 years in Group 2. The mean PSA level was 10.57 ng/mL in Group 1 and 8.16 ng/mL in Group 2. Digital rectal examination findings were abnormal in 15 patients of Group 1 and in 13 patients of Group 2 who had not clinical T3 or T4 disease. Table 1 shows the demographic characteristics of the patients. There were no statistically significant differences in terms of age, PSA, biopsy, or final pathologic Gleason sum scores between the two groups.
Table 1.
Demographics of the two groups
| Group 1 (n=30) | Group 2 (n=30) | p | |
|---|---|---|---|
| Age (year) | 62.1 | 61.9 | 0.239 |
| PSA (ng/mL) | 10.57 | 8.16 | 0.160 |
| Positive surgical margin (n, %) | 5 (16%) | 4 (13%) | 0.720 |
| Biopsy Gleason sum | 7.06 | 6.50 | 0.473 |
| Prostatectomy Gleason sum | 7.06 | 6.53 | 0.090 |
PSA: prostate-specific antigen
There were 5 patients in Group 1 with a positive surgical margin of which four of them involved to nerve sparing route and one of them were focal area and three of them were significant. Four patients in Group 2 had positive focal surgical margins. Statistically, there were no significant differences between the two groups in terms of surgical margin positivity (p>0.05). Before the operation, patients were stratified according to the D’Amico risk classification as low, intermediate, or high risk (Table 2).
Table 2.
D’Amico risk classification of the groups
| Preoperative | Low risk | Intermediate risk | High risk |
|---|---|---|---|
| Group 1 (n=30) | 7 (23%) | 13 (43%) | 10 (33%) |
| Group 2 (n=30) | 14 (46%) | 14 (46%) | 2 (6%) |
mp-MRI predicted 90% of the primary index tumor localization in the final pathology of the specimens. After the final pathology, ECE (pathologic T3a) was reported in eight patients from Group 1 who had abnormal digital rectal examination findings, and three out of five patients from Group 2 had abnormal digital rectal examination findings (Table 3). The initial planned dissection technique was changed to the nerve-sparing technique (intra- or interfascial) following mp-MRI evaluation for at least one side for 17 (56%) of the 30 patients. The initial planned dissection technique was changed to the non-nerve sparing technique (extrafascial) following mp-MRI evaluation for at least one side for 1 (3%) of the 30 patients. The mp-MRI and final pathologic ECE findings and their sensitivity, specificity, positive, and negative predictive values are presented in Table 4.
Table 3.
Pathological stages of the groups according to final pathology
| Pathologic stage | T2 | T3 |
|---|---|---|
| Group 1 (n=30) | 18 (60%) | 12 (40%) |
| Group 2 (n=30) | 25 (83%) | 5 (17%) |
Table 4.
Comparison of ECE and mp-MRI with final pathology in Group 1
| Final pathology-ECE | Final Pathology-No ECE | |
|---|---|---|
| mp-MRI-ECE | 2 | 1 |
| mp-MRI-No ECE | 6 | 21 |
| Sensitivity (%) | 25 | ---- |
| Specificity (%) | --- | 95 |
| Positive Predictive Value (%) | 66 | --- |
| Negative Predictive Value (%) | --- | 77 |
mp-MRI: multiparametric prostate magnetic resonance imaging; ECE: extracapsular extension
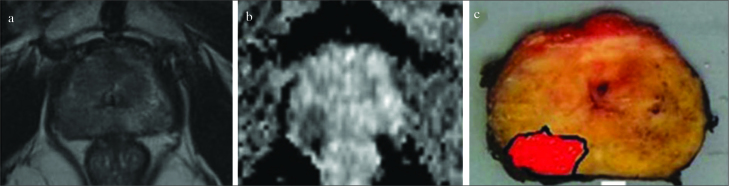
The mp-MRI and pathologic images of one case in which the dissection plan was changed to the nerve-sparing technique after mp-MRI are shown in Figure 1. According to the final pathology results, 46% of the patients in Group 1 and 50% of the patients in Group 2 had higher Gleason sum scores (Tables 5 and 6).
Figure 1. a–c.
Example of dissection plan that was changed according to the nerve-sparing (interfascial) technique. Images of a 58-year-old man with a PSA level of 5.8 ng/mL and biopsy (Gleason score 4+4=8) performed for five of the ten cores on the right. Digital rectal examination revealed abnormalities on the right side of the prostate. Non-nerve sparing dissection was initially planned on the right side in accordance with the biopsy. MRI showed no involvement of the neurovascular bundles or seminal vesicles but revealed a tumor adjacent to the capsule. MRI images of T2-weighted (a) and diffusion phases (b) were focused on the tumor at the right posterolateral gland. The right interfascial dissection plan was performed, and the final pathology (c) had a Gleason score of 4+4, confirming the images that showed no extracapsular extension on the right posterolateral gland with negative surgical margins
Table 5.
Biopsy and prostatectomy Gleason scores in Group1
| Group1 | Final Gleason score | Nerve-sparing surgery | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Biopsy GS | No. of Patients | 3+3 | 3+4 | 4+3 | 4+4 | 4+5 | 5+4 | 5+5 | Initial plan | Active surgery |
| 3+3 | 9 | 5 | 3 | 1 | -- | -- | -- | -- | 8 | 1 |
|
| ||||||||||
| 3+4 | 9 | -- | 4 | 5 | -- | -- | -- | -- | 2 | 7 |
| 4+3 | 5 | -- | 1 | 3 | -- | 1 | -- | -- | 1 | 4 |
| 4+4 | 4 | -- | 1 | 1 | 2 | -- | -- | -- | 1 | 3 |
| 4+5 | 3 | -- | 1 | 1 | -- | -- | 1 | -- | 1 | 2 |
Table 6.
Biopsy and prostatectomy Gleason scores in Group 2
| Group 2 | Final Gleason score | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Biopsy GS | No. of Patients | 3+3 | 3+4 | 4+3 | 4+4 | 4+5 |
| 3+3 | 15 | 8 | 7 | -- | -- | -- |
|
| ||||||
| 3+4 | 9 | 1 | 2 | 5 | 1 | -- |
| 4+3 | 2 | 1 | 1 | -- | -- | -- |
| 3+5 | 2 | -- | -- | 1 | -- | 1 |
| 4+5 | 1 | -- | -- | -- | -- | 1 |
Discussion
Radical prostatectomy is the most common treatment for clinically localized PCa, and the number of RARP procedures has been rapidly increasing all over the world since its introduction in 2000.[18] The neurovascular bundles that mediate erectile function and continence lay posterolateral to the prostatic capsule and adjacent tissues, and they are the most important anatomic landmarks to spare in RARP for the achievement of the trifecta outcomes. According to the latest studies, three different dissection plans can be utilized, two of which are for nerve-sparing techniques (intrafascial and interfascial) that can be used around the periprostatic region.[15] ECE predicts the survival and influences the management of localized PCa, and before surgery, the patient’s ECE must be identified in order to make a decision regarding whether to spare the neurovascular bundles. Partin tables were constructed to assess the risk of ECE before surgery based on the patient’s PSA level, biopsy Gleason score, and clinical stage.[19] ECE can also be predicted based on the results of prostate biopsy, such as the percentage of biopsy cores involved with cancer and perineural invasion.[20] However, these models are not completely reliable predictors of ECE.
Surgeons performing open radical prostatectomy (ORP) demonstrate that tactile feedback enables intraoperative decisions to be made regarding dissection plans for controlling cancer, reducing positive surgical margins, and maintaining good postoperative erectile function.[21] During RARP, a lack of tactile feedback is the main disadvantage of dissecting the neurovascular bundles that are adjacent to the tumor. Prostate MRI has been used for staging for many years. Due to the implementation of diffusion-weighted and dynamic contrast-enhanced MRI, tumor diagnosis and localization has been improved. mp-MRI may be a good tool for detecting prostatic capsule invasion before RARP. Current specialized mp-MRI techniques have been shown to accurately detect the extent and localization of tumors, thus helping the surgeon to choose an appropriate dissection plan for radical prostatectomy.[5–9,22–24] In addition, mp-MRI has been reported to have a sensitivity of 35–58% and a specificity of 89–92% for predicting ECE.[4,5,9–11] In our study, the specificity, sensitivity, and positive and negative predictive values of ECE were 95%, 25%, 66%, and 77%, respectively. We evaluated the patients who had RARP with or without preoperative MRI-guided dissection planning. To our knowledge, this is the first study to compare the oncologic results of RARP performed with and without mp-MRI-guided dissection techniques.
In our study, for the patients in Group 1, who underwent mp-MRI, suspicion of ECE was the main factor influencing dissection plans. For the patients in Group 2, dissection plans were determined based on digital rectal examination findings, and the D’Amico risk classification. McClure et al.[25] reported a series of cases involving mp-MRI-guided preservation of the NVB in RARP. In their study, the initial dissection plan was changed for 28 of 104 patients (27%) based on MRI findings. Radkte et al.[26] reported changes in the dissection plans for 41 of 132 patients (31%). However, in our study, the initial plan was changed for 18 of 30 patients (60%). The higher percentage of changes in our study may be due to our more limited study population. In our study, there were more intermediate- and high-risk patients than in the previous two studies, which meant that mp-MRI was necessary more often; in the previous two studies, nerve-sparing surgery was more appropriate given the patients’ risk groups. In our study, we changed the dissection plan from non-nerve-sparing to nerve-sparing for 17 of 30 patients (56%), while McClure et al.[25] reported this change for 16% (n=17) of their patients. They also reported that the surgical technique was changed from nerve-sparing to non-nerve-sparing for 10% of their 11 patients while in our study we made this change for only one patient (3%). In McClure et al.[25] study, no positive surgical margins were reported in patients for which the surgical plan was changed to nerve-sparing based on the results of mp-MRI. However, in our study, five patients had positive surgical margins (16%)-one of which was focal involvement of the NVB dissection plan. Of these, three patients were in the high-risk group.
MRI has been shown to improve the accuracy of the surgeon’s decision to preserve or resect the NVB during ORP. Schlomm et al.[27] reported in their retrospective study that intraoperative frozen section biopsy of NVB has positive surgical margin less often for all stages (15% vs. 22%) and for pathologic T3 (21% vs. 32%). Biopsy was positive on that side of the NVB, another wide resection of NVB was performed. According to Radtke et al.[26], in high-risk patients, ECE score based on mp-MRI is an independent predictor of pT3 and may help surgeons plan for radical prostatectomy with oncologic security by intraoperatively obtaining and freezing sections from patients. In contrast, another study that focused on intraoperative frozen sections as a way to spare NVB demonstrated a high rate of false negatives, potentially resulting in unjustified NVB resection, time consumption, and no significant effect on prognosis.[28,29] We did not obtain routine intraoperative frozen sections from our high-risk patients, but for a few patients we performed surgical margin biopsy, which was negative for all patients. This technique is appropriate only for certain high-risk patients. In addition, the cost- effectiveness of intraoperative frozen sections must be studied in the future.
Five patients (14%) in Group 1 whose dissection plans were changed to nerve-sparing had positive surgical margins, only one of which was focal. One patient underwent adjuvant radiotherapy after 18 months, and the others are undergoing close follow-up.
Previous studies reported that mp-MRI had 73–95% specificity regarding the differentiation of T2 from T3 disease.[11,25,30] Our results showed that mp-MRI had 95% specificity, 80% positive predictive value for ECE, and 91% specificity regarding primary tumor localization. A previous study reported that the positive predictive value of mp-MRI for ECE was highest in the intermediate- and high-risk groups of patients.[11] Our study confirmed this finding with a positive predictive value of 91%. The final pathology reports were highly concordant with the primary lesions reported by mp-MRI. Smaller (<5 mm) cancer foci reported in the final pathology, were not detected and reported by the mp-MRI, in concordance with the literature. Previous studies also concluded that prostate MRI is highly correlated with final pathology among intermediate- and high-risk patients.[11,25]
This is a preliminary study showing the efficacy of preoperative imaging for surgical planning. The study has several limitations. This is a prospective non-randomized study. Our study had a limited patient population, and thus it has a lower statistical power. For some of our patients, mp-MRI was performed after prostate biopsy, which may have resulted in hemorrhagic distortion and thus a misleading Prostate Imaging Reporting and Data System (PIRADS) score. Since our follow- up period was rather limited, we could not determine the rate of patients’ long-term biochemical recurrence. We performed a two-arm study with proportionate numbers of patients in each risk group. For instance, Group 2 had a larger number of patients at low risk preoperatively (46%) than Group 1 (20%). Regarding the final pathology, Group 1 had 18 (53%), and Group 2 had 25 (83%) patients with pathologic T2. Based on this, it seems as though there was a bias toward performing mp-MRI in the intermediate- and high-risk groups, but we offered mp-MRI to all our patients regardless of their preoperative risk group. All final pathologies were investigated in accordance with the International Society of Urological Pathology (ISUP) in our hospital’s pathology clinic, but the preoperative biopsy pathologies were not standard, and some were performed at other centers.
The functional results of the two groups were not reported in this manuscript because of the limited data and short follow-up period, but we observed early continence and erectile function rates in the third month in the MRI-guided operation group.
In conclusion, we recommend performing mp-MRI before RARP for intermediate- and high-risk patients as it can be used to determine which dissection technique to use during RARP to achieve the ultimate trifecta without compromising the short-term oncological outcome.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of İstanbul Bilim University School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – H.H.T.; Design – H.H.T., F.A.; Supervision – H.K., N.C.B., F.A.; Resources – Ö.A., H.H.T.; Materials – Ö.A., H.H.T.; Data Collection and/or Processing – Ö.A., H.H.T.; Analysis and/or Interpretation – Ö.A., H.H.T., H.K., N.C.B., F.A.; Literature Search – Ö.A., H.H.T.; Writing Manuscript – Ö.A., H.H.T.; Critical Review – H.K., N.C.B., F.A.; Other – Ö.A., H.H.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473–85. doi: 10.1002/pros.2990040506. https://doi.org/10.1002/pros.2990040506. [DOI] [PubMed] [Google Scholar]
- 2.Tollefson MK, Karnes RJ, Rangel LJ, Bergstralh EJ, Boorjian SA. The impact of clinical stage on prostate cancer survival following radical prostatectomy. J Urol. 2013;189:1707–12. doi: 10.1016/j.juro.2012.11.065. https://doi.org/10.1016/j.juro.2012.11.065. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Carmichael MJ, Pizov G, Walsh PC. Influence of capsular penetration on progression following radical prostatectomy: a study of 196 cases with long-term followup. J Urol. 1993;150:135–41. doi: 10.1016/s0022-5347(17)35415-0. https://doi.org/10.1016/S0022-5347(17)35415-0. [DOI] [PubMed] [Google Scholar]
- 4.Roethke MC, Lichy MP, Kniess M, Werner MK, Claussen CD, Stenzl A, et al. Accuracy of preoperative endorectal MRI in predicting extracapsular extension and influence on neurovascular bundle sparing in radical prostatectomy. World J Urol. 2013;31:1111–6. doi: 10.1007/s00345-012-0826-0. https://doi.org/10.1007/s00345-012-0826-0. [DOI] [PubMed] [Google Scholar]
- 5.Chandra RV, Heinze S, Dowling R, Shadbolt C, Costello A, Pedersen J. Endorectal magnetic resonance imaging staging of prostate cancer. ANZ J Surg. 2007;77:860–5. doi: 10.1111/j.1445-2197.2007.04259.x. https://doi.org/10.1111/j.1445-2197.2007.04259.x. [DOI] [PubMed] [Google Scholar]
- 6.Kozlowski P, Chang SD, Jones EC, Berean KW, Chen H, Goldenberg SL. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis--correlation with biopsy and histopathology. J Magn Reson Imaging. 2006;24:108–13. doi: 10.1002/jmri.20626. https://doi.org/10.1002/jmri.20626. [DOI] [PubMed] [Google Scholar]
- 7.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. https://doi.org/10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Hricak H, Kattan MW, Chen HN, Scardino PT, Kuroiwa K. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology. 2006;238:597–603. doi: 10.1148/radiol.2382041905. https://doi.org/10.1148/radiol.2382041905. [DOI] [PubMed] [Google Scholar]
- 9.Yu KK, Scheidler J, Hricak H, Vigneron DB, Zaloudek CJ, Males RG, et al. Prostate cancer: prediction of extracapsular extension with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology. 1999;213:481–8. doi: 10.1148/radiology.213.2.r99nv26481. https://doi.org/10.1148/radiology.213.2.r99nv26481. [DOI] [PubMed] [Google Scholar]
- 10.Cerantola Y, Valerio M, Kawkabani Marchini A, Meuwly JY, Jichlinski P. Can 3T multiparametric magnetic resonance imaging accurately detect prostate cancer extracapsular extension? Can Urol Assoc J. 2013;7:E699–703. doi: 10.5489/cuaj.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somford DM, Hamoen EH, Futterer JJ, van Basten JP, Hulsbergen-van de Kaa CA, Vreuls W, et al. The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low, intermediate and high risk prostate cancer. J Urol. 2013;190:1728–34. doi: 10.1016/j.juro.2013.05.021. https://doi.org/10.1016/j.juro.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Raskolnikov D, George AK, Rais-Bahrami S, Turkbey B, Siddiqui MM, Shakir NA, et al. The Role of Magnetic Resonance Image Guided Prostate Biopsy in Stratifying Men for Risk of Extracapsular Extension at Radical Prostatectomy. J Urol. 2015;194:105–11. doi: 10.1016/j.juro.2015.01.072. https://doi.org/10.1016/j.juro.2015.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boesen L, Chabanova E, Logager V, Balslev I, Mikines K, Thomsen HS. Prostate cancer staging with extracapsular extension risk scoring using multiparametric MRI: a correlation with histopathology. Eur Radiol. 2015;25:1776–85. doi: 10.1007/s00330-014-3543-9. https://doi.org/10.1007/s00330-014-3543-9. [DOI] [PubMed] [Google Scholar]
- 14.Feng TS, Sharif-Afshar AR, Wu J, Li Q, Luthringer D, Saouaf R, et al. Multiparametric MRI Improves Accuracy of Clinical Nomograms for Predicting Extracapsular Extension of Prostate Cancer. Urology. 2015;86:332–7. doi: 10.1016/j.urology.2015.06.003. https://doi.org/10.1016/j.urology.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179–92. doi: 10.1016/j.eururo.2009.11.009. https://doi.org/10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Hatiboglu G, Teber D, Hohenfellner M. Robot-assisted prostatectomy: the new standard of care. Langenbecks Arch Surg. 2012;397:343–52. doi: 10.1007/s00423-011-0743-5. https://doi.org/10.1007/s00423-011-0743-5. [DOI] [PubMed] [Google Scholar]
- 17.Egevad L, Srigley JR, Delahunt B. International society of urological pathology consensus conference on handling and staging of radical prostatectomy specimens. Adv Anat Pathol. 2011;18:301–5. doi: 10.1097/PAP.0b013e3182211ce0. https://doi.org/10.1097/PAP.0b013e3182211ce0. [DOI] [PubMed] [Google Scholar]
- 18.Hu JC, Gandaglia G, Karakiewicz PI, Nguyen PL, Trinh QD, Shih YC, et al. Comparative effectiveness of robot-assisted versus open radical prostatectomy cancer control. Eur Urol. 2014;66:666–72. doi: 10.1016/j.eururo.2014.02.015. https://doi.org/10.1016/j.eururo.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Eifler JB, Feng Z, Lin BM, Partin MT, Humphreys EB, Han M, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2013;111:22–9. doi: 10.1111/j.1464-410X.2012.11324.x. https://doi.org/10.1111/j.1464-410X.2012.11324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankin A, Tareen B, Lepor H. Side-specific factors associated with extracapsular extension and seminal vesicular invasion in men undergoing open radical retropubic prostatectomy. Prostate Cancer Prostatic Dis. 2009;12:204–8. doi: 10.1038/pcan.2009.2. https://doi.org/10.1038/pcan.2009.2. [DOI] [PubMed] [Google Scholar]
- 21.Hubanks JM, Umbreit EC, Karnes RJ, Myers RP. Open radical retropubic prostatectomy using high anterior release of the levator fascia and constant haptic feedback in bilateral neurovascular bundle preservation plus early postoperative phosphodiesterase type 5 inhibition: a contemporary series. Eur Urol. 2012;61:878–84. doi: 10.1016/j.eururo.2011.11.046. https://doi.org/10.1016/j.eururo.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 22.desouza NM, Reinsberg SA, Scurr ED, Brewster JM, Payne GS. Magnetic resonance imaging in prostate cancer: the value of apparent diffusion coefficients for identifying malignant nodules. Br J Radiol. 2007;80:90–5. doi: 10.1259/bjr/24232319. https://doi.org/10.1259/bjr/24232319. [DOI] [PubMed] [Google Scholar]
- 23.Scheidler J, Hricak H, Vigneron DB, Yu KK, Sokolov DL, Huang LR, et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging--clinicopathologic study. Radiology. 1999;213:473–80. doi: 10.1148/radiology.213.2.r99nv23473. https://doi.org/10.1148/radiology.213.2.r99nv23473. [DOI] [PubMed] [Google Scholar]
- 24.Yu KK, Hricak H, Alagappan R, Chernoff DM, Bacchetti P, Zaloudek CJ. Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: multivariate feature analysis. Radiology. 1997;202:697–702. doi: 10.1148/radiology.202.3.9051019. https://doi.org/10.1148/radiology.202.3.9051019. [DOI] [PubMed] [Google Scholar]
- 25.McClure TD, Margolis DJ, Reiter RE, Sayre JW, Thomas MA, Nagarajan R, et al. Use of MR imaging to determine preservation of the neurovascular bundles at robotic-assisted laparoscopic prostatectomy. Radiology. 2012;262:874–83. doi: 10.1148/radiol.11103504. https://doi.org/10.1148/radiol.11103504. [DOI] [PubMed] [Google Scholar]
- 26.Radtke JP, Hadaschik BA, Wolf MB, Freitag MT, Schwab C, Alt C, et al. The Impact of Magnetic Resonance Imaging on Prediction of Extraprostatic Extension and Prostatectomy Outcome in Patients with Low-, Intermediate- and High-Risk Prostate Cancer: Try to Find a Standard. J Endourol. 2015;29:1396–405. doi: 10.1089/end.2015.0358. https://doi.org/10.1016/j.eururo.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 27.Schlomm T, Tennstedt P, Huxhold C, Steuber T, Salomon G, Michl U, et al. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients. Eur Urol. 2012;62:333–40. doi: 10.1016/j.eururo.2012.04.057. https://doi.org/10.1148/radiol.14140044. [DOI] [PubMed] [Google Scholar]
- 28.Petralia G, Musi G, Padhani AR, Summers P, Renne G, Alessi S, et al. Robot-assisted radical prostatectomy: Multiparametric MR imaging-directed intraoperative frozen-section analysis to reduce the rate of positive surgical margins. Radiology. 2015;274:434–44. doi: 10.1148/radiol.14140044. https://doi.org/10.1111/j.1464-410X.2010.09591.x. [DOI] [PubMed] [Google Scholar]
- 29.Gillitzer R, Thuroff C, Fandel T, Thomas C, Thuroff JW, Brenner W, et al. Intraoperative peripheral frozen sections do not significantly affect prognosis after nerve-sparing radical prostatectomy for prostate cancer. BJU Int. 2011;107:755–9. doi: 10.1111/j.1464-410X.2010.09591.x. https://doi.org/10.1111/j.1464-410X.2010.09599.x. [DOI] [PubMed] [Google Scholar]
- 30.Brajtbord JS, Lavery HJ, Nabizada-Pace F, Senaratne P, Samadi DB. Endorectal magnetic resonance imaging has limited clinical ability to preoperatively predict pT3 prostate cancer. BJU Int. 2011;107:1419–24. doi: 10.1111/j.1464-410X.2010.09599.x. https://doi.org/10.1111/j.1464-410X.2010.09599.x. [DOI] [PubMed] [Google Scholar]