Abstract
Objective
Minimally invasive techniques are increasingly evolving and preferred to reduce surgical induced morbidity and mortality and minimize the challenges of surgical techniques. Especially radical perineal prostatectomy (RPP) includes some challenges like working in a deep and narrow space and challenging ergonomics for the surgeons. Because of these issues open RPP is still performed in experienced centers. In order to reduce these difficulties, robot- assisted radical perineal prostatectomy (r-RPP) is developed. In this study, we report our first clinical results for r-RPP.
Material and methods
Between November 2016 and February 2017, 15 patients underwent r-RPP in our center. Multiparametric magnetic resonance imaging was performed for all patients to exclude locally advanced disease. The patients with chronic obstructive pulmonary disease and locally advanced prostate cancer were not chosen for r-RPP method. The patient was positioned in the exaggerated lithotomy with 15 degrees of Trendelenburg position. After incision and dissection of subcutaneous tissue, dissection was advanced to the margin of posterior recto-urethral muscle fibers. Then a GelPOINT® device was placed and robotic system was docked.
Results
The mean age of the patients was 60.2±7.8 years. The mean body mass index of the patients was 28.8±1.9 kg/m2. Four patients had previous major abdominal surgeries. Preoperative mean prostate specific antigen value was 7.3±2.4 ng/mL. The mean prostate volume was 40.8±12.4 cc. Mean perineal dissection time was 60±10.1 minutes. Mean console time and total operation time was 95±11.3 and 167±37.4 minutes, respectively. The mean time of postoperative catheterization was 8.3±1.7 days. Early continence rate was 40% after urethral catheter removal and at 3rd month of the surgery mean continence rate was 94% for all patients.
Conclusion
We demonstrate that r-RPP is a feasible and efficient method. But still this method needs for further studies in this area.
Keywords: Perineal prostatectomy, radical prostatectomy, robotic surgery
Introduction
The first radical perineal prostatectomy (RPP) was reported in 1905.[1] Belt et al. [2] modified this technique to describe the RPP through the superficial fascia of the anal sphincter and approach from the inferior aspect of the prostate. Millin described retropubic radical prostatectomy (RP) in 1940.[3] Walsh described anatomic retropubic RP in the 1980s. This method has become widespread among urologists.[4] RP is the gold standard treatment for localized prostate cancer (PCa). Laparoscopic RP was first performed by Schuessler in 1991, and the first series was reported in 1997.[5] Although initially long operative times and increased undesired side effects appear to prevent the spread of laparoscopy, laparoscopic RP has been successfully applied in many centers since 1999 for the treatment of localized PCa.[6] Robotic technology was first used in 1994 by Kavoussi et al. to assist laparoscopic surgeries.[7] The first robot-assisted RP operation was performed in Frankfurt in May 2000 by Binder and Kramer.[8] In the USA, this operation was first performed by Menon et al in October 2000 in Detroit.[9] Kaouk et al.[10] described a laparoendoscopic single-site surgery technique and presented a series of single-port robotic RP. Asimakopoulos et al.[11] described retzius sparing robotic RP in 2013 and presented the series. RP techniques are continuously being developed dynamically and rapidly.
Kaouk et al.[12] from Cleveland Clinic, USA, defined the first technique of robot- assisted radical perineal prostatectomy (r-RPP). They experimentally tested this model on cadavers. The same team reported the first clinical experience of four patiens who underwent r-RPP successfully.
With the assistance of Dr. Akca from Dr. Kaouk’s team, we initiated the first r-RPP in our clinic in November 2016. We aimed to report the preoperative, peroperative and postoperative outcomes of our initial experience in respect to this novel technique.
Material and methods
The study was approved by the University of Health Sciences Bakirkoy Dr. Sadi Konuk Research and Training Hospital Ethics Committee and written informed consent was taken from all patients. Between November 2016 and February 2017, we have performed 15 r-RPPs in University of Health Sciences, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Urology Clinic, Istanbul. Multiparametric magnetic resonance imaging (mp-MRI) was performed for all patients to exclude locally advanced disease prior to operation. The patients with chronic obstructive pulmonary disease and locally advanced prostate cancer were not chosen for r-RPP method. But there was an exception for a patient who had locally advanced disease with a Gleason score of 4+4=8 prostatic adenocarcinoma preoperatively. This was the first patient in our r-RPP series. We preferred perineal approach for this patient because of the past surgical history of prostate-sparing radical cystectomy including orthotopic neobladder surgery ten years ago. For that patient, bilateral pelvic lymph node dissection had already been performed during radical cystectomy. The previous surgery was performed via transperitoneal approach and the novel imaging had not demonstrated pathologic lymph nodes. Thus, pelvic lymph node dissection was not planned and performed for this patient.
Surgical technique
The DaVinci XI robotic platform was used in the three arms setting.
Preoperative preparation
We preferred to wait at least one month following the prostate biopsy to perform the surgery. The patient’s all antiaggregant medications were stopped 1 week before the surgery. The bowel preparation was made one day before the operation. The oral intake of the patients was stopped from the night before the surgery. The bowel preparation is very important in this operation. Because, a sterile glove is placed in the rectum for further digital rectal examination and rectal damage may happen during the surgery.
Patient position, initial perineal dissection and single port placement
The patient was brought to the exaggerated lithotomy position with 15 degrees of Trendelenburg. A urethral catheter was placed and the bladder is emptied. A sterile glove was placed in the rectum and the sides of the glove were stitched to the perineal skin. Thus, we aimed to avoid rectal damage by using digital rectal examination during perineal dissections. A 6 cm semilunar incision was made between bilateral tuberculum ischiadicum (Figure 1).
Figure 1.
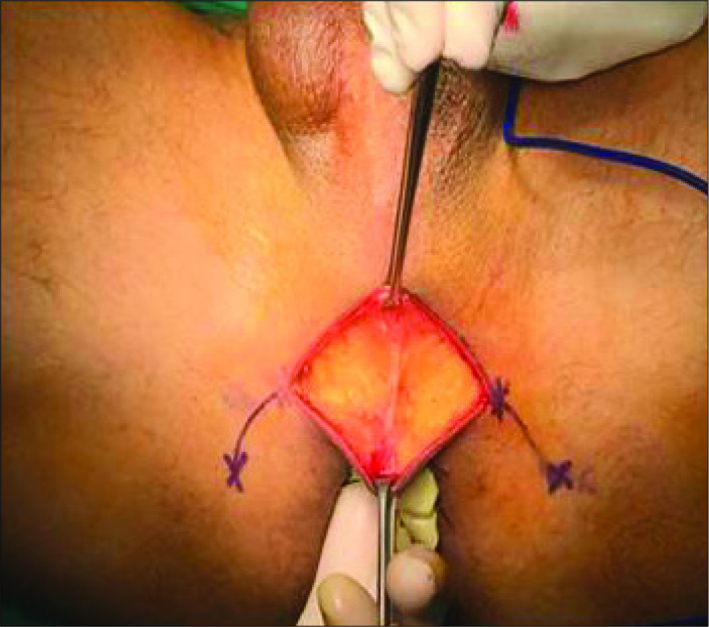
Perineal incision
The posterior fibers of the perineal body were dissected and cut. The bilateral Ischio-rectal fossas were dissected bluntly. The dissection was maintained up to the apex of the prostate from the inferior aspect. The puborectal muscle groups were incised transversely. The perineal dissection was terminated when the dissection margin reached to the membraneous urethra and the apex of the prostate was visible. Subcutaneous tissue laying under the incision borders was dissected deeply over the superficial perineal fascia to place the GelPOINT® (Applied Medical, Rancho Santa Margarita, CA, USA). The tissue hanging down from the upper side of the perineal incision was suspended using a vicryl suture passing under the skin through the bottom of scrotum. That suture was fixed by a Hem-o-Lock® Clip (Teleflex Medical, Research Triangle Park, North Carolina, USA) over the skin to enhance the optical view during robotic dissections. The trocars were placed on GelPOINT® prior to placement of it in to the perineal incision. For the camera, an 8mm robotic trocar was placed at 12 o’clock, and the other two robotic 8mm trocars were placed at 5 and 7 o’clock positions on GelPOINT®. For the assistance, a 10mm trocar was placed at 6 o’clock position (Figure 2). Insuflation was initiated and maintained during the entire procedure with a 10–12 mmHg pressure level. For the camera, 30-degree robotic scope was used.
Figure 2.
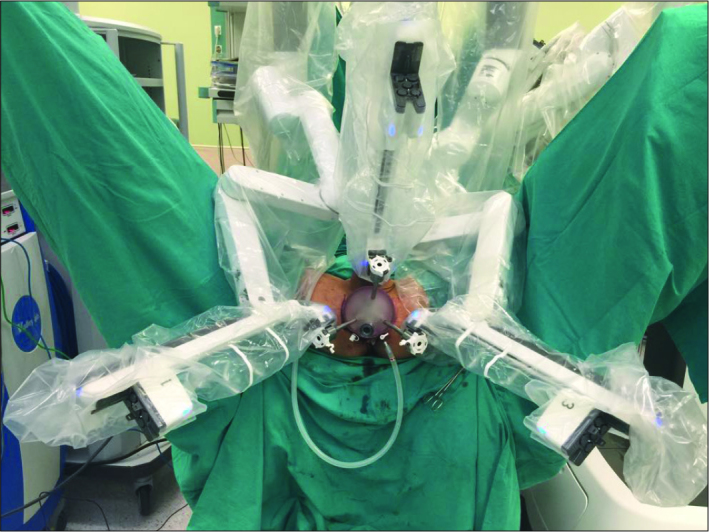
The docking of the three robotic arms
Robotic prostatectomy
Dissection was started from the prostate apex (Figure 3) and extended up to the lateral sides of the prostate (Figure 4) and then deeped inferiorly to reveal the Denonvilliers’ fascia covering the seminal vesicle compartment. Once the Denonvilliers’ fascia was incised vas deferences were exposed, dissected and and cut bilaterally. Seminal vesicles were completely dissected and freed. Then the membranous urethra was dissected and cut. A Hem-o-Lock® Clip was placed on the urethral catheter to keep the ballon inflated inside the bladder and to handle it for further dissections. The catheter then was cut from the urethral side using a laparoscopic scissors. The ‘Veil of the Aphrodite’ was swept up keeping the dorsal venous complex intact. The lateral prostatic pedicles are dissected and controlled using Hem-o-Lock® Clips. After completing the lateral dissections of prostate bilaterally, the bladder neck was identified and incised with monopolar scissors. The urethral catheter balloon was cut by scissors and catheter was pulled out after anterior bladder neck incision. If the prostate had a median lobe, we used a 2.0 vicryl to stretch it for an easy dissection. Once the bladder neck dissection was completed, the robot was undocked and the prostate was removed from the surgical field. Then the robotic system was redocked for the vesico-urethral anastomosis.
Figure 3.
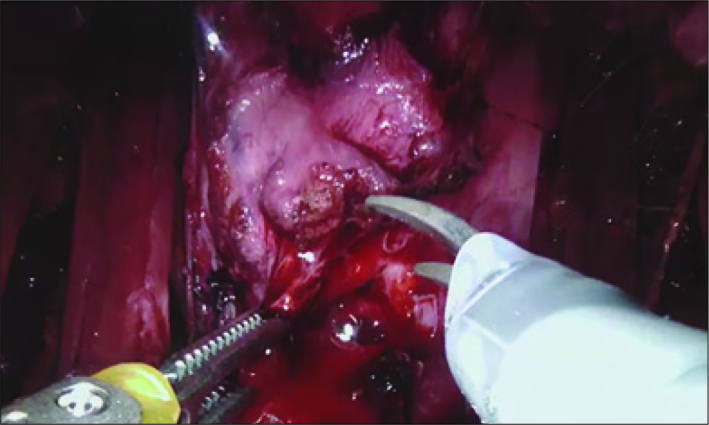
Dissection of the apex
Figure 4.
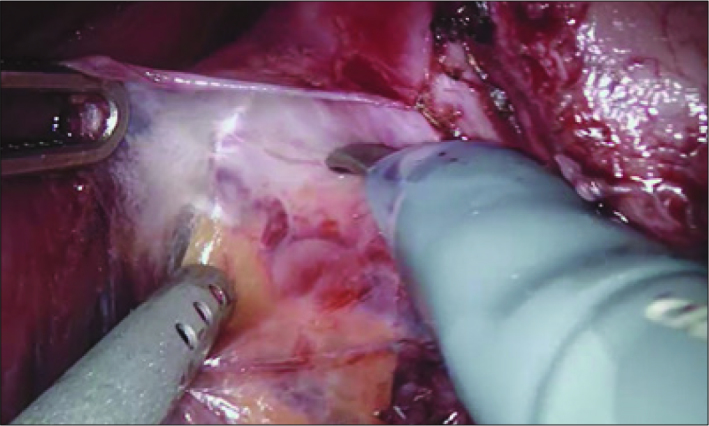
Dissection of lateral lobes
Vesico-urethral anastomosis
The two of 4/0 V-Loc™ (Covidien, Mansfield, MA, USA) sutures was used in a running fashion starting from the retzius side to rectal side of the bladder neck (Figure 5). The first suture was put at 12 o’clock on the bladder neck from outside to inside and then continued to the urethra from inside to outside clockwise down to 6 o’clock. A second barbed suture was used in the same setting but in reverse clockwise direction. While the anastomotic sutures were being tigthened, the insufflation pressure was kept under 10 mmHg to place the bladder neck and the urethra closer to each other which facilitated the approximation. Once the anastomosis was completed a 22 Ch urethral catheter was replaced. The bladder was filled by 200 cc saline to test the anastomosis for leakage. After observing the watertightness of the anastomosis, robotic system was undocked and a Jackson Pratt drain was placed (Figure 6).
Figure 5.
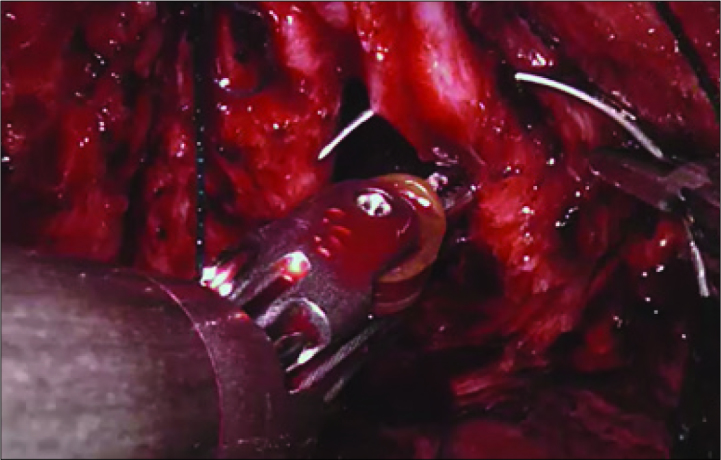
Vesico-urethral anastomosis
Figure 6.
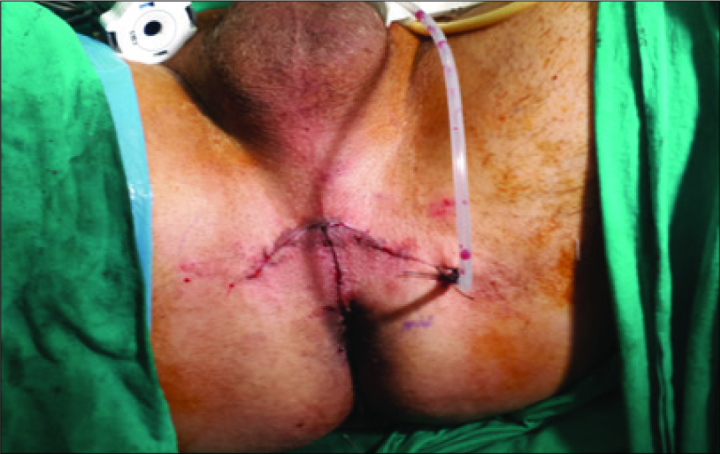
The Jackson Pratt drain is placed
Results
The mean age of the patients was 60±8 years. The mean body mass index (BMI) of the patients was 28.8±1.9 kg/m2. Four patients had previous major abdominal surgeries. Preoperative mean prostate specific antigen value was 7.3±2.4 ng/mL. The mean prostate volume was 40±12 cc in transrectal ultrasonography. The demographic and the preoperative data of the patients are summarized in Table 1.
Table 1.
Preoperative demographic data
| Age (year) | BMI (kg/m2) | Charlson score | Previous Surgery | Prostate Volume (cc) | Preop. PSA (ng/mL) | Preop. Pathology (Gleason Score) | mp-MRI | |
|---|---|---|---|---|---|---|---|---|
| 1st case | 68 | 31 | 2 | Yes | 30 | 8.4 | 4+4 | PIRADS V |
| 2nd case | 67 | 33 | 1 | Yes | 30 | 4.8 | 3+3 | PIRADS IV |
| 3rd case | 54 | 32 | 3 | Yes | 35 | 9.9 | 3+4 | PIRADS II |
| 4th case | 66 | 30 | 1 | Yes | 40 | 7.8 | 3+4 | PIRADS IV |
| 5thcase | 65 | 29 | 1 | Yes | 65 | 6.7 | 3+3 | PIRADS III |
| 6th case | 58 | 29 | 2 | Yes | 60 | 6.8 | 3+3 | PIRADS IV |
| 7th case | 61 | 27 | 3 | Yes | 55 | 11.2 | 3+3 | PIRADS IV |
| 8th case | 46 | 27 | 1 | Yes | 30 | 4.42 | 3+3 | PIRADS III |
| 9th case | 53 | 28 | 1 | Yes | 56 | 3.36 | 3+3 | PIRADS II |
| 10th case | 60 | 27 | 2 | No | 40 | 12.1 | 3+3 | PIRADS III |
| 11th case | 70 | 27 | 1 | Yes | 40 | 8.03 | 3+4 | PIRADS III |
| 12th case | 60 | 29 | 3 | No | 25 | 4.85 | 3+3 | PIRADS II |
| 13th case | 58 | 27 | 1 | No | 40 | 6.6 | 3+4 | PIRADS IV |
| 14th case | 47 | 28 | 1 | Yes | 37 | 6.5 | 3+4 | PIRADS II |
| 15th case | 71 | 29 | 1 | Yes | 30 | 8.2 | 3+3 | PIRADS II |
BMI: body mass index; mp-MRI: multiparametric magnetic resonance imaging; PSA: prostate specific antigen
Mean perineal dissection time was 60±10 minutes. Mean console time and total operation time was 95±11 and 167±37 minutes, respectively. Mean blood loss was 65±20 cc. The final pathology of 3 patients in the RP specimens was upgraded while the pathology of a patient was downgraded. Positive surgical margin was found in three patients. One patient was converted to open surgery because of carbon dioxide retention. Postoperative wound infection developed in the perineal region of a patient and followed up with daily wound care. No additional treatment was needed. There were not any peroperative and postoperative complications for the other patients. The peroperative data are reported in Table 2. The mean postoperative urethral catheterization time was 8±2 days. All patients were continent before the surgery. Immediate continence rate was 40% after urethral catheter removal and it was 67% and 94% at the first and the third months respectively. PSA recurrence was not detected in any patient.
Table 2.
Peroperative and postoperative follow-up data
| OR Time (minute) | Blood loss (cc) | Hospital Stay (day) | Postop. Pathology (Gleason) | Perop. Complication | Postop. Complication | Catheter Removal | |
|---|---|---|---|---|---|---|---|
| 1st case | 255 | 60 | 5 | 4+5 (SM+) | Yes* | No | 14 |
| 2nd case | 230 | 50 | 3 | 4+3 (SM− ) | No | No | 9 |
| 3rd case | 210 | 30 | 3 | 3+4 (SM−) | No | No | 8 |
| 4th case | 190 | 40 | 3 | 3+3 (SM+) | No | Yes** | 7 |
| 5th case | 170 | 55 | 3 | 3+3 (SM−) | No | No | 7 |
| 6th case | 140 | 35 | 2 | 3+3 (SM−) | No | No | 8 |
| 7th case | 150 | 45 | 2 | 3+4 (SM−) | No | No | 9 |
| 8th case | 140 | 40 | 2 | 3+3 (SM+) | No | No | 7 |
| 9th case | 160 | 30 | 2 | 3+3 (SM−) | No | No | 8 |
| 10th case | 140 | 60 | 2 | 4+3 (SM−) | No | No | 7 |
| 11th case | 150 | 50 | 2 | 4+3 (SM−) | No | No | 9 |
| 12th case | 145 | 45 | 2 | 3+3 (SM−) | No | No | 7 |
| 13th case | 155 | 50 | 2 | 3+3 (SM−) | No | No | 8 |
| 14th case | 130 | 60 | 2 | 3+4 (SM−) | No | No | 9 |
| 15th case | 140 | 30 | 2 | 3+3 (SM−) | No | No | 8 |
Converted to open surgery because of carbon dioxide retention,
Wound infection developed in the perineal region.
SM: surgical margin
Discussion
Many urological operations have been done with different techniques with the development of the technology and using urology practice. The RP technique has also developed rapidly. A lot of techniques are defined. R-RPP is a newly developed technique. In order to practice this surgery, it is first necessary to choose patients with localized PCa. Most urologists advocate that patients who have localized PCa do not need definitive treatment.[13] There is not yet a classification that assesses which cancers need or do not need definitive treatment.[14] RPP is a well- defined and successful technique for organ-confined disease. In a study done by Albayrak et al.[15] it was emphasized that the patients who has clinical stage T1b, T1c or T2 diseases should be chosen for RPP. In addition, it has been reported that a predictive instrument such as the Partin normogram can be used to exclude patients at risk for extracapsular spread. We also selected organ- confined PCa cases for r-RPP technique in our series. One patient did not have localized PCa. In this case, preoperative pathology revealed Gleason score of 4 + 4 = 8 adenocarcinoma. The patient had a past surgical history of prostate-sparing radical cystectomy with orthotopic neobladder and extended pelvic lymph node dissection. We performed r-RPP in order to make a more appropriate uretro-ileal anastomosis and to keep the neobladder intact.
Another important issue for the patient selection is the presence of previous major abdominal and pelvic surgeries. Four patients had previous major abdominal surgeries and especially for those patients, perineal approach might be the more appropriate anatomical way to do a RP.
With respect to BMI, obesity might a disadvantage for retropubic approach and it would be more difficult to apply this method. The mean BMI of the patients was 28.8±1.9 kg/m2 in our series. Leung and Melman[16] reported that RPP is an advantage in obese patients. However, robot-assisted laparoscopic transperitoneal RP was reported to have satisfactory results.
Prostate volume has been also reported to be important in RPP technique. Eden et al.[17] have proposed the highest prostate volume of 60 cc for RPP. However, prostate volume is not a major factor if the surgeon’s experience is promosing. The largest prostate volume was 65 cc and the mean prostate volume was 40±12 cc in our series. The surgeon might have difficulty with the increased prostate volume, since dissection of a large volume prostate while working in a narrow field is particularly challenging. Especially in respect to this statement, Da vinci XI robotic system is offering a more comfortable surgery for the surgeons by including the 7 DOF instruments which can easily work in narrow and deep operation fields compared to open RPP. In addition, the insufflation of the surgical site reveals a great exposure for anatomical dissection. The vision is very limited and it is necessary using surgical rigid instruments for retraction within the open RPP technique, which causes severe postoperative pain. The disadvantages of working with CO2 are that venous structures open during pedicle dissection which causes rapid absorption of CO2 and leads to CO2 retention. Thus, the end-tidal CO2 pressures of the patient should be closely monitored in coordination with anesthesia team. If the end-tidal CO2 pressure passes over 33 mmHg, care must be taken and the insufflation pressure must be reduced. Only one patient developed CO2 retention in our series and the operation was continued with open procedure. The patient was followed up in intensive care unit for one day.
Limited number of studies have indicated that the RPP shortens the operation time. In a study conducted by Harris et al.[18], mean duration of open RPP was reported to be 120 minutes. In a study by Hu et al.[19], longevities of laparoscopic exraperitoneal RP, and robot- assisted laparoscopic transperitoneal RP were reported as 246, and 186 minutes, respectively. In our series, mean duration of operation was 167±37 minutes which was comparable to the operating time of standard transperitoneal robotic RP reported in the literature.
In the study performed by Resnick[20], the mean amount of bleeding in open RPP was reported as 150 cc. Martis et al.[21] reported that the mean amount of blood loss in open RRP was 200 cc. In a study by Porpiglia et al.[22], the average amount of bleeding in robot-assisted laparoscopic transperitoneal RP was reported as 200 cc, whereas the amount of bleeding in laparoscopic extraperitoneal RP was reported as 234 cc. The mean amount of bleeding in our series was 62.9±18.5 cc. We found that the bleeding was lower than other methods in the literature which could be explained in as follows; 1) positive CO2 pressure over the venous system pressure in the perineal region might be blocking the venous bleeding, 2) the magnification capability of the robotic scope might be allowing the surgeon to maintain a superior bleeding control in a narrow space. Another advantage of robotic RP using perineal way is that the Santorini’s venous plexus is out of the resection area. Thus, there is no need to dissect the deep dorsal venous complex and to control bleeding.
Although many intraoperative complications can develop, the most frightening complication for urologists is rectal injury. All techniques have the potential to cause rectal injury. In a study conducted by Korman and Harris[23], rectal injury varying between 1–11% was reported in open RPP. They reported no difference in rectal injury between RRP and open RPP techniques. Amorim et al.[24] reported that rectal injury in 2.2–2.8% of laparoscopic extraperitoneal RP operations. Tewari et al.[25] reported the risk of rectal injury as 0.5–1.5% in robot- assisted laparoscopic RP operations. In our series, rectal injury did not happen. At the beginning of the operation, a sterilized glove was placed into rectum and it was fixed to the perianal skin. Thus, it enabled digital rectal examination peroperatively under sterile conditions and a safe dissection was possible. At the end of the operation, a catheter was placed into the rectum and the operation site was filled with the saline, air was then delivered through the rectal catheter and the possible bubbles were watched on the surgical field. By that way, rectal injury was checked. No intraoperative complications were detected in our series. We believe that appropriate patient selection and the experience of the surgeon are important factors.
In a study by Song et al.[26] the duration of median hospitalization for open RPP was reported as 1.1 days. In a study by Ku and Ha[27] the duration of median hospitalization for robot-assisted laparoscopic transperitoneal RP was 4 days and the duration of median hospitalization for laparoscopic extraperitoneal RP was 4 days. In our series, the drainage tube was removed within the first or second day and the patients were discharged within a mean period of 1.61±0.5 days. We think that postoperative pain is less in perineal approach. Absence of abdominal involvement makes it possible for the patient to gain bowel movements earlier. Early mobilization of the patient might also influence early discharge. We also think that our perineal incision is small which can not be seen by the patient, which makes sense in terms of positive psychological effect on the patient.
It is emphasized that the postoperative urethral catheter removal time depends on surgeon’s choice in all procedures. In a study conducted by Asimakopoulos et al.[28], in patients who underwent robot- assisted laparoscopic transperitoneal RP or laparoscopic extraperitoneal RP catheters were removed on postoperative 7.45, and 7.25 days, respectively. In this study, early catheter removal time was recommended. In our series, postoperative mean catheter removing time was 8.3±1.7 days. In a study conducted by Trabulsi et al.[29] continence was recovered in 80, and 62% of the patients who underwent robot-assisted laparoscopic transperitoneal RP or laparoscopic extraperitoneal RP within the first postoperative 3 months, respectively. Steiner et al.[30] reported an early continence rate of 50% following catheter removal after open RPP. Their rate of continence at the third month was 94%. In our study, early continence rate was 40% following urethral catheter removal and continence rate was 94% at 3 months. Urethro-vesical anastomosis is one of the great advantages of this technique. Even though the anastomosis is performed in a narrow field, this technique provides excellent anatomical exposure to perform urethro-vesical anastomosis. We think that high continence rates at an early postoperative period might be associated with the anastomotic technique via perineal approach.
In a study conducted by Comploj and Pycha[31] presence of positive surgical margin was reported in 16–24% of the patients who underwent RPP. In a study conducted by Koutlidis et al.[32] the presence of a positive surgical margin was reported in 10–24% of the patients who underwent robot-assisted laparoscopic transperitoneal RP and in 12–46% of the patients undergoing laparoscopic extraperitoneal RP. In our study, the positive surgical margin was detected in three patients. PSA recurrence was not observed in these three patients within the three months after surgery.
In conclusion, robotic RP through perineal approach is a new and promising method especially for the patients whose choice is definitive surgical treatment for their localized PCa disease. Even it is a small series, we demonstrated that r-RPP is a feasible and effective surgical method. However, further series of randomized controlled studies with higher number of patients are needed to compare this technique with its well defined and accepted counterparts of open retropubic and robotic transperitoneal RP approaches.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Health Science Universty Bakırköy Dr. Sadi Konuk Training and Research Hospital (2017/42).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – O.A., V.T., A.Ş.; Design – A.Ş., İ.Y.; Supervision – V.T., S.Ş., İ.Y., A.İ.T.; Resources – V.T., A.Ş., S.Ş., İ.Y.; Materials – V.T., A.İ.T.; Data Collection and/or Processing – İ.Y., A.Ş.; Analysis and/or Interpretation – V.T., A.Ş., İ.Y., O.A., S.Ş., A.İ.T.; Literature Search – İ.Y., S.Ş.; Writing Manuscript – O.A., V.T., İ.Y.; Critical Review – V.T., A.Ş., O.A., İ.Y., A.Ş., S.Ş., A.İ.T.; Other – V.T., A.Ş., O.A., İ.Y., A.Ş., S.Ş., A.İ.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Young HH. The early diagnosis and radical cure of carcinoma of the prostate. Being a study of 40 cases and presentation of a radical operation which was carried out in four cases. J Urol. 2002;168:914–21. doi: 10.1016/S0022-5347(05)64542-9. https://doi.org/10.1016/S0022-5347(05)64542-9. [DOI] [PubMed] [Google Scholar]
- 2.Belt E, Turner RD. A study of 229 consecutive cases of total perineal prostatectomy for cancer of the prostate. J Urol. 1957;77:62–77. doi: 10.1016/S0022-5347(17)66523-6. https://doi.org/10.1016/S0022-5347(17)66523-6. [DOI] [PubMed] [Google Scholar]
- 3.Millin T. Retropubic prostatectomy. J Urol. 1948;59:267–80. doi: 10.1016/S0022-5347(17)69374-1. https://doi.org/10.1016/S0022-5347(17)69374-1. [DOI] [PubMed] [Google Scholar]
- 4.Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473–85. doi: 10.1002/pros.2990040506. https://doi.org/10.1002/pros.2990040506. [DOI] [PubMed] [Google Scholar]
- 5.Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: Initial short-term experience. J Urology. 1999;50:854–7. doi: 10.1016/S0090-4295(97)00543-8. https://doi.org/10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- 6.Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: Montsouris technique. J Urol. 2000;163:1643–8. doi: 10.1016/s0022-5347(05)67512-x. https://doi.org/10.1016/S0022-5347(05)67512-X. [DOI] [PubMed] [Google Scholar]
- 7.Kavoussi LR, Moore RG, Partin AW, Bender JS, Zenilman ME, Satava RM. Telerobotic assisted laparoscopic surgery: initial laboratory and clinical experience. Urology. 1994;44:15–9. doi: 10.1016/s0090-4295(94)80003-0. https://doi.org/10.1016/S0090-4295(94)80227-0. [DOI] [PubMed] [Google Scholar]
- 8.Binder J, Kramer W. Robotically assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408–10. doi: 10.1046/j.1464-410x.2001.00115.x. https://doi.org/10.1046/j.1464-410x.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 9.Menon M, Shrivastava A, Tewari A, Sarle R, Hemal A, Peabody JO, et al. Laparoscopic and robot assistedradical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. 2002;168:945–9. doi: 10.1016/S0022-5347(05)64548-X. https://doi.org/10.1097/00005392-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Kaouk JH, Goel RK, Haber GP, Crouzet S, Desai MM, Gill IS. Single-port laparoscopic radical prostatectomy. Urology. 2008;72:1190–3. doi: 10.1016/j.urology.2008.06.010. https://doi.org/10.1016/j.urology.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Asimakopoulos AD, Miano R, Galfano A, Bocciardi AM, Vespasiani G, Spera E, et al. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: Critical appraisal of the anatomic landmarks for a complete intrafascial approach. Clin Anat. 2015;28:896–902. doi: 10.1002/ca.22576. https://doi.org/10.1002/ca.22576. [DOI] [PubMed] [Google Scholar]
- 12.Kaouk JH, Akca O, Zargar H, Caputo P, Ramirez D, Andrade H, et al. Descriptive technique and ınitial results for robotic radical perineal prostatectomy. Urology. 2016;94:129–38. doi: 10.1016/j.urology.2016.02.063. https://doi.org/10.1016/j.urology.2016.02.063. [DOI] [PubMed] [Google Scholar]
- 13.Salomon L, Levrel O, Anastasiadis AG, Saint F, de La Taille A, Cicco A, et al. Outcome and complications of radical prostatectomy in patients with PSA <10 ng/ml: comparison between the retropubic, perineal and laparoscopic approach. Prostate Cancer Prostatic Dis. 2002;5:285–90. doi: 10.1038/sj.pcan.4500605. https://doi.org/10.1038/sj.pcan.4500605. [DOI] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Odisho AY, Carroll PR. Outcomes for Radical Prostatectomy: Is It the Singer, the Song, or Both? J Clin Oncol. 2012;30:476–8. doi: 10.1200/JCO.2011.38.9593. https://doi.org/10.1200/JCO.2011.38.9593. [DOI] [PubMed] [Google Scholar]
- 15.Albayrak S, Horuz R, Göktaş C, Cangüven Ö, Çetinel C. Radical perineal prostatectomy: our experiences on 40 cases. Turk J Urol. 2007;33:398–404. [Google Scholar]
- 16.Leung AC, Melman A. Radical perineal prostatectomy: a more optimal treatment approach than laparoscopic radical prostatectomy in obese patients? Rev Urol. 2005;7:48–52. [PMC free article] [PubMed] [Google Scholar]
- 17.Eden CG. Minimal access radical prostatectomy: how is it shaping up? BJU Int. 2008;101:791–2. doi: 10.1111/j.1464-410X.2008.07452.x. https://doi.org/10.1111/j.1464-410X.2008.07452.x. [DOI] [PubMed] [Google Scholar]
- 18.Harris MJ. Radical perineal prostatectomy: cost efficient, outcome effective, minimally invasive prostate cancer management. Eur Urol. 2003;44:303–8. doi: 10.1016/s0302-2838(03)00298-7. https://doi.org/10.1016/S0302-2838(03)00298-7. [DOI] [PubMed] [Google Scholar]
- 19.Hu JC, Nelson RA, Wilson TG, Kawachi MH, Ramin SA, Lau C, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006;175:541–6. doi: 10.1016/S0022-5347(05)00156-4. https://doi.org/10.1016/S0022-5347(05)00156-4. [DOI] [PubMed] [Google Scholar]
- 20.Resnick MI. Radical perineal prostatectomy. BJU Int. 2003;92:522–3. doi: 10.1046/j.1464-410x.2003.04423.x. https://doi.org/10.1046/j.1464-410X.2003.04423.x. [DOI] [PubMed] [Google Scholar]
- 21.Martis G, Diana M, Ombres M, Cardi A, Mastrangeli R, Mastrangeli B. Retropubic versus perineal radical prostatectomyin early prostate cancer: eight-year experience. J Surg Oncol. 2007;95:513–8. doi: 10.1002/jso.20714. https://doi.org/10.1002/jso.20714. [DOI] [PubMed] [Google Scholar]
- 22.Porpiglia F, Morra I, Lucci Chiarissi M, Manfredi M, Mele F, Grande S, et al. Randomised controlledtrial comparing laparoscopic and robot-assisted radicalprostatectomy. Eur Urol. 2013;63:606–14. doi: 10.1016/j.eururo.2012.07.007. https://doi.org/10.1016/j.eururo.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Korman HJ, Harris MJ. Prostate Cancer-Radical Perineal Prostatectomy. Medscape.com. 2004;7:249–52. doi: 10.1038/sj.pcan.4500723. [DOI] [PubMed] [Google Scholar]
- 24.Amorim GL, Cruz GM, Veloso DF, Kartabil JD, Vieira JC, Alves PR. Comparative analysis of radical prostatectomy techniques using perineal or suprapubic approach in the treatment of localizedprostate cancer. Einstein. 2010;8:200–4. doi: 10.1590/S1679-45082010AO1592. https://doi.org/10.1590/s1679-45082010ao1592. [DOI] [PubMed] [Google Scholar]
- 25.Tewari A, Sooriakumaran P, Bloch DA, Seshadri-Kreaden U, Hebert AE, Wiklund P. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–15. doi: 10.1016/j.eururo.2012.02.029. https://doi.org/10.1016/j.eururo.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Song W, Park JH, Jeon HG, Jeong BC, Seo SI, Jeon SS, et al. Comparison of Oncologic Outcomes and Complications According to Surgical Approach to Radical Prostatectomy: Special Focus on the Perineal Approach. Clin Genitourin Cancer. 2017;15:e645–52. doi: 10.1016/j.clgc.2017.01.015. https://doi.org/10.1016/j.clgc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Ku JY, Ha HK. Learning curve of robot-assisted laparoscopicradical prostatectomy for a single experienced surgeon: comparison with simultaneous laparoscopic radical prostatectomy. World J Mens Health. 2015;33:30–5. doi: 10.5534/wjmh.2015.33.1.30. https://doi.org/10.5534/wjmh.2015.33.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asimakopoulos AD, Pereira Fraga CT, Annino F, Pasqualetti P, Calado AA, Mugnier C. Randomized comparison betweenlaparoscopic and robot-assisted nerve-sparing radical prostatectomy. J Sex Med. 2015;8:1503–12. doi: 10.1111/j.1743-6109.2011.02215.x. https://doi.org/10.1111/j.1743-6109.2011.02215.x. [DOI] [PubMed] [Google Scholar]
- 29.Trabulsi EJ, Zola JC, Gomella LG, Lallas CD. Transitionfrom pure laparoscopic to robotic-assisted radical prostatectomy:a single surgeon institutional evolution. Urol Oncol. 2010;28:81–5. doi: 10.1016/j.urolonc.2009.07.002. https://doi.org/10.1016/j.urolonc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Steiner MS, Morton RA, Walsh PC. Impact of anatomicalradical prostatectomy on urinary continence. J Urol. 1991;145:512–4. doi: 10.1016/s0022-5347(17)38382-9. https://doi.org/10.1016/S0022-5347(17)38382-9. [DOI] [PubMed] [Google Scholar]
- 31.Comploj E, Pycha A. Experience with radical perineal prostatectomy in the treatment of localized prostate cancer. Ther Adv Urol. 2012;4:125–31. doi: 10.1177/1756287212441497. https://doi.org/10.1177/1756287212441497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koutlidis N, Mourey E, Champigneulle J, Mangin P, Cormier L. Robot-assisted or pure laparoscopic nerve-sparing radicalprostatectomy: what is the optimal procedure for the surgicalmargins? A single center experience. Int J Urol. 2012;19:1076–81. doi: 10.1111/j.1442-2042.2012.03102.x. https://doi.org/10.1111/j.1442-2042.2012.03102.x. [DOI] [PubMed] [Google Scholar]


