Abstract
Objective
It has been shown in previous studies that inadequate empirical treatment is associated with mortality in a variety of infections caused by Gram-negative bacteria. In this study, the clinical effect of discordance in empirical treatment was investigated in patients with urinary tract infection (UTI) accompanied by bacteremia.
Material and methods
We retrospectively reviewed the files of adult (>18 years old) patients who were diagnosed with UTI in our clinic between January 2014 and December 2015. Cases in which the same causative microorganism grew in both blood and urine cultures were included in the study. Patients using ceftriaxone and carbapenem as empirical antibiotic therapy (EAT) were compared as two different groups. In cases that the ethiologic agents were extended- spectrum beta lactamase (ESBL)-producing Klebsiella pneumoniae and Escherichia coli isolates, if the microorganism was resistant to initial antibiotic treatment the situation was defined as EAT discordance, and if it was sensitive it was defined as EAT concordance.
Results
After the exclusion criteria were applied, 65 of the 266 cases examined were taken into the study. Clinical and laboratory features of cases of ceftriaxone and carbapenem groups were similar. There was no statistically significant difference between the two groups in terms of hospital stay and survival (p>0.05). Of 28 cases of ESBL-producing E. coli and K. pneumoniae, 18 were EAT discordant and 10 were EAT concordant. Clinical and laboratory features of EAT concordant and EAT discordant groups were similar. No statistically significant difference was found between the two groups in terms of hospital stay and survival (p>0.05).
Conclusion
It was considered that ceftriaxone can still be a viable option in the EAT of UTI, which is accompanied by bacteremia without severe sepsis and septic shock findings. It was concluded that EAT discordance may not have a negative effect on the duration of hospital stay and survival rates in neither total cases nor ESBL positive ones.
Keywords: Bacteremia, empirical antibiotic therapy, urinary tract infection
Introduction
Urinary tract infection (UTI) is the most frequently seen bacterial infection in adults, and bacteremia accompanies this infection in 20–40% of the cases.[1–3] Bacterial agent is most often Escherichia coli, while Gram-negative cocci, enterococci, and Staphylococcus saprophyticus may also cause this infection.[4] In recent studies, extended- spectrum beta-lactamase (ESBL) producing strains have been detected in E.coli isolates at a higher frequency, both in the world, and in our country.[5,6] In the treatment of the infections caused by ESBL -producing strains, inadequacy of the antibiotics other than carbanepems in addition to their higher mortality rates have been also indicated.[7] However, together with increased resistance of members of Enterobacteriaceae family, and P. aeruginosa isolates against carbapenems, ways of using carbapenems at a lesser frequency have been investigated.[8] In infections caused by Gram-negative bacteria, inadequacy of initial empirical treatment have been associated with high rates of mortality in some studies.[9] However these studies have encompassed not only cases with UTI, but also bloodstream infections, and pneumonia with higher mortality rates. It has been reported that in cases with acute pyelonephritis discordance in empirical antibiotherapy (EAT) has no effect on treatment outcomes, and mortality, however it has unfavourable effects on early clinical response, and hospital stay.[10]
In this study, in cases with UTI coursing with bacteremia, the impact of discordance in empirical treatment on treatment outcomes, survival, and hospital stay was investigated.
Material and methods
The study was performed after written approval (01.19.2016 decree # 1178) from the Ethics Committee of Clinical Research Center of Şişli Etfal Training and Research Hospital was obtained. Medical files of adult patients (>18 years) hospitalized with the diagnosis of UTI between January 2014, and December 2015 in The Clinics of Infectious Diseases, and Clinical Microbiology of our tertiary training and research hospital with 700 patient beds were reviewed. The cases whose blood, and urine cultures demonstrated the growth of the same agent were included in the study. The patients whose cultures did not reveal the presence of any causative agent or only in urine cultures bacterial growth was detected, in cases who had more than one bacterial agent in their culture media, and those with another different comorbidity were excluded from the study.
Before antibiotherapy, urine and blood samples were obtained from the patients twice for antibiotic susceptibility tests. For the identification of bacteria BD Phoenix™ automated system (Becton Dickinson, USA) or Matrix-assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) (Bruker Daltonics, Germany) was used. Antibiotic susceptibility tests were performed using BD Phoenix™ automated system or Kirby Bauer disk diffusion method in compliance with the criteria established by Clinical Laboratory Standarts Institute (CLSI).[11]
The decision to initiate EAT treatment with carbanepem or ceftriaxone was made by the clinician. The cases were divided into two groups based on their EAT as ceftriaxone and carbapenem (ertapenem, imipenem, and meropenem) users. Clinical and laboratory findings of both groups were compared as for their hospital stay, and survival rates.
If the microbial agent is found to be in vitro resistant against initial antibiotherapy, then EAT was defined as discordant. In cases where bacterial agent was found to be in vitro resistant against initial antibiotherapy, then EAT was defined as discordant antibiotherapy. In cases where ESBL –producing, ceftriaxone –resistant K. pneumoniae and E. coli isolates were found, ceftriaxone was replaced by ertapenem on the third day of treatment. These cases were classified as discordant EAT group. Cases where these bacteria were the causative pathogen, and so carbapenem was initiated as an empirical treatment were classied as EAT concordant group.
Improvement in clinical symptoms, and signs, and sterile urine culture obtained 7–9 days after completion of treatment was defined as criterion of treatment response.[12] Hospital records of the patients were examined retrospectively to determine the reasons of the patients for their presentation to the hospital after termination of their treatment, and the treatment they received. Development of UTI caused by a different pathogen 28–31 days after the treatment was defined as reinfection.[13] If the causative agent was the same as previously treated, then this condition was termed as relapse. Isolation of one or more than one different agent in the culture of the patient during treatment or a short time after the treatment was defined as superinfection.[14] Urinary system catheterization, urinary system malignancies, diabetes mellitus, neurogenic bladder, benign prostatic hyperplasia, and nephrolithiasis were evaluated as complicating factors.
Statistical analysis
For statistical analysis Statistical Package for the Social Sciences (SPSS Inc.; Chicago, IL, USA) 15.0 Windows program was used. Descriptive statistics were expressed as numbers, and percentages for categorical variables, and means, and standard deviation for numerial variables. In comparisons between two independent groups for numerical variables with normal distribution Student t test, for those with non-normal distribution Mann-Whitney U test were used. Chi-square analysis was employed to test rates of categorical variables between groups. When required conditions could not be achieved Monte Carlo Simulation was applied. Level of statistical significance was accepted as p<0.05.
Results
Characteristic features of 266 cases followed up within the specified time interval were retrospectively examined and in 97 cases causative microorganism was not detected. From the remaining 169 cases, 104 patients met the exclusion criteria, and finally the study was conducted with 30 female, and 35 male patients. Clinical, and demographic findings of the patients in the ceftriaxone (n=47), and carbapenem (n=18) groups were comparable. A total of 21 (32%) cases [9 (25.5%) in the carbanepem, and 12 (50%) in the ceftriaxone group] were hospitalized within the last 3 months without any statistically significant intergroup difference (p=0.059). In 12 (32%) cases a complicating factor as urinary system malignancy, benign prostatic hyperplasia, urinary catheterization, neurogenic bladder, and urolithiasis was detected. As a comorbidity diabetes mellitus was detected in 4 (22.2%) carbapenem, and 14 (29.8%) ceftriaxone users (p=0.758). Any statistically significant was not found between ceftriaxone, and carbanepem groups as for comorbidities, and urologic complicating factors (Table 1). Besides, hospital stay, and survival rates were comparable between groups (p=0.586, and p=1.000, respectively).
Table 1.
Comparison of ceftriaxone, and carbapenem groups
| Characteristics of the cases | Carbapenem group (n=18) | Ceftriaxone group (n=47) | p |
|---|---|---|---|
| Age Mean SD (year) | 74.5±12.0 | 71.9±14.7 | 0.607 |
|
| |||
| Gender, n (%) | |||
| Female, n (%) | 8 (44.4) | 22 (46.8) | 0.864 |
| Male, n (%) | 10 (55.6) | 25 (53.2) | |
|
| |||
| Admission complaints, n (%) | |||
| Fever, n (%) | 15 (83.3) | 41 (87.2) | 0.699 |
| Confusion, n (%) | 6 (33.3) | 14 (29.8) | 0.782 |
| Dysuria, n (%) | 7 (38.9) | 14 (29.8) | 0.782 |
| Flank pain, n (%) | 3 (16.7) | 11 (23.4) | 0.740 |
| Vomiting, n (%) | 4 (22.2) | 18 (28.3) | 0.220 |
|
| |||
| History | |||
| Hospitalization within the last 3 months, n (%) | 9 (50.0) | 12 (25.5) | 0.059 |
| Antibiotic use within the last 10 days, n (%) | 5 (27.8) | 9 (19.1) | 0.507 |
|
| |||
| Physical examination | |||
| Tachycardia (HR>100 bpm), n (%) | 8 (44.4) | 18 (38.4) | 0.651 |
| Tachypnea (>24/min), n (%) | 7 (38.9) | 12 (25.5) | 0.289 |
| Hypotension (SBP<90), n (%) | 0 (0) | 5 (10.6) | 0.311 |
| Costovertebral angle tenderness, n (%) | 8 (44.4) | 15 (31.9) | 0.344 |
| Suprapubic tenderness, n (%) | 0 (0) | 5 (10.6) | 0.311 |
|
| |||
| Complicating factors | |||
| Urolithiasis, n (%) | 0 (0) | 6 (12.8) | 0.175 |
| Urinary system malignancy, n (%) | 1 (5.6) | 3 (6.4) | 1.000 |
| Benign prostatic hyperplasia, n (%) | 1 (5.6) | 4 (8.5) | 1.000 |
| Nephrostomy, n (%) | 1 (5.6) | 1 (2.1) | 0.480 |
| Neurogenic bladder, n (%) | 0 (0) | 2 (4.3) | 1.000 |
| Indwelling urinary catheterization, n (%) | 0 (0) | 1 (2.1) | 1.000 |
| Intermittent catheterization, n (%) | 0 (0) | 1 (2.1) | 1.000 |
| Diabetes mellitus, n (%) | 4 (2.2) | 14 (29.8) | 0.758 |
|
| |||
| Laboratory parametres | |||
| Pyuria, n (%) | 18 (100) | 44 (93.6) | 0.555 |
| Leucocytes, median | 15.977.20 | 14.454.40 | 0.525 |
| Neutrophils, median | 13.696.10 | 12.523.80 | 0.758 |
| Platelets, median | 218444.4 | 217297.9 | 0.964 |
| Hemoglobin, median | 11.8 | 11.1 | 0.211 |
| CRP, median | 180.4 | 215.4 | 0.195 |
|
| |||
| Hospital stay (days) Mean±SD | 11.1±6.1 | 10.0±5.3 | 0.586 |
|
| |||
| Health state at discharge n (%) | |||
| Cure | 17 (94.4) | 43 (91.5) | 1.000 |
| Referral to ICU | 0 (0) | 2 (4.3) | |
| Death | 1 (5.6) | 2 (4.3) | |
SD: standard deviation; SBP: Systolic blood pressure; CRP: C-reactive protein; ICU: intensive care unit
Most frequently growth of E.coli (63%) was detected as a causative agent, and 43% of Gram-negative agents consisted of ESBL-producing bacteria. Forty and 44.6% of Gram-negative agents were resistant to ciprofloxacin, and cotrimoxazole, respectively (Table 2). ESBL-positivity was detected in 19 (19/41) E. coli isolates, while ESBL-positivity was detected in 7 (58%) out of 12 K. pneumoniae isolates. Eighteen (44%) E. coli isolates were resistant to ciprofloxacin, and cotrimoxazole. Three (3/12) K. pneumoniae isolates were resistant to ciprofloxacin, and 7 of them were resistant to cotrimoxazole. Resistance against ampicillin (6.1%) was not detected in patients whose cultures demonstrated growth of Enterococcus faecalis. Three of these cases were in ceftriaxone, and one in carbepenem group. Resistance against ceftriaxone was not detected in patients whose cultures demonstrated growth of Proteus mirabilis (n=4) or Enterobacter cloacae (n=1). All of ESBL producing E. coli and K. pneumoniae isolates were resistant to ceftriaxone.
Table 2.
Distribution of gram-negative agents, and resistance rates
| Carbapenem group (%) | Ceftriaxone group (%) | Total | ||
|---|---|---|---|---|
| Microorganisms | E. coli | 9 (50) | 32 (68) | 41 (63.1) |
| K. pneumoniae | 5 (27) | 7 (16) | 12 (18.4) | |
| Proteus mirabilis | 2 (11) | 4 (8) | 6 (9.2) | |
| P. aeruginosa | 0 (0) | 1 (2) | 1 (1.5) | |
| E. cloacae | 1 (6) | 0 (0) | 1 (1.5) | |
| ESBL | ||||
| Resistance against: | 10 (62.5) | 18 (40.0) | 28 (43.1) | |
| Carbapenem | 0 (0) | 2 (4.4) | 2 (3.1) | |
| Quinolone | 4 (25.0) | 20 (44.4) | 24 (40.0) | |
| Cotrimoxazole | 9 (56.3) | 20 (44.4) | 29 (44.6) | |
ESBL: extended-spectrum beta-lactamase
In the ceftriaxone group EAT of 24 (51.0%) cases was discordant, while concordance was detected in EAT in 23 (49.0%) cases (Figure 1). In two patients growth of K. pneumoniae resistant to carbapenems was detected. One of these patients was transferred to intensive care unit (ICU), and the other one died on the 5. day of colistin+meropenem treatment. Severe sepsis developed in 5 cases. The characteristic features of these cases are shown in Table 3. One of the cases in the carbapenem group, disease relapsed.
Figure 1.
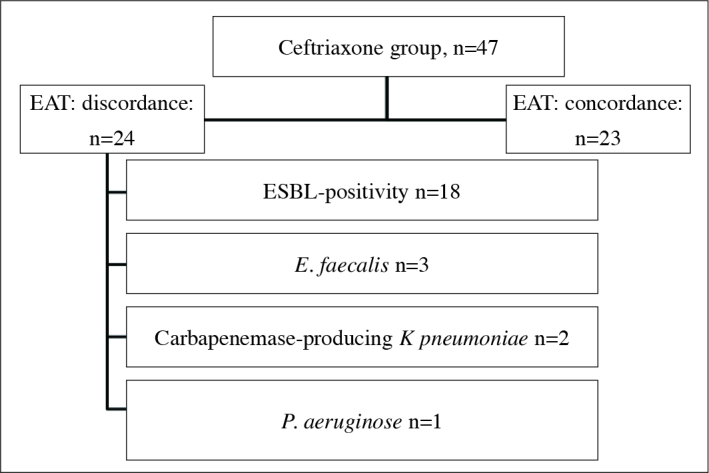
Causes of discordance in EAT among cases in the ceftriaxone group
EAT: empirical antibiotherapy; ESBL: extended-spectrum beta- lactamase
Table 3.
Characteristics of the patients who were referred to intensive care units (ICUs), and those exited
| Patients, n | Concordance to EAT | Age (year)/gender | Agent | The state of resistance | Concomitant disease | Outcome |
|---|---|---|---|---|---|---|
| 1 | Concordant | 81/Female | K. pneumonia | ESBL producing | None | Exitus |
| 2 | Discordant | 98/Male | Enterococcus faecalis | Susceptible to penicilline | None | ICU |
| 3 | Discordant | 49/Male | K. pneumonia | Resistant to all antibiotics | Bladder carcinoma | Exitus |
| 4 | Discordant | 69/Male | K. pneumonia | Carbapenemase | DM, cardiovascular disease | ICU |
| 5 | Discordant | 83/Female | E. coli | ESBL producing | DM | Exitus |
ESBL: extended–spectrum beta-lactamase; EAT: empirical antibiotherapy; DM: diabetes mellitus; ICU: intensive care unit
In a total of 28 cases whose cultures revealed the presence of ESBL producing E. coli, and K. pneumoniae EAT was discordant in 18, and concordant in 10 patients. Clinical, and demographic characteristics of both groups were similar (p>0.05). Hospital stay and survival rates of these cases were not statistically significantly different (p=0.765, and p=1.000, respectively). One patient from each group died, while one case from discordant EAT group was transferred into intensive care unit (Table 4).
Table 4.
Comparisons among ESBL-positive cases
| Characteristics of the cases | Discordant empirical treatment (n=18) | Concordant empirical treatment (n=10) | p |
|---|---|---|---|
| Age Mean.±SD (year) | 66.8±13.8 | 71.2±13.8 | 0.424 |
|
| |||
| Gender, n (%) | |||
| Female | 7 (38.9) | 3 (30.0) | 0.703 |
| Male | 11 (61.1) | 7 (70.0) | |
|
| |||
| Admission complaint, n (%) | |||
| Fever | 17 (94.4) | 9 (90.0) | 1.000 |
| Confusion | 3 (16.7) | 2 (20.1) | 1.000 |
| Dysuria | 5 (27.8) | 4 (40.0) | 0.677 |
| Flank pain | 5 (27.8) | 3 (30.0) | 1.000 |
| Vomiting | 10 (55.6) | 4 (40.0) | 0.430 |
|
| |||
| History | |||
| Hospitalization within the last 3 months | 9 (50.0) | 4 (40.0) | 0.705 |
| Antibiotic use within the last 10 days | 7 (38.9) | 1 (10.0) | 0.194 |
|
| |||
| Physical examination | |||
| Tachycardia (100 bpm) | 9 (50.0) | 5 (50.0) | 1.000 |
| Tachypnea (>24/min) | 5 (27.8) | 4 (40.0) | 0.677 |
| Hypotension (SKB<90) | 3 (16.7) | 0 (0) | 0.533 |
| Costovertebral angle tenderness | 4 (22.2) | 6 (60.0) | 0.097 |
| Suprapubic tenderness | 4 (22.2) | 0 (0) | 0.265 |
|
| |||
| Complicating factors | |||
| Urolithiasis | 2 (11.1) | 0 (0) | 0.524 |
| Urinary system malignancies | 3 (16.7) | 0 (0) | 0.533 |
| Benign prostatic hyperplasia | 0 (0) | 1 (10.0) | 0.357 |
| Nephrostomy | 1 (5.6) | 1 (10.0) | 1.000 |
| Neurogenic bladder | 1 (5.6) | 0 (0) | 1.000 |
| Indwelling catheterization | 1 (5.6) | 0 (0) | 1.000 |
| Intermittent catheterization | 0 (0) | 0 (0) | |
| Diabetes mellitus | 7 (38.3) | 2 (20.0) | 0.417 |
|
| |||
| Laboratory parametres | |||
| Pyuria | 17 (94.4) | 10 (100) | 1.000 |
| Leucocyte, median | 13490 | 16687 | 0.338 |
| Neutrophil, median | 11833.9 | 13996 | 0.533 |
| Platelet, median | 232777.8 | 231400 | 0.968 |
| Hemoglobin, median | 11.1 | 12.1 | 0.268 |
| CRP, median | 208.4 | 220.1 | 0.749 |
|
| |||
| Hospital stay (days) Mean±SD | 12.7±5.2 | 12.0±7.4 | 0.765 |
|
| |||
| Health state at discharge n (%) | |||
| Cure | 16 (88.9) | 9 (90.0) | 1.000 |
| Referral to ICU | 1 (5.6) | 0 (0) | |
| Death | 1 (5.6) | 1 (10.0) | |
SS: standard deviation; ESBL: extended–spectrum beta-lactamase; SBP: Systolic blood pressure; CRP: C-reactive protein; ICU: intensive care unit
Discussion
Bacteremia is an important indicator in the approach to the cases with UTI which demonstrates the severity of the disease. Bacteremia is more frequently associated with cases manifesting symptoms of severe sepsis, and septic shock. In some studies, risk factors for bacteremia have been determined. In cases with complicated acute pyelonephritis, advanced age has been detected as a risk factor for bacteremia.[2] On the other hand, malignancy, and indwelling urinary catheter have been demonstrated as independent risk factors for bacteremia.[15] These risk factors for bacteremia were also observed in our cases. Our series consisted of relatively older patients (median ages were 74.5, and 71.9 years in ceftriaxone, and carbapenem groups, respectively), and 8 patients had also concomitant urinary system malignancies, and indwelling urinary catheters.
In cases with urinary system infections E. coli is the most frequently detected microbial agent, and in 20–27% of them it has ESBL-producing strains.[4,5,16] Based on the 2013 data released by National Antimicrobial Resistance Surveillance System (UAMDS) in Turkey, ESBL-producing strains were detected in 44.9% of E. coli, and 49.9% of K. pneumoniae isolates. In our study the most frequent microbial agent was E. coli (63.1%). Overall ESBL-producing rate among E. coli, and K. pneumoniae isolates was detected as 43.1 percent. In Turkey increasingly higher rates of carbanepem resistance among Enterobacteriaceae strains have been reported in recent years.[17] In our study, carbanepem- resistant K. pneumoniae strains were detected in 2 cases in the ceftriaxone group. Resistance rates against quinolone antibiotics are gradually increasing among Gram-negative urinary pathogens.[16] Based on 2013 UAMDS data rates of resistance against ciprofloxacin among E.coli and K. pneumoniae isolates were 44.0, and 43.0%, respectively. Similarly, in our study 40%, and 44.6% of all Gram-negative agents were resistant to ciprofloxacin, and cotrimoxazole, respectively. According to Infectious Diseases Society of America guidelines antibiotics with regional resistance rates above 20 % are not recommended in empirical treatment of UTIs.[18] In this case, use of ciprofloxacin in our country for empirical treatment of cases with UTI associated with bacteremia will not be an appropriate choice.
In a study by Tamma et al.[7] carbapenem, and tazobactam (TZB) were compared in the treatment of cases with bacteremia caused by ESBL-producing microorganisms. In this study TZB was found to be less effective, and 20% of sources of bacteremia were UTIs. However in some recent studies, effectiveness of antibiotics other than carbapenems in the treatment of UTIs caused by ESBL-producing bacteria has been reported.[19,20] In another study, in patients with severe sepsis, and septic shock caused by resistant Gram-negative microorganisms, inappropriate initial empirical antibiotics was demonstrated to be related to mortality. In this study, as a source of sepsis, cases with UTI constituted 19.5% of all cases.[9] All of our study population consisted of cases with UTI, and did not include patients with symptoms of severe sepsis, and septic shock at admission.
A study which investigated clinical effects of inappropriate empirical antibiotherapy, demonstrated that discordance in empirical treatment in cases with community-acquired UTI had no effect on treatment outcome, and mortality, however it exerted unfavorable effects on hospital stay, and early clinical response.[10] In our study EAT was found to be effective in 23 (49.0%) cases in the ceftriaxone group, so the same EAT was maintained. While in the remaining 24 (51.0%) cases EAT was ineffective, so antibiotherapy was changed. Based on the treatment outcomes, in 2 out of 5 cases who developed severe sepsis, growth of carbapenemase-producing K. pneumoniae was detected. Advanced age, and an complicating factor were noted in 3 exited cases. Still in 2 cases transferred to the intensive care unit advanced age, and inappropriate EAT were observed. Relapse developed in one patient in the carbapenem group. However, in the carbapenem, and ceftriaxone groups, hospital stays, and survival rates of the cases were nearly identical. Discordance in EAT had no effect on survival, and hospital stay. Besides, we compared patients with USI caused by ESBL-producing agents which received appropriate, and inappropriate EAT, and we couldn’t find any significant difference as for longevity of hospital stay, and survival rates. In our study since relapse rates were estimated based on registration data recorded at admission, we think that these data did not reflect actual relapse rates. Retrospective design, dubious relapse rates, and relatively small number of cases are limitations of our study.
In conclusion, considering gradually increasing number of carbapenem –resistant Enterobacteriaceae isolates, one may think of decreasing the use of carbapenems in the empirical treatment of UTI. In cases with UTI without any evidence of severe sepsis, and septic shock and concomitant bacteremia, ceftriaxone may be an alternative. It has been detected that in all cases, and also in ESBL-positive cases discordant EAT might not effect hospital stay and survival rates unfavorably. Prospective randomized studies should be performed on this issue with higher number of case series.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Şişli Hamidiye Etfal Training and Research Hospital.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – İ.D., A.A.H.; Design – İ.D., A.A.H.; Supervision – İ.D., A.A.H., N.U.; Resources – İ.D., A.A.H., M.E.B., S.Ç.; Materials – İ.D., A.A.H., M.E.B., S.Ç., A.Ö., N.U.; Data Collection and/or Processing – İ.D., A.A.H., M.E.B., S.Ç., A.Ö.; Analysis and/or Interpretation – İ.D., A.A.H., N.U.; Literature Search – İ.D., A.A.H., M.E.B., S.Ç., A.Ö., N.U.; Writing Manuscript – İ.D., A.A.H.; Critical Review – İ.D., A.A.H., M.E.B., S.Ç., A.Ö., N.U.; Other – İ.D., A.A.H., M.E.B., S.Ç., A.Ö., N.U.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Nicolle LE. Update in adult urinary tract infection. Curr Infect Dis Rep. 2011;13:552–60. doi: 10.1007/s11908-011-0212-x. https://doi.org/10.1007/s11908-011-0212-x. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, Fang HC, Chou KJ, Chen CL, Lee PT, Chung HM. The clinical impact of bacteremia in complicated acute pyelonephritis. Am J Med Sci. 2006;332:175–80. doi: 10.1097/00000441-200610000-00004. https://doi.org/10.1097/00000441-200610000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ledochowski S, Abraham PS, Jacob X, Dumitrescu O, Lina G, Lepape A, et al. Relevance of blood cultures in acute pyelonephritis in a single-center retrospective study. Intern Emerg Med. 2015;10:607–12. doi: 10.1007/s11739-015-1223-7. https://doi.org/10.1007/s11739-015-1223-7. [DOI] [PubMed] [Google Scholar]
- 4.Czaja CA, Scholes D, Hooton TM, Stamm WE. Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis. 2007;45:273–80. doi: 10.1086/519268. https://doi.org/10.1086/519268. [DOI] [PubMed] [Google Scholar]
- 5.Yılmaz N, Ağuş N, Bayram A, Şamlıoğlu P, Şirin MC, Derici YK, et al. Antimicrobial susceptibilities of Escherichia coli isolates as agents of community-acquired urinary tract infection (2008–2014) Turk J Urol. 2016;42:32–6. doi: 10.5152/tud.2016.90836. https://doi.org/10.5152/tud.2016.90836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazaz R, Chapman AL, Winstanley TG. Ertapenem administered as outpatient parenteral antibiotic therapy for urinary tract infections caused by extended-spectrum-beta-lactamase-producing Gram-negative organisms. J Antimicrob Chemother. 2010;65:1510–3. doi: 10.1093/jac/dkq152. https://doi.org/10.1093/jac/dkq152. [DOI] [PubMed] [Google Scholar]
- 7.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, et al. Antibacterial resistance leadership group. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrumβ-lactamase bacteremia. Clin Infect Dis. 2015;60:1319–25. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, et al. Ceftazidime-avibactam Versus Doripenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: RECAPTURE, a Phase 3 Randomized Trial Program. Clin Infect Dis. 2016;63:754–62. doi: 10.1093/cid/ciw378. https://doi.org/10.1093/cid/ciw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multidrug resistance, inappropriate initial antibiotic therapy and mortality in gram negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596. doi: 10.1186/s13054-014-0596-8. https://doi.org/10.1186/s13054-014-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SS, Kim Y, Chung DR. Impact of discordant empirical therapy on outcome of community-acquired bacteremic acute pyelonephritis. J Infect. 2011;62:159–64. doi: 10.1016/j.jinf.2010.10.009. https://doi.org/10.1016/j.jinf.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 11.CLSI. 24th Informational Supplement, M100-S24. Clinical and Laboratory Standards Institute; Wyane, PA: 2014. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 12.Rubin RH, Shapiro ED, Andriole VT, Davis RJ, Stamm WE. Evaluation of new anti-infective drugs for the treatment of urinary tract infection. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992;15:216–27. doi: 10.1093/clind/15.supplement_1.s216. https://doi.org/10.1093/clind/15.Supplement_1.S216. [DOI] [PubMed] [Google Scholar]
- 13.Wells WG, Woods GL, Jiang Q, Gesser RM. Treatment of complicated urinary tract infection in adults: combined analysis of two randomized, double-blind, multicentre trials comparing ertapenem and ceftriaxone followed by appropriate oral therapy. J Antimicrob Chemother. 2004;53:67–74. doi: 10.1093/jac/dkh208. https://doi.org/10.1093/jac/dkh208. [DOI] [PubMed] [Google Scholar]
- 14.Tasbakan MI, Pullukcu H, Sipahi OR, Yamazhan T, Ulusoy S. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554–6. doi: 10.1016/j.ijantimicag.2012.08.003. https://doi.org/10.1016/j.ijantimicag.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 15.van Nieuwkoop C, Bonten TN, Wout JW, Becker MJ, Groeneveld GH, Jansen JL, et al. Risk factors for bacteremia with uropathogen not cultured from urine in adults with febrile urinary tract infection. Clin Infect Dis. 2010;50:69–72. doi: 10.1086/652657. https://doi.org/10.1086/652657. [DOI] [PubMed] [Google Scholar]
- 16.Çelikbilek N, Gözalan A, Özdem B, Kırca F, Açıkgöz ZC. Extended-spectrum beta-lactamase production by Enterobacteriaceae isolates from urine cultures of outpatients: results of a 7-year follow-up. Mikrobiyol Bul. 2015;49:259–65. doi: 10.5578/mb.9031. https://doi.org/10.5578/mb.9031. [DOI] [PubMed] [Google Scholar]
- 17.Çakar A, Akyön Y, Gür D, Karatuna O, Öğünç D, Özhak Baysan B, et al. Investigation of carbapenemases in carbapenem-resistant Escherichia coli and Klebsiella pneumoniae strains isolated in 2014 in Turkey. Mikrobiyol Bul. 2016;50:21–33. doi: 10.5578/mb.10695. https://doi.org/10.5578/mb.10695. [DOI] [PubMed] [Google Scholar]
- 18.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:103–20. doi: 10.1093/cid/ciq257. https://doi.org/10.1093/cid/cir102. [DOI] [PubMed] [Google Scholar]
- 19.Asakura T, Ikeda M, Nakamura A, Kodera S. Efficacy of empirical therapy with non-carbapenems for urinary tract infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int J Infect Dis. 2014;29:91–5. doi: 10.1016/j.ijid.2014.08.018. https://doi.org/10.1016/j.ijid.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Mambie A, Vuotto F, Poitrenaud D, Weyrich P, Cannesson O, Dessein R, et al. Cefoxitin: An alternative to carbapenems in urinary tract infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. Med Mal Infect. 2016;46:215–9. doi: 10.1016/j.medmal.2016.04.008. https://doi.org/10.1016/j.medmal.2016.04.008. [DOI] [PubMed] [Google Scholar]


