Abstract
Purpose
Overdose of acetaminophen (APAP) is a major cause of acute liver failure. This study was aimed to identify pathways related to hepatotoxicity and potential biomarkers of liver injury.
Experimental design
Rats were treated with low (100 mg/kg) and high (1250 mg/kg) doses of APAP, and liver tissues at 6 and 24 h post-treatment were analyzed using a proteomic approach of 16O/18O labeling and 2D-LC-MS/MS.
Results
Molecular pathways evolved progressively from scattered and less significant perturbations to more focused and significant alterations in a dose- and time-dependent manner upon APAP treatment. Imbalanced expression of hemeoxygenase 1 (HMOX1) and biliverdin reductase A (BLVRA) was associated with hepatotoxicity. Protein abundance changes of a total of 31 proteins were uniquely correlated to liver damage, among which a dramatic increase of HMOX1 levels in plasma was observed. Liver injury-associated significant elevation of plasma HMOX1 was further validated in mice treated with APAP.
Conclusions and clinical relevance
This study unveiled molecular changes associated with APAP-induced liver toxicity at the pathway levels and identified HMOX1 as a potential plasma biomarker of liver injury.
Keywords: Acetaminophen, Heme oxygenase 1 (HMOX1), Hepatotoxicity, MS
1 Introduction
Drug-induced liver injury (DILI) is an adverse event that has been one of the most frequent causes leading to cessation of drug testing in clinical trials, restrictions on drug use, and the withdrawal of approved drugs [1]. The overall occurrence of DILI is between 1 in 10 000 to 1 in 1 000 000 patient-years based on the assessment of individual drugs relevant to DILI [2,3]. Acetaminophen (paracetamol; N-acetyl-p-aminophenol, APAP), an over-the-counter drug, has been commonly used for nearly six decades for pain and fever relief. Overdose of APAP is well known to cause clinical hepatotoxicity with a potential of acute liver failure, accounting for 50% of all the acute liver failure cases with a 30% mortality rate in United States [4,5]. Nearly half of the liver transplantation cases caused by drug-induced liver failure in the United States are attributed to APAP alone or in combination with other drugs [6]. The clinical symptoms of liver damage caused by severe overdose of APAP appear within 24–48 h after ingestion [7].
In clinic practice, other than plasma APAP concentration, potential APAP-induced liver injury is monitored by the measurement of serum alanine and aspartate aminotransferases (ALT and AST, respectively) and bilirubin. Although these diagnostic biomarkers provide reasonable indications for DILI and liver abnormality [8], they lack needed specificity, and provide no information on the severity of the lesion or its likelihood of resolution without the need for liver transplantation. The rise of serum ALT and AST could be due to damage to nonliver organs or diseases [9]. For better risk assessment, efforts have been made to discover more specific biomarkers of hepatotoxicity, for example, 3-(cystein-S-yl) acetaminophen derivatives [10], plasma advanced oxidation protein products concentration [11], urinary miRNAs [12], single nucleotide polymorphisms [13], toxicity-associated metabolites in APAP-treated mice [14], metabolomics biomarkers of hepatotoxicity in rat urine [15–17], and pharmacokinetic monitoring of APAP protein adducts in blood [18]. However, these biomarker candidates have not been qualified.
Recently, omics technologies have been utilized to analyze APAP-induced hepatotoxicity, including proton nuclear magnetic resonance profiling of APAP metabolites in human urine [19–21], genomic profiling of mouse liver specific to APAP toxicity [22], 2DE analysis of protein targets of APAP reactive metabolites in mouse liver [23, 24], and overall proteomic profiling of APAP-induced hepatotoxicity in rat and mouse livers [25–28]. Due to technical limitations of the techniques used in these studies (e.g., 2D-GE), they did not provide sufficient information for full understanding of the molecular changes under APAP-induced hepatotoxicity.
MS-based quantitative proteomics provides a useful tool for the identification of protein biomarkers of toxicity and potential therapeutic targets, and it also provides quantitative information to unveil the mechanisms of drug toxicity [29, 30]. A few studies have been published using quantitative MS-based approaches to investigate APAP-induced hepatotoxicity. Label-free quantitative LC-MS/MS method was utilized to examine mouse liver mitochondrial proteome changes after a single intraperitoneal (i.p.) dose of APAP [31]. ICAT labeling in combination with LC-MS/MS was employed to measure mouse whole liver proteome changes after APAP treatment [32]. In a very recent study, Sun et al. [33] examined the blood proteome in a mouse model of APAP-induced hepatotoxicity (a single dose administered through i.p. injection) using a quantative LC-MS/MS approach based on amine-specific isotope labeling. However, protein biomarkers that are able to detect liver injury at early stages are highly needed and the work to date has not been successful in identifying such biomarkers. This unmet need continues to drive further studies on biomarkers of APAP-induced hepatotoxicity.
In this study, we investigated the liver proteome changes after a single oral dose (100 mg/kg or 1250 mg/kg) of APAP at two time points (6- and 24-h posttreatment) in a rat model. By using trypsin-catalyzed 16O/18O labeling and MS-based quantitative proteomic approaches, we were able to identify protein changes in each dose at each time point after APAP treatment. Protein changes uniquely associated with liver damage were also identified. Pathway analysis of the quantitative proteome data unveiled the molecular changes associated with liver toxicity.
2 Materials and methods
2.1 Chemicals and reagents
Ammonium bicarbonate (NH4HCO3), ammonium formate (NH4HCO2), APAP, methyl cellulose, and urea were purchased from Sigma-Aldrich (St. Louis, MO). Formic acid and TFA were from Fluka (Milwaukee, WI). HPLC grade ACN (CH3CN) and water were obtained from ThermoFisher Scientific (Fair Lawn, NJ). Excellulose desalting columns (5K MWCO) and bicinchonic acid (BCA) protein assay reagent kit were purchased from Pierce (Rockford, IL).
2.2 Animal treatment and assessment of liver damage
Male Sprague–Dawley rats were provided by the National Center for Toxicological Research (Jefferson, AR) breeding colony. Rats 6–7 weeks of age and an average body weight of ≈300 g were used. The treatment of the rats was approved by the Institutional Animal Care and Use Committee at this American Association for Laboratory Animal Science accredited facility. In each dose/time group, four to six animals were employed. The rats were fasted overnight for at least 12 h prior to dosing. A single dose of APAP (100 or 1250 mg/kg) or control vehicle (0.5% methylcellulose) was administered via oral gavage. Feed was provided to the animals ad libitum 4 h after dosing. The rats were euthanized at 6 or 24 h after dosing by exposure to carbon dioxide. Liver samples were collected and immediately frozen in an isopentane/dry ice slurry for omics studies. Among other hematological parameters assayed were serum levels of ALT and AST. A portion of the left lateral lobes of liver from each animal was fixed in 10% neutral buffered formalin, paraffin-embedded, sectioned at approximately 5 μm, stained with hematoxylin and eosin, and examined by light microscopy for histopathology.
To verify the potential biomarkers discovered from the rat study in different species, mouse experiment was performed in accordance with the guidelines for small animals of the National Institutes of Health. Age-matched female mice (4–5 months of age) on a 129/SvJ background were maintained in a temperature-controlled room (23–25°C) on a 12-h light/12-h dark cycle at the National Institute on Alcohol Abuse and Alcoholism and were fed standard rodent chow ad libitum. Handling and APAP treatment procedures were approved by the Institute Animal Care and Use Committee. Mice were treated with a single i.p. injection of 300 mg/kg APAP dissolved in warm isotonic saline solution (n = 6). Control mice were treated with equal volumes of sterile saline solution (n = 5). Mice were euthanized at 24 h after dosing, and the trunk blood and liver from each mouse were collected immediately. A small liver section from the largest lobe of each mouse was fixed in 10% formalin in PBS and subjected to hematoxylin and eosin staining by American HistoLabs (Gaithersburg, MD) for histopathology examination. Grades of liver damage and necrosis were characterized according to the criteria as previously reported [34]. The level of ALT was measured in the plasma of each mouse using the clinical IDEXX Vet Test chemistry analyzer system (IDEXX Laboratories, West Brook, ME).
2.3 Rat liver proteome extraction and trypsin digestion
A liver tissue sample from each rat was subjected for proteome extraction. Briefly, the tissue was mixed with ~1–2 mL of the lysis buffer (8 M urea, 50 mM NH4HCO3, pH 8.0) and was ground on ice using a Dounce tissue grinder (Wheaton Science International, Millville, NJ). The mixture was further disrupted using a Branson Sonifier equipped with a microtip probe (Branson, Danbury, CT) on ice. The resulting lysate was centrifuged at 15 000 × g for 20 min at 4°C, and the resulting supernatant was collected. The protein concentration was measured by the BCA protein assay. An aliquot of cell lysate of 1.5 mg protein was diluted fourfold using ~1 mL of 25 mM NH4HCO3 solution, and then digested with trypsin (Promega, Madison, WI) at 37°C for 16 h using a protein to enzyme ratio of 50:1 (w/w). The resulting peptides were further cleaned with Alltech Extract-Clean SPE C18 HC columns (Grace, Deerfield, IL). Peptide concentration was determined by the BCA assay. The peptide samples were then aliquoted, lyophilized, and stored at −80°C. The above procedure was performed for four rat liver tissue samples from the 6 h (control), 6 h (100 mg/kg APAP), 6 h (1250 mg/kg APAP), 24 h (control), and 24 h (100 mg/kg APAP) groups, respectively, and six rat liver tissue samples from the 24 h (1250 mg/kg APAP) group. Additional samples were processed in this latter group due to the heterogeneity of the liver damage.
2.4 Trypsin-catalyzed 16O/18O labeling
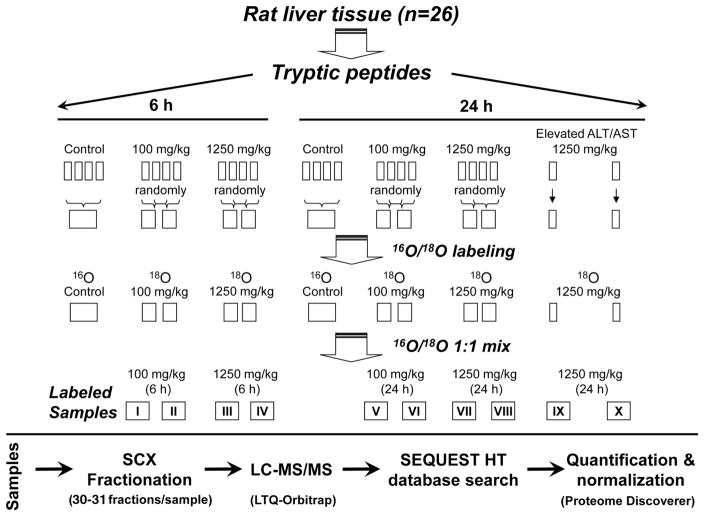
Prior to labeling, equal amounts of tryptic peptides from each rat in each dose/time group were pooled as follows (Fig. 1): control samples were combined as one pool for each time point (6 and 24 h) and samples from APAP-treated rats were pooled according to the treatment dose and time point but randomly within each treatment group. Briefly, every two samples were combined randomly for the APAP treatment groups of 6 h (100 mg/kg APAP), 6 h (1250 mg/kg APAP), and 24 h (100 mg/kg APAP). For the 24 h (1250 mg/kg APAP) group, two rats showed significant elevation of serum ALT and AST; therefore, these two samples were kept separate, but the remaining four samples were pooled into two samples as described above. Peptide C-terminal 16O/18O labeling was performed as described previously [35], which generated two 16O-labeled control samples and ten 18O-labeled APAP-treatment samples as a result of pooling strategy. The 16O- and 18O-labeled samples were stored separately at −80°C prior to further analysis.
Figure 1.
Rat tissue sample pooling strategy, 16O/18O peptide C-terminal labeling, and LC-MS/MS experiment workflow. Liver samples from the two rats with elevated levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were not pooled.
2.5 Strong cation exchange liquid chromatographic fractionation and nanoflow RP LC-MS/MS analysis
Prior to strong cation exchange liquid chromatographic fractionation, the 16O-labeled controls and 18O-labeled APAP-treated samples were dissolved in 25% CH3CN/0.1% formic acid and combined in pairs according to their dose and time points, resulting in ten 16O/18O sample pairs totally as specified in Fig. 1. Sample fractionation was performed following the method described previously [36], and 30–31 fractions were collected at 2-min intervals for each sample. The fractions were lyophilized and stored at −80°C for LC-MS/MS analysis. Nanoflow RPLC separation of peptides was conducted using the 9 cm long × 75 μm inner diameter C18 capillary ESI column that was coupled online to an Orbitrap mass spectrometer (LTQ-Orbitrap XL; Thermo Electron, San Jose, CA) for MS/MS analysis of each strong cation exchange liquid chromatographic fraction according to the method described previously [36].
2.6 Peptide identification, quantitation, normalization, and statistical analysis
The raw MS/MS data were searched using the SEQUEST HT running under Proteome Discoverer (version 1.4; Thermo Scientific, San Jose, CA) against a rat IPI proteome database (version 3.78, containing 39 473 protein sequence entries) downloaded from the European Bioinformatics Institute (http://www.ebi.ac.uk). Reversed protein sequences of all the protein entries were added to the same database for evaluation of the peptide false discovery rate (FDR). Peptide mass tolerance of 10 ppm and fragment ion tolerance of 1 Da were set with tryptic specificity allowing two missed cleavages. A dynamic 4.0085 Da modification on the C-terminus was set in a single search to identify both 18O-labeled peptides and peptides with normal C-termini. In addition, dynamic oxidation of Met by the addition of one oxygen (+15.9949 Da) was included. SEQUEST HT search criteria were Xcorr ≥ 1.7 for [M + H]1+ ions, ≥ 2.5 for [M + 2H]2+ ions, ≥ 3.2 for [M + 3H]3+ ions, and ≥ 3.5 for [M + 4H]4+ ions, and FDR < 1 % for identification of fully tryptic peptides. Legitimately identified proteins were quantified using Proteome Discoverer, which calculated the relative abundance (ratio of 18O/16O, heavy/light) of each identified peptide pair based on the areas of their extracted ion chromatograms. The abundance ratio of a protein was then calculated based on the median ratio of the uniquely quantitated peptides from the same protein. Proteins were grouped to represent unique proteins. Only the peptides that were unique to a specific protein were considered for protein quantification. The protein ratios were normalized to the median protein ratio of all the proteins quantified within the control-treatment pair. Next, the replicate datasets from each dose and time point were combined, and this resulted in five final proteome quantification datasets: Dataset 1 (6 h, 100 mg/kg APAP), Dataset 2 (6 h, 1250 mg/kg APAP), Dataset 3 (24 h, 100 mg/kg APAP), Dataset 4 (24 h, 1250 mg/kg APAP without elevated serum ALT and AST levels), and Dataset 5 (24 h, 1250 mg/kg APAP with elevated serum ALT and AST levels). An average protein ratio and coefficient of variation (CV, %) of each unique protein were calculated among the replicates, and Student’s t-test was performed. The average of CV was 10–16% for quantified datasets. To improve accuracy, the peptides carrying Cys residues were not included in quantification to avoid potential interference from APAP adducts on Cys residues (APAP-Cys). In addition, peptides carrying Met residues or miss cleavage sites were discarded, and protein quantification data were considered valid only if the total count of quantification (heavy/light count) was two or more in each dataset. The threshold to define differentially expressed proteins was set as 1.4-fold change with p < 0.05. Thus, the percentage change in protein abundance was more than two times of average quantification CV.
2.7 Pathway and molecular pathology analysis
To investigate the pathways and toxic pathologies at the molecular level, the differentially expressed liver proteins between the control and APAP-treated rats were analyzed using the MetaCore (GeneGo, St. Joseph, MI) program. MetaCore is an integrated software suite for functional analysis of experimental data and contains curated protein interaction networks on the basis of manually curated database of human, mouse, rat protein–protein, protein–DNA, protein–RNA, and protein–compound interactions.
2.8 Western blot validation
Several proteins of significant interest were selected for verification by Western blotting. Equivalent amounts of liver tissue lysates from rats were separated by SDS-PAGE using NuPAGE Novex 4–12% Bis-Tris precast gels (Life Technologies, Grand Island, NY). After separation, proteins were transferred onto PVDF membranes (Life Technologies, Grand Island, NY) using a Mini Trans-Blot Cell device (Bio-Rad, Hercules, CA). The membranes were incubated with primary antibodies against rat carbonyl reductase [NADPH] 1 (CBR1), lymphocyte cytosolic protein 1 (plastin L) (LCP1), biliverdin reductase A (BLVRA), heme oxygenase 1 (HMOX1) (Abcam Inc., Cambridge, MA), or β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), and followed by incubation with the appropriate secondary IgG-antibodies conjugated with HRP (Santa Cruz Biotechnology, Santa Cruz, CA). Protein abundance in blots was measured using ECL detection substrate (Pierce, Rockford, IL) by a FluorChem E System (ProteinSimple, Santa Clara, CA). Protein band intensities were normalized by β-actin as the loading and membrane transfer control, and statistical analysis was performed using Student’s t-test.
2.9 ELISA measurement of plasma HMOX1
The levels of HMOX1 protein in the rat and mouse plasma were quantified using a rat HMOX1 ELISA kit (Enzo Life Sciences, Farmingdale, NY) and a mouse HMOX1 SimpleStep ELISA kit (Abcam, Cambridge, MA), respectively. The absorbance was measured at 450 nm on a synergy H4 Hybrid microplate reader (Bio-Tek, Winooski, VT).
2.10 Measurement of serum levels of the major APAP metabolites
The serum levels of major APAP metabolites in rats were measured following the method described previously [37]. A 3 μL aliquot of serum supernatant after methanol precipitation was introduced into an Acquity Ultra Performance Liquid Chromatography (UPLC) system (Waters, Milford, MA) equipped with a Waters bridged ethyl hybrid C8 column (1.7 μm particles) with a dimension of 2.1 mm × 10 cm. The mass spectrometric data were collected with a Q-Tof Premier mass spectrometer (Waters) operated in negative ionization electrospray. Raw UPLC-MS/MS data were analyzed using MarkerLynx XS Application Version 4.1 (Waters, Milford, MA) with extended statistical tools. APAP metabolites were identified based on the accurate m/z measurement, fragmentation mass spectra, and isotope patterns. The metabolites were semiquantitatively measured based on the detected intensity.
3 Results
3.1 Histopathology and changes in serum ALT and AST levels in rats
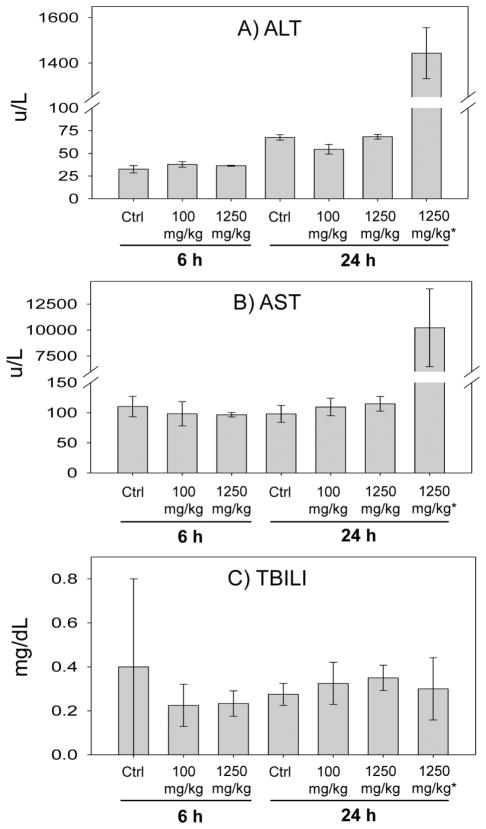
Serum ALT and AST levels were measured to evaluate liver toxicity as the result of APAP treatment in rats at different doses and time points. As shown in Fig. 2, the levels of ALT (1444 ± 112 U/L) and AST (10 230 ± 3748 U/L) were significantly elevated in two of the rats in the 24 h, 1250 mg/kg treated group. The levels of ALT and AST in the remaining rats were not changed significantly compared to the corresponding control groups regardless of posttreatment time and APAP dose. No obvious changes in total bilirubin (TBILI) level were observed in any rat. Histopathology showed centrilobular necrosis of a moderate severity in the liver of the two rats (Fig. 3, Table 1) with elevated serum ALT and AST levels. One of these two rats had a moderate severity of centrilobular degeneration.
Figure 2.
Levels of serum ALT, AST, and total bilirubin (TBILI) in the control and the rats at 6- and 24-h posttreatment with low (100 mg/kg) and high (1250 mg/kg) doses of APAP. *The two rats with elevated serum ALT and AST levels are displayed separately from the other rats treated with the same high dose of APAP (1250 mg/kg). N = 4 except for the two rats in the 24 h, 1250 mg/kg treatment group with elevated serum ALT and AST levels.
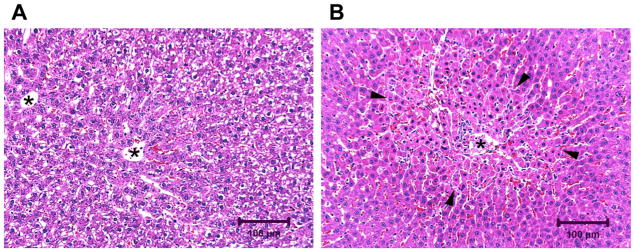
Figure 3.
Representative microscopic liver tissue images from the rats at 24-h post-APAP treatment with a dose of 1250 mg/kg. (A) Liver from a rat without elevation of serum ALT and AST. Note the absence of hepatocyte necrosis and the presence of vacuolated cytoplasm in hepatocytes consistent with postprandial glycogen accumulation (rats were fed ad libitum 4 h after dosing). (B) Liver from a rat with elevation of serum ALT and AST levels. Note the band of necrotic hepatocytes (black arrowheads) surrounding the central vein. Central veins are labeled with an asterisk (hematoxylin and eosin stain).
Table 1.
Histopathology analysis of rat liver tissues used for proteomic analysis
| Hours after dosing (h) | APAP dose (mg/kg) | Degeneration (centrilobular) | Necrosis (centrilobular) | ALT and AST |
|---|---|---|---|---|
| 6 | 0 | 0/4 | 0/4 | Low |
| 100 | 2/4 (minimal) | 0/4 | Low | |
| 1250 | 3/4 (minimal) | 0/4 | Low | |
| 24 | 0 | 0/4 | 0/4 | Low |
| 100 | 0/4 | 0/4 | Low | |
| 1250 | 2/4 (minimal) | 1/4 (minimal) 1/4 (mild) |
Low | |
| 1250 | 1/2 (moderate) | 2/2 (moderate) | High |
Data in the table are presented as no. of affected rats/no. of examined rats (severity of affected animals).
3.2 Proteomic changes of rat liver at 6-h post-APAP treatment
To identify protein abundance changes in the liver associated with APAP treatment, trypsin-catalyzed 16O/18O labeling was employed as described previously to achieve reliable and confident protein quantitation. The analysis resulted in quantitation of 1362 and 1387 unique proteins from liver tissues of rats at 6-h posttreatment with the low (100 mg/kg, Dataset 1) and high (1250 mg/kg, Dataset 2) doses of APAP, respectively (Supporting Information Tables S1 and S2 and S6 and S7). Among them, 204 and 217 proteins were differentially expressed with a fold change of ≥ 1.4 and p < 0.05 in the low and high APAP-dosed animals, respectively. Pathway analysis of these differentially expressed proteins using the MetaCore program indicated that diverse pathways were slightly changed. Among them, NF-E2-related transcription factor 2 (NRF2) regulation of oxidative stress response, oxidative phosphorylation, granzyme A signaling on apoptosis and survival, and APAP metabolism were the mostly affected pathways, although the perturbation was not significant (FDR < 0.01). These results suggest that an early stage response of proteins to APAP treatment is scattered at the pathway level. Molecular toxicity analysis using the MetaCore software revealed that liver necrosis was associated only with the high dose of APAP treatment, although it was not prominent (Supporting Information Fig. S1).
3.3 Proteomic changes in rat liver at 24-h post-APAP treatment
Quantitative proteomic analysis of rat liver tissues at 24-h post-APAP treatment resulted in reliable quantitation of 1097, 969, and 960 proteins from the low dose (100 mg/kg, Dataset 3), high dose (1250 mg/kg, Dataset 4), and high dose (1250 mg/kg with elevated serum ALT and AST levels, Dataset 5) treatments (Supporting Information Tables S3–S5 and S8–S10). Using a 1.4-fold cutoff and p < 0.05, 65, 106, and 156 proteins were differentially expressed in Datasets 3, 4, and 5, respectively.
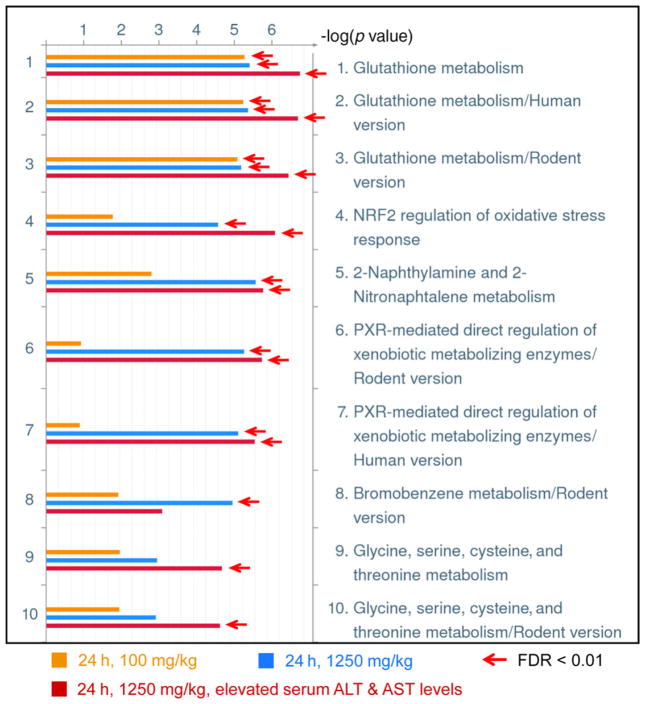
As expected, pathway analysis of differentially expressed proteins from the datasets of 24-h post-APAP treatment revealed that multiple pathways were significantly impacted by APAP treatment (FDR < 0.01). The most significantly changed pathways were from the high-dose treated animals with elevated serum ALT and AST, followed by high-dose treatment without serum ALT and AST elevation and low-dose treatment (Fig. 4). The glutathione metabolism was the most significantly affected pathway in all the 100 and 1250 mg/kg APAP-treated animal groups (Fig. 4). In comparison to the animals at 6-h post-APAP treatment, many enzymes catalyzing glutathione conjugation or synthesis were down-regulated at 24 h (Table 2); suggesting cellular detoxifying capability was decreased at 24 h after APAP treatment. NRF2 regulation of oxidative stress response, 2-naphthylamine and 2-nitronaphtalene metabolism, and PXR-mediated direct regulation of xenobiotic metabolizing enzymes were significantly impacted only by the high-dose treatment. Interestingly, change in the glycine, serine, cysteine, and threonine metabolism pathway was associated only with the high-dose treatment group that showed elevated serum ALT and AST. All these data indicate molecular pathway changes are closely associated with the degree of liver injury and pathological changes.
Figure 4.
GeneGo pathway/map analysis of significantly changed liver proteins (≥ 1.4-fold and p < 0.05) in the rats at 24-h posttreatment with low (100 mg/kg) and high (1250 mg/kg) doses of APAP. The arrows denote the experiment mapping passed the threshold of FDR < 0.01.
Table 2.
Expression changes in proteins involved in the glutathione metabolism pathway
| Gene symbol | Protein name | 100 mg/kg, 6 h | 1250 mg/kg, 6 h | 100 mg/kg, 24 h | 1250 mg/kg, 24 h | |
|---|---|---|---|---|---|---|
|
|
|
|
|
|||
| 1 | 2 | 3 | 4 | 5a) | ||
| OPLAH | 5-Oxoprolinase (ATP-hydrolysing) | +1.02 | +1.28 | −1.07 | – | – |
| GLCLC | Glutamate-cysteine ligase, catalytic subunit | +1.56* | +1.41 | – | – | −9.74* |
| GLCLR | Glutamate-cysteine ligase regulatory subunit | +1.23 | +2.38 | −1.20 | +1.55* | +1.69* |
| GCTG | γ-Glutamylcyclotransferase | +1.15 | – | – | – | – |
| GSHB | Glutathione synthetase | – | – | – | – | −2.05 |
| AMPN | Alanyl (membrane) aminopeptidase | – | +1.62 | – | – | – |
| GSHR | Glutathione reductase | – | +1.19 | −1.01 | +1.27 | +1.06 |
| G6PD | Glucose-6-phosphate dehydrogenase | −1.10 | – | – | – | – |
| GPX1 | Glutathione peroxidase 1 | −1.12 | −1.28 | +1.01 | +1.01 | −1.01 |
| GPX4 | Glutathione peroxidase 4 | – | – | −1.05 | – | −1.27 |
| Glutathione S-transferase | ||||||
| MGST1 | Microsomal glutathione S-transferase 1 | +1.20 | +1.48* | −1.36 | −1.27 | −1.26 |
| GSTA5 | Glutathione S-transferase α 5 | +1.34 | – | −3.31* | −1.67* | −2.79* |
| GSTA1 | Glutathione S-transferase α 1 | – | +1.17 | −1.93 | −1.83* | −1.61* |
| GSTA2 | Glutathione S-transferase α 2 | +1.06 | – | −1.52* | – | – |
| GSTM2 | Glutathione S-transferase, mu 2 | – | – | +1.33 | +1.41* | +1.34 |
| GSTM3 | Glutathione S-transferase, mu 3 | – | – | −2.80* | – | – |
| GSTM1 | Glutathione S-transferase, mu 1 | −1.23 | −1.64 | −1.16 | +1.12 | −1.07 |
| MAAI | Maleylacetoacetate isomerase | – | +1.18 | −1.57 | −1.36 | −2.26* |
| GSTO1 | Glutathione S-transferase ω 1 | – | – | −1.74* | −2.68* | −2.43* |
| GSTK1 | Glutathione S-transferase κ 1 | −1.02 | +1.02 | +1.31 | +1.39 | +1.20 |
| GSTT2 | Glutathione S-transferase ϑ 2 | −1.22 | −1.32 | −1.05 | −1.26 | −1.42* |
| GSTT1 | Glutathione S-transferase ϑ 1 | +1.16 | +1.09 | +1.19 | +1.17 | −1.00 |
| GSTP1 | Glutathione S-transferase P | – | −1.06 | −1.06 | −1.11 | +1.25 |
The group showing elevated serum ALT and AST levels. A fold change of ≥ 1.4 (treatment/control) and p-value < 0.05 is considered to be significant and marked with an asterisk (*). A positive fold change represents upregulation and a negative fold change indicates downregulation upon APAP treatment.
“—”, not detected or quantified by LC-MS/MS.
Molecular toxicity analysis for the differentially expressed proteins revealed that liver necrosis, increased mitosis, microvesicular lipid accumulation, bile duct injury, lipid peroxidation, and hyperplasia were the top pathological responses, which were associated only with the rats treated with high dose of APAP (Supporting Information Fig. S2). No statistically significant pathological responses (FDR < 0.01) were observed at the molecular level for the low-dose treatment. In addition, liver macrovesicular lipid accumulation and liver inflammation were only associated with high-dose treated rats with elevated serum ALT and AST (Supporting Information Fig. S2). Taken together, these data show that molecular pathology responses are well correlated with APAP doses and the degree of liver toxicity.
Since two rats had elevated serum ALT and AST levels and liver tissue necrosis, protein changes unique in these animals could be more closely associated with liver damage and an indication of such pathology. To this end, the significantly regulated proteins from the group of rats treated with the high dose and with elevated serum ALT and AST (Dataset 5) were compared with the quantitative proteome data from the groups of low- and high-dosed rats without serum ALT and AST elevation at 24-h post-APAP treatment (Dataset 3 and 4). After statistical analysis using Student’s t-test, 31 proteins were significantly differentiated in the rats with elevated serum ALT and AST from the other two groups based on p < 0.05 (Table 3). These proteins were associated with observed liver injury in rats.
Table 3.
Proteins with expression change correlated to elevation of serum ALT and AST levels
| Accession | Protein | Gene symbol | Fold changea) | p-value | Biomarker applicationb) | Hepatic disease relatedb) |
|---|---|---|---|---|---|---|
| D3ZN29 | AHNAK nucleoprotein isoform 1 | AHNAK | 2.10 | 5.90 × 10−5 | ||
| P13601 | Aldehyde dehydrogenase, cytosolic 1 | ALDH1A7 | 2.01 | 3.39 × 10−2 | ||
| D3Z9Z0 | Ankyrin 1 | ANK1 | 2.23 | 4.31 × 10−5 | ||
| P47727 | Carbonyl reductase [NADPH] 1 | CBR1 | 1.62 | 3.74 × 10−2 | ||
| Q66SY1 | Clathrin-assembly lymphoid leukemia protein | PICALM | 1.43 | 1.41 × 10−3 | ||
| P45592 | Cofilin-1 | CFL1 | 2.49 | 1.73 × 10−2 | ||
| A2RRT9 | Cytochrome P450 4V3 | CYP4V2 | −1.52 | 1.49 × 10−3 | ||
| Q9WTN7 | Cytochrome P450, family 8, subfamily b, polypeptide 1 | CYP8B1 | 1.80 | 2.00 × 10−2 | ||
| Q9Z122 | Fatty acid desaturase 2 | FADS2 | −7.61 | 2.16 × 10−2 | ||
| A1L114 | Fga protein | FGA | 2.71 | 6.90 × 10−8 | ||
| Q66HT1 | Fructose-bisphosphate aldolase | ALDOB | −1.76 | 1.20 × 10−13 | × | |
| P09606 | Glutamine synthetase | GLUL | −1.45 | 3.79 × 10−9 | ||
| P0DMW0 | Heat-shock 70 kDa protein 1A/1B | HSPA1A | 55.81 | 3.40 × 10−10 | ||
| P42930 | Heat-shock protein beta-1 | HSPB1 | 50.27 | 2.89 × 10−6 | Diagnosis, efficacy | × |
| P82995 | Heat-shock protein HSP 90-alpha | HSP90AA1 | 1.45 | 7.87 × 10−6 | Safety | × |
| P06762 | Heme oxygenase 1 | HMOX1 | 23.63 | 2.77 × 10−5 | Efficacy, safety | × |
| Q0ZHH6-1 | Isoform 1 of Atlastin-3 | ATL3 | −2.10 | 4.48 × 10−4 | ||
| Q63945-2 | Isoform 2 of protein SET | SET | 1.57 | 2.20 × 10−3 | Prognosis | |
| Q3MIB4 | Lon protease homolog 2, peroxisomal | LONP2 | 1.54 | 2.39 × 10−5 | ||
| Q920P0 | L-xylulose reductase | DCXR | −1.80 | 8.45 × 10−6 | ||
| Q5XI38 | Lymphocyte cytosolic protein 1 | LCP1 | 2.35 | 9.61 × 10−3 | Disease progression | |
| D3ZS58 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2 | NDUFA2 | 1.47 | 3.03 × 10−2 | ||
| P50116 | Protein S100-A9 | S100A9 | 11.86 | 1.18 × 10−4 | Diagnosis | |
| Q499R7 | Pyrophosphatase 1 | PPA1 | −2.21 | 8.56 × 10−5 | ||
| D3ZA81 | SAM domain and HD domain-containing protein 1 | SAMHD1 | 1.74 | 8.88 × 10−3 | ||
| Q6AYR8 | Secernin-2 | SCRN2 | 1.65 | 1.11 × 10−3 | ||
| Q5M878 | Serum amyloid A protein | HPS5 | 2.41 | 1.05 × 10−4 | ||
| Q9WUW9 | Sulfotransferase 1C2A | SULT1C2A | −2.61 | 1.54 × 10−2 | ||
| Q63199 | Tumor necrosis factor receptor superfamily member 6 | FAS | 1.41 | 4.30 × 10−2 | Diagnosis, disease progression, efficacy | × |
| P08430 | UDP-glucuronosyltransferase 1–6 | UGT1A6 | 1.44 | 3.33 × 10−2 | ||
| Q5I034 | Uncharacterized protein C12orf43 homolog | RGD1311899 | 2.04 | 4.17 × 10−3 |
Fold change listed was from dataset Dataset 5 (24 h, 1250 mg/kg APAP, elevated serum ALT and AST levels) (see Section 2). A positive fold change represents upregulation and a negative fold change indicates downregulation upon APAP treatment.
Annotation from ingenuity pathway analysis.
3.4 Validation of uniquely changed proteins from damaged rat liver
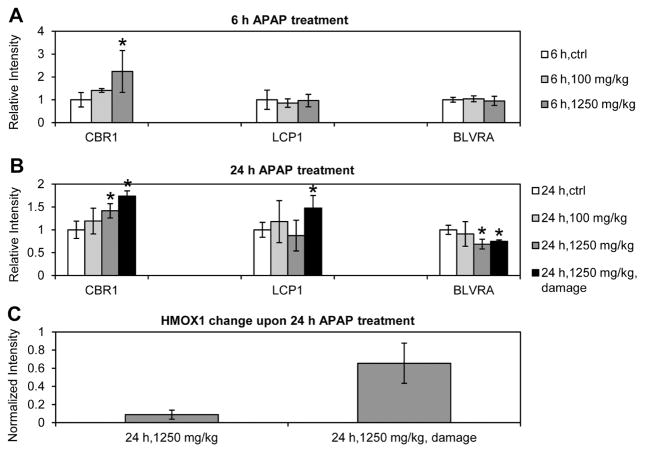
HMOX1, carbonyl reductase [NADPH] 1 (CBR1), and LCP1 (L-plastin) were selected for further validation from the list of 31 uniquely changed proteins in the rats with damaged liver and elevated serum ALT and AST levels (Table 3). Since BLVRA is another essential enzyme involved in heme catabolism downstream of HMOX1 [38], this protein was also included in the verification experiment. Western blot was performed for the four proteins in all the individual rats treated with low and high doses of APAP at both 6- and 24-h time points. Although the proteins were not quantitated in one or more time points during MS measurement, Western blot provided relative abundance of those four proteins at 6- and 24-h time points. For those quantifiable by MS, the Western blot (Fig. 5 and Supporting Information Fig. S3) were consistent with the corresponding 16O/18O LC-MS/MS data (Supporting Information Table S11). HMOX1 was detected by Western blot in liver tissues only in the rats at 24-h post-APAP high-dose treatment. It was elevated in all these rats but was dramatically increased in the animals with elevated serum ALT and AST, which was consistent with the MS results. In addition, BLVRA was found to be decreased at 24-h posttreatment with a high dose of APAP (1250 mg/kg).
Figure 5.
Western blot analysis of protein expression changes upon APAP treatment. Expression of CBR1, LCP1, and BLVRA at 6-h (A) and 24-h (B) post-APAP treatment. (C) HMOX1 expression at 24-h post-APAP treatment with a dose of 1250 mg/kg. Protein band intensity was normalized by the loading control (β-actin). The normalized intensity was compared to the APAP treatment control and expressed as a relative intensity or fold change to the control. Standard deviation was calculated, and t-test was performed comparing to the control sample. *p < 0.05. N = 2 in the 24 h, 1250 mg/kg treatment group with elevated serum ALT and AST levels (24 h, 1250 mg/kg, damage). For all the other treatment and control groups, n = 4. HMOX1 protein was detected only in the high dose groups at 24-h post-APAP treatment, thus only normalized intensities are displayed.
3.5 Abundance levels of HMOX1 in plasma
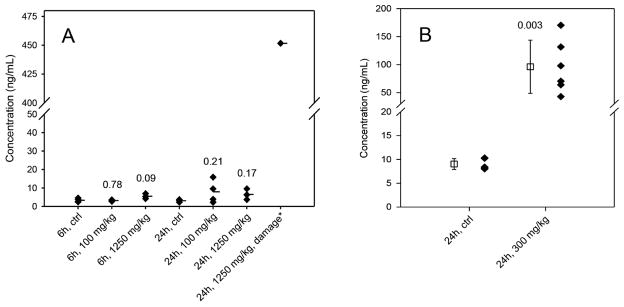
A rat HMOX1 ELISA was used to quantify levels of this protein in rat plasma to examine whether APAP overdose caused abundance changes that could suggest this protein be a plasma biomarker of liver toxicity. For the rats subjected to APAP treatment for 24 h, an ≈ 150-fold increase in plasma HMOX1 (451.7 ng/mL) was observed in the rat with elevated serum ALT and AST levels as compared to the control (3.0 ng/mL) (Fig. 6A). However, the plasma HMOX1 levels were 7.9 ng/mL and 6.5 ng/mL, respectively, in other rats subjected to APAP treatment for 24 h with low and high doses that did not exhibit a significant increase in ALT and AST levels, and this change was not statistically significant from the corresponding control (Fig. 6A). For the rats at 6-h post-APAP treatment, the fold change in plasma HMOX1 level (≈1.7-fold, 5.5 ng/mL) resulted from high-dosage (1250 mg/kg) treatment was not statistically significant (p = 0.09) compared to the control (3.3 ng/mL), and no HMOX1 changes were observed in the low-dose (100 mg/kg) treated rats (3.2 ng/mL). Overall, the change in plasma HMOX1protein level was correlated with its expression in liver tissue and the degree of liver damage upon APAP treatment.
Figure 6.
The plasma protein concentrations (ng/mL) of HMOX1 measured by ELISA. (A) Plasma HMOX1 levels from the control and the rats at 6- or 24-h post-APAP treatment with a dose of 100 or 1250 mg/kg. The average concentrations are marked as the horizontal bars (−). *The group with elevated serum ALT and AST levels. (B) Plasma HMOX1 levels from the control and the mice at 24-h post-APAP injection with a dose of 300 mg/kg. The square with error bars is mean ± SD for each group. The numbers displayed in the graph A and B are the p-values from the t-test comparing to the control groups.
To further verify HMOX1 as a potential plasma biomarker of liver injury and toxicity in response to APAP, 129/SvJ mice were i.p. injected with either saline solution (control, n = 5) or 300 mg/kg APAP (n = 6) and liver injury was evaluated at 24-h posttreatment. We intentionally chose this toxic dose and APAP delivery approach to ensure increased liver damage with elevated ALT levels based on the rat’s study results where there was a positive correlation between the increased levels of HMOX1 in rats and the liver toxicity. Injection of APAP resulted in significant elevation of plasma ALT in all the treated mice with an average level of 2833 ± 1339 IU/L, which was ~88-fold increase as compared to that in the control (32 ± 11 IU/L) (Supporting Information Fig. S4). Accordingly, grade 2 and grade 3 liver damage (Supporting Information Table S12) and hepatocyte necrosis were observed in the liver tissues from APAP-treated mice but not from the control mice (Supporting Information Fig. S5). ELISA measurement showed that HMOX1 was dramatically increased in the plasma of all APAP-treated mice with an average level of 96.3 ng/mL, which corresponds to an average of ~11-fold increase as compared to the control (9.0 ng/mL) (Fig. 6B). A complete separation of the liver-injured mice from the control mice was achieved based on the plasma HMOX1 levels. Together, these data are in agreement with the results from rats exposed to low and high doses of APAP and further support the notion of HMOX1 as a potential biomarker for APAP-induced liver injury.
4 Discussion
4.1 Histopathology and molecular toxicity analyses
In this study, groups of four to six rats were treated with low (100 mg/kg) and high (1250 mg/kg) doses of APAP for either 6 or 24 h via oral administration. Results indicate that different rats did not uniformly respond to the same dose of APAP. Histopathology analysis of the rats in the high-dose group at 24 h found two rats with moderate centrilobular necrosis in the liver, two with minimal to mild centrilobular necrosis, and the remaining two rats did not have obvious liver damage (Table 1). Consistent with histopathology, significant increases in serum ALT and AST levels were only observed in the two rats with moderate liver necrosis (Fig. 2). These results indicate that the liver injury was induced by oral APAP treatment in these two rats.
In line with the histopathology, molecular toxicity analysis of liver tissues based on differentially expressed proteins revealed that liver toxicity was observed only in the rats treated with a high dose of APAP, especially at 24-h posttreatment (Supporting Information Figs. S1 and S2). However, molecular analysis did indicate liver pathology (e.g., necrosis) occurred at 6-h posttreatment and in the rats at 24-h posttreatment without elevation of serum ALT and AST levels, suggesting molecular toxicity analysis is more sensitive than traditional histopathology.
Hepatic necrosis, rather than hepatic apoptosis, seems to be the main outcome in response to toxic doses of APAP [39], which is in accordance with the massive necrosis we monitored in response to the high doses of APAP. The large increase in serum ALT and AST levels is usually indicative of lysis of hepatocytes (liver necrosis) [40], a process in which plasma membrane disrupts accompanied by leakage of ALT and AST into bloodstream [41]. Results from this study showed that ALT was not changed significantly in rat liver tissues upon APAP treatment at different time points and doses, while AST change in liver (1.56-fold increase) was observed only in the rats with liver damage at 24-h posttreatment with a high dose of APAP, when administered orally (Supporting Information Table S13). These data further confirmed that a large increase in serum ALT and AST was largely relied on the release of these enzymes into blood due to hepatocellular necrosis. There are several events through which the mechanism of APAP -mediated hepatic necrosis occurs. The APAP is first metabolized by cytochrome P450 including 2E1, 1A2, 3A4, and 2D6 to the major reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI). This reactive metabolite can then decrease the levels of glutathione while it increases covalently binding to many proteins [39,42]. The decreased levels of glutathione are also associated with increased levels of oxidative stress and nitrative stress [39, 43, 44]. The hepatic areas with high oxidative stress were found to undergo necrotic changes [39]. The increased oxidative stress can also lead to disturbance in calcium homeostasis as well as the activation of cell-death associated JNK signaling pathways, both of which can lead to mitochondrial membrane transition and increased mitochondrial permeability, ultimately leading to decreased levels of ATP and hepatic necrosis [39,45,46]. Thus, mitochondrial dysfunction in response to oxidative stress, at least partially, seems to play a central role in hepatic cell necrosis [44].
4.2 Time- and dose-dependent molecular pathway changes
Over 200 proteins changed abundance in liver at 6-h post-treatment with the low or high dose of APAP, although these proteins were scattered among a variety of pathways and there was no significant impact at the pathway levels. However, more focused pathway changes were observed at 24 h (Fig. 4), including regulation of glutathione metabolism and NRF2 regulation of oxidative stress [47]. The results suggest that a progressive change in molecular pathways took place in the rat liver over time and doses, and a significant focused alteration of pathways at 24-h posttreatment was more related to hepatotoxicity.
NRF2 is a transcription factor that regulates the expression of anti-oxidative and phase II detoxifying enzymes. As an early response to APAP treatment, NRF2 could be activated to protect the liver from oxidative stress induced damage, as illustrated by the increase in antioxidative and detoxifying enzymes (i.e., SOD1, GLCLC, and NQO1) 6-h post-APAP treatment (Supporting Information Table S14). In addition to NRF2 activation by ROS, studies also supported the hypothesis that generation of NAPQI, the major APAP metabolite, could activate NRF2 expression by consuming GSH or modifying cysteine residues in Kelch-like ECH-associated protein 1 (KEAP1) [47,48]. However, a recent study indicated that NRF2 amplifies oxidative stress through induction of Kruppel-like factor 9 (Klf9) in response to elevation of intracellular ROS above a critical threshold [49]. Therefore, the cellular protection function of NRF2 could be limited to a certain threshold. NRF2 could lose the ability to protect cells from injury due to excessive ROS, as the results from this study revealed that the levels of NRF2-targeted antioxidative and detoxifying enzymes (e.g., SOD1, GLCLC, GSTA1, and GSTA5) were decreased in the rats at 24-h post-APAP treatment compared to either the control or 6-h post-APAP treatment. This transition of the NRF2 signaling could eventually lead to liver damage.
As discussed earlier, the NRF2 target NQO1 was upregulated in rats at 6 h but downregulated at 24-h post-APAP treatment. Metabolism of APAP to form NAPQI by the cytochrome P450 enzymes is believed to cause APAP hepatotoxicity [50, 51]. NAPQI could be converted enzymatically back to APAP by NQO1 [52]. Decreased NQO1 might suggest that the liver lacked the ability to convert NAPOI back to APAP. This study also examined metabolites in these rats and found that serum levels of all the three major APAP-metabolized products, namely, APAP O-glucuronide, APAP sulfate, and N-acetyl-L-cysteine APAP were significantly higher in the high-dose treatment at both 6- and 24-h time points than in the low-dose group (Supporting Information Fig. S6). APAP-NAC was the end product of (acetaminophen-2-hydroxyphenyl)-glutathione, which is the intermediate metabolite formed through the NAPQI and glutathione conjugation. We found glutathione S-transferases (GSTA5, GSTA1, GSTA2, GSTM3, MAAI, GSTO1, and GSTT2) and catalytic subunit of glutamate-cysteine ligase (GLCLC) were all downregulated in the rats at 24 h post-APAP treatment, especially in the high-dose group (Table 2). These data suggest cellular detoxifying capacities were decreased after 24 h of APAP treatment. Taken together, functional transition of NRF2, exhaust of antioxidants such as glutathione, and excessive ROS impaired the cellular antioxidant defense system, which would ultimately contribute to the APAP-induced liver injury.
4.3 Proteins uniquely changed in liver injury
In this 16O/18O labeling based LC-MS/MS study of rat tissues, 31 proteins were classified as the proteins with expression changes correlated to moderate liver necrosis (Table 3). In other words, these proteins were associated with APAP-induced hepatotoxicity and could be potential biomarkers of acute liver injury because the differentially expressed proteins that were associated with adaptive response or noninjury related physiological response were eliminated in this study. By comparing to the differentially expressed proteins in damaged rat livers from the APAP adaptation study by Eakins et al. [28], it was found that 20 proteins were identified solely from this study. The remaining 11 proteins (cofilin-1, fructose-bisphosphate aldolase, glutamine synthetase, HSP70, HSP beta-1, HSP 90-alpha, HMOX1, protein SET, L-xylulose reductase, sulfotransferase 1C2A, and UDP-glucuronosyltransferase 1–6) were also identified previously by Eakins et al. The abundance changes in all the 11 proteins were consistent between the two studies, suggesting reliable protein quantification of both studies. By comparing to the APAP toxicity study in rats by Kikkawa et al. [27], fructose-bisphosphate aldolase and HSP70, which were among the 11 proteins, were identified in Kikkawa’s study as well. These 11 previously identified proteins (identified in this study as well) and 20 newly identified proteins constitute a potential novel panel of biomarkers of acute liver injury. From Ingenuity Systems database (www.ingenuity.com), seven of the proteins were reported as biomarkers of diseases and treatment or have such potential applications, and five proteins are known to be related to hepatic diseases. Proteins CBR1, LCP1, HMOX1, and BLVRA were selected and confirmed by Western blot experiment.
Carbonyl reductases metabolize reactive carbonyls substrates, such as environmental quinones and pharmacologically relevant metabolites [53]. In our study, CBR1 was induced by high-dose APAP treatment in the rat liver at both 6-and 24-h posttreatment (Fig. 5). By comparing the high-dose APAP-treated rats at 24 h, the level of CBR1 was higher in the group with elevated serum ALT and AST levels. Since the toxic APAP metabolite NAPQI has carbonyl groups [50], increased expression of CBR1, which metabolizes reactive carbonyl substrates [53], could represent a compensatory mechanism in response to increased NAPQI.
LCP1 is an actin-binding protein. Its expression correlates with tumor stages in several cancers [54]. In addition, LCP1 was reported upregulated in the liver from mice with hepatosplenic schistosomiasis [55], and LCP1 mRNA was reported to be increased by 300% in the liver samples from patients with nonalcoholic fatty liver disease [56]. In this study, LCP1 was upregulated only in the 24-h APAP-treated rats that showed high serum ALT and AST levels (Fig. 5), suggesting overexpression of LCP1 is associated with liver damage.
4.4 HMOX1 is a potential plasma biomarker of liver injury
As defined by the 2009 FDA’s Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation (http://www.fda.gov/downloads/Drugs/.../Guidances/UCM174090.pdf), elevated levels of serum ALT, AST, and bilirubin are reasonable indications of liver damage or altered function. Heme oxygenase and biliverdin reductase are two essential enzymes involved in heme catabolism [38]. Heme oxygenase catalyzes heme degradation to form biliverdin, and biliverdin reductase converts biliverdin to bilirubin. Expression of HMOX1 has been reported to be upregulated in rat liver between 12 and 36 hours after APAP overdose [57, 58]. In this study, HMOX1 was identified by LC-MS/MS only in the liver samples from the rats with elevated serum ALT and AST levels, and its abundance was increased ~24-fold compared to the control. Western blot confirmed the expression and also detected elevated levels of HMOX1 in liver tissues from other high-dose rats at 24 h in the absence of classical signs of liver injury, as compared to the control. However, the protein abundance in these animals was approximately six- to sevenfold lower than the rats with elevated serum ALT and AST levels (Fig. 5). HMOX1 was not identified in liver tissues from all the other treated rats. In contrast, BLVRA was found, for the first time in this study, to be decreased at 24-h posttreatment with the high-dose APAP. This accounts for the similar total blood bilirubin levels between APAP-treated groups and the control rats (Fig. 2), although HMOX1 is increased. BLVRA is the major physiological antioxidant [59]. As the only inducible heme oxygenase isoform, the induction of HMOX1 is well known to be a cytoprotective response against liver injuries by suppressing oxidative stress and inflammation [60–62]. A recent study showed that a moderate increase in bilirubin had a protective effect against ethanol-induced hepatotoxicity [63]. In this study, decreased expression of BLVRA could counteract HMOX1 increase, thus the cytoprotective effects of HMOX1 and bilirubin could be attenuated. In addition, other reports suggested that the benefit of cytoprotection provided by overexpression of HMOX1 could be reversed by side products of heme degradation, such as iron ions, biliverdin, and carbon monoxide [64, 65]. Taken together, the results from this study indicated that an imbalanced expression of HMOX1 and BLVRA could diminish their cytoprotection functions and even could enhance cytotoxicity due to increased side products of heme degradation.
Besides the potential mechanistic involvement of HMOX1 in liver toxicity, it is interesting to examine if the plasma HMOX1 level increased as a result of its overexpression in the liver following APAP treatment. To explore the potential utility of detecting HMOX1 as a blood biomarker of liver injury, we measured plasma HMOX1 in rats using ELISA. Our results revealed a dramatic increase in plasma HMOX1 (~150-fold) in the group that showed elevated serum ALT and AST levels and liver injury. Plasma HMOX1 had a mild but not statistically significant increase (approximately twofold) in the other rats at 24-h post-APAP treatment (Fig. 6A). Cross-species analysis of HMOX1 in a mouse model of acute liver injury (Fig. 6B) verified that elevation of this protein in plasma was closely correlated with the severity of hepatotoxicity and histopathological changes, although APAP doses, administration routes, ages, and gender were all different between the rats and mice under our study. These discrepancies might account for the differences of exact concentrations and fold changes in plasma HMOX1 between rats and mice, but not affect the potential diagnostic value of HMOX1 as a biomarker of liver injury. In this animal study, liver injury was detected by plasma HMOX1 with 100% sensitivity. Early studies suggested mRNA and protein levels of HMOX1 as potential biomarkers for Alzheimer’s disease [66, 67], renal disease [68, 69], lung injury [70], and inorganic arsenic toxicity [71,72]. To our knowledge, the use of HMOX1 as a plasma biomarker of liver toxicity or injury has not been reported. In this study, we showed for the first time that HMOX1 was detectable in plasma samples. Furthermore, we validated this protein as a promising biomarker of liver injury at the cross-species level, although its clinical specificity and sensitivity require further investigation with a large number of samples.
5 Conclusions
A quantitative proteomic analysis based on 16O/18O labeling and 2D-LC-MS/MS was performed in the liver tissues collected from the rats at 6- and 24-h posttreatment with low (100 mg/kg) and high (1250 mg/kg) doses of APAP. Approximately 1000–1400 proteins were confidently quantified from each treatment group. Molecular toxicity analysis of differentially expressed proteins was found to be more sensitive than traditional histopathology to characterize pathological changes of liver tissues. Molecular pathway analysis revealed that abundance-changed proteins were scattered among diverse pathways at the early time point, but more focused pathway alterations were observed at the later time point, including regulation of glutathione metabolism and NRF2 regulation of oxidative stress. A total of 31 proteins were identified as their expression was uniquely correlated to liver damage, of which 20 were identified only in this study. These proteins could be candidate biomarkers of liver injury. Western blot analysis confirmed hepatic expression changes in CBR1, BLVRA, HMOX1, and LCP1 in rats following APAP treatment. Imbalanced expression of HMOX1 and BLVRA was associated with hepatotoxicity. Elevated plasma HMOX1 was correlated with the degree of liver damage, and marked increase in HMOX1 in plasma was observed in both rats and mice with damaged liver. The results from this study provided insights into the molecular changes in APAP-induced liver toxicity and identified HMOX1 as a potential plasma biomarker of liver injury.
Supplementary Material
Clinical Relevance.
Drug-induced liver injury (DILI) is one of the most frequent causes of drug development failure and withdrawal of approved drugs. Overdose of APAP is well known to cause clinical hepatotoxicity with a potential of acute liver failure. It is critical to detect potential liver injury at an early stage prior to onset of liver failure. By using a rat model treated with different doses of acetaminophen (APAP, a model drug for hepatotoxicity) for different periods of time, we found not only the molecular pathway alterations associated with the degree of liver injury but also the abundance changes of 31 proteins that were correlated to liver damage. Furthermore, we discovered HMOX1 could be a potential plasma biomarker of liver injury via cross-species verification and validation between plasma and liver tissues. Since dramatic increase in HMOX1 in plasma can be detected at moderate liver injury with great sensitivity, this protein could be an early biomarker of liver injury and its clinical diagnostic values could be further explored.
Acknowledgments
This study was supported with funds from the National Center for Toxicological Research, U.S. Food and Drug Administration (NCTR/FDA), Jefferson, Arkansas, and in part with the Intramural funds from National Institute on Alcohol Abuse and Alcoholism, Bethesda, Maryland. The information in this article is not a formal dissemination of information by FDA and does not represent agency position or policy.
Abbreviations
- APAP
N-acetyl-p-aminophenol
- BCA
bicinchonic acid
- BLVRA
biliverdin reductase A
- DILI
drug-induced liver injury
- FDR
false discovery rate
- HMOX1
heme oxygenase 1
- i.p
intraperitoneal
- LCP1
lymphocyte cytosolic protein 1
- NAPQI
N-acetyl-p-benzoquinone imine
- NRF2
NF-E2-related transcription factor 2
Footnotes
The authors have declared no conflict of interest.
Additional supporting information may be found in the online version of this article at the publisher’s web-site
References
- 1.Lee WM. Medical progress: drug-induced hepatotoxicity. New Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 2.Fontana RJ. Approaches to the study of drug-induced liver injury. Clin Pharmacol Ther. 2010;88:416–419. doi: 10.1038/clpt.2010.100. [DOI] [PubMed] [Google Scholar]
- 3.Sgro C, Clinard F, Ouazir K, Chanay H, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 4.Lee WM. Acetaminophen-related acute liver failure in the United States. Hepatol Res. 2008;38:S3–S8. doi: 10.1111/j.1872-034X.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Larson AM, Polson J, Fontana RJ, Davern TJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 6.Russo MW, Galanko JA, Shrestha R, Fried MW, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transplant. 2004;10:1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 7.Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342–349. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 8.Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30:277–294. doi: 10.2165/00002018-200730040-00001. [DOI] [PubMed] [Google Scholar]
- 9.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC Public Policy Committee American A. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 10.Roberts DW, Benson RW, Pumford NR, Potter DW, et al. Sensitive immunochemical assays for monitoring acetaminophen toxicity in humans. ACS Symp Ser. 1991;451:314–326. [Google Scholar]
- 11.Sun J, Sugiyama A, Masuda A, Ochi T, et al. Expressions of protein oxidation markers, dityrosine and advanced oxidation protein products in acetaminophen-induced liver injury in rats. J Vet Med Sci. 2011;73:1185–1190. doi: 10.1292/jvms.11-0088. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Greenhaw J, Shi Q, Su Z, et al. Identification of urinary microRNA profiles in rats that may diagnose hepatotoxicity. Toxicol Sci. 2012;125:335–344. doi: 10.1093/toxsci/kfr321. [DOI] [PubMed] [Google Scholar]
- 13.Moyer AM, Fridley BL, Jenkins GD, Batzler AJ, et al. Acetaminophen-NAPQI hepatotoxicity: a cell line model system genome-wide association study. Toxicol Sci. 2011;120:33–41. doi: 10.1093/toxsci/kfq375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Krausz KW, Idle JR, Gonzalez FJ. Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J Biol Chem. 2008;283:4543–4559. doi: 10.1074/jbc.M706299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KB, Chung MW, Um SY, Oh JS, et al. Metabolomics and biomarker discovery: NMR spectral data of urine and hepatotoxicity by carbon tetrachloride, acetaminophen, and D-galactosamine in rats. Metabolomics. 2008;4:377–392. [Google Scholar]
- 16.Sun J, Schnackenberg LK, Holland RD, Schmitt TC, et al. Metabonomics evaluation of urine from rats given acute and chronic doses of acetaminophen using NMR and UPLC/MS. J Chromatogr B. 2008;871:328–340. doi: 10.1016/j.jchromb.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Schnackenberg LK, Beger RD. Studies of acetaminophen and metabolites in urine and their correlations with toxicity using metabolomics. Drug Metab Lett. 2009;3:130–136. doi: 10.2174/187231209789352139. [DOI] [PubMed] [Google Scholar]
- 18.James LP, Chiew A, Abdel-Rahman SM, Letzig L, et al. Acetaminophen protein adduct formation following low-dose acetaminophen exposure: comparison of immediate-release vs extended-release formulations. Eur J Clin Pharmacol. 2013;69:851–857. doi: 10.1007/s00228-012-1410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton TA, Baker D, Lindon JC, Everett JR, et al. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci USA. 2009;106:14728–14733. doi: 10.1073/pnas.0904489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loo RL, Coen M, Ebbels T, Cloarec O, et al. Metabolic profiling and population screening of analgesic usage in nuclear magnetic resonance spectroscopy-based large-scale epidemiologic studies. Anal Chem. 2009;81:5119–5129. doi: 10.1021/ac900567e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bales JR, Nicholson JK, Sadler PJ. Two-dimensional proton nuclear magnetic-resonance maps of acetaminophen metabolites in human-urine. Clin Chem. 1985;31:757–763. [PubMed] [Google Scholar]
- 22.Kang JS, Jeong YK, Suh SK, Kim JH, et al. Assessment of feasibility for developing toxicogenomics biomarkers by comparing in vitro and in vivo genomic profiles specific to liver toxicity induced by acetaminophen. Mol Cell Toxicol. 2007;3:177–184. [Google Scholar]
- 23.Qiu YC, Benet LZ, Burlingame AL. In: Biological Reactive Intermediates Vi: Chemical and Biological Mechanisms in Susceptibility to and Prevention of Environmental Diseases. Dansette PM, Snyder R, Delaforge M, Gibson GG, et al., editors. 2001. pp. 663–673. [Google Scholar]
- 24.Qiu YC, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940–17953. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- 25.Ruepp SU, Tonge RP, Shaw J, Wallis N, et al. Genomics and proteomics analysis of acetaminophen toxicity in mouse liver. Toxicol Sci. 2002;65:135–150. doi: 10.1093/toxsci/65.1.135. [DOI] [PubMed] [Google Scholar]
- 26.Fountoulakis M, Berndt P, Boelsterli UA, Crameri F, et al. Two-dimensional database of mouse liver proteins: changes in hepatic protein levels following treatment with acetaminophen or its nontoxic regioisomer 3-acetamidophenol. Electrophoresis. 2000;21:2148–2161. doi: 10.1002/1522-2683(20000601)21:11<2148::AID-ELPS2148>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Kikkawa R, Fujikawa M, Yamamoto T, Hamada Y, et al. In vivo hepatotoxicity study of rats in comparison with in vitro hepatotoxicity screening system. J Toxicol Sci. 2006;31:23–34. doi: 10.2131/jts.31.23. [DOI] [PubMed] [Google Scholar]
- 28.Eakins R, Walsh J, Randle L, Jenkins RE, et al. Adaptation to acetaminophen exposure elicits major changes in expression and distribution of the hepatic proteome. Sci Rep. 2015;5:16423. doi: 10.1038/srep16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, Holland RD, Yu LR. Quantitative proteomics for drug toxicity. Brief Funct Genomic Proteomic. 2009;8:158–166. doi: 10.1093/bfgp/elp006. [DOI] [PubMed] [Google Scholar]
- 30.Yu L-R, Gao Y, Mendrick DL. In: Handbook of Systems Toxicology. Daniel A, Casciano SCS, editors. John Wiley & Sons, Ltd; Chichester, UK: 2011. pp. 177–196. [Google Scholar]
- 31.Stamper BD, Mohar I, Kavanagh TJ, Nelson SD. Proteomic analysis of acetaminophen-induced changes in mitochondrial protein expression using spectral counting. Chem Res Toxicol. 2011;24:549–558. doi: 10.1021/tx1004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welch KD, Wen B, Goodlett DR, Yi EC, et al. Proteomic identification of potential susceptibility factors in drug-induced liver disease. Chem Res Toxicol. 2005;18:924–933. doi: 10.1021/tx050011b. [DOI] [PubMed] [Google Scholar]
- 33.Sun BY, Utleg AG, Hu ZY, Qin SZ, et al. Glycocapture-assisted global quantitative proteomics (gagQP) reveals multiorgan responses in serum toxicoproteome. J Proteome Res. 2013;12:2034–2044. doi: 10.1021/pr301178a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blazka ME, Elwell MR, Holladay SD, Wilson RE, et al. Histopathology of acetaminophen-induced liver changes: role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol. 1996;24:181–189. doi: 10.1177/019262339602400206. [DOI] [PubMed] [Google Scholar]
- 35.Yu LR, Zhu ZY, Chan KC, Issaq HJ, et al. Improved titanium dioxide enrichment of phosphopeptides from HeLa cells and high confident phosphopeptide identification by cross-validation of MS/MS and MS/MS/MS spectra. J Proteome Res. 2007;6:4150–4162. doi: 10.1021/pr070152u. [DOI] [PubMed] [Google Scholar]
- 36.Gao Y, Gopee NV, Howard PC, Yu LR. Proteomic analysis of early response lymph node proteins in mice treated with titanium dioxide nanoparticles. J Proteomics. 2011;74:2745–2759. doi: 10.1016/j.jprot.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Ando Y, Ahlbory-Dieker D, Schnackenberg LK, et al. Systems biology investigation to discover metabolic biomarkers of acetaminophen-induced hepatic injury using integrated transcriptomics and metabolomics. J Mol Biomarkers Diagn. 2013 doi: 10.4172/2155-9929.S1-002. [DOI] [Google Scholar]
- 38.Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab. 2009;10:579–594. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 39.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts DW, Bucci TJ, Benson RW, Warbritton AR, et al. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 1991;138:359–371. [PMC free article] [PubMed] [Google Scholar]
- 41.Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul Toxicol Pharmacol. 1998;27:119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- 42.Wang K. Molecular mechanisms of liver injury: apoptosis or necrosis. Exp Toxicol Pathol. 2014;66:351–356. doi: 10.1016/j.etp.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, et al. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, et al. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic Biol Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latchoumycandane C, Seah QM, Tan RC, Sattabongkot J, et al. Leflunomide or A77 1726 protect from acetaminophen-induced cell injury through inhibition of JNK-mediated mitochondrial permeability transition in immortalized human hepatocytes. Toxicol Appl Pharmacol. 2006;217:125–133. doi: 10.1016/j.taap.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Jang S, Yu LR, Abdelmegeed MA, Gao Y, et al. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015;6:552–564. doi: 10.1016/j.redox.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldring CEP, Kitteringham NR, Elsby R, Randle LE, et al. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 2004;39:1267–1276. doi: 10.1002/hep.20183. [DOI] [PubMed] [Google Scholar]
- 48.Copple IM, Goldring CE, Jenkins RE, Chia AJL, et al. The hepatotoxic metabolite of acetaminophen directly activates the KEAP1-Nrf2 cell defense system. Hepatology. 2008;48:1292–1301. doi: 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- 49.Zucker SN, Fink EE, Bagati A, Mannava S, et al. Nrf2 amplifies oxidative stress via induction of Klf9. Mol Cell. 2014;53:916–928. doi: 10.1016/j.molcel.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Disposition. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 51.Hinson JA, Reid AB, McCullough SS, James LP. Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev. 2004;36:805–822. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- 52.Moffit JS, Aleksunes LM, Kardas MJ, Slitt AL, et al. Role of NAD(P)H: quinone oxidoreductase 1 in clofibrate-mediated hepatoprotection from acetaminophen. Toxicology. 2007;230:197–206. doi: 10.1016/j.tox.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oppermann U. Carbonyl reductases: the complex relationships of mammalian carbonyland quinone-reducing enzymes and their role in physiology. Annu Rev Pharmacol Toxicol. 2007;47:293–322. doi: 10.1146/annurev.pharmtox.47.120505.105316. [DOI] [PubMed] [Google Scholar]
- 54.Samstag Y, Klemke M. Ectopic expression of L-plastin in human tumor cells: diagnostic and therapeutic implications. Adv Enzyme Regul. 2007;47:118–126. doi: 10.1016/j.advenzreg.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Manivannan B, Rawson P, Jordan TW, Secor WE, et al. Differential patterns of liver proteins in experimental murine Hepatosplenic Schistosomiasis. Infect Immun. 2010;78:618–628. doi: 10.1128/IAI.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams LA, White SW, Marsh JA, Lye SJ, et al. Association between liver-specific gene polymorphisms and their expression levels with nonalcoholic fatty liver disease. Hepatology. 2013;57:590–600. doi: 10.1002/hep.26184. [DOI] [PubMed] [Google Scholar]
- 57.Chiu H, Brittingham JA, Laskin DL. Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicol Appl Pharmacol. 2002;181:106–115. doi: 10.1006/taap.2002.9409. [DOI] [PubMed] [Google Scholar]
- 58.Bauer I, Vollmar B, Jaeschke H, Rensing H, et al. Transcriptional activation of heme oxygenase-1 and its functional significance in acetaminophen-induced hepatitis and hepatocellular injury in the rat. J Hepatol. 2000;33:395–406. doi: 10.1016/s0168-8278(00)80275-5. [DOI] [PubMed] [Google Scholar]
- 59.Florczyk UM, Jozkowicz A, Dulak J. Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance. Pharmacol Rep. 2008;60:38–48. [PMC free article] [PubMed] [Google Scholar]
- 60.Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60:1121–1128. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 61.Morse D, Choi AMK. Heme oxygenase-1—The “emerging molecule” has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 62.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 63.Jie Q, Tang Y, Deng Y, Li Y, et al. Bilirubin participates in protecting of heme oxygenase-1 induction by quercetin against ethanol hepatotoxicity in cultured rat hepatocytes. Alcohol. 2013;47:141–148. doi: 10.1016/j.alcohol.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 65.Bauer M, Bauer I. Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal. 2002;4:749–758. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- 66.Schipper HM. Biomarker potential of heme oxygenase-1 in Alzheimer’s disease and mild cognitive impairment. Biomarkers Med. 2007;1:375–385. doi: 10.2217/17520363.1.3.375. [DOI] [PubMed] [Google Scholar]
- 67.Schipper HM, Chertkow H, Mehindate K, Frankel D, et al. Evaluation of heme oxygenase-1 as a systemic biological marker of sporadic AD. Neurology. 2000;54:1297–1304. doi: 10.1212/wnl.54.6.1297. [DOI] [PubMed] [Google Scholar]
- 68.Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23:17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zager RA, Johnson ACM, Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol. 2012;23:1048–1057. doi: 10.1681/ASN.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato T, Takeno M, Honma K, Yamauchi H, et al. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-induced lung injury. Am J Respir Crit Care Med. 2006;174:906–914. doi: 10.1164/rccm.200508-1237OC. [DOI] [PubMed] [Google Scholar]
- 71.Kitchin KT, Del Razo LM, Brown JL, Anderson WL, et al. An integrated pharmacokinetic and pharmacodynamic study of arsenite action. 1. Heme oxygenase induction in rats. Teratog Carcinog Mutagen. 1999;19:385–402. [PubMed] [Google Scholar]
- 72.Menzel DB, Rasmussen RE, Lee E, Meacher DM, et al. Human lymphocyte heme oxygenase 1 as a response biomarker to inorganic arsenic. Biochem Biophys Res Commun. 1998;250:653–656. doi: 10.1006/bbrc.1998.9363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.