Abstract
Complex social life is thought to be a major driver of complex cognition in primates, but few studies have directly tested the relationship between a given primate species’ social system and their social cognitive skills. We experimentally compared life span patterns of a foundational social cognitive skill (following another’s gaze) in tolerant Barbary macaques, Macaca sylvanus, and despotic rhesus macaques, Macaca mulatta. Semi-free-ranging monkeys (N = 80 individuals from each species) followed gaze more in test trials where an actor looked up compared to control trials. However, species differed in ontogenetic trajectories: both exhibited high rates of gaze following as juveniles, but rhesus monkeys exhibited declines in social attention with age, whereas Barbary macaques did not. This pattern indicates that developmental patterns of social attention vary with social tolerance, and that diversity in social behaviour can lead to differences in social cognition across primates.
Keywords: cognitive evolution, comparative development, gaze following, primate, social cognition
A fundamental question regarding the evolution of intelligence concerns how variation in social systems shapes cognitive abilities. Although many theories propose that variation in social cognition stems from the challenges of social life (Dunbar, 1998; Dunbar & Shultz, 2007), little work has tested the specific kind of social interactions that promote sophisticated cognitive capacities. Some proposals have linked complex social cognition to political or ‘Machiavellian’ social interactions (Byrne & Whiten, 1988; de Waal, 1982; Hare, 2001). Under this competition hypothesis, individuals use social cognitive skills to outcompete or deceive others. Yet other proposals argue that societies characterized by cooperative relationships exhibit more robust social cognition (Burkart, Hrdy, & van Schaik, 2009; Hare, 2017). This tolerance hypothesis is particularly focused on explaining uniquely human cognition. To test the importance of tolerant versus competitive systems for social cognition, we compared the life span development of gaze following abilities in two closely related species with different social styles: more tolerant Barbary macaques, Macaca sylvanus, and more despotic rhesus macaques, Macaca mulatta.
Social attention, or detection of the locus of another’s gaze direction, provides a strong test of the evolutionary relationship between social behaviour and cognition for several reasons. First, social attention is a foundational component of human social cognition: attending to where and at what others are looking underpins such abilities as joint attention and theory of mind (Emery, 2000; Flom, Lee, & Muir, 2007; Langton, Watt, & Bruce, 2000; Puce & Bertenthal, 2015). That is, information about where others are directing their gaze is potent cue to what they are seeing or thinking, and is therefore important for more complex mentalizing abilities. Second, a basic sensitivity to others’ gaze direction is also widely shared across primates; species ranging from strepsirrhine lemurs to monkeys to great apes tend to co-orient with conspecifics or humans, at least in some situations (Rosati & Hare, 2009; Shepherd, 2010). Finally, evidence for both the competition and tolerance hypotheses directly invoke cognitive skills that capitalize on such gaze sensitivity.
Under the competition hypothesis, for example, successful competition may require exploitation of information about others’ gaze and visual perspective. Along these lines, more competitive primate species have quite sophisticated abilities to model the perspective of others. For example, chimpanzees, Pan troglodytes, and rhesus monkeys engage in visual (and auditory) perspective taking to obtain hidden food when competing with a human or conspecific (Flombaum & Santos, 2005; Hare, 2011; Hare, Call, Agnetta, & Tomasello, 2000; Hare, Call, & Tomasello, 2001, 2006; Kaminski, Call, & Tomasello, 2008; Melis, Call, & Tomasello, 2006; Santos, Nissen, & Ferrugia, 2006). In contrast, behaviour-reading strategies, where individuals do not directly reason about the subject mental states of others, seems to account for the performance of more tolerant species, including marmosets (Callithrix jacchus), capuchins (Cebus apella) and Tonkean macaques, Macaca tonkeana, in similar contexts (Burkhart & Heschl, 2007; Canteloup, Piraux, Poulin, & Meunier, 2016; Costes-Thire, Leve, Uhlrich, de Marco, & Thierry, 2015; Hare, Addessi, Call, Tomasello, & Visalberghi, 2003). Additional evidence for this claim comes from direct comparisons of different lemur species. Ringtailed lemurs, Lemur catta, which typically live in large groups with anthropoid-like dominance hierarchies, exhibit more robust performance on perspective-taking tasks, outperforming other lemur species that live in smaller family groups (Bray, Krupenye, & Hare, 2014; MacLean et al., 2013; Sandel, MacLean, & Hare, 2011). Finally, chimpanzees are more successful at exploiting social cues specifically in competitive contexts compared to cooperative contexts (Hare & Tomasello, 2004; Herrmann & Tomasello, 2006; but see MacLean & Hare, 2015).
Under the tolerance hypothesis, in contrast, tolerant species should be especially sensitive to gaze cues because they facilitate cooperative interactions. Social tolerance has been specifically linked to robust comprehension of communicative signals, including gaze cues, in domesticated animals. For example, in social tasks in which a human experimenter communicatively informs the participant of the location of hidden food by looking or pointing at its location, domesticated dogs, Canis familiaris, and experimentally domesticated silver foxes, Vulpes vulpes, outperform wolves, Canis lupus, and a control line of undomesticated foxes. Both dogs and the domesticated foxes exhibit greater social tolerance towards humans (Hare, Brown, Williamson, & Tomasello, 2002; Hare et al., 2005; Hare & Tomasello, 2005). Along the same lines, relatively tolerant bonobos, Pan paniscus, are more interested in viewing eyes than are chimpanzees (Kano & Call, 2014; Kano, Hirata, & Call, 2015), although they do exhibit more comparable performance in some gaze-following contexts (Braeuer, Call, & Tomasello, 2005; Okamoto-Barth, Call, & Tomasello, 2007; see also MacLean & Hare, 2012). More generally, tolerance seems to facilitate the emergence of cooperative interactions: more tolerant chimpanzee dyads are more cooperative than less tolerant dyads, and bonobos outperform chimpanzees in cooperative tasks (Hare, Melis, Woods, Hastings, & Wrangham, 2007; Melis, Hare, & Tomasello, 2006). Finally, humans are characterized by extreme tolerance, joint attention and high levels of cooperation in which gaze cues communicate information about an actor’s intentions (Csibra, 2010; Csibra & Gergely, 2009; Senju & Csibra, 2008; Tomasello & Carpenter, 2007; Tomasello, Carpenter, Call, Behne, & Moll, 2005; Tomasello, Hare, Lehmann, & Call, 2007). Indeed, some views argue that competition may be the most important driver of complex social cognitive abilities in other primates, whereas cooperation is specifically important for the emergence of human-unique cognition (Moll & Tomasello, 2007; Tomasello, 2014; Tomasello & Call, 1997).
To test the importance of tolerant versus competitive systems for social cognition in nonhuman primates, we compared the life span development of gaze following in two species of macaques. The genus Macaca is a radiation of closely related species that share a similar basic social organization (multimale-multifemale groups, where females stay in their natal group and males disperse), but diverge in social style (Thierry, 2000, 2004). Some macaque species exhibit greater despotism, characterized by steep dominance hierarchies, more intense aggression and formalized submission signals. In contrast, other species are characterized by a relaxed dominance hierarchy, reconciliation after aggression and more affiliative social signals. Across macaque species, this suite of behavioural traits related to tolerance are strongly linked and tend to covary (Thierry, 2013). Indeed, Thierry (2007) classed macaques into four ‘grades’ of social styles of increasing tolerance based on this cluster of characteristics. Thus, comparisons of different macaque species can isolate variation in social tolerance across species with otherwise similar social organizations. In the current work, we therefore compared more despotic rhesus macaques (grade 1, the most despotic) with more tolerant Barbary macaques (grade 3). We predicted that rhesus monkeys should exhibit more robust gaze following if competition spurs complex social cognition, whereas Barbary macaques should exhibit more robust gaze following if social tolerance promotes this skill.
We further examined developmental changes in gaze following across the life span of these macaques. Across primate species, younger individuals tend to exhibit greater social tolerance, whereas mature individuals show higher rates of aggression and competition (Pereira & Fairbanks, 2002). Similarly, developmental shifts in social tolerance seem to track developmental shifts in some social cognitive abilities in chimpanzees and bonobos (Wobber, Wrangham, & Hare, 2010). Consequently, patterns of cognitive development provide a second test of the relationship between social tolerance and social cognition. While some prior work has examined the emergence of gaze following within a single primate species (Rosati, Arre, Platt, & Santos, 2016; Simpson, Miller, Ferrari, Suomi, & Pauker, 2015; Teufel, Gutmann, Pirow, & Fischer, 2010; Tomasello, Hare, & Fogleman, 2001), no study has directly compared life span patterns of social attention across different primates. Moreover, qualitative comparisons of different species’ ontogenetic patterns in different studies are also somewhat contradictory. Rhesus macaques show high levels of gaze following in the juvenile period that declines with age (Rosati et al., 2016; Tomasello et al., 2001). Yet pigtail macaques, Macaca nemestrina, which are somewhat more tolerant than rhesus macaques (Thierry, 2007), exhibit relatively delayed development of gaze following and have been hypothesized to need more social experience to acquire this skill (Ferrari, Coude, Gallese, & Fogassi, 2008; Ferrari, Kohler, Fogassi, & Gallese, 2000). Our study, comparing cognitive development across species with identical methods, allows us to disentangle this issue. We predicted that any species difference should be exacerbated with age, as variation in tolerance is most pronounced in mature individuals.
METHODS
Ethics Statement
All noninvasive behavioural tests were approved by the Institutional Animal Care and Use Committee (IACUC) for Yale University (Barbary: number 2014-11615: rhesus: number 2014-11624), as well as the Cayo Santiago IACUC (rhesus: number 8310106) administered through the University of Puerto Rico Medical Sciences Campus. All tests adhered to site guidelines for animal research. Monkeys who participated in this study live in natural social groups, are provisioned daily (in addition to access to plants growing at their respective sites) and have ad libitum access to water.
Subjects
We tested 80 rhesus monkeys living at Cayo Santiago in Puerto Rico (41 females and 39 males, ranging in age from 1.4 to 22 years), and 80 Barbary macaques living at Trentham Monkey Forest, Stoke-on-Trent, U.K. (41 females and 39 males, ranging in age from 2.1 to 29 years); sample size for age cohorts in each species are shown in Fig. 1. Rhesus data were partially reported in previous work (see Study 2 in Rosati et al., 2016). Both populations are semi-free-ranging and highly habituated to humans. At Cayo Santiago, monkeys have been habituated to human observers since the founding of the population, and a variety of researchers work at the site. At Trentham, monkeys have been habituated to tourists, who can walk through the monkeys’ habitat in a situation similar to that of Cayo Santiago.
Figure 1.
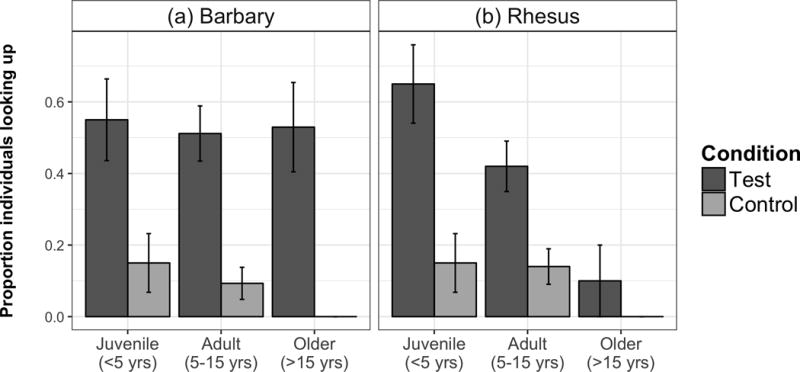
First trial responses. Barbary and rhesus performance on their first test and control trial, split by age cohorts; error bars represent SE.
Monkeys are also individually identifiable with known ages in both populations. At Cayo Santiago, monkeys are identifiable by unique combinations of tattoos and ear notches. Exact birth dates for all rhesus monkeys are known through a long-term census maintained at the site. At Trentham, monkeys are identifiable by tattoos and facial and body features. Exact dates of birth are known for all Barbary macaques born in 2005 or later. Before 2005, only individuals’ birth years were known. Macaques are seasonal breeders, so we therefore assigned these individuals a birth date in the middle of their birth season (which lasts for 3 months from April to June at this site).
Procedure
Monkeys completed up to four trials in a gaze-following task in which they could follow a human’s look upwards. We used a human demonstrator to ensure that the gaze stimulus was tightly controlled, given that previous work has clearly demonstrated that macaques follow the gaze of both conspecifics and humans (Ferrari et al., 2000; Goossens, Dekleva, Read, Sterck, & Bolhuis, 2008; Rosati & Hare, 2009; Teufel et al., 2010; Tomasello, Call., & Hare, 1998; Tomasello et al., 2001). The demonstrator alternated test trials, in which she looked up, and control trials, in which her behaviour was identical except that she looked down; the first trial type experienced was counterbalanced across subjects. We used an upward look as our experimental demonstration as this has generated robust responses in prior work with primates (Tomasello et al., 1998; Tomasello et al., 2001; Tomasello et al., 2007); monkeys in these populations often look around scanning the environment (as they were free ranging during the test), so a ‘sideways’ look (e.g. Emery, Lorincz, Perrett, Oram, & Baker, 1997) would have been more difficult to detect experimentally. Prior work has also contrasted an upwards look with a ‘no look’ control that involves either no social demonstration (Tomasello et al., 1998), or an experimenter that gazes directly at the subject (Braeuer et al., 2005; Tomasello et al., 2001). However, because extended direct gaze from a human is generally perceived as a threat in macaques, we instead used a ‘downwards look’ in order to provide a fair contrast: the experimenter captured the monkey’s attention and then looked in a specific direction, without introducing additional confounds such as increased arousal due to the human’s directed gaze. This control therefore served as a baseline measure of how often monkeys looked up, as the control procedure was thus identical except that the experimenter looked in a different direction.
In tests, two experimenters approached a calmly sitting monkey (approximately 1–2 m away). The demonstrator, experimenter 1 (E1), first attracted the monkey’s attention to her face (by calling ‘monkey’ and/or clapping her hands). When the monkey was looking, E1 said ‘now’ and then looked straight up or down, rotating her entire head with her eyes open for 10 s (following the same procedure in Rosati et al. (2016) (see Supplementary Material 1, Fig. S1). E1 did not look at an actual target, but to make the experimenter’s action appear plausible, we tested monkeys sitting in the vicinity of a tree. We therefore refrained from testing in locations when another monkey was actually present above the subject, to avoid any potential visual and auditory confounds. The cameraperson, experimenter 2 (E2), stood next to E1 and filmed the monkey’s response (see Supplementary Videos S1–S2). After the 10 s were up, E2 said ‘stop’ to end the trial. E1 tried to attract the monkey’s attention for the next trial as soon as the previous one concluded. The same actor served as E1 for both populations.
Exclusions
As the monkeys were free ranging during the tests, some individuals moved away of their own volition before completing all four trials. Monkeys therefore had to successfully complete at least two trials (e.g. one test and one control trial) to be included in the study because of the within-subjects design. Consequently, additional monkeys were approached by the experimenters for testing but were not included in the analyses because they only completed one trial (N = 10 rhesus, N = 7 Barbary), or because the coders scored that the monkey was not looking when the primary experimenter called ‘now’ on one of the first two trials (N = 4 rhesus monkeys). An additional four trials (2 from rhesus monkeys, 2 from Barbary macaques) were excluded; these trials occurred in the second half of the test, so these monkeys were included in the final sample as they had successfully completed at least the first two trials prior to these excluded trials. In the final sample, both species exhibited similar mean trial completion rates of 3.54 trials, with no difference between species (t158 = 0.001, P > 0.99; see Supplementary Material 1, Table S1 for means by cohort). If the monkey ran away or moved to an inaccessible location before the entire four trials could be completed, E1 (who was blind to the monkey’s previous responses, as she had been looking up) decided whether to end the session.
Video Processing and Coding
The same two independent coders scored both species’ responses on all trials. We first clipped out individual trials from longer session videos, and then randomized the order of trials (assigning a new, random trial ID) to blind coders to condition and trial number. Each coder independently identified the start of the trial (e.g. when E1 said ‘now’) and examined the subsequent 10 s period frame by frame in the program MPEG Streamclip (http://www.squared5.com/). Following previous work (Rosati et al., 2016), we coded: (1) whether the monkey looked up, using only his/her eyes or entire head (binomial response); (2) total duration of looking upwards (in seconds); (3) latency to look up (in seconds); and (4) number of discrete, independent looks up within the trial (a count response). The last measure therefore examined whether monkeys looked up multiple times within a trial, echoing previous work examining whether primates ‘check back’ with actors to assess their true line of sight (Braeuer et al., 2005; Tomasello et al., 2001). In this naturalistic context, however, we could only assess whether monkeys made multiple independent looks (e.g. looked up, looked away, and then looked up again). The coders had high reliability for all measures (the Kappa value for whether or not the monkey looked was 0.92 (agreement on 97% of trials); Pearson correlations were 0.94 for total time spent looking up, 0.95 for latency to look up and 0.91 for the total number of discrete looks).
Statistical Analyses
When modelling propensity to follow gaze as a binary response measure, we implemented generalized linear mixed models (GLMM) in R version 3.3.3 (R Development Core Team, 2017). We used the glmer function from the lme4 software package (Bates, 2010), fitting binomial models with a logit link function using maximum likelihood. We included random subject intercepts to account for repeated trials within subjects. GLMM can account for unequal repeats across subjects (Baayen, 2008), which is important since subjects did not always complete all four trials (as they were free ranging during tests). We then compared the fit of different models using likelihood ratio tests (Bolker et al., 2008). We used the glht function in the multcomp package for post hoc pairwise comparisons of model factors (Hothorn, Bretz, & Westfall, 2008). Other characteristics of the monkeys’ gaze following had distributions with positive skew (e.g. duration of total looking and latency to look upwards). We therefore used an inverse Gaussian distribution (inverse link function) for these GLMMs (following recommendations for similiar reaction time data; Baayen & Milin, 2010; Lo & Andrews, 2015). Finally, when modelling the number of discrete looks within a trial (a count response), we used a Poisson distribution. Across analyses, graphs showing predicted effects and confidence intervals (CIs) from these models were calculated using the effects package in R (Fox et al., 2016).
Across analyses, we generally accounted for condition order (the first trial type the individual experienced), trial number (1–4) and subject (as a random factor accounting for repeated measures). We then tested the importance of additional predictors in subsequent models, including trial type (whether the experimenter looked up in test trials or down in control trials), species (rhesus or Barbary macaques), sex (male or female) and the subject’s age (in years), as relevant. We included sex as a predictor because prior work has demonstrated that female rhesus monkeys show interest in social stimuli (Simpson, Nicolini, et al., 2016) and exhibit greater responsivity to gaze cues (Rosati et al., 2016; Simpson, Paukner, et al., 2016) than do males. We analysed data with age in years as a continuous predictor, but in some figures we split individuals into age cohorts based on life history transitions in this species (following those used in Rosati et al., 2016): juveniles up to 5 years (sexual maturity); adults up to 15 years; and older monkeys over 15 years (monkeys in this population have a median life span of 15 years, only rarely exceeding 25 years; see Hoffman, Higham, Mas-Rivera, Ayala, & Mastripieri, 2010).
RESULTS
Performance on Test versus Control Trials
In our first set of analyses, we confirmed that both macaque species responded to the experimental manipulation. Overall, 43.8 ± 5.6% (mean ± SE) of rhesus macaques looked up on their first test trial, whereas only 12.5 ± 3.7% did on their first control trial; 52.5 ± 5.6% of Barbary macaques looked up on their first test trial, whereas only 8.8 ± 3.2% did on their first control trial (see Fig. 1 for breakdown by species and age cohort). For each species, we created a basic GLMM model, with response as a binary outcome, accounting for condition order (first trial type), trial number (1–4) and subject (as a random factor accounting for repeated measures). We then tested the importance of three additional predictors for each species’ responses in subsequent models: trial type (test versus control), age and sex.
In rhesus macaques, model fit was improved by including trial type in the second model (χ21 = 33.82, P < 0.001): they looked up more on test trials than on control trials. In a third model, fit was further improved by adding age (χ21 = 17.87, P < 0.001): younger rhesus monkeys looked up more than older monkeys. Finally, fit was further improved in the fourth model by adding sex (χ21 = 8.87, P < 0.005): female rhesus looked up more than male rhesus (see Supplementary Material 1, Table S2 for parameters from the full rhesus model). In contrast, model fit for Barbary macaque responses was only improved by including trial type as a predictor (χ21 = 59.96, P < 0.001). Subsequent models that included age (χ21 = 1.64, P > 0.20) and sex (χ21 = 1.00, P > 0.31) did not improve model fit (see Supplementary Material 1, Table S3 for parameters from the full Barbary model). Thus, Barbary macaques were more likely to look up in test trials when the experimenter initially looked up, compared to control trials where she looked down, but they did not exhibit any developmental changes in responses like those seen in rhesus macaques. These results indicate that both species exhibited a robust gaze-following response on test trials relative to control trials, but they also showed potential variation in developmental trajectories.
Developmental Patterns
In our second set of analyses, we directly compared the two species’ responses to test trials to ascertain whether they differed in their propensity to gaze-follow across the life span (see Fig. 2). To do so, we first created a basic GLMM model, with response as a binary outcome, again with accounting for condition order, session half (as there were only two possible test trials per monkey), sex, age and subject (as a random factor). In a second model, we added species as a predictor, which marginally improved fit (χ21 = 3.41, P = 0.065). In the third model, we then added a species*age interaction, the main test of our prediction that any species differences would become exacerbated with increasing age. This improved model fit compared to the second model (χ21 = 9.17, P < 0.005): social attention declined with age in rhesus but not in Barbary macaques. Finally, we added a species*sex interaction, as our initial analyses suggested different sex effects in these two species. This further increased fit (χ21 = 7.63, P < 0.01): females followed gaze more often than males in rhesus macaques, whereas there was no sex difference in Barbary macaques (Table 1). This shows that, whereas rhesus macaque gaze following declines with age, Barbary macaques sustain high rates of gaze following across their life span.
Figure 2.
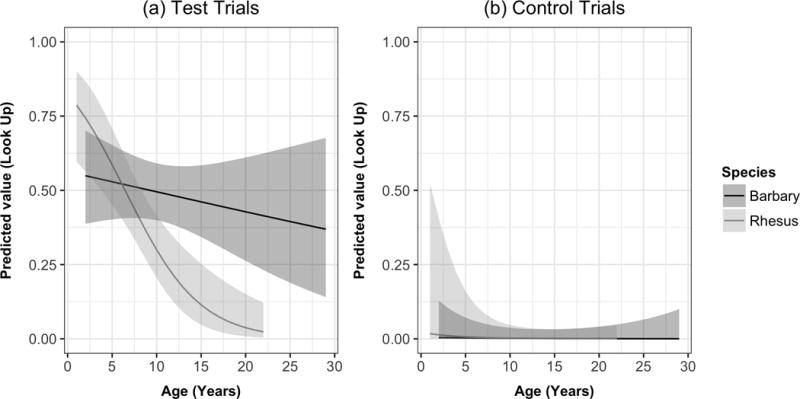
Developmental changes in gaze-following responses across macaque species. Estimated values for each species’ response across age in (a) test trials and (b) control trials. Estimates are derived from generalized linear mixed models also controlling for sex, session-half, condition order and subject identity. Ribbons indicate 95% confidence intervals; lines are truncated to tested age range of each species.
Table 1.
Factors influencing propensity to look up in test trials
| Factor | Estimate | SE | Z | P |
|---|---|---|---|---|
| Condition order (control first = reference) | −0.434 | 0.298 | −1.455 | 0.14 |
| Session half (1–2) | −0.358 | 0.277 | −1.295 | 0.19 |
| Species (Barbary = reference) | 2.141 | 0.765 | 2.798 | <0.01 |
| Age: Barbary | −0.027 | 0.033 | −0.824 | 0.87 |
| Age: Rhesus | −0.240 | 0.061 | −3.944 | <0.001 |
| Sex: Barbary (female = reference) | 0.095 | 0.401 | 0.237 | 0.99 |
| Sex: Rhesus (female = reference) | −1.609 | 0.486 | −3.309 | <0.005 |
Predictors from the full (best-fit) model. Condition order, session half and a random subject factor were included across models. Species and the interactions between species*age and species*sex were added to successive models to test their importance. Significant P values are shown in bold.
To confirm that developmental effects were specific to test trials involving actual gaze-following responses, we then performed the same analyses on the monkeys’ behaviour in control trials. In general, both species responded with upward looks infrequently on control trials. Barbary macaques looked up on 9.2 ± 2.4% of all control trials, and rhesus looked up on 14.1 ± 2.9% of all control trials. As with the analysis of test trials, we first created a basic model of control trial performance accounting for condition order (first trial test versus control), session half, sex, age (as a covariate) and random subject intercepts to account for repeated measures. We added species as a predictor in a second model, which did not improve fit (χ21 = 0.16, P > 0.68). In the third model, we then added the species*age interaction, which also did not improve fit compared to the second model (χ21 = 0.35, P > 0.55). Finally, we added a species*sex interaction, which also did not increase fit compared to the third model (χ21 = 1.16, P > 0.28). These results therefore stand in contrast to the results from the test trials, where interactions between species and age, as well as between species and sex, significantly increased model fit (see Fig. 1b and Table 2 for parameters from the full control trial model).
Table 2.
Factors influencing propensity to look up in control trials
| Factor | Estimate | SE | Z | P |
|---|---|---|---|---|
| Condition order (control first = reference) | −0.212 | 0.585 | −0.362 | 0.71 |
| Session half (1–2) | 0.193 | 0.449 | 0.430 | 0.66 |
| Species (Barbary = reference) | 2.515 | 1.509 | 1.666 | 0.10 |
| Age: Barbary | −0.113 | 0.089 | −1.272 | 0.58 |
| Age: Rhesus | −0.261 | 0.126 | −2.062 | 0.14 |
| Sex: Barbary (female = reference) | 1.243 | 0.904 | 1.376 | 0.51 |
| Sex: Rhesus (female = reference) | −0.577 | 0.830 | −0.695 | 0.92 |
Predictors from the full model. Condition order, session half and a random subject factor were included across models. Species and the interactions between species*age and species*sex were added to successive models to test their importance.
Characteristics of Gaze-following Response
Our final analyses examined differences in other characteristics of the macaques’ gaze-following responses. Across three sets of models, we compared the two species’ responses across three dependent variables indexing different aspects of their looking patterns: their total duration of time spent looking upwards, their latency to initially look up and the number of discrete looks that monkeys produced to identify the (absent) target. In these analyses, we only analysed test trials in which monkeys produced a gaze-following response. This allowed us to assess whether rhesus and Barbary macaques also differed in other aspects of their gazing patterns beyond their overall propensity to produce a response at all.
Barbary macaques spent an average duration of 1.50 ± 0.17 s looking upwards when they responded; rhesus macaques looked for 1.61 ± 0.18 s. We first fitted a base GLMM with an inverse-Gaussian distribution and inverse link (as this data had positive skew), accounting for condition order (first trial test versus control), session half, sex, age and random subject intercepts to account for repeated measures. We then added species to test whether rhesus and Barbary macaques differed in their duration of looking, but this did not improve model fit (χ21 = 0.09, P > 0.76; see Fig. 3a for estimated duration of looking across the two species, derived from this second model). In the third model, we added a species*age interaction (χ21 = 0.97, P > 0.32), which also did not improve fit. Finally, we added a species*sex interaction (χ21 = 0.01, P > 0.98), which again did not improve fit. Thus, the two species did not differ in their duration of time spent looking upwards in test trials.
Figure 3.
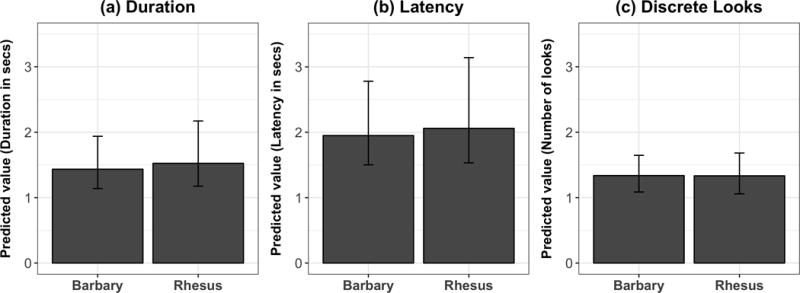
Characteristics of rhesus and Barbary macaques’ gaze-following responses. Estimated values for (a) duration of total looking upwards, (b) response latency and (c) number of discrete looks for each species when monkeys produced a gaze-following response. Estimates are derived from generalized linear mixed models of each response variable also controlling for sex, age, session half, condition order and subject identity; error bars indicate 95% confidence intervals.
We used the same procedure to analyse the monkeys’ latency to make a gaze-following response. On average, Barbary macaques had a response latency of 2.12 ± 0.26 s; rhesus macaques had a latency of 1.98 ± 0.30 s. We first created a base model with an inverse-Gaussian distribution (inverse link), and then added species, the species*age interaction and the species*sex interaction to test their importance as predictors. In fact, neither species (χ21 = 0.05, P > 0.81; see Fig. 3b for predicted latency to respond across the two species, derived from this second model), the species*age interaction (χ21 = 1.10, P > 0.29), nor the species*sex interaction (χ21 = 0.03, P > 0.86) improved model fit. Thus, the two species did not differ in their latency to look on test trials in which they did respond.
Finally, we used the same general procedure to analyse monkeys’ number of discrete looks to identify the (absent) target of the actor’s gaze. As this was a count response, we implemented a mixed model with a Poisson distribution. On average, Barbary macaques made 1.36 ± 0.08 discrete looks upwards; rhesus macaques made 1.32 ± 0.08 looks. For the number of discrete looks, neither the addition of species (χ21 = 0.001, P > 0.98; see Fig. 3c for predicted number of looks across the two species, derived from this second model), a species*age interaction (χ21 = 0.16, P > 0.69) nor a species*sex interaction (χ21 = 0.46, P > 0.49) improved model fit. Thus, the two species did not differ in the number of discrete looks they made when they did respond.
DISCUSSION
Tolerant Barbary macaques exhibited more robust social attention across the life span than despotic rhesus macaques. Both species followed gaze at high rates as juveniles, but Barbary macaques continued to do so in old age, whereas rhesus monkeys did not, aligning with our prediction that these species would exhibit great divergence in social cognition with increasing age. Importantly, both species exhibited low rates of baseline looking in control trials, showing that this pattern was not due to general changes in vigilance unrelated to gaze following. In contrast, other characteristics of these species’ gaze-following responses, such as latency and duration of looking, were quite similar across species when they did follow gaze. This pattern indicates that the major difference between the two species involved their overall propensity to follow gaze. Together, these results show that tolerant macaques maintain high sensitivity to gaze signals across the life span, whereas more competitive rhesus macaques do not. These results support the hypothesis that species characterized by tolerant relationships exhibit more robust social cognition (Burkart et al., 2009; Hare, 2017).
Comparisons of cognitive development across species are a powerful method for understanding how cognition evolves, as shifts in development are thought to be a potential evolutionary mechanism for generating variation in mature traits across species (Rosati, Wobber, Hughes, & Santos, 2014; Wobber, Herrmann, Hare, Wrangham, & Tomasello, 2013). Our results demonstrate that social attention declines with age in despotic rhesus macaques relative to tolerant Barbary macaques, paralleling shifts in tolerance in the transition to adulthood seen in primates more generally (Pereira & Fairbanks, 2002; Wobber et al., 2010). This represents the largest study to date comparing the development of gaze following across multiple primate species. While some developmental work has tracked early gaze following in single species (Ferrari et al., 2000; Rosati et al., 2016; Simpson et al., 2015; Teufel et al., 2010; Tomasello et al., 2001), or compared gaze following across ape species (Braeuer et al., 2005; Kano & Call, 2014; Kano et al., 2015; MacLean & Hare, 2012; Okamoto-Barth et al., 2007), no prior studies have tested this skill across the entire life span of multiple species. Our developmental results do align with prior work showing that rhesus monkeys exhibit high rates of gaze following in the juvenile period but show declining social attention with age (Rosati et al., 2016; Tomasello et al., 2001), as well as prior work indicating that female rhesus macaques are more sensitive to social information than are males (Rosati et al., 2016; Simpson, Nicolini, et al., 2016; Simpson, Paukner, et al., 2016). This pattern mirrors that seen in humans, where adult females are more sensitive to gaze cues than are adult males (Alwall, Johansson, & Hansen, 2010; Bayliss, di Pellegrino, & Tipper, 2005; Deaner, Shepherd, & Platt, 2007; Mundy et al., 2007). In contrast, we found that Barbary macaques do not exhibit such developmental changes or sex differences in patterns of social attention. While there have been no prior examinations of life span patterns of Barbary gaze following (see Teufel et al., 2010, for patterns of gaze following in early development), old Barbary macaques do maintain high interest in other types of social stimuli, such as photographs or vocal playbacks of conspecifics (Almeling, Hammerschmidt, Senn-Reulen, Freund, & Fischer, 2016), suggesting that this species may maintain other aspects of social cognition during ageing as well. Taken together, our results suggest that social cognition in Barbary macaques may be relatively preserved during the ageing process compared to that of rhesus monkeys.
Importantly, our results are unlikely to be due to different developmental experiences with humans across the two species, as both study populations are highly habituated to human observers. Moreover, the experimental evidence mentioned above has shown that adult rhesus monkeys also exhibit declines in gaze following relative to juveniles at other sites despite having different experience with humans (Tomasello et al., 2001). In addition, our work aligns with previous studies showing that Barbary macaques exhibit continued interest in conspecific social stimuli with age (Almeling et al., 2016) even though they have different experiences with humans and other monkeys. Finally, previous work directly tested this possibility, by presenting a human actor directing their gaze and then examining monkeys’ subsequent gaze-following responses in an encounter sometime later, and found that this experience did not affect the monkeys’ subsequent performance (Ferrari et al., 2008). Together, these findings suggest that greater experience with humans does not necessarily impact gaze-following patterns in these kinds of situations.
We examined monkeys’ gaze following in a neutral context that was not specifically cooperative or competitive in nature. One important open question is therefore whether nonhuman primate species’ cognitive skills are tailored to their typical social interactions. For example, competitive species might exhibit more robust gaze following when actually competing with others (e.g. Hare & Tomasello, 2004), whereas tolerant primate species might exhibit even greater sensitivity to gaze signals during cooperation. Along these lines, there is evidence that tolerant crested macaques, Macaca nigra, are faster to respond to the gaze cues of conspecifics who are close friends versus nonfriends (Micheletta & Waller, 2012), highlighting the potential importance of gaze sensitivity in their affiliative interactions. In contrast, less tolerant longtailed macaques, Macaca fascicularis, exhibit more gaze following in response to a human producing a ‘bare teeth’ expression (as signal of submission) than one producing a ‘lip smack’ expression (a signal of affiliation) (Goossens et al., 2008). This finding may point to the possibility that gaze signals are more meaningful to despotic species in the context of agonistic interactions. Thus, an important next step would involve directly comparing how species varying in tolerance respond to gaze cues across both kinds of contexts.
Another question concerns how species variation in gaze-following propensity affects other aspects of social cognition. In humans, co-orienting is a foundational social skill that emerges during early infancy (D’Entremont, Hains, & Muir, 1997) and then scaffolds the development of more sophisticated social capacities. For example, co-orienting is an important cognitive component for establishing joint attention with others (Butterworth & Cochran, 1980; Carpenter, Nagell, & Tomasello, 1998), communicative and linguistic development (Brooks & Meltzoff, 2005, 2008; Csibra, 2010; Senju & Csibra, 2008) and perspective taking and theory of mind more generally (Charman et al., 2000; Flom et al., 2007; Moll & Tomasello, 2004, 2006; Wellman, 2011). Furthermore, older adult humans show a decline in their propensity to respond to gaze cues (Kuhn, Pagano, Maani, & Bunce, 2015; Slessor, Phillips, & Bull, 2008; Slessor et al., 2014) as well as impairments in more complex theory of mind skills such as perspective taking and false-belief attribution (Moran, 2013; Phillips et al., 2011; Slessor, Phillips, & Bull, 1007). Given that gaze is such a potent source of information into other minds, it is possible that the reduced sensitivity to gaze cues that we found in older rhesus monkeys may feed forward and lead to relative decrements in other aspects of their social cognition as well.
The relationship between variation in tolerance and social cognition may be important for understanding the origins of human cognition as well. Some theories suggest that competition shapes social cognition in nonhuman primates, whereas cooperation is important for human cognition specifically (Moll & Tomasello, 2007; Tomasello, 2014; Tomasello & Call, 1997). Indeed, several proposals have specifically invoked our species’ high levels of social tolerance as an important evolutionary precursor to our exceptional social intelligence (Burkart et al., 2009; Hare, 2017). Along these lines, children with calmer or more tolerant temperaments exhibit more sophisticated theory of mind capacities relative to children with more aggressive or reactive temperaments (Lane et al., 2013; Wellman, Lane, LaBounty, & Olson, 2010). Our results show that tolerance may also have powerful effects on the social cognitive development of nonhuman primates, therefore providing a window into the evolutionary and developmental processes that may have shaped our own species. Given that humans are not just an extremely tolerant species, but also an ultra-social species that can routinely interact and cooperate with others in large groups, understanding the relationship between variation in social systems and complex cognition is critical to address how human cognition evolved.
Supplementary Material
Video S1. Barbary macaque following gaze (test trial). The demonstrator (E1) and the cameraperson (E2) stood next to each other, 1–2 m away from the monkey. E1 can be heard calling the monkey to capture the monkey’s attention. When the monkey looks at E1’s face, E1 says ‘now’ and looks straight up. She holds that position for the rest of the 10 s trial. This monkey makes two discrete looks upwards.
Video S2. Rhesus macaque following gaze (test trial). The demonstrator (E1) and the cameraperson (E2) stood next to each other, 1–2 m away from the monkey. When the monkey looks at E1’s face, E1 says ‘now’ and looks straight up. She holds that position for the rest of the 10 s trial. This monkey makes one discrete look upwards.
Highlights.
We examined the development of gaze following in two macaque species.
Both juvenile rhesus and juvenile Barbary macaques followed gaze at high rates.
Only Barbary macaques showed high levels of social attention in old age.
Tolerant social systems may promote more robust social cognition across primate species.
Acknowledgments
We thank Alyssa Arre and Lindsey Drayton for assistance with data collection, Linda Chang for assistance with coding and Steve Worthington at Harvard’s Institute for Quantitative Social Science for statistical advice. At Cayo Santiago, we thank Angelina Ruiz Lambides, Nahiri Rivera Barreto and Giselle Caraballo Cruz for research support. At Trentham, we thank Sue Wiper, Matt Lovatt Anna Smith and Diane Floyd for research support. Cayo Santiago research was supported by the National Institute of Mental Health (R01MH096875), an NCRR CM-5-P40RR003640-13 award to the Caribbean Primate Research Center and the University of Puerto Rico and an Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health (NIH) through grant number 5P40OD012217 to the Caribbean Primate Research Center and the University of Puerto Rico. L.R.S. was supported by the James S. McDonnell Foundation (grant number 220020242) and Yale University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary Material associated with this article is available, in the online version, at doi:
References
- Almeling L, Hammerschmidt K, Senn-Reulen H, Freund AM, Fischer J. Motivational shifts in aging monkeys amd the origins of social selectivity. Current Biology. 2016;26:1744–1749. doi: 10.1016/j.cub.2016.04.066. [DOI] [PubMed] [Google Scholar]
- Alwall N, Johansson D, Hansen S. The gender difference in gaze-cueing: Associations with empathizing and systemizing. Personality and Individual Differences. 2010;49:729–732. [Google Scholar]
- Baayen RH. Analyzing linguistic data A practical introduction to statistics. Cambridge, U.K: Cambridge University Press; 2008. [Google Scholar]
- Baayen RH, Milin P. Analyzing reaction times. International Journal of Psychlogical Research. 2010;3:12–28. [Google Scholar]
- Bates D. The LME4 package: Linear mixed-effects models using S4 classes. 2010 http://www.R-project.org.
- Bayliss AP, di Pellegrino G, Tipper SP. Sex differences in eye gaze and symbolic cueing of attention. Quarterly Journal of Experimental Psychology. 2005;58A:631–650. doi: 10.1080/02724980443000124. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, et al. Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology & Evolution. 2008;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Braeuer J, Call J, Tomasello M. All great ape species follow gaze to distant locations and around barriers. Journal of Comparative Psychology. 2005;119:145–154. doi: 10.1037/0735-7036.119.2.145. [DOI] [PubMed] [Google Scholar]
- Bray J, Krupenye C, Hare B. Ring-tailed lemurs (Lemur catta) exploit information about what others can see but not what they can hear. Animal Cognition. 2014;17:735–744. doi: 10.1007/s10071-013-0705-0. [DOI] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The development of gaze following and its relation to language. Developmental Science. 2005;8:535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. Infant gaze following and pointing predict accelerated vocabulary growth through two years of age: A longitudinal, growth curve modeling study. Journal of Child Language. 2008;35:207–220. doi: 10.1017/s030500090700829x. [DOI] [PubMed] [Google Scholar]
- Burkart JM, Hrdy SB, van Schaik CP. Cooperative breeding and human cognitive evolution. Evolutionary Anthropology. 2009;18:175–186. [Google Scholar]
- Burkhart J, Heschl A. Understanding visual access in common marmosets, Callithix jacchus: Perspective taking or behaviour reading? Animal Behaviour. 2007;73:457–469. [Google Scholar]
- Butterworth G, Cochran E. Towards a mechanism of joint visual attention in human infancy. International Journal of Behavioral Development. 1980;3:253–272. [Google Scholar]
- Byrne RW, Whiten AW. Machiavellian intelligence: Social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford, U.K: Clarendon Press; 1988. [Google Scholar]
- Canteloup C, Piraux E, Poulin N, Meunier H. Do Tonkean macaques (Macaca tonkeana) perceive what conspecifics do and do not see? Peer J. 2016;4:e1693. doi: 10.7717/peerj.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63:1–143. [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development. 2000;15:481–498. [Google Scholar]
- Costes-Thire M, Leve M, Uhlrich P, de Marco, Thierry B. Lack of evidence that Tonkean macaques understand what others can hear. Animal Cognition. 2015;18:251–258. doi: 10.1007/s10071-014-0795-3. [DOI] [PubMed] [Google Scholar]
- Csibra G. Recognizing communicative intentions in infancy. Mind & Language. 2010;25:141–168. [Google Scholar]
- Csibra G, Gergely G. Natural pedagogy. Trends in Cognitive Sciences. 2009;13:148–153. doi: 10.1016/j.tics.2009.01.005. [DOI] [PubMed] [Google Scholar]
- D’Entremont B, Hains SMJ, Muir DW. A demonstration of gaze following in 3- to 6-month-olds. Infant Behavior and Development. 1997;20:569–572. [Google Scholar]
- de Waal FBM. Chimpanzee politics: Power and sex among apes. New York, NY: Harper & Row; 1982. [Google Scholar]
- Deaner RO, Shepherd SV, Platt ML. Familiarity accentuates gaze cuing in women but not men. Biology Letters. 2007;2:64–67. doi: 10.1098/rsbl.2006.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RI. The social brain hypothesis. Evolutionary Anthropology. 1998;6:178–190. [Google Scholar]
- Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317(5843):1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: The neuroethology, function, and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24:581–601. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI. Gaze following and joint attention in rhesus monkeys (Macaca mulatta) Journal of Comparative Psychology. 1997;111:286–293. doi: 10.1037/0735-7036.111.3.286. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Coude G, Gallese V, Fogassi L. Having access to others’ mind through gaze: The role of ontogenetic and learning processes in gaze-following behavior of macaques. Social Neuroscience. 2008;3:239–249. doi: 10.1080/17470910701429065. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Kohler E, Fogassi L, Gallese V. The ability to follow eye gaze and its emergence during development in macaque monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3997–14002. doi: 10.1073/pnas.250241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom R, Lee K, Muir D, editors. Gaze-following: Its development and significance. Mahwah, NJ: L. Erlbaum; 2007. [Google Scholar]
- Flombaum JI, Santos S. Rhesus monkeys attribute perceptions to others. Current Biology. 2005;15:447–452. doi: 10.1016/j.cub.2004.12.076. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S, Friendly M, Hong J, Andersen R, Firth D, et al. Package ‘effects’: Effect displays for linear, generalized linear, and other models. 2016 http://www.R-project.org.
- Goossens BMA, Dekleva M, Read SM, Sterck EHM, Bolhuis JJ. Gaze following in monkeys is modulated by observed facial expressions. Animal Behaviour. 2008;75:1673–1681. [Google Scholar]
- Hare B. Can competitive paradigms increase the validity of experiments on primate scial cognition? Animal Cognition. 2001;4:269–280. doi: 10.1007/s100710100084. [DOI] [PubMed] [Google Scholar]
- Hare B. From hominoid to hominid mind: What changed and why? Annual Review of Anthropology. 2011;40:293–309. [Google Scholar]
- Hare B. Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Annual Review of Psychology. 2017;68:155–186. doi: 10.1146/annurev-psych-010416-044201. [DOI] [PubMed] [Google Scholar]
- Hare B, Addessi E, Call J, Tomasello M, Visalberghi E. Do capuchin monkeys, Cebus apella, know what conspecifics do and do not see? Animal Behaviour. 2003;65:131–142. [Google Scholar]
- Hare B, Brown M, Williamson C, Tomasello M. The domestication of social cognition in dogs. Science. 2002;298:1634–1636. doi: 10.1126/science.1072702. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Agnetta B, Tomasello M. Chimpanzees know what conspecifics do and do not see. Animal Behaviour. 2000;59:771–785. doi: 10.1006/anbe.1999.1377. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know? Animal Behaviour. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Chimpanzees deceive a human competitor by hiding. Cognition. 2006;101:495–514. doi: 10.1016/j.cognition.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Current Biology. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Hare B, Plyusnina I, Ignacio N, Schepina A, Wrangham R, Trut L. Social cognitive evolution in captive foxes is a correlated by-product of experimental domestication. Current Biology. 2005;3:226–230. doi: 10.1016/j.cub.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Hare B, Tomasello M. Chimpanzees are more skillful in competitive than cooperative cognitive tasks. Animal Behaviour. 2004;68:571–581. [Google Scholar]
- Hare B, Tomasello M. Human-like social skills in dogs? Trends in Cognitive Sciences. 2005;9:439–444. doi: 10.1016/j.tics.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Tomasello M. Apes’ and children’s understanding of cooperative and competitive motives in a communicative situation. Developmental Science. 2006;9:518–529. doi: 10.1111/j.1467-7687.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, Mastripieri D. Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behavioral Ecology. 2010;21:972–978. doi: 10.1093/beheco/arq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical Journal. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Kaminski J, Call J, Tomasello M. Chimpanzees know what others know, but not what they believe. Cognition. 2008;109:224–234. doi: 10.1016/j.cognition.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Kano F, Call J. Cross-species variation in gaze following and conspecific preference among great apes, human infants and adults. Animal Behaviour. 2014;91:137–150. [Google Scholar]
- Kano F, Hirata S, Call J. Social attention in the two species of Pan: Bonobos make more eye contact than chimpanzees. PLoS One. 2015;10:e0129684. doi: 10.1371/journal.pone.0129684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn G, Pagano A, Maani S, Bunce D. Age-related decline in the reflexive component of overt gaze following. Quarterly Journal of Experimental Psychology. 2015;68(6):1073–1081. doi: 10.1080/17470218.2014.975257. [DOI] [PubMed] [Google Scholar]
- Lane JD, Wellman HM, Olson SL, Miller AL, Wang L, Tardif T. Relations between temperament and theory of mind development in the United States and China: Biological and behavioral correlates of preschoolers’ false-belief understanding. Developmental Psychology. 2013;49:825–836. doi: 10.1037/a0028825. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Watt RJ, Bruce V. Do the eyes have it? Cues to the direction of social attention. Trends in Cognitive Sciences. 2000;4:50–59. doi: 10.1016/s1364-6613(99)01436-9. [DOI] [PubMed] [Google Scholar]
- Lo S, Andrews S. To transform or not to transform: Using generalized linear mixed models to analyse reaction time data. Frontiers in Psychology. 2015;6:1171. doi: 10.3389/fpsyg.2015.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean E, Hare B. Bonobos and chimpanzees infer the target of another’s attention. Animal Behaviour. 2012;83:345–353. [Google Scholar]
- MacLean E, Hare B. Bonobos and chimpanzees exploit helpful but not prohibitive gestures. Behaviour. 2015;152:493–520. [Google Scholar]
- MacLean E, Sandel A, Bray J, Oldenkamp R, Reddy R, Hare B. Group size predicts social but not nonsocial cognition in lemurs. PLoS One. 2013;8:e66359. doi: 10.1371/journal.pone.0066359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis AP, Call J, Tomasello M. Chimpanzees conceal visual and auditory information from others. Journal of Comparative Psychology. 2006;120:154–162. doi: 10.1037/0735-7036.120.2.154. [DOI] [PubMed] [Google Scholar]
- Melis AP, Hare B, Tomasello M. Engineering cooperation in chimpanzees: Tolerance constraints on cooperation. Animal Behaviour. 2006;72:275–286. [Google Scholar]
- Micheletta J, Waller BM. Friendship affects gaze following in a tolerant species of macaque, Macaca nigra. Animal Behaviour. 2012;83:459–467. [Google Scholar]
- Moll H, Tomasello M. 12- and 18-month-old infants follow gaze to spaces behind barriers. Developmental Science. 2004;7:F1–F9. doi: 10.1111/j.1467-7687.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- Moll H, Tomasello M. Level 1 perspective-taking at 24 months of age. British Journal of Developmental Psychology. 2006;24:603–613. [Google Scholar]
- Moll H, Tomasello M. Cooperation and human cognition: The Vygotskian intelligence hypothesis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1480):639–648. doi: 10.1098/rstb.2006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM. Lifespan development: The effects of typical aging on theory of mind. Behavioual Brain Research. 2013;237:32–40. doi: 10.1016/j.bbr.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, van Hecke AV, Parlade MV. Individual differences and the development of joint attention in infancy. Child Development. 2007;78:938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto-Barth S, Call J, Tomasello M. Great apes’ understanding of other individuals’ line of sight. Psychological Science. 2007;18:462–468. doi: 10.1111/j.1467-9280.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- Pereira M, Fairbanks LA. Juvenile primates: Life history, development and behavior. Chicago, IL: University of Chicago Press; 2002. [Google Scholar]
- Phillips LH, Bull R, Allen R, Insch PM, Burr K, Ogg W. Lifespan aging and belief reasoning: Influences of executive function and social cue decoding. Cognition. 2011;120:236–247. doi: 10.1016/j.cognition.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Puce A, Bertenthal BI, editors. The many faces of social attention. New York, NY: Springer; 2015. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. http://www.R-project.org. [Google Scholar]
- Rosati AG, Arre AM, Platt ML, Santos LR. Rhesus monkeys show human-like changes in gaze following across the lifespan. Proceedings of the Royal Society B: Biological Sciences. 2016;283:20160376. doi: 10.1098/rspb.2016.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Hare B. Looking past the model species: Diversity in gaze-following skills across primates. Current Opinion in Neurobiology. 2009;19:45–51. doi: 10.1016/j.conb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Wobber V, Hughes K, Santos LR. Comparative developmental psychology: How is human cognitive development unique? Evolutionary Psychology. 2014;12:448–473. [PubMed] [Google Scholar]
- Sandel AA, MacLean EL, Hare B. Evidence from four lemur species that ringtailed lemur social cognition converges with that of haplorhine primates. Animal Behaviour. 2011;81:925–931. [Google Scholar]
- Santos LR, Nissen AG, Ferrugia JA. Rhesus monkeys, Macaca mulatta, know what others can and cannot hear. Animal Behaviour. 2006;71:1175–1181. [Google Scholar]
- Senju A, Csibra G. Gaze following in human infants depends on communicative signals. Current Biology. 2008;18:668–671. doi: 10.1016/j.cub.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Shepherd SV. Following gaze: Gaze-following behaviors as a window into social cognition. Frontiers in Integrative Neuroscience. 2010;4:5. doi: 10.3389/fnint.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Miller GM, Ferrari PF, Suomi SJ, Pauker A. Neonatal imitation and early social experience predict gaze following abilities in infant monkeys. Scientific Reports. 2015;6:20233. doi: 10.1038/srep20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Nicolini Y, Shetler M, Suomi SJ, Ferrari PF, Pauker A. Experience-independent sex differences in newborn macaques: Females are more social than males. Scientific Reports. 2016;6:19669. doi: 10.1038/srep19669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Paukner A, Sclafani V, Kaburu SSK, Suomi SJ, Ferrari PF. Acute oxytocin improves memory and gaze following in male but not female nursery-reared infant macaques. Psychopharmacology. 2016;234:497–506. doi: 10.1007/s00213-016-4480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slessor G, Phillips LH, Bull R. Exploring the specificity of age-related differences in theory of mind tasks. Psychological Science. 1007;22:639–643. doi: 10.1037/0882-7974.22.3.639. [DOI] [PubMed] [Google Scholar]
- Slessor G, Phillips LH, Bull R. Age-related declines in basic social perception: Evidence from tasks assessing eye-gaze processing. Psychology and Aging. 2008;23:812–822. doi: 10.1037/a0014348. [DOI] [PubMed] [Google Scholar]
- Slessor G, Venturini C, Bonny EJ, Insch PM, Rokaszewicz A, Finnerty AN. Specificity of age-related differences in eye-gaze following: Evidence from social and nonsocial stimuli. Journals of Gerontology B: Psychological Sciences and Social Sciences. 2014;71(1):11–22. doi: 10.1093/geronb/gbu088. [DOI] [PubMed] [Google Scholar]
- Teufel C, Gutmann A, Pirow R, Fischer J. Facial expressions modulate the ontogenetic trajectory of gaze-following among monkeys. Developmental Science. 2010;13:913–922. doi: 10.1111/j.1467-7687.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- Thierry B. Covariation of conflict management patterns across macaque species. In: Aureli F, de Waal FBM, editors. Natural conflict resolution. Berkely, CA: University of California Press; 2000. pp. 106–128. [Google Scholar]
- Thierry B. Social epigenesis. In: Thierry MSWKB, editor. Macaque societies. Cambridge, UK: Cambridge University Press; 2004. pp. 267–290. [Google Scholar]
- Thierry B. Unity in diversity: Lessons from macaque societies. Evolutionary Anthropology. 2007;16:224–238. [Google Scholar]
- Thierry B. Identifying constraints in the evolution of primate societies. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368:20120342. doi: 10.1098/rstb.2012.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. A natural history of human thinking. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- Tomasello M, Call J. Primate cognition. Oxford, U.K: Oxford University Press; 1997. [Google Scholar]
- Tomasello M, Call C, Hare B. Five primate species follow the visual gaze of conspecifics. Animal Behaviour. 1998;55:1063–1069. doi: 10.1006/anbe.1997.0636. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M. Shared intentionality. Developmental Science. 2007;10:121–125. doi: 10.1111/j.1467-7687.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences. 2005;28:675–735. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Fogleman T. The ontogeny of gaze following in chimpanzee, Pan troglodytes, and rhesus macaques, Macaca mulatta. Animal Behaviour. 2001;61:335–343. [Google Scholar]
- Tomasello M, Hare B, Lehmann H, Call J. Reliance on head versus eyes in the gaze following of great apes and human infants: The cooperative eye hypothesis. Journal of Human Evolution. 2007;52:314–320. doi: 10.1016/j.jhevol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Wellman HM. Developing a theory of mind. In: Goswami U, editor. The Wiley-Blackwell handbook of childhood cognitive development. 2nd. Oxford, U.K: Wiley-Blackwell; 2011. pp. 258–284. [Google Scholar]
- Wellman HM, Lane JD, LaBounty J, Olson SL. Observant, nonaggressive temperament predicts theory-of-mind development. Developmental Science. 2010;14:319–326. doi: 10.1111/j.1467-7687.2010.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Herrmann E, Hare B, Wrangham R, Tomasello M. Differences in the early cognitive development of children and great apes. Developmental Psychobiology. 2013;56:574–573. doi: 10.1002/dev.21125. [DOI] [PubMed] [Google Scholar]
- Wobber V, Wrangham R, Hare B. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Current Biology. 2010;20:226–230. doi: 10.1016/j.cub.2009.11.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Barbary macaque following gaze (test trial). The demonstrator (E1) and the cameraperson (E2) stood next to each other, 1–2 m away from the monkey. E1 can be heard calling the monkey to capture the monkey’s attention. When the monkey looks at E1’s face, E1 says ‘now’ and looks straight up. She holds that position for the rest of the 10 s trial. This monkey makes two discrete looks upwards.
Video S2. Rhesus macaque following gaze (test trial). The demonstrator (E1) and the cameraperson (E2) stood next to each other, 1–2 m away from the monkey. When the monkey looks at E1’s face, E1 says ‘now’ and looks straight up. She holds that position for the rest of the 10 s trial. This monkey makes one discrete look upwards.


