Abstract
The use of nitroglycerin in the treatment of angina pectoris began not long after its original synthesis in 1847. Since then, the discovery of nitric oxide as a biological effector and better understanding of its roles in vasodilation, cell permeability, platelet function, inflammation, and other vascular processes have advanced our knowledge of the hemodynamic (mostly mediated through vasodilation of capacitance and conductance arteries) and non-hemodynamic effects of organic nitrate therapy, via both nitric oxide-dependent and independent mechanisms. Nitrates are rapidly absorbed from mucous membranes, the gastrointestinal tract, and the skin, and, thus, nitroglycerin is available in several preparations for delivery via several routes: oral tablets, sublingual tablets, buccal tablets, sublingual spray, transdermal ointment, and transdermal patch; it is also available in intravenous formulations. The organic nitrates are commonly used in the treatment of cardiovascular disease, but clinical data limit their use mostly to the treatment of angina. They are also used in the treatment of subsets of patients with heart failure and pulmonary hypertension. One major problem with the use of nitrates is the development of tolerance. Although several agents have been studied for use in the prevention of nitrate tolerance, none are currently recommended owing to a paucity of supportive clinical data. Only one method of preventing nitrate tolerance remains widely accepted: the use of a dosing strategy that provides an interval of no or low nitrate exposure during each 24-hour period. Nitric oxide’s important role in several cardiovascular disease mechanisms continues to drive research toward finding novel ways to affect both endogenous and exogenous sources of this key molecular mediator.
Keywords: nitroglycerin, nitrate, nitric oxide, soluble guanylyl cyclase, nitrate-nitrite-NO pathway, angina
Introduction
In this review, we detail the discovery of nitroglycerin and its early use in the treatment of angina; the history of the discovery of nitric oxide, its sources, and its roles; the mechanism of action, preparations, and hemodynamic and non-hemodynamic effects of the organic nitrates, as well as their biotransformation; and the clinical uses and adverse effects of nitrate therapy, including tolerance. We conclude by outlining current and future work investigating novel modulators of the NO-sGC-cGMP pathway that may result in new therapeutic options in the treatment of cardiovascular disease.
Early History of the Use of Nitrates in Coronary Artery Disease
William Heberden, MD, is credited with coining the term “angina pectoris” in 1768. Joseph Priestley, an English theologian and chemist, discovered nitric oxide six years later. It would not be until the next century, however, that nitric oxide and its congeners, organic nitrates, would be linked to the treatment of angina (1). Glyceryl trinitrate, or nitroglycerin, was first synthesized by Ascanio Sombrero in Turin, Italy. In 1847, he noted that “a very minute quantity put on the tongue produced a violent headache for several hours” (2,3). Three year earlier, the French chemist Antoine Balard synthesized amyl nitrite (2). Frederick Guthrie, an English chemist, explored the actions of amyl nitrite and published in 1859 that when it was held near the nostrils, “after a lapse of about 50 seconds, a sudden throbbing of the arteries of the neck is felt, immediately followed by a flushing of neck, temples, and forehead and an acceleration action of the heart”(4).
T. Lauder Brunton, a Scottish physician and medical scientist, first described the clinical effectiveness of amyl nitrite (2,3,5). Brunton began caring for patients with angina pectoris as a house physician at the Edinburgh Royal Infirmary (5). At the time, many treatments, including therapeutic bleeding, were being used to treat angina, largely unsuccessfully. In 1867, Brunton published the first report of the use of amyl nitrite in the treatment of angina pectoris (5,6). He explain that he believed “the relief produced by [therapeutic] bleeding to be due to the diminution it occasioned in the arterial tension” and “that a substance which possesses the power of lessening it in such an eminent degree as nitrite of amyl would probably produce the same effect, and might be repeated as often as necessary without detriment to the patient’s health”(5,6). In 1903, Charles-Émile François-Franck, a French physiologist, first suggested that amyl nitrite was a coronary vasodilator in 1903 (5).
In 1879, William Murrell described the symptomatic effects of placing drops of one percent solution of nitroglycerin in alcohol on the tongue (7). In addition to reporting that it relieved angina and prevented subsequent attacks, he also reported the symptoms he felt when he “[tried] its action on [himself]” (7,8). He described a “violent pulsation in [his] head” and noticed that his pulse was “much fuller than natural” (7). In 1914, Brunton, who originally thought angina was caused by hypertension, acknowledged that the “dilating action of amyl nitrite and nitroglycerine upon the coronary vessels would readily explain the relief they offer in angina pectoris, even in cases where the blood-pressure is normal” (5).
Nitric Oxide in the Cardiovascular System
History of Nitric Oxide
In 1975, Diamond and Holmes showed that tissue levels of cyclic adenosine monophosphate (cAMP) were increased in rat myometrial strips maintained in a state of sustained contracture. They demonstrated that nitroglycerin was able to relax the depolarized muscles without significantly increasing cAMP levels and, therefore, concluded that changes in total tissue levels of cAMP were not responsible for the uterine relaxation caused by nitroglycerin (9). These investigators went on to show that nitroglycerin increased cyclic guanosine monophosphate (cGMP) levels in the depolarized muscles (9). The following year, Diamond and Blisard showed that 200 μM nitroglycerin increased levels of cGMP by more than 15-fold during relaxation of isolated strips of phenylephrine-contracted canine femoral arteries while having no significant effect on cAMP levels (10). In 1977, the pharmacologist, Ferid Murad, and his colleagues published their seminal work on modulating contractility in bovine tracheal smooth muscle showing that the guanylyl cyclase activators, sodium nitrite, nitroglycerin, and sodium nitroprusside, increased cGMP levels and relaxed tracheal smooth muscle (11). They went on to demonstrate that solutions of nitric oxide (NO) gas increased cGMP activity in soluble and particulate preparations from various tissues in a dose-dependent fashion, and that NO alone and in combination with sodium azide, sodium nitrite, hydroxylamine, and sodium nitroprusside increased cGMP levels to approximately the same degree and concluded that they activate guanylyl cyclase through a “similar but undefined mechanism” (12). Later, Murad and colleagues postulated that “while the precise mechanism of guanylate cyclase activation by these agents is not known, activation may be due to the formation of NO or another reactive material since NO also increased guanylate cyclase activity” (13).
Working independently, Robert Furchgott and John Zawadzki published their observations on the importance of the endothelium in blood vessel relaxation in 1980 (2,14). They reported that acetylcholine did not always produce blood vessel relaxation in vitro, even though it was a potent vasodilator in vivo. They found that loss of relaxation in vitro was due to “unintentional rubbing of [the rabbit thoracic aorta’s] intimal surface against foreign surfaces during its preparation.” When this mechanical injury was avoided during preparation of the tissue, it always relaxed in response to acetylcholine. Therefore, they concluded that acetylcholine-mediated relaxation of vascular smooth muscle required the presence of endothelial cells (14). Furchgott and his colleagues eventually proposed that bradykinin and other molecules acting on the endothelium caused relaxation of vascular smooth muscle via a substance they termed endothelium-derived relaxing factor (EDRF) (15). The link to NO, however, was not yet appreciated (2).
Louis Ignarro and Salvador Moncada independently discovered that EDRF is NO in 1987 (2,16). After a series of experiments, Ignarro and colleagues stated that “EDRF released from artery and vein possesses identical biological and chemical properties as NO” (17,18). Moncada and colleagues suggested that EDRF and NO were “identical” as “NO released from endothelial cells is indistinguishable from EDRF in terms of biological activity, stability, and susceptibility to an inhibitor and to a potentiator” (19). Based on their collective contributions to the field, the Nobel Prize in Physiology or Medicine was awarded to Furchgott, Ignarro, and Murad in 1998 “for their discoveries concerning NO as a signaling molecule in the cardiovascular system” (2).
Sources of Nitric Oxide
Nitric oxide is a free radical that is synthesized by the family of NO synthases from L-arginine and oxygen, yielding L-citrulline as a co-product (Figure 1) (20–22). In the blood vessel wall, NO is mainly produced by endothelial NO synthase (eNOS) (22,23). However, there are two other isoforms of NO synthase (NOS) that also produce NO from L-arginine: neuronal NOS (nNOS) and cytokine-inducible NOS (iNOS) (24–26). Although eNOS is the major NOS isoform that regulates vascular function, both nNOS and iNOS are implicated as sources in certain tissues and environments (22). For example, vascular injury induces expression of nNOS in the neointima and medial smooth muscle cells, and the production of iNOS within vascular smooth muscle cells after exposure to pro-inflammatory cytokines is a major cause of vasodilation in sepsis (27,28).
Figure 1. Sources of Nitric Oxide.
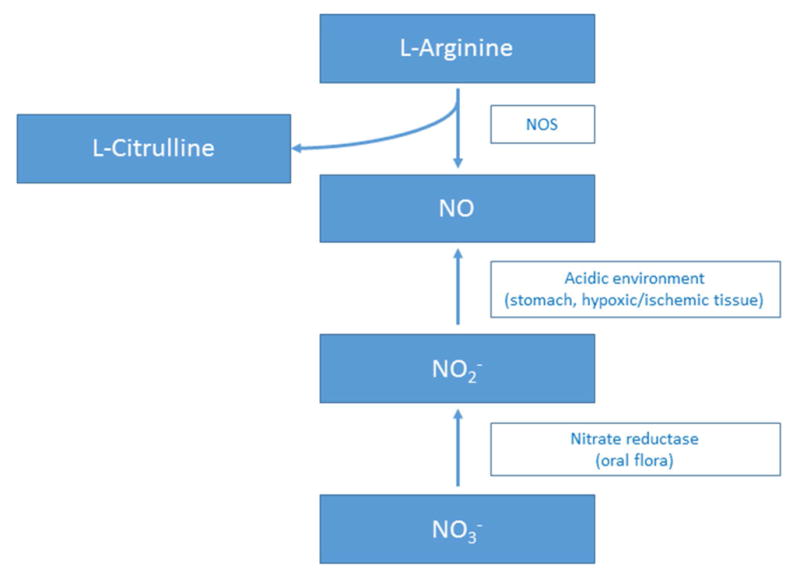
Nitric oxide is a free radical that is synthesized by the family of NO synthases from L-arginine and oxygen, yielding L-citrulline as a co-product. In the blood vessel wall, NO is mainly produced by endothelial NOS (eNOS). There are two other isoforms of NOS that also produce NO from L-arginine: neuronal NOS (nNOS) and cytokine-inducible NOS (iNOS). Additionally, bacterial flora present in the mammalian oral cavity is rich in nitrate reductases and can convert dietary sources of nitrate to nitrite. In the extremely acidic environment (pH ≤ 3) of the gastric lumen, protonation of nitrite can, in turn, produce nitrous acid, which can spontaneously decompose to nitric oxide. This pathway of NO generation is referred to as the enterosalivary nitrate circulation (nitrate-nitrite-NO pathway). NOS, nitric oxide synthase; NO, nitric oxide; NO2−, inorganic nitrite; NO3−, inorganic nitrate.
S-nitrosothiols, such as S-nitrosoglutathione and S-nitrosohemoglobin, are also sources of NO (22,23). Under certain conditions, such as in the presence of trace transition metal ions or photolysis, S-nitrosothiols decompose to liberate nitric oxide (29). In addition, S-nitrosothiols can undergo trans-S-nitrosation with other thiol groups and thereby modify cell or protein function (30–32).
Several proteins, such as hemoglobin, cytochrome P450 reductase, and cytochrome P450, can catalyze the reduction of nitrite or nitrate to generate NO (22). Hemoglobin has enzymatic behavior as a nitrite reductase under hypoxic conditions and is a sensor and effector of hypoxic vasodilatation (33). Hemoglobin’s maximal nitrite reduction rate occurs when hemoglobin is 40–60% saturated with oxygen (22,33). Cytochrome P450 reductase causes NO release by reducing nitrate, and can also facilitate generation of S-nitrosothiols (34).
Bacterial flora present in the mammalian oral cavity is rich in nitrate reductases and can convert dietary sources of nitrate to nitrite. In the extremely acidic environment (pH ≤ 3) of the gastric lumen, protonation of nitrite can, in turn, produce nitrous acid, which can spontaneously decompose to nitric oxide (35,36). This pathway of NO generation is referred to as the enterosalivary nitrate circulation (nitrate-nitrite-NO pathway) (Figure 1) (37,38).
Nitric Oxide’s Roles
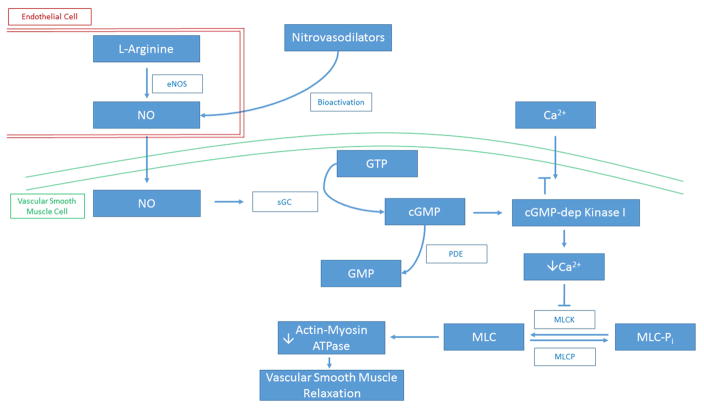
Nitric oxide is a powerful vasodilator that induces formation of cGMP by activating soluble gyanylyl cyclase (sGC) in vascular smooth muscle cells (Figure 2) (12). cGMP can bind to and enhance protein kinase G activity, cGMP-gated ion channels, and cGMP-sensitive phosphodiesterases (39). Protein kinase G promotes reuptake of cytosolic calcium into the sarcoplasmic reticulum, the movement of calcium from the intracellular to the extracellular environment, and the opening of calcium-activated potassium channels (22). These changes result in relaxation of vascular tone as the reduction in intracellular calcium impairs myosin light chain kinase’s ability to phosphorylate myosin, resulting in smooth muscle cell relaxation (40).
Figure 2. Regulation of Vascular Tone by Nitric Oxide.
Nitric oxide is a powerful vasodilator that induces formation of cGMP by activating soluble sGC in vascular smooth muscle cells. cGMP can bind to and enhance protein kinase G activity, cGMP-gated ion channels, and cGMP-sensitive phosphodiesterases. Protein kinase G promotes reuptake of cytosolic calcium into the sarcoplasmic reticulum, the movement of calcium from the intracellular to the extracellular environment, and the opening of calcium-activated potassium channels. These changes result in relaxation of vascular tone as the reduction in intracellular calcium impairs myosin light chain kinase’s ability to phosphorylate myosin, resulting in smooth muscle cell relaxation. eNOS, endothelial nitric oxide synthase; NO, nitric oxide; sGC, soluble guanylyl cyclase; GTP, guanosine triphosphate; cGMP, cyclic guanosine monophosphate; GMP, guanosine monophosphate; PDE, phosphodiesterase; Ca2+, calcium ion; cGMP-dep Kinase I, cyclic guanosine monophosphate-dependent protein kinase I; MLCK, myosin light-chain kinase; MLCP, myosin-light-chain phosphatase; MLC-Pi, phosphorylated myosin light chain; MLC, myosin light chain.
Nitric oxide has several other important roles in the vasculature. For example, when vascular endothelial growth factor (VEGF) binds to VEGF receptor-2, eNOS is activated and NO is formed (41–43). This NO is essential for VEGF function as eNOS-deficient mice have markedly reduced vascular permeability and angiogenesis (44). S-nitrosylation of β-catenin by NO modulates intercellular contacts between endothelial cells likely accounting for NO-dependent changes in endothelial permeability (41). Nitric oxide and organic nitrates also impair platelet activation and aggregation by activating platelet guanylyl cyclase and increasing intraplatelet cGMP (45–48).
Finally, better understanding of the consequences of impaired NO synthesis can yield more information regarding the importance of NO’s role in a well-functioning cardiovascular system. For example, when low levels of its essential cofactor (6R)-5,6,7,8-tetrahydrobiopterin are present, eNOS produces superoxide anion instead of NO in a process denoted enzymatic uncoupling (49). eNOS uncoupling has been observed in animal models of cardiovascular disease and in patients with cardiovascular risk factors, such as hypertension and diabetes mellitus (50,51).
Mechanism of Action of Nitrates
Organic nitrates, such as nitroglycerin, isosorbide dinitrate, and isosorbide mononitrate, are rapidly absorbed from several sites, such as the gastrointestinal tract, mucous membranes, and skin, depending on the preparation. These compounds are prodrugs with a nitrooxy (-O-NO2) moiety and are metabolized to produce bioactive metabolites (52,53). The bioactivation of organic nitrates causes liberation of NO, allowing them to serve as NO donors (53,54). Nitric oxide then causes vasodilation via its effect on vascular smooth muscle cells, and impairs platelet activation as previously discussed (12).
Nitrates also cause vasodilation by other indirect mechanisms. For example, recent data have shown that there is epigenetic regulation of organic nitrate-induced smooth muscle cell relaxation (55,56). Nitroglycerin has been shown to increase the activity of histone acetylases with nitroglycerin-dependent vascular responses influenced by Nε-lysine acetylation of contractile proteins (56).
Preparations
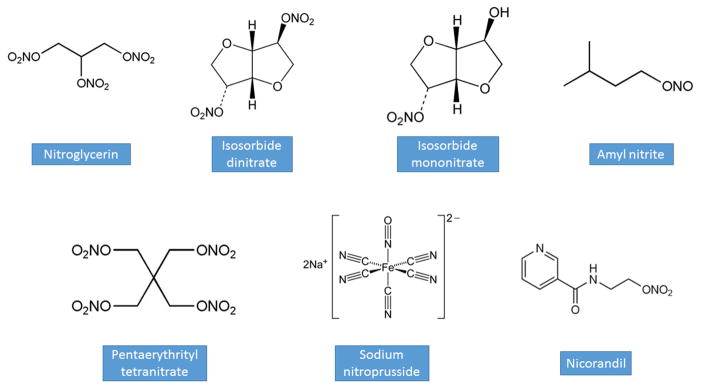
Nitrates are rapidly absorbed from mucous membranes, the gastrointestinal tract, and the skin (53). Thus, nitroglycerin is available in several preparations for delivery via several routes: oral tablets, sublingual tablets, buccal tablets, sublingual spray, transdermal ointment, and transdermal patch; it is also available in intravenous formulations, which are used in hospitalized patients with angina, hypertension, or heart failure (Figure 3, Table 1) (8,57). Nitroglycerin has a plasma half-life of approximately one to four minutes; following hepatic and intravascular metabolism, its biologically active metabolites have a half-life of approximately 40 minutes (8,58).
Figure 3.
Chemical Structures of Key Nitrovasodilators.
Table 1.
Properties of Key Nitrovasodilators.
| Drug | Route/Formulation | Dose | Time until onset of action | Duration of action |
|---|---|---|---|---|
| Nitroglycerin | SL spray | 0.4 mg | 1 to 3 minutes | 25 minutes |
| SL tablet | 0.3 to 0.8 mg | 1 to 3 minutes | 25 minutes | |
| TD patch | 0.2 to 0.8 mg/hour | 30 minutes | 10 to 12 hours | |
| IV infusion | 5 to 400 mcg/minute | Within seconds | 3 to 5 minutes | |
|
| ||||
| Isosorbide dinitrate | PO IR tablet | 5 to 80 mg | 60 minutes | 8 hours |
| PO SR tablet | 40 to 160 mg | 60 minutes | 12 hours | |
|
| ||||
| Isosorbide mononitrate | PO IR tablet | 5 to 20 mg | 30 to 45 minutes | 6 hours |
| PO SR tablet | 30 to 240 mg | 30 to 45 minutes | 12 to 24 hours | |
|
| ||||
| Amyl nitrite | Inhalation of ampule | 0.3 mL | 30 seconds | 3 to 15 minutes |
|
| ||||
| Pentaerythrityl tetranitrate | PO tablet | 10 to 80 mg | 10 to 20 minutes | 8 to 12 hours |
|
| ||||
| Sodium nitroprusside | IV infusion | 0.3 to 10 mcg/kg/minute | 1 minute | 6 to 12 minutes |
|
| ||||
| Nicorandil | PO tablet | 5 to 20 mg | 30 to 60 minutes | 12 hours |
SL, sublingual; TD, transdermal; IV, intravenous; mg, milligram; mcg, microgram; PO, oral; IR, immediate release; SR, sustained release; mL, milliliter; kg, kilogram.
Isosorbide dinitrate is rapidly absorbed and undergoes extensive first-pass metabolism by the liver, which results in low bioavailability (8,53). Sustained-release formulations have a slower rate of absorption and can provide therapeutic plasma concentrations of the drug for up to 12 hours (8). Oral isosorbide mononitrate is completely absorbed and has 100% bioavailability as it avoids first pass metabolism. This leads to a more predictable dose-response and plasma levels with less variation when compared to other nitrates (53,59). Pentaerythrityl tetranitrate is a long-acting organic nitrate available in an oral formulation that acts within 10 to 20 minutes and has a duration of action of eight to 12 hours (55). Pre-clinical data suggested that pentaerythrityl tetranitrate may not cause nitrate tolerance (see below) or endothelial dysfunction. However, the drug is not currently commonly used as clinical studies have not shown any clear benefit in patients with chronic, stable angina (60).
Nicorandil is a nicotinamide-nitrate ester whose chemical structure consists of a nicotinamide derivative combined with a nitrate moiety (53). Nicorandil acts as both a NO donor and a K+ATP channel opener to provide anti-anginal effects (61). The bioactivation of nicorandil occurs via the nicotinamide/nicotinic acid pathway and involves NO generation via denitration (62). Nicorandil also dilates the coronary microvasculature and peripheral resistance arterioles by causing vascular smooth cell hyperpolarization and closure of L-type voltage-gated calcium channels via its action on K+ATP channels; it is rapidly absorbed via the gastrointestinal tract, does not undergo first-pass metabolism, reaches maximal plasma concentration after 30 to 60 minutes, and has a half-life of approximately 52 minutes (53,63,64). Nicorandil has an oral bioavailability of >75%, and its anti-anginal effects last approximately 12 hours (53,62,65).
Sodium nitroprusside, which is 44% cyanide by weight, is comprised of a ferrous ion center complexed with five cyanic moieties and a nitrosyl group (66). It is available intravenously and interacts with oxyhemoglobin to produce methemoglobin and spontaneously release NO and cyanide (67,68). Sodium nitroprusside causes direct venous and arterial vasodilation, is a potent pulmonary vasodilator, and is an inhibitor of hypoxia-induced pulmonary vasoconstriction (66). It has an almost immediate onset of action and a very short half-life, its effects dissipating within one to two minutes (66).
Finally, there are several direct NO donors that are already being used clinically, or have been synthesized and studied for use particularly in biomaterials. Inhaled NO gas diffuses rapidly across the alveolar-capillary membrane and activates sGC in the subjacent smooth muscle cells in the pulmonary vasculature (69). N-diazeniumdiolates (NONOates) are NO donors that are synthesized by reacting primary or secondary amines with NO gas at high pressure and low temperatures; hydrolysis causes spontaneous decomposition under physiological conditions and releases NO (70–72). Nitric oxide (as N2O3 or −ONOO) can react with thiols in vivo to form S-nitrosothiols, such as S-nitrosocysteine and S-nitrosoglutathione (73). S-nitrosothiol compounds can also be synthesized chemically by reacting thiols with nitrous acid. When exposed to physiological fluids, S-nitrosothiols decompose to release NO (70). Other newer classes of direct NO donors that are actively being studied include furoxans, benzofuroxans, and zeolites (74,75).
Biotransformation of Organic Nitrates
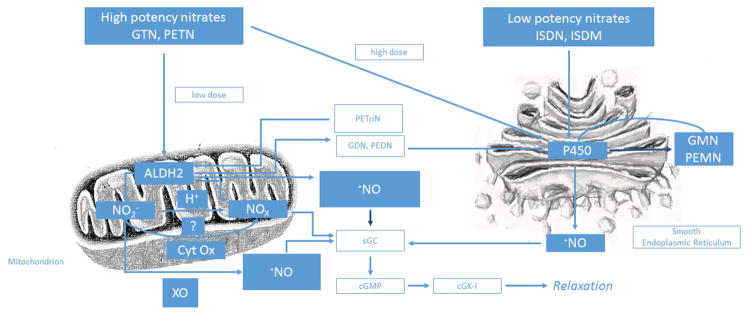
Organic nitrates must undergo biotransformation to release a vasoactive molecule (76). The detailed study of the biotransformation of nitroglycerin has suggested a high potency pathway mediated by aldehyde dehydrogenase-2 (ALDH-2) and a low potency pathway mediated by other enzymes or low-molecular-weight reductants (Figure 4) (76).
Figure 4. Bioactivation of Organic Nitrates.
Organic nitrates must undergo biotransformation to release a vasoactive molecule via one of two pathways: a high potency pathway mediated by ALDH-2, or a low potency pathway mediated by other enzymes or low-molecular-weight reductants. The high potency pathway is important at clinically relevant nitroglycerin concentrations (< 1 μM) and is localized to the mitochondria. The mitochondrial isoform of aldehyde dehydrogenase, ALDH-2, was found to be a key enzyme in the biotransformation of nitroglycerin via this pathway as it generates inorganic nitrite (NO2−) and 1,2-glyceryl dinitrate from nitroglycerin. There are three proposed mechanisms for the release of the vasoactive molecule via this pathway: nitrogen oxide(s) is(are) formed via reduction of inorganic nitrite; nitric oxide is formed directly in response to interaction with ALDH-2; and inorganic nitrite released from mitochondria may be reduced by xanthine oxidase in the cytoplasm to form NO. The low potency pathway is important at suprapharmacological nitroglycerin concentrations (> 1 μM), is found in the smooth endoplasmic reticulum, and leads to formation of measurable amounts of NO in vascular tissues. In this pathway, nitroglycerin is biotransformed by proteins such as deoxyhemoglobin, deoxymyoglobin, cytrochrome P450, xanthine oxidase, glutathione-S-transferase, glyceraldehyde-3-phosphate dehydrogenase, or other ALDH isoforms; and by low-molecular-weight reductants such as cysteine, N-acetyl-cysteine, thiosalicylic acid, and ascorbate. GTN, glyceryl trinitrate (nitroglycerin); PETN, pentaerythrityl tetranitrate; ALDH2, aldehyde dehydrogenase-2; NO2−, inorganic nitrite; H+, hydrogen ion; Cyt Ox, cytochrome c oxidase; XO, xanthine oxidase; NOx, nitrogen oxides; ·NO, nitric oxide; PETriN, pentaerythrityl trinitrate; GDN, 1,2-glyceryl dinitrate; PEDN, pentaerythrityl dinitrate; sGC, soluble gyanylyl cyclase; cGMP, cyclic guanosine monophosphate; cGK-I, cyclic guanosine monophosphate-dependent protein kinase I; ISDN, isosorbide dinitrate; ISDM, isosorbide mononitrate; P450, cytochrome P450 enzyme(s); GMN, 1,2-glyceryl mononitrate; PEMN, pentaerythrityl mononitrate. Reproduced with permission from Munzel, T., Daiber, A. Pharmacology of Nitrovasodilators. In: Bryan, N., and Loscalzo, J., editors. Nitrite and Nitrate in Human Health and Disease. 2nd Ed., New York, Humana Press, 2017.
The high potency pathway is important at clinically relevant nitroglycerin concentrations (< 1 μM) and is localized to the mitochondria. The mitochondrial isoform of aldehyde dehydrogenase, ALDH-2, was found to be a key enzyme in the biotransformation of nitroglycerin via this pathway as it generates inorganic nitrite (NO2−) and 1,2-glyceryl dinitrate from nitroglycerin (77). There are three proposed mechanisms for the release of the vasoactive molecule via this pathway: nitrogen oxide(s) is(are) formed via reduction of inorganic nitrite; nitric oxide is formed directly in response to interaction with ALDH-2; and inorganic nitrite released from mitochondria may be reduced by xanthine oxidase in the cytoplasm to form NO (76).
The low potency pathway is important at suprapharmacological nitroglycerin concentrations (> 1 μM), is found in the smooth endoplasmic reticulum, and leads to formation of measurable amounts of NO in vascular tissues (78,79). In this pathway, nitroglycerin is biotransformed by proteins such as deoxyhemoglobin, deoxymyoglobin, cytrochrome P450, xanthine oxidase, glutathione-S-transferase, glyceraldehyde-3-phosphate dehydrogenase, or other ALDH isoforms; and by low-molecular-weight reductants such as cysteine, N-acetyl-cysteine, thiosalicylic acid, and ascorbate (55,80–82).
Hemodynamic Effects of Nitrates
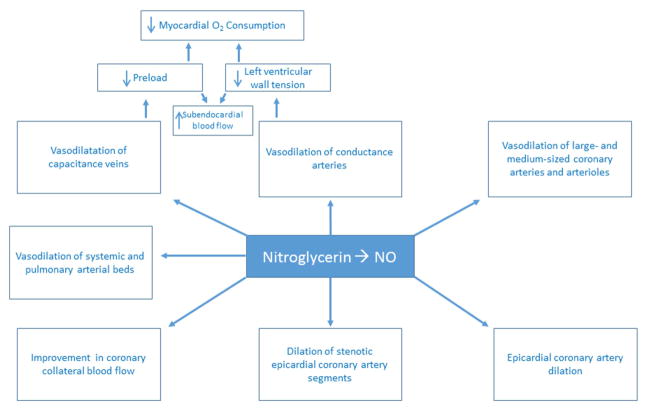
The organic nitrates have several hemodynamic effects that are mostly mediated through vasodilation of capacitance veins and conductance arteries (Central Illustration) (8). In 1971, Parker and colleagues published the results of a study of hemodynamic indices and coronary blood flow at rest and during exercise before and after administration of nitroglycerin in 15 patients with coronary artery disease. With nitroglycerin, they found that only two of these patients had angina, and their hemodynamic response to exercise was normal; however, coronary blood flow was normal at rest and during exercise in both groups of patients before and after nitroglycerin. They, therefore, concluded that nitroglycerin acted primarily by reducing left ventricular oxygen requirements through a reduction in left ventricular volume (83). Nitric oxide-mediated dilation of capacitance veins decreases ventricular preload, which results in reduction in myocardial oxygen demand. In 1975, Greenberg and colleagues, after angiographic and hemodynamic assessment of ten patients, concluded that the mechanism of action of nitroglycerin “seems to relate best to the decrease in systolic wall tension” (84). They also found that the end-diastolic wall tension decreased by 57%, “suggesting the possibility that diastolic coronary blood flow may be augmented by diminished extravascular resistance to flow” (84).
Central Illustration. Hemodynamic Effects of Nitroglycerin.
The organic nitrates have several hemodynamic effects that are largely mediated through vasodilation of capacitance veins and conductance arteries. Seminal work in the 1970s, as detailed in the main text, showed that nitroglycerin acted primarily by reducing left ventricular oxygen requirements through a reduction in left ventricular volume. Nitric oxide-mediated dilation of capacitance veins decreases ventricular preload, which results in reduction in myocardial oxygen demand and left ventricular wall tension. This results in an increase in subendocardial myocardial blood flow. Nitrates also dilate large and medium sized coronary arteries and arterioles > 100 micrometers in diameter. This effect reduces left ventricular systolic wall tension via decreasing afterload and, therefore, also decreases myocardial oxygen demand. The hemodynamic effects of nitrates on the coronary vasculature also relieve angina. Nitrates dilate the epicardial coronary arteries, including stenotic segments, and also improve blood flow in coronary collaterals via decreasing resistance to collateral flow. Nitrates, particularly sodium nitroprusside, can also dilate the systemic arterial bed, and NO gas and sodium nitroprusside dilate the pulmonary vascular bed and inhibit hypoxia-induced pulmonary vasoconstriction. NO, nitric oxide; O2, oxygen.
Nitrates also dilate large and medium sized coronary arteries and arterioles > 100 micrometers in diameter. This effect reduces left ventricular systolic wall tension via decreasing afterload and, therefore, also decreases myocardial oxygen demand (8,85). The hemodynamic effects of nitrates on the coronary vasculature of atherosclerotic patients also relieve angina. Nitrates dilate the epicardial coronary arteries, including stenotic segments, and also improve blood flow in coronary collaterals via decreasing resistance to collateral flow (85,86). Importantly, the organic nitrates do not dilate coronary microvessels that are < 100 micrometers in diameter, which reduces the risk of ischemia from coronary steal (87). Nitrates, particularly sodium nitroprusside, can also dilate the systemic arterial bed, and NO gas and sodium nitroprusside dilate the pulmonary vascular bed and inhibit hypoxia-induced pulmonary vasoconstriction (66).
Non-Hemodynamic Effects of Nitrates
The organic nitrates have also been found to have several important, non-hemodynamic effects. Inhibition of platelet function by organic nitrates was first reported in 1967 by Hampton and colleagues, who showed that nitroglycerin impairs platelet aggregation in vitro (88). Subsequently, the organic nitrates have been shown to inhibit platelet aggregation and function by increasing intracellular cGMP and forming S-nitrosothiols, which are potent activators of sGC and inhibitors of platelet aggregation (45,89). Organic nitrates also have other antithrombotic effects as activation of sGC is accompanied by inhibition of agonist-mediated calcium flux, which results in a reduction of fibrinogen binding to the glycoprotein Ilb/IIIa receptor of platelets (90).
Nitrates have also been found to have anti-inflammatory effects via NO’s role in the inflammatory process (91). Nitric oxide inhibits neutrophil adhesion and chemotaxis in acute inflammation and modulates microvascular permeability (92–95). However, increased expression of iNOS has been implicated in chronic inflammatory conditions, as high-flux NO from iNOS or its derivates in the inflammatory milieu (e.g., peroxynitrite) promotes adhesion molecule expression and leukocyte-endothelial cell interactions, among other pro-inflammatory mechanisms (95).
Finally, nitroglycerin has been shown to induce a protective phenotype that limits damage after ischemia and reperfusion. Nitroglycerin protects against post-ischemic endothelial dysfunction in particular, in part, by impairing the opening of the mitochondrial permeability transition pore (96,97).
Clinical Uses of Nitrates
Organic nitrates, in intravenous, sublingual, and oral formulations, are often used in the management of acute coronary syndrome. Treatment with nitrates causes vasodilatation of the capacitance veins and results in reduced ventricular filling pressure, wall tension, and myocardial oxygen demand (8). As previously discussed, nitrates also dilate the epicardial coronary arteries, improving coronary blood flow, particularly in ischemic zones (85,86,98,99).
These well-demonstrated physiological effects notwithstanding, randomized control data supporting a clinical outcome benefit for the use of nitrates in acute coronary syndrome are lacking. The GISSI-3 trial randomly assigned 19,394 patients with acute myocardial infarction in a two-by-two factorial design to intravenous nitroglycerin followed by a nitrate patch or placebo, as well as to lisinopril or placebo. The primary study end point of mortality at six weeks demonstrated no significant benefit of nitrate therapy. The subset of patients receiving combination therapy with lisinopril and nitrates, however, had the lowest mortality in the trial (100). At six months, there was no difference in mortality in patients who received nitrate therapy (101). The ISIS-4 trial randomized 58,050 patients presenting up to 24 hours after the onset of a suspected acute myocardial infarction in a two-by-two factorial design that involved treatment with captopril, isosorbide mononitrate, magnesium sulfate, and/or placebo. There was no significant reduction in mortality attributed to nitrate therapy (102). Of note, both the GISSI-3 and ISIS-4 trials were conducted in the fibronolytic era (i.e., prior to the percutaneous coronary intervention era). However, in the pre-fibrinolytic era, meta-analyses show of ten trials predating the GISSI-3 and ISIS-4 trials of patients randomized to intravenous nitroglycerin or sodium nitroprusside in acute myocardial infarction showed a reduction in mortality of 35% associated with nitrate therapy with the greatest reduction in mortality occurring during the first week of follow-up (103).
Nitroglycerin is the most frequently used drug to treat acute episodes of angina. It is usually given as a sublingual tablet, but is also available as a sublingual spray. Sublingual nitroglycerin or nitroglycerin oral spray can also be used prior to angina-inducing activities to prevent the occurrence of acute angina (8,104,105).
Winsor and Berger studied 53 patients with documented angina and concluded in 1975 that controlled-release oral nitroglycerin provided statistically significant clinical improvement of angina (106). In 1974, Reichek and colleagues published their work on 14 patients with angina pectoris and concluded that nitroglycerin ointment produced a significant increase in exercise capacity which persisted for at least 3 hours (107). Several studies have also shown an improvement in exercise capacity without angina via use of transdermal preparations (108–112). Taken together, these many studies support the use of oral organic nitrates to increase exercise tolerance, prevent angina, and improve chronic stable angina. In current practice, isosorbide dinitrate and isosorbide mononitrate are often used for these indications (8).
Although not available in the United States, nicorandil is used in the treatment of chronic, stable angina in other countries. In the Impact of Nicorandil in Angina (IONA) trial, treatment with nicorandil in patients with stable angina statistically significantly reduced the primary end point of coronary death, non-fatal myocardial infarction, or unplanned hospitalization for angina (113).
The established treatment for Prinzmetal variant angina, or coronary artery spasm, is therapy with calcium-channel blockers. Long-acting oral nitrates have also been found to be helpful, and it is thought that their vasodilatory effects are additive to calcium-channel blockade in this setting (114,115).
Intravenous nitroglycerin and sodium nitroprusside are used to treat patients hospitalized with hypertensive urgency and emergency with preference to sodium nitroprusside as it induces more arterial vasodilatation than nitroglycerin. Although nitrates have generally not been shown to be of use in the management of hypertension, they are considered the drug class-of-choice for patients with hypertension and stable angina (116). They should ideally be used in combination with beta-blockers, or alone if beta-blockers are contraindicated or cause unacceptable side effects (116).
Nitrates were previously considered first-line therapy for patients with acute heart failure with normal or high blood pressures due to their previously described hemodynamic effects on the venous and arterial systems, preload, and afterload (117). A recent Cochrane review, however, concluded that there appears to be no significant difference between nitrate vasodilator therapy and alternative interventions for the treatment of acute heart failure syndromes with regard to symptom relief and hemodynamic variables (118). However, due to the striking results of the African-American Heart Failure Trial, the combination of hydralazine and isosorbide dinitrate is recommended together with angiotensin converting enzyme inhibitors, beta-blockers, and aldosterone antagonists by current guidelines for African-Americans who require further blood pressure control and relief of symptoms from New York Heart Association Class III or Class IV heart failure (116,119,120).
Inhaled NO gas has several clinical uses in adults. It has a well-established role in vasoreactivity testing in patients with pulmonary arterial hypertension. This testing is helpful in identifying patients who may respond to therapy with calcium-channel blockers (69,121). Although robust data are lacking, it is also used in patients both with and without a prior diagnosis of pulmonary hypertension with acute hypoxemic respiratory failure to improve ventilation-perfusion matching (122). Inhaled NO has an established role in the treatment of severe persistent pulmonary hypertension of the newborn, and a recently published Cochrane review also supports its use in the treatment of term and near-term infants with hypoxic respiratory failure who do not have a diaphragmatic hernia (123,124). By contrast, a recent Cochrane review does not support its use for respiratory failure in preterm infants (125).
Nitrate Tolerance
One major problem with the use of nitrates is the development of tolerance, which is defined as “the loss of hemodynamic and anti-anginal effects during sustained therapy” (8). Tolerance occurs following chronic exposure to all nitrates and results in “complete or markedly diminished anti-anginal and anti-ischemic effects throughout the 24-hour period of long-term therapy” (126). In 1980, Thadani and colleagues reported their studies of tolerance to oral isosorbide dintrate and showed that both partial circulatory tolerance to isosorbide dinitrate and cross-tolerance to nitroglycerin developed rapidly during treatment with isosorbide dinitrate given every 6 hours (127). They went on to report similar findings with regard to its anti-anginal effect two years later (128). Subsequent studies would report similar findings to different formulations of nitrate therapy (59,111,126,129–134).
The cause of nitrate tolerance is still incompletely understood, but several hypotheses exist. One hypothesis argues that chronic treatment with organic nitrates triggers supersensitivity to vasoconstrictors, which attenuates the vasodilator effects of nitrates. This action is mediated by increased autocrine levels of endothelin within the vasculature, with the subsequent activation of phospholipase C and protein kinase C. These pathways lead to increased actomyosin activity and myocyte contractility. Additionally, agonist-driven calcium-dependent activation of the RhoA/Rho kinase pathway contributes to vasoconstriction via inhibition of myosin light chain phosphatase (135–137).
Another hypothesis for the mechanism of nitrate tolerance is that chronic therapy with organic nitrates desensitizes sGC (138,139). S-nitrosylation of sGC is a means by which “memory” of NO exposure is retained in smooth muscle cells, resulting in decreased responsiveness to NO, and could be a mechanism of NO tolerance (135,140). Nitroglycerin metabolism also promotes the production of reactive oxygen species. Oxidation of thiol groups in the active site of ALDH-2 by these reactive derivatives has been observed during chronic nitroglycerin treatment. This post-translational modification may cause inhibition of ALDH-2 enzyme activity, which can lead to both reduced nitroglycerin biotransformation and efficacy (135,141–143).
Additionally, continuous treatment with nitroglycerin has been shown to cause nitric oxide synthase dysfunction, likely through the reduced bioavailability of tetrahydrobiopterin (144). Nitric oxide synthase dysfunction can cause superoxide anion formation. If not effectively mitigated by superoxide dismutases, increased superoxide anion formation can lead to the formation of peroxynitrite anion (through reaction with NO), which is a highly reactive intermediate that promotes oxidant stress and reduces NO bioavailability (135,145,146).
Nitrate therapy-induced increases in phosphodiesterase activity have also been implicated in the development of tolerance (147). As previously discussed, nitroglycerin is metabolized to NO, which activates sGC to increase cGMP. Phosphodiesterase decreases cGMP levels, and, therefore, NO-induced vasodilation is attenuated by its activity.
Finally, pseudotolerance is also an issue complicating chronic treatment with organic nitrates. This adaptive phenomenon is not considered true vascular tolerance because it occurs in response to every form of vasodilator therapy. Pseudotolerance is marked by neurohormonal activation, increased catecholamine release rates and circulating catecholamine levels, sodium retention, and intravascular volume expansion (135,148).
Although several agents have been studied for use in the prevention of nitrate tolerance, none are currently recommended owing to a paucity of supportive clinical data (126,132,149–156). Only one method of preventing nitrate tolerance remains widely accepted: the use of a dosing strategy that provides an interval of no or low nitrate exposure during each 24-hour period (112,126,133,157–159). Nitrate tolerance is rapidly reversed during a nitrate-free interval (8,160).
Adverse Effects of Nitrates
In addition to the development of tolerance, there are other important considerations to treatment with organic nitrates. Common side effects of nitrate therapy include headache, flushing, lightheadedness, and postural hypotension. When sodium nitroprusside is infused, it interacts with oxyhemoglobin to form methemoglobin and releases NO and cyanide. Therefore, patients receiving sodium nitroprusside must be monitored for manifestations of both cyanide toxicity, such as altered mental status, seizure, and metabolic acidosis, and methemoglobinemia, such as cyanosis, headache, fatigue, and lethargy (66,67,161,162). Cyanide toxicity from sodium nitroprusside therapy is rare and is unlikely if the cumulative dose of nitroprusside does not exceed 0.5 mg/kg/hr. Large infusion doses of nitroglycerin can also cause methemoglobinemia (163). There are several antidotes available to treat cyanide toxicity, including sodium thiosulfate, sodium nitrite, amyl nitrite, and hydroxycobalamin, as well as potential antidotes that are undergoing development, such as sulfanegen sodium. Sodium thiosulfate is available intravenously, and by increasing thiosulfate concentration in plasma, acts as a sulfur donor to rhodanase, an enzyme that transforms cyanide to thiocyanate, which is non-toxic (164). Intravenous sodium nitrite and inhaled amyl nitrite are methemoglobin generators, and methemoglobin’s strong affinity for cyanide binding is the rationale for their use as antidotes (165). Hydroxycobalamin is a vitamin B derivative that is available intravenously and contains a cobalt moiety that avidly binds to intracellular cyanide forming cyanocobalamin (165–167). Sulfanegen sodium is a prodrug of 3-mercaptopyruvate that converts cyanide to thiocyanate (164,168). It was shown to be effective in reversing cyanide toxicity in a juvenile pig model, but work is ongoing to develop it into a antidote that is safe for use in humans (169).
Additionally, there are certain clinical situations in which nitrates should not be used, or should be used with extreme caution. Nitrates should not be given to patients who have used a phosphodiesterase inhibitor, such as sildenafil or tadalafil, within 24–48 hours owing to the risk of severe hypotension (170,171). Nitrates should also be avoided in suspected causes of right ventricular infarction as nitrate-induced dilatation of the venous capacitance beds could cause hypotension given the need for high filling pressures in this setting (172). Patients with hypertrophic cardiomyopathy should not be placed on nitrate therapy as it can increase outflow tract obstruction by decreasing preload and ventricular volumes (173).
Novel Modulators of the NOsGC-cGMP Pathway
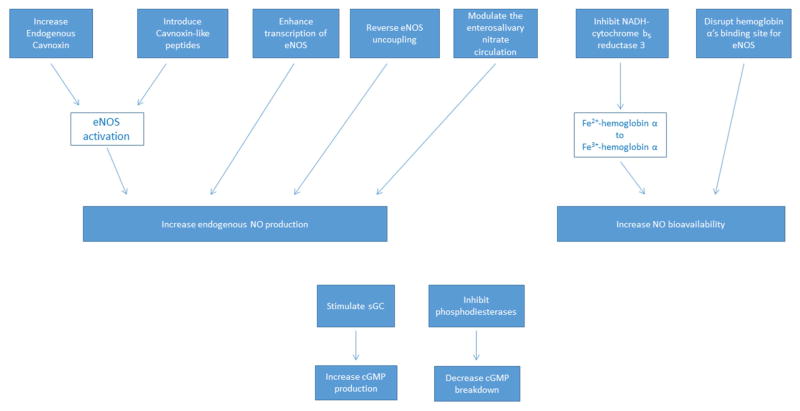
The organic nitrates have been and continue to be mainstays in the treatment of several acute and chronic cardiovascular conditions. Although many of the organic nitrates have been used clinically for decades, there are several new drugs and drug classes that have been approved recently or are currently being studied that have the potential to add to the list of modulators of the NO-sGC-cGMP pathway (174). Their methods of modulation of this pathway are summarized in Figure 5.
Figure 5. Novel Ways of Modulating the NOsGC-cGMP Pathway.
Several novel modulators of the NO-sGC-cGMP pathway are undergoing active investigation, and the details are summarized in the main text. Methods of increasing endogenous cavnoxin production or introducing synthesized cavnoxin-like peptides could increase endogenous NO production. Transcriptional enhances of eNOS, the small molecules AVE3085 and AVE9488, have increased endogenous NO production, reversed eNOS uncoupling, and decreased eNOS production of superoxide anion in mice. The enterosalivary nitrate circulation (nitrate-nitrite-NO pathway) is a source of NO that is derived from dietary inorganic nitrate intake and production of NO from this pathway is enhanced by hypoxia and acidosis. Research dedicated to exploring therapeutic options, such as prebiotics, probiotics, and antimicrobial agents, that can modulate the microbiome and the nitrate-nitrite-NO pathway in heart failure, pulmonary hypertension, hypertension, obesity, and other cardiovascular disease states is ongoing. Increasing the bioavailability of endogenous NO is another potential approach to modulating the NO-sGC-cGMP pathway. The oxidized form of hemoglobin-α has a much lower affinity for NO than the reduced form, and, therefore, allows eNOS-generated NO to diffuse to underlying vascular smooth muscle cells. Since NADH-cytrochrome b5 reductase 3 reduces Fe3+-hemoglobin-α to Fe2+-hemoglobin-α, a potential way to increase NO bioavailability would be to inhibit NADH-cytochrome b5 reductase 3. Another possible strategy would be to disrupt the binding site of hemoglobin-α for eNOS, and a small peptide has been developed, hemoglobin-αX, as just such an inhibitor. Finally, modulating the NO-sGC-cGMP pathway in an NO-independent fashion is also being study. The ciguats modulate sGC activity to increase cGMP production independently of NO. The phosphodiesterases hydrolyze the phosphodiester bond of cGMP. Inhibition of these enzymes decreases cGMP breakdown. eNOS, endothelial nitric oxide synthase; NO, nitric oxide; NADH, nicotinamide adenine dinucleotide; Fe, iron; sGC, soluble gyanylyl cyclase; cGMP, cyclic guanosine monophosphate.
Caveolin-1 (CAV1) is the main coat protein of caveolae, invaginations in the plasma membrane implicated in several biological processes, including signaling, in endothelial cells (174–178). eNOS activity is decreased when bound to CAV1 (179,180). Cavnoxin, a peptide that activates eNOS, was identified by studying the key residues in CAV1 responsible for inhibition of eNOS function (181). In wild-type mice, cavnoxin increases NO levels, causes a reduction in vascular tone, and lowers systemic blood pressure (181). Therefore, novel methods of increasing endogenous cavnoxin production or introducing synthesized cavnoxin-like peptides could be a promising way to increase endogenous NO production.
Another approach to increasing endogenous NO is to increase NO bioavailability by affecting hemoglobin-α’s ability to complex with eNOS. In the microcirculation, hemoglobin α forms a macromolecular complex with eNOS and controls the flux of bioavailable NO (174,182). The reduced Fe2+-O2-hemoglobin-α reacts with NO rapidly and generates nitrate and the oxidized Fe3+-hemoglobin-α. This oxidized form of hemoglobin-α has a much lower affinity for NO than the reduced form, and, therefore, allows eNOS-generated NO to diffuse to underlying vascular smooth muscle cells (174,183). Nicotinamide adenine dinucleotide (NADH)-cytrochrome b5 reductase 3 reduces Fe3+-hemoglobin-α to Fe2+-hemoglobin-α. Therefore, yet another potential way to increase NO bioavailability would be to inhibit NADH-cytochrome b5 reductase 3. Still another possible strategy would be to disrupt the binding site of hemoglobin α for eNOS, and a small peptide has been developed, hemoglobin-αX, as just such an inhibitor. Hemoglobin αX has been found to decrease blood pressure in mice and dilate constricted arterioles isolated from patients with hypertension (182,184).
Modulating the transcription, and, therefore, translation, of NOS is another focus of research and development efforts to augment levels of endogenous NO. Two small molecules, AVE3085 and AVE9488, have been identified as transcriptional enhancers of eNOS that bind its promoter (174,185). In apolipoprotein E-knockout mice, 12 weeks of treatment with AVE9488 or AVE3085 reduced atherosclerotic plaque formation. Treatment of these mice with AVE9488 also reversed eNOS uncoupling, increased vascular content of the essential eNOS cofactor BH4, and reduced vascular cuff-induced neointima formation (185). Four weeks of treatment with AVE3085 was shown to attenuate cardiac remodeling in an experimental mouse model involving aortic banding to induce remodeling (186). Finally, nine weeks of treatment with AVE9488 was shown to improve left ventricular remodeling and contractile dysfunction in an experimental myocardial infarction rat model (187).
As referred to above with regard to treatment with AVE9488, reversing eNOS uncoupling is desired as it decreases eNOS production of superoxide anion (49). Tetrahydrobiopterin has been shown to improve endothelial dysfunction in patients with type II diabetes mellitus and in patients on cyclosporine A after cardiac transplantation (51,188). However, a study of 49 patients randomized to receive 400 mg/day of BH4, 700mg/day of BH4, or placebo for two to six weeks before coronary artery bypass graft surgery found no effect of BH4 treatment on vascular function or superoxide production (189). More research is needed to explore alternative ways to reverse eNOS uncoupling and, thereby, increase NO production and decrease superoxide anion production.
Ignarro and colleagues showed that sGC activity could be modulated in an NO-independent fashion by protoporphyrin IX, an sGC activator (190). Protoporphyrin IX was never used clinically owing to its severe photosensitizing effect (174). However, a recently developed class of drugs called the ciguats also modulate the NO-sGC-cGMP pathway similarly in an NO-independent fashion (174). There are two subgroups of ciguats: NOsGC stimulators (which bind NOsGC and act through allosteric regulation) and NOsGC activators (which occupy the heme binding site of NOsGC and work additively with NO) (174). Lificiguat was the first NOsGC stimulator that was identified when it was found to stimulate sGC in rabbit platelets during a small molecule screen designed to identify novel platelet aggregation inhibitors (191). Further work to improve the solubility and efficacy of lificiguat led to the development of riociguat, which is the only NOsGC stimulator that is approved for clinical use (192). Riociguat is currently approved for the treatment of pulmonary arterial hypertension and inoperable chronic thromboembolic pulmonary hypertension (174,193,194). There are ongoing trials of riociguat’s potential role in the treatment of pulmonary hypertension and heart failure. Other NOsGC-stimulating ciguats that are being studied include vericiguat, which was shown to decreased circulating levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) in patients with worsening chronic systolic heart failure, as well as nelociguat, IW-1973, and IW-1701 (174,195,196).
Cinaciguat was identified as a NOsGC activator via high-throughput screening (197,198). Its clinical use has been limited due to significant hypotension. In a placebo-controlled trial of 139 patients admitted with acute, decompensated, systolic heart failure, cinaciguat significantly decreased pulmonary capillary wedge pressure, right atrial pressure, pulmonary vascular resistance, and systemic vascular resistance, and also significantly increase cardiac index. However, the trial was stopped prematurely due to an increased occurrence of hypotension at cinaciguat doses greater than 200 micrograms/hour (199). At lower doses, cinaciguat has been shown to decrease systemic blood pressure without improving dyspnea or cardiac index in patients with acute heart failure, therefore limiting its use in this population (200). Another NOsGC activator that is under development is ataciguat (174).
Another class of NOsGC-cGMP pathway modulators is the phosphodiesterases. These enzymes inhibit the pathway by hydrolyzing the phosphodiester bond of cGMP (174,201). There are four phosphodiesterase 5 inhibitors in current clinical use that inhibit cGMP breakdown: sildenafil, vardenafil, tadalafil, and avanafil. All four are approved for use in erectile dysfunction. Sildenafil and tadalafil are also used for pulmonary arterial hypertension, and tadalafil is approved for use in benign prostatic hyperplasia (174). Work is ongoing to develop additional phosphodiesterase inhibitors, including inhibitors of non-selective phosphodiesterases and cGMP-selective phosphodiesterases (phosphodiesterase 5, phosphodiesterase 6, and phosphodiesterase 9) (174).
Finally, as discussed above, the enterosalivary nitrate circulation (nitrate-nitrite-NO pathway) is a source of NO that is derived from dietary inorganic nitrate intake (37,38,202). Production of NO from this pathway is enhanced by hypoxia and acidosis (203,204). Therefore, there is interest in studying the use of inorganic nitrates to improve exercise capacity, particularly in patients with heart failure with preserved left ventricular ejection fraction (202). There is also ongoing research dedicated to exploring therapeutic options, such as prebiotics, probiotics, and antimicrobial agents, that can modulate the microbiome and the nitrate-nitrite-NO pathway in heart failure, pulmonary hypertension, hypertension, obesity, and other cardiovascular disease states (38).
Conclusion
The use of nitrates in cardiovascular disease has a long and storied history. They continue to play a major role in current clinical practice to improve symptoms despite the paucity of evidence of benefit on hard clinical endpoints. As research and development of new ways to modulate the NO-sGC-cGMP pathway continue, it may be time to revisit the study of nitrates in large, prospective clinical trials in the percutaneous coronary intervention era.
Perspectives.
Nitric oxide is an important biological effector with roles in vasodilation, cell permeability, platelet function, inflammation, and other vascular processes. The organic nitrates, as sources of nitric oxide, are commonly used in the treatment of cardiovascular disease, but clinical data limit their use primarily to the treatment of angina. As research and development of new ways to modulate the NO-sGC-cGMP pathway continue, it may be time to revisit the study of nitrates in large, prospective clinical trials in the percutaneous coronary intervention era.
Acknowledgments
Funding: This work was supported in part by NIH grants HL61795 and GM107618 to J.L. The authors have no relationship with industry relevant to this work.
The authors wish to thank Ms. Stephanie Tribuna for expert technical assistance. This work was supported in part by NIH grants HL61795 and GM107618 to J.L.
Abbreviations
- ALDH-2
Aldehyde dehydrogenase-2
- Ca2+
Calcium ion
- CAV1
Caveolin-1
- cAMP
Cyclic adenosine monophosphate
- cGMP
Cyclic guanosine monophosphate
- cGK-I
Cyclic guanosine monophosphate-dependent protein kinase I
- iNOS
Cytokine-inducible NOS
- Cyt Ox
Cytochrome c oxidase
- P450
Cytochrome P450 enzyme(s)
- eNOS
Endothelial nitric oxide synthase
- EDRF
Endothelium-derived relaxing factor
- eNOS
Endothelial NO synthase
- GDN
1,2-glyceryl dinitrate
- GMN
1,2-glyceryl mononitrate
- GTN
Glyceryl trinitrate (nitroglycerin)
- GTP
Guanosine triphosphate
- GMP
Guanosine monophosphate
- H+
Hydrogen ion
- NO3−
Inorganic nitrate
- NO2−
Inorganic nitrites
- ISDN
Isosorbide dinitrate
- ISDM
Isosorbide mononitrate
- MLC
Myosin light chain
- MLCK
Myosin light-chain kinase
- MLCP
Myosin-light-chain phosphatase
- nNOS
Neuronal NOS
- NO
Nitric oxide
- NOx
Nitrogen oxides
- NOS
NO synthase
- NONOates
N-diazeniumdiolates
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- PEDN
Pentaerythrityl dinitrate
- PEMN
Pentaerythrityl mononitrate
- PETN
Pentaerythrityl tetranitrate
- PETriN
Pentaerythrityl trinitrate
- PDE
Phosphodiesterase
- MLC-Pi
Phosphorylated myosin light chain
- sGC
Soluble gyanylyl cyclase
- BH4
Tetrahydro-L-biopterin
- VEGF
Vascular endothelial growth factor
- XO
Xanthine oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinhorn BS, Loscalzo J, Michel T. Nitroglycerin and Nitric Oxide--A Rondo of Themes in Cardiovascular Therapeutics. N Engl J Med. 2015;373:277–80. doi: 10.1056/NEJMsr1503311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh N, Marsh A. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin Exp Pharmacol Physiol. 2000;27:313–9. doi: 10.1046/j.1440-1681.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- 3.Berlin R. Historical aspects of nitrate therapy. Drugs. 1987;33(Suppl 4):1–4. doi: 10.2165/00003495-198700334-00003. [DOI] [PubMed] [Google Scholar]
- 4.Guthrie F. Contributions to the knowledge of the amyl group. 1. Nitryl of amyl and its derivatives. J Chem Soc. 1859;11:245–52. [Google Scholar]
- 5.Fye WB. T. Lauder Brunton and amyl nitrite: a Victorian vasodilator. Circulation. 1986;74:222–9. doi: 10.1161/01.cir.74.2.222. [DOI] [PubMed] [Google Scholar]
- 6.Brunton TL. On the use of nitrite of amyl in angina pectoris. Lancet. 1867;2:97. [Google Scholar]
- 7.Murrell W. Nitro-Glycerine as a Remedy for Angina Pectoris. The Lancet. 1879;113:80–81. [Google Scholar]
- 8.Parker JD, Parker JO. Nitrate therapy for stable angina pectoris. N Engl J Med. 1998;338:520–31. doi: 10.1056/NEJM199802193380807. [DOI] [PubMed] [Google Scholar]
- 9.Diamond J, Holmes TG. Effects of potassium chloride and smooth muscle relaxants on tension and cyclic nucleotide levels in rat myometrium. Can J Physiol Pharmacol. 1975;53:1099–107. doi: 10.1139/y75-153. [DOI] [PubMed] [Google Scholar]
- 10.Diamond J, Blisard KS. Effects of stimulant and relaxant drugs on tension and cyclic nucleotide levels in canine femoral artery. Mol Pharmacol. 1976;12:668–92. [PubMed] [Google Scholar]
- 11.Katsuki S, Murad F. Regulation of adenosine cyclic 3′,5′-monophosphate and guanosine cyclic 3′,5′-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol. 1977;13:330–41. [PubMed] [Google Scholar]
- 12.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–7. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15.Cherry PD, Furchgott RF, Zawadzki JV, Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci U S A. 1982;79:2106–10. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–79. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 19.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 20.Loscalzo J. The identification of nitric oxide as endothelium-derived relaxing factor. Circ Res. 2013;113:100–3. doi: 10.1161/CIRCRESAHA.113.301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Vanhoutte PM, Leung SW. Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10:1185–98. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–31. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 25.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morishita T, Tsutsui M, Shimokawa H, et al. Vasculoprotective roles of neuronal nitric oxide synthase. FASEB J. 2002;16:1994–6. doi: 10.1096/fj.02-0155fje. [DOI] [PubMed] [Google Scholar]
- 28.Spink J, Cohen J, Evans TJ. The cytokine responsive vascular smooth muscle cell enhancer of inducible nitric oxide synthase. Activation by nuclear factor-kappa B. J Biol Chem. 1995;270:29541–7. doi: 10.1074/jbc.270.49.29541. [DOI] [PubMed] [Google Scholar]
- 29.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem. 1996;271:18596–603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 30.Scharfstein JS, Keaney JF, Slivka A, et al. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994;94:1432–9. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Rudd MA, Freedman JE, Loscalzo J. S-Transnitrosation reactions are involved in the metabolic fate and biological actions of nitric oxide. J Pharmacol Exp Ther. 1998;284:526–34. [PubMed] [Google Scholar]
- 32.Handy DE, Loscalzo J. Nitric oxide and posttranslational modification of the vascular proteome: S-nitrosation of reactive thiols. Arterioscler Thromb Vasc Biol. 2006;26:1207–14. doi: 10.1161/01.ATV.0000217632.98717.a0. [DOI] [PubMed] [Google Scholar]
- 33.Huang Z, Shiva S, Kim-Shapiro DB, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Liu X, Cui H, Chen YR, Cardounel AJ, Zweier JL. Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem. 2006;281:12546–54. doi: 10.1074/jbc.M511803200. [DOI] [PubMed] [Google Scholar]
- 35.Maron BA, Tang SS, Loscalzo J. S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system. Antioxid Redox Signal. 2013;18:270–87. doi: 10.1089/ars.2012.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14:545–8. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 37.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 38.Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–20. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 40.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27:201–6. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- 41.Thibeault S, Rautureau Y, Oubaha M, et al. S-nitrosylation of beta-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol Cell. 2010;39:468–76. doi: 10.1016/j.molcel.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–9. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziche M, Morbidelli L, Choudhuri R, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–34. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukumura D, Gohongi T, Kadambi A, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–9. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loscalzo J. N-Acetylcysteine potentiates inhibition of platelet aggregation by nitroglycerin. J Clin Invest. 1985;76:703–8. doi: 10.1172/JCI112024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendelsohn ME, O’Neill S, George D, Loscalzo J. Inhibition of fibrinogen binding to human platelets by S-nitroso-N-acetylcysteine. J Biol Chem. 1990;265:19028–34. [PubMed] [Google Scholar]
- 47.Pigazzi A, Heydrick S, Folli F, Benoit S, Michelson A, Loscalzo J. Nitric oxide inhibits thrombin receptor-activating peptide-induced phosphoinositide 3-kinase activity in human platelets. J Biol Chem. 1999;274:14368–75. doi: 10.1074/jbc.274.20.14368. [DOI] [PubMed] [Google Scholar]
- 48.Folts JD, Stamler J, Loscalzo J. Intravenous nitroglycerin infusion inhibits cyclic blood flow responses caused by periodic platelet thrombus formation in stenosed canine coronary arteries. Circulation. 1991;83:2122–7. doi: 10.1161/01.cir.83.6.2122. [DOI] [PubMed] [Google Scholar]
- 49.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 50.Higashi Y, Sasaki S, Nakagawa K, et al. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15:326–32. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 51.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–8. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 52.Bogaert MG. Pharmacokinetics of organic nitrates in man: an overview. Eur Heart J. 1988;9(Suppl A):33–7. doi: 10.1093/eurheartj/9.suppl_a.33. [DOI] [PubMed] [Google Scholar]
- 53.Tarkin JM, Kaski JC. Vasodilator Therapy: Nitrates and Nicorandil. Cardiovasc Drugs Ther. 2016;30:367–78. doi: 10.1007/s10557-016-6668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fung HL, Chung SJ, Bauer JA, Chong S, Kowaluk EA. Biochemical mechanism of organic nitrate action. Am J Cardiol. 1992;70:4B–10B. doi: 10.1016/0002-9149(92)90588-p. [DOI] [PubMed] [Google Scholar]
- 55.Daiber A, Münzel T. Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress. Antioxid Redox Signal. 2015;23:899–942. doi: 10.1089/ars.2015.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colussi C, Scopece A, Vitale S, et al. P300/CBP associated factor regulates nitroglycerin-dependent arterial relaxation by N(ε)-lysine acetylation of contractile proteins. Arterioscler Thromb Vasc Biol. 2012;32:2435–43. doi: 10.1161/ATVBAHA.112.254011. [DOI] [PubMed] [Google Scholar]
- 57.den Uil CA, Brugts JJ. Impact of intravenous nitroglycerin in the management of acute decompensated heart failure. Curr Heart Fail Rep. 2015;12:87–93. doi: 10.1007/s11897-014-0230-8. [DOI] [PubMed] [Google Scholar]
- 58.Armstrong PW, Moffat JA, Marks GS. Arterial-venous nitroglycerin gradient during intravenous infusion in man. Circulation. 1982;66:1273–6. doi: 10.1161/01.cir.66.6.1273. [DOI] [PubMed] [Google Scholar]
- 59.Thadani U, Hamilton SF, Olson E, et al. Duration of effects and tolerance of slow-release isosorbide-5-mononitrate for angina pectoris. Am J Cardiol. 1987;59:756–62. doi: 10.1016/0002-9149(87)91087-3. [DOI] [PubMed] [Google Scholar]
- 60.Münzel T, Meinertz T, Tebbe U, et al. Efficacy of the long-acting nitro vasodilator pentaerithrityl tetranitrate in patients with chronic stable angina pectoris receiving anti-anginal background therapy with beta-blockers: a 12-week, randomized, double-blind, placebo-controlled trial. Eur Heart J. 2014;35:895–903. doi: 10.1093/eurheartj/eht384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taira N. Nicorandil as a hybrid between nitrates and potassium channel activators. Am J Cardiol. 1989;63:18J–24J. doi: 10.1016/0002-9149(89)90200-2. [DOI] [PubMed] [Google Scholar]
- 62.Frydman A. Pharmacokinetic profile of nicorandil in humans: an overview. J Cardiovasc Pharmacol. 1992;20(Suppl 3):S34–44. doi: 10.1097/00005344-199206203-00008. [DOI] [PubMed] [Google Scholar]
- 63.Brodmann M, Lischnig U, Lueger A, Stark G, Pilger E. The effect of the K+ agonist nicorandil on peripheral vascular resistance. Int J Cardiol. 2006;111:49–52. doi: 10.1016/j.ijcard.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 64.Akai K, Wang Y, Sato K, et al. Vasodilatory effect of nicorandil on coronary arterial microvessels: its dependency on vessel size and the involvement of the ATP-sensitive potassium channels. J Cardiovasc Pharmacol. 1995;26:541–7. doi: 10.1097/00005344-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Frydman AM, Chapelle P, Diekmann H, et al. Pharmacokinetics of nicorandil. Am J Cardiol. 1989;63:25J–33J. doi: 10.1016/0002-9149(89)90201-4. [DOI] [PubMed] [Google Scholar]
- 66.Friederich JA, Butterworth JF. Sodium nitroprusside: twenty years and counting. Anesth Analg. 1995;81:152–62. doi: 10.1097/00000539-199507000-00031. [DOI] [PubMed] [Google Scholar]
- 67.Smith RP, Kruszyna H. Nitroprusside produces cyanide poisoning via reaction with hemoglobin. J Pharmacol Exp Ther. 1974;191:557–63. [PubMed] [Google Scholar]
- 68.Ivankovich AD, Miletich DJ, Tinker JH. Sodium nitroprusside: metabolism and general considerations. Int Anesthesiol Clin. 1978;16:1–29. [PubMed] [Google Scholar]
- 69.Ichinose F, Roberts JD, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–11. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 70.Naghavi N, de Mel A, Alavijeh OS, Cousins BG, Seifalian AM. Nitric oxide donors for cardiovascular implant applications. Small. 2013;9:22–35. doi: 10.1002/smll.201200458. [DOI] [PubMed] [Google Scholar]
- 71.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev. 2002;102:1135–54. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 72.Varu VN, Tsihlis ND, Kibbe MR. Basic science review: nitric oxide--releasing prosthetic materials. Vasc Endovascular Surg. 2009;43:121–31. doi: 10.1177/1538574408322752. [DOI] [PubMed] [Google Scholar]
- 73.Al-Sa’doni H, Ferro A. S-Nitrosothiols: a class of nitric oxide-donor drugs. Clin Sci (Lond) 2000;98:507–20. [PubMed] [Google Scholar]
- 74.Scatena R, Bottoni P, Pontoglio A, Giardina B. Pharmacological modulation of nitric oxide release: new pharmacological perspectives, potential benefits and risks. Curr Med Chem. 2010;17:61–73. doi: 10.2174/092986710789957841. [DOI] [PubMed] [Google Scholar]
- 75.Serafim RA, Primi MC, Trossini GH, Ferreira EI. Nitric oxide: state of the art in drug design. Curr Med Chem. 2012;19:386–405. doi: 10.2174/092986712803414321. [DOI] [PubMed] [Google Scholar]
- 76.Munzel T, Daiber A. Pharmacology of Nitrovasodilators. In: Bryan N, Loscalzo J, editors. Nitrite and Nitrate in Human Health and Disease. 2. New York: Humana Press; 2017. p. 349. [Google Scholar]
- 77.Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2002;99:8306–11. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kleschyov AL, Oelze M, Daiber A, et al. Does nitric oxide mediate the vasodilator activity of nitroglycerin? Circ Res. 2003;93:e104–12. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- 79.Mülsch A, Bara A, Mordvintcev P, Vanin A, Busse R. Specificity of different organic nitrates to elicit NO formation in rabbit vascular tissues and organs in vivo. Br J Pharmacol. 1995;116:2743–9. doi: 10.1111/j.1476-5381.1995.tb17236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Münzel T, Daiber A, Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–28. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 81.Daiber A, Wenzel P, Oelze M, Münzel T. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clin Res Cardiol. 2008;97:12–20. doi: 10.1007/s00392-007-0588-7. [DOI] [PubMed] [Google Scholar]
- 82.Daiber A, Münzel T, Gori T. Organic nitrates and nitrate tolerance--state of the art and future developments. Adv Pharmacol. 2010;60:177–227. doi: 10.1016/B978-0-12-385061-4.00007-6. [DOI] [PubMed] [Google Scholar]
- 83.Parker JO, West RO, Di Giorgi S. The effect of nitroglycerin on coronary blood flow and the hemodynamic response to exercise in coronary artery disease. Am J Cardiol. 1971;27:59–65. doi: 10.1016/0002-9149(71)90083-x. [DOI] [PubMed] [Google Scholar]
- 84.Greenberg H, Dwyer EM, Jameson AG, Pinkernell BH. Effects of nitroglycerin on the major determinants of myocardial oxygen consumption. An angiographic and hemodynamic assessment. Am J Cardiol. 1975;36:426–32. doi: 10.1016/0002-9149(75)90889-9. [DOI] [PubMed] [Google Scholar]
- 85.Brown BG, Bolson E, Petersen RB, Pierce CD, Dodge HT. The mechanisms of nitroglycerin action: stenosis vasodilatation as a major component of the drug response. Circulation. 1981;64:1089–97. doi: 10.1161/01.cir.64.6.1089. [DOI] [PubMed] [Google Scholar]
- 86.Goldstein RE, Stinson EB, Scherer JL, Seningen RP, Grehl TM, Epstein SE. Intraoperative coronary collateral function in patients with coronary occlusive disease. Nitroglycerin responsiveness and angiographic correlations. Circulation. 1974;49:298–308. doi: 10.1161/01.cir.49.2.298. [DOI] [PubMed] [Google Scholar]
- 87.Harrison DG, Bates JN. The nitrovasodilators. New ideas about old drugs. Circulation. 1993;87:1461–7. doi: 10.1161/01.cir.87.5.1461. [DOI] [PubMed] [Google Scholar]
- 88.Hampton JR, Harrison MJ, Honour AJ, Mitchell JR. Platelet behaviour and drugs used in cardiovascular disease. Cardiovasc Res. 1967;1:101–7. doi: 10.1093/cvr/1.2.101. [DOI] [PubMed] [Google Scholar]
- 89.Schafer AI, Alexander RW, Handin RI. Inhibition of platelet function by organic nitrate vasodilators. Blood. 1980;55:649–54. [PubMed] [Google Scholar]
- 90.Loscalzo J. Antiplatelet and antithrombotic effects of organic nitrates. Am J Cardiol. 1992;70:18B–22B. doi: 10.1016/0002-9149(92)90590-u. [DOI] [PubMed] [Google Scholar]
- 91.Kumar S, Singh RK, Bhardwaj TR. Therapeutic role of nitric oxide as emerging molecule. Biomed Pharmacother. 2017;85:182–201. doi: 10.1016/j.biopha.2016.11.125. [DOI] [PubMed] [Google Scholar]
- 92.Lefer AM, Lefer DJ. Nitric oxide. II. Nitric oxide protects in intestinal inflammation. Am J Physiol. 1999;276:G572–5. doi: 10.1152/ajpgi.1999.276.3.G572. [DOI] [PubMed] [Google Scholar]
- 93.Cherla RP, Ganju RK. Stromal cell-derived factor 1 alpha-induced chemotaxis in T cells is mediated by nitric oxide signaling pathways. J Immunol. 2001;166:3067–74. doi: 10.4049/jimmunol.166.5.3067. [DOI] [PubMed] [Google Scholar]
- 94.Adachi R, Matsui S, Kinoshita M, et al. Nitric oxide induces chemotaxis of neutrophil-like HL-60 cells and translocation of cofilin to plasma membranes. Int J Immunopharmacol. 2000;22:855–64. doi: 10.1016/s0192-0561(00)00045-x. [DOI] [PubMed] [Google Scholar]
- 95.Granger DN, Kubes P. Nitric oxide as antiinflammatory agent. Methods Enzymol. 1996;269:434–42. doi: 10.1016/s0076-6879(96)69044-2. [DOI] [PubMed] [Google Scholar]
- 96.Gori T, Di Stolfo G, Dragoni S, et al. The mechanism of nitrate-induced preconditioning. Clin Hemorheol Microcirc. 2008;39:191–6. [PubMed] [Google Scholar]
- 97.Gori T, Di Stolfo G, Sicuro S, et al. Nitroglycerin protects the endothelium from ischaemia and reperfusion: human mechanistic insight. Br J Clin Pharmacol. 2007;64:145–50. doi: 10.1111/j.1365-2125.2007.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karlberg KE, Saldeen T, Wallin R, Henriksson P, Nyquist O, Sylvén C. Intravenous nitroglycerin reduces ischaemia in unstable angina pectoris: a double-blind placebo-controlled study. J Intern Med. 1998;243:25–31. doi: 10.1046/j.1365-2796.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 99.Curfman GD, Heinsimer JA, Lozner EC, Fung HL. Intravenous nitroglycerin in the treatment of spontaneous angina pectoris: a prospective, randomized trial. Circulation. 1983;67:276–82. doi: 10.1161/01.cir.67.2.276. [DOI] [PubMed] [Google Scholar]
- 100.GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell’infarto Miocardico. Lancet. 1994;343:1115–22. [PubMed] [Google Scholar]
- 101.Six-month effects of early treatment with lisinopril and transdermal glyceryl trinitrate singly and together withdrawn six weeks after acute myocardial infarction: the GISSI-3 trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. J Am Coll Cardiol. 1996;27:337–44. [PubMed] [Google Scholar]
- 102.ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–85. [PubMed] [Google Scholar]
- 103.Yusuf S, Collins R, MacMahon S, Peto R. Effect of intravenous nitrates on mortality in acute myocardial infarction: an overview of the randomised trials. Lancet. 1988;1:1088–92. doi: 10.1016/s0140-6736(88)91906-x. [DOI] [PubMed] [Google Scholar]
- 104.Kimchi A, Lee G, Amsterdam E, Fujii K, Krieg P, Mason DT. Increased exercise tolerance after nitroglycerin oral spray: a new and effective therapeutic modality in angina pectoris. Circulation. 1983;67:124–7. doi: 10.1161/01.cir.67.1.124. [DOI] [PubMed] [Google Scholar]
- 105.SANDLER G, ILAHI MA, LAWSON CW. Glyceryl trinitrate in angina pectoris. Lancet. 1963;1:1130–6. doi: 10.1016/s0140-6736(63)91802-6. [DOI] [PubMed] [Google Scholar]
- 106.Winsor T, Berger HJ. Oral nitroglycerin as a prophylactic antianginal drug: clinical, physiologic, and statistical evidence of efficacy based on a three-phase experimental design. Am Heart J. 1975;90:611–26. doi: 10.1016/0002-8703(75)90226-4. [DOI] [PubMed] [Google Scholar]
- 107.Reichek N, Goldstein RE, Redwood DR, Epstein SE. Sustained effects of nitroglycerin ointment in patients with angina pectoris. Circulation. 1974;50:348–52. doi: 10.1161/01.cir.50.2.348. [DOI] [PubMed] [Google Scholar]
- 108.Scardi S, Camerini F, Pandullo C, Pollavini G. Efficacy of continuous and intermittent transdermal treatment with nitroglycerin in effort angina pectoris: a multicentric study. The Collaborative Nitro Group. Int J Cardiol. 1991;32:241–8. doi: 10.1016/0167-5273(91)90331-i. [DOI] [PubMed] [Google Scholar]
- 109.Klemsdal TO, Gjesdal K. The effect of transdermal nitroglycerin on exercise tolerance in relation to patch application time--a meta-analysis. Cardiovasc Drugs Ther. 1992;6:641–9. doi: 10.1007/BF00052566. [DOI] [PubMed] [Google Scholar]
- 110.Parker JO, VanKoughnett KA, Fung HL. Transdermal isosorbide dinitrate in angina pectoris: effect of acute and sustained therapy. Am J Cardiol. 1984;54:8–13. doi: 10.1016/0002-9149(84)90296-0. [DOI] [PubMed] [Google Scholar]
- 111.Thadani U, Hamilton SF, Olson E, et al. Transdermal nitroglycerin patches in angina pectoris. Dose titration, duration of effect, and rapid tolerance. Ann Intern Med. 1986;105:485–92. doi: 10.7326/0003-4819-105-4-485. [DOI] [PubMed] [Google Scholar]
- 112.DeMots H, Glasser SP. Intermittent transdermal nitroglycerin therapy in the treatment of chronic stable angina. J Am Coll Cardiol. 1989;13:786–95. doi: 10.1016/0735-1097(89)90216-7. [DOI] [PubMed] [Google Scholar]
- 113.Group IS. Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet. 2002;359:1269–75. doi: 10.1016/S0140-6736(02)08265-X. [DOI] [PubMed] [Google Scholar]
- 114.Stern S, Bayes de Luna A. Coronary artery spasm: a 2009 update. Circulation. 2009;119:2531–4. doi: 10.1161/CIRCULATIONAHA.108.843474. [DOI] [PubMed] [Google Scholar]
- 115.Lombardi M, Morales MA, Michelassi C, Moscarelli E, Distante A, L’Abbate A. Efficacy of isosorbide-5-mononitrate versus nifedipine in preventing spontaneous and ergonovine-induced myocardial ischaemia. A double-blind, placebo-controlled study. Eur Heart J. 1993;14:845–51. doi: 10.1093/eurheartj/14.6.845. [DOI] [PubMed] [Google Scholar]
- 116.Rosendorff C, Lackland DT, Allison M, et al. Treatment of Hypertension in Patients With Coronary Artery Disease: A Scientific Statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Coll Cardiol. 2015;65:1998–2038. doi: 10.1016/j.jacc.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 117.Singh A, Laribi S, Teerlink JR, Mebazaa A. Agents with vasodilator properties in acute heart failure. Eur Heart J. 2017;38:317–325. doi: 10.1093/eurheartj/ehv755. [DOI] [PubMed] [Google Scholar]
- 118.Wakai A, McCabe A, Kidney R, et al. Nitrates for acute heart failure syndromes. Cochrane Database Syst Rev. 2013:CD005151. doi: 10.1002/14651858.CD005151.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 120.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 121.Barst RJ, Gibbs JS, Ghofrani HA, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S78–84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hill NS, Preston IR, Roberts KE. Inhaled Therapies for Pulmonary Hypertension. Respir Care. 2015;60:794–802. doi: 10.4187/respcare.03927. discussion 802–5. [DOI] [PubMed] [Google Scholar]
- 123.Barrington KJ, Finer N, Pennaforte T, Altit G. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2017;1:CD000399. doi: 10.1002/14651858.CD000399.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kinsella JP, Steinhorn RH, Krishnan US, et al. Recommendations for the Use of Inhaled Nitric Oxide Therapy in Premature Newborns with Severe Pulmonary Hypertension. J Pediatr. 2016;170:312–4. doi: 10.1016/j.jpeds.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 125.Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2017;1:CD000509. doi: 10.1002/14651858.CD000509.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thadani U. Challenges with nitrate therapy and nitrate tolerance: prevalence, prevention, and clinical relevance. Am J Cardiovasc Drugs. 2014;14:287–301. doi: 10.1007/s40256-014-0072-5. [DOI] [PubMed] [Google Scholar]
- 127.Thadani U, Manyari D, Parker JO, Fung HL. Tolerance to the circulatory effects of oral isosorbide dinitrate. Rate of development and cross-tolerance to glyceryl trinitrate. Circulation. 1980;61:526–35. doi: 10.1161/01.cir.61.3.526. [DOI] [PubMed] [Google Scholar]
- 128.Thadani U, Fung HL, Darke AC, Parker JO. Oral isosorbide dinitrate in angina pectoris: comparison of duration of action an dose-response relation during acute and sustained therapy. Am J Cardiol. 1982;49:411–9. doi: 10.1016/0002-9149(82)90518-5. [DOI] [PubMed] [Google Scholar]
- 129.Acute and chronic antianginal efficacy of continuous twenty-four-hour application of transdermal nitroglycerin. Steering Committee, Transdermal Nitroglycerin Cooperative Study. Am J Cardiol. 1991;68:1263–73. doi: 10.1016/0002-9149(91)90229-e. [DOI] [PubMed] [Google Scholar]
- 130.Parker JO, Fung HL. Transdermal nitroglycerin in angina pectoris. Am J Cardiol. 1984;54:471–6. doi: 10.1016/0002-9149(84)90233-9. [DOI] [PubMed] [Google Scholar]
- 131.Thadani U, Lipicky RJ. Ointments and transdermal nitroglycerin patches for stable angina pectoris. Cardiovasc Drugs Ther. 1994;8:625–33. doi: 10.1007/BF00877416. [DOI] [PubMed] [Google Scholar]
- 132.Thadani U, Lipicky RJ. Short and long-acting oral nitrates for stable angina pectoris. Cardiovasc Drugs Ther. 1994;8:611–23. doi: 10.1007/BF00877415. [DOI] [PubMed] [Google Scholar]
- 133.Chrysant SG, Glasser SP, Bittar N, et al. Efficacy and safety of extended-release isosorbide mononitrate for stable effort angina pectoris. Am J Cardiol. 1993;72:1249–56. doi: 10.1016/0002-9149(93)90292-k. [DOI] [PubMed] [Google Scholar]
- 134.Zimrin D, Reichek N, Bogin KT, et al. Antianginal effects of intravenous nitroglycerin over 24 hours. Circulation. 1988;77:1376–84. doi: 10.1161/01.cir.77.6.1376. [DOI] [PubMed] [Google Scholar]
- 135.Münzel T, Steven S, Daiber A. Organic nitrates: update on mechanisms underlying vasodilation, tolerance and endothelial dysfunction. Vascul Pharmacol. 2014;63:105–13. doi: 10.1016/j.vph.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 136.Münzel T, Giaid A, Kurz S, Stewart DJ, Harrison DG. Evidence for a role of endothelin 1 and protein kinase C in nitroglycerin tolerance. Proc Natl Acad Sci U S A. 1995;92:5244–8. doi: 10.1073/pnas.92.11.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oelze M, Knorr M, Kröller-Schön S, et al. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur Heart J. 2013;34:3206–16. doi: 10.1093/eurheartj/ehs100. [DOI] [PubMed] [Google Scholar]
- 138.Mülsch A, Busse R, Bassenge E. Desensitization of guanylate cyclase in nitrate tolerance does not impair endothelium-dependent responses. Eur J Pharmacol. 1988;158:191–8. doi: 10.1016/0014-2999(88)90066-0. [DOI] [PubMed] [Google Scholar]
- 139.Molina CR, Andresen JW, Rapoport RM, Waldman S, Murad F. Effect of in vivo nitroglycerin therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J Cardiovasc Pharmacol. 1987;10:371–8. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- 140.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci U S A. 2007;104:12312–7. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sydow K, Daiber A, Oelze M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004;113:482–9. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Daiber A, Oelze M, Coldewey M, et al. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol. 2004;66:1372–82. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- 143.Wenzel P, Hink U, Oelze M, et al. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity. Implications for mitochondrial oxidative stress and nitrate tolerance. J Biol Chem. 2007;282:792–9. doi: 10.1074/jbc.M606477200. [DOI] [PubMed] [Google Scholar]
- 144.Gori T, Burstein JM, Ahmed S, et al. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance: a human in vivo study. Circulation. 2001;104:1119–23. doi: 10.1161/hc3501.095358. [DOI] [PubMed] [Google Scholar]
- 145.Hink U, Oelze M, Kolb P, et al. Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J Am Coll Cardiol. 2003;42:1826–34. doi: 10.1016/j.jacc.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 146.Warnholtz A, Mollnau H, Heitzer T, et al. Adverse effects of nitroglycerin treatment on endothelial function, vascular nitrotyrosine levels and cGMP-dependent protein kinase activity in hyperlipidemic Watanabe rabbits. J Am Coll Cardiol. 2002;40:1356–63. doi: 10.1016/s0735-1097(02)02133-2. [DOI] [PubMed] [Google Scholar]
- 147.Kim D, Rybalkin SD, Pi X, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–43. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- 148.Parker JD, Farrell B, Fenton T, Cohanim M, Parker JO. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation. 1991;84:2336–45. doi: 10.1161/01.cir.84.6.2336. [DOI] [PubMed] [Google Scholar]
- 149.Thadani U. Nitrate tolerance, rebound, and their clinical relevance in stable angina pectoris, unstable angina, and heart failure. Cardiovasc Drugs Ther. 1997;10:735–42. doi: 10.1007/BF00053031. [DOI] [PubMed] [Google Scholar]
- 150.Katz RJ, Levy WS, Buff L, Wasserman AG. Prevention of nitrate tolerance with angiotension converting enzyme inhibitors. Circulation. 1991;83:1271–7. doi: 10.1161/01.cir.83.4.1271. [DOI] [PubMed] [Google Scholar]
- 151.Boden WE, Finn AV, Patel D, Peacock WF, Thadani U, Zimmerman FH. Nitrates as an integral part of optimal medical therapy and cardiac rehabilitation for stable angina: review of current concepts and therapeutics. Clin Cardiol. 2012;35:263–71. doi: 10.1002/clc.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dakak N, Makhoul N, Flugelman MY, et al. Failure of captopril to prevent nitrate tolerance in congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1990;66:608–13. doi: 10.1016/0002-9149(90)90489-n. [DOI] [PubMed] [Google Scholar]
- 153.Dupuis J, Lalonde G, Lemieux R, Rouleau JL. Tolerance to intravenous nitroglycerin in patients with congestive heart failure: role of increased intravascular volume, neurohumoral activation and lack of prevention with N-acetylcysteine. J Am Coll Cardiol. 1990;16:923–31. doi: 10.1016/s0735-1097(10)80342-0. [DOI] [PubMed] [Google Scholar]
- 154.Münzel T, Holtz J, Mülsch A, Stewart DJ, Bassenge E. Nitrate tolerance in epicardial arteries or in the venous system is not reversed by N-acetylcysteine in vivo, but tolerance-independent interactions exist. Circulation. 1989;79:188–97. doi: 10.1161/01.cir.79.1.188. [DOI] [PubMed] [Google Scholar]
- 155.Parker JO, Farrell B, Lahey KA, Rose BF. Nitrate tolerance: the lack of effect of N-acetylcysteine. Circulation. 1987;76:572–6. doi: 10.1161/01.cir.76.3.572. [DOI] [PubMed] [Google Scholar]
- 156.Packer M, Lee WH, Kessler PD, Gottlieb SS, Medina N, Yushak M. Prevention and reversal of nitrate tolerance in patients with congestive heart failure. N Engl J Med. 1987;317:799–804. doi: 10.1056/NEJM198709243171304. [DOI] [PubMed] [Google Scholar]
- 157.Fox KM, Dargie HJ, Deanfield J, Maseri A. Avoidance of tolerance and lack of rebound with intermittent dose titrated transdermal glyceryl trinitrate. The Transdermal Nitrate Investigators. Br Heart J. 1991;66:151–5. doi: 10.1136/hrt.66.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Freedman SB, Daxini BV, Noyce D, Kelly DT. Intermittent transdermal nitrates do not improve ischemia in patients taking beta-blockers or calcium antagonists: potential role of rebound ischemia during the nitrate-free period. J Am Coll Cardiol. 1995;25:349–55. doi: 10.1016/0735-1097(94)00416-n. [DOI] [PubMed] [Google Scholar]
- 159.Thadani U, Maranda CR, Amsterdam E, et al. Lack of pharmacologic tolerance and rebound angina pectoris during twice-daily therapy with isosorbide-5-mononitrate. Ann Intern Med. 1994;120:353–9. doi: 10.7326/0003-4819-120-5-199403010-00001. [DOI] [PubMed] [Google Scholar]
- 160.Parker JO, Fung HL, Ruggirello D, Stone JA. Tolerance to isosorbide dinitrate: rate of development and reversal. Circulation. 1983;68:1074–80. doi: 10.1161/01.cir.68.5.1074. [DOI] [PubMed] [Google Scholar]
- 161.Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth. 2014;28:1043–7. doi: 10.1053/j.jvca.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 162.Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf. 1996;14:394–405. doi: 10.2165/00002018-199614060-00005. [DOI] [PubMed] [Google Scholar]
- 163.Kaplan KJ, Taber M, Teagarden JR, Parker M, Davison R. Association of methemoglobinemia and intravenous nitroglycerin administration. Am J Cardiol. 1985;55:181–3. doi: 10.1016/0002-9149(85)90324-8. [DOI] [PubMed] [Google Scholar]
- 164.Hottinger DG, Beebe DS, Kozhimannil T, Prielipp RC, Belani KG. Sodium nitroprusside in 2014: A clinical concepts review. J Anaesthesiol Clin Pharmacol. 2014;30:462–71. doi: 10.4103/0970-9185.142799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cummings TF. The treatment of cyanide poisoning. Occup Med (Lond) 2004;54:82–5. doi: 10.1093/occmed/kqh020. [DOI] [PubMed] [Google Scholar]
- 166.Petrikovics I, Budai M, Kovacs K, Thompson DE. Past, present and future of cyanide antagonism research: From the early remedies to the current therapies. World J Methodol. 2015;5:88–100. doi: 10.5662/wjm.v5.i2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thompson JP, Marrs TC. Hydroxocobalamin in cyanide poisoning. Clin Toxicol (Phila) 2012;50:875–85. doi: 10.3109/15563650.2012.742197. [DOI] [PubMed] [Google Scholar]
- 168.Nagasawa HT, Goon DJ, Crankshaw DL, Vince R, Patterson SE. Novel, orally effective cyanide antidotes. J Med Chem. 2007;50:6462–4. doi: 10.1021/jm7011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Belani KG, Singh H, Beebe DS, et al. Cyanide toxicity in juvenile pigs and its reversal by a new prodrug, sulfanegen sodium. Anesth Analg. 2012;114:956–61. doi: 10.1213/ANE.0b013e31824c4eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kloner RA. Cardiovascular effects of the 3 phosphodiesterase-5 inhibitors approved for the treatment of erectile dysfunction. Circulation. 2004;110:3149–55. doi: 10.1161/01.CIR.0000146906.42375.D3. [DOI] [PubMed] [Google Scholar]
- 171.Kostis JB, Jackson G, Rosen R, et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference) Am J Cardiol. 2005;96:85M–93M. doi: 10.1016/j.amjcard.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 172.Ondrus T, Kanovsky J, Novotny T, Andrsova I, Spinar J, Kala P. Right ventricular myocardial infarction: From pathophysiology to prognosis. Exp Clin Cardiol. 2013;18:27–30. [PMC free article] [PubMed] [Google Scholar]
- 173.Zemanek D, Tomasov P, Bìlehrad M, et al. Comparison of sublingual isosorbide dinitrate and Valsalva maneuver for detection of obstruction in hypertrophic cardiomyopathy. Arch Med Sci. 2015;11:751–5. doi: 10.5114/aoms.2015.47096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kraehling JR, Sessa WC. Contemporary Approaches to Modulating the Nitric Oxide-cGMP Pathway in Cardiovascular Disease. Circ Res. 2017;120:1174–1182. doi: 10.1161/CIRCRESAHA.117.303776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335:41–7. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- 176.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408–17. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 177.Yu J, Bergaya S, Murata T, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–91. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–94. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 179.García-Cardeña G, Martasek P, Masters BS, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–40. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 180.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–5. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 181.Bernatchez P, Sharma A, Bauer PM, Marin E, Sessa WC. A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J Clin Invest. 2011;121:3747–55. doi: 10.1172/JCI44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Keller TC, Butcher JT, Broseghini-Filho GB, et al. Modulating Vascular Hemodynamics With an Alpha Globin Mimetic Peptide (HbαX) Hypertension. 2016;68:1494–1503. doi: 10.1161/HYPERTENSIONAHA.116.08171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Straub AC, Lohman AW, Billaud M, et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 2012;491:473–7. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Straub AC, Butcher JT, Billaud M, et al. Hemoglobin α/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arterioscler Thromb Vasc Biol. 2014;34:2594–600. doi: 10.1161/ATVBAHA.114.303974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wohlfart P, Xu H, Endlich A, et al. Antiatherosclerotic effects of small-molecular-weight compounds enhancing endothelial nitric-oxide synthase (eNOS) expression and preventing eNOS uncoupling. J Pharmacol Exp Ther. 2008;325:370–9. doi: 10.1124/jpet.107.128009. [DOI] [PubMed] [Google Scholar]
- 186.Chen Y, Chen C, Feng C, et al. AVE 3085, a novel endothelial nitric oxide synthase enhancer, attenuates cardiac remodeling in mice through the Smad signaling pathway. Arch Biochem Biophys. 2015;570:8–13. doi: 10.1016/j.abb.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 187.Fraccarollo D, Widder JD, Galuppo P, et al. Improvement in left ventricular remodeling by the endothelial nitric oxide synthase enhancer AVE9488 after experimental myocardial infarction. Circulation. 2008;118:818–27. doi: 10.1161/CIRCULATIONAHA.107.717702. [DOI] [PubMed] [Google Scholar]
- 188.Ramzy D, Rao V, Tumiati LC, et al. Tetrahydrobiopterin prevents cyclosporine-induced vasomotor dysfunction. Transplantation. 2005;79:876–81. doi: 10.1097/01.tp.0000157364.80712.45. [DOI] [PubMed] [Google Scholar]
- 189.Cunnington C, Van Assche T, Shirodaria C, et al. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation. 2012;125:1356–66. doi: 10.1161/CIRCULATIONAHA.111.038919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ignarro LJ, Wood KS, Wolin MS. Activation of purified soluble guanylate cyclase by protoporphyrin IX. Proc Natl Acad Sci U S A. 1982;79:2870–3. doi: 10.1073/pnas.79.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–33. [PubMed] [Google Scholar]
- 192.Frey R, Mück W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521: an ascending-dose study in healthy male volunteers. J Clin Pharmacol. 2008;48:926–34. doi: 10.1177/0091270008319793. [DOI] [PubMed] [Google Scholar]
- 193.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–40. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 194.Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 195.Breitenstein S, Roessig L, Sandner P, Lewis KS. Novel sGC Stimulators and sGC Activators for the Treatment of Heart Failure. Handb Exp Pharmacol. 2017;243:225–247. doi: 10.1007/164_2016_100. [DOI] [PubMed] [Google Scholar]
- 196.Gheorghiade M, Greene SJ, Butler J, et al. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA. 2015;314:2251–62. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 197.Stasch JP, Schmidt P, Alonso-Alija C, et al. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol. 2002;136:773–83. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wunder F, Stasch JP, Hütter J, Alonso-Alija C, Hüser J, Lohrmann E. A cell-based cGMP assay useful for ultra-high-throughput screening and identification of modulators of the nitric oxide/cGMP pathway. Anal Biochem. 2005;339:104–12. doi: 10.1016/j.ab.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 199.Erdmann E, Semigran MJ, Nieminen MS, et al. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur Heart J. 2013;34:57–67. doi: 10.1093/eurheartj/ehs196. [DOI] [PubMed] [Google Scholar]
- 200.Gheorghiade M, Greene SJ, Filippatos G, et al. Cinaciguat, a soluble guanylate cyclase activator: results from the randomized, controlled, phase IIb COMPOSE programme in acute heart failure syndromes. Eur J Heart Fail. 2012;14:1056–66. doi: 10.1093/eurjhf/hfs093. [DOI] [PubMed] [Google Scholar]
- 201.Uzunov P, Weiss B. Separation of multiple molecular forms of cyclic adenosine-3′,5′-monophosphate phosphodiesterase in rat cerebellum by polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1972;284:220–6. doi: 10.1016/0005-2744(72)90060-5. [DOI] [PubMed] [Google Scholar]
- 202.Chirinos JA, Zamani P. The Nitrate-Nitrite-NO Pathway and Its Implications for Heart Failure and Preserved Ejection Fraction. Curr Heart Fail Rep. 2016;13:47–59. doi: 10.1007/s11897-016-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Maher AR, Milsom AB, Gunaruwan P, et al. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–7. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 204.Modin A, Björne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]