Abstract
Background and Purpose
Our goal is to determine the added value of intracranial vessel wall MRI (IVWI) in differentiating non-occlusive vasculopathies compared to luminal imaging alone.
Methods
We retrospectively reviewed images from patients with both luminal and IVWI to identify cases with clinically defined intracranial vasculopathies: atherosclerosis (ICAD), reversible cerebral vasoconstriction syndrome (RCVS) and inflammatory vasculopathy (IVas). Two neuroradiologists blinded to clinical data reviewed the luminal imaging of defined luminal stenoses/irregularities and evaluated the pattern of involvement to make a presumed diagnosis with diagnostic confidence. Six weeks later, the 2 raters re-reviewed the luminal imaging in addition to IVWI for the pattern of wall involvement, presence and pattern of post-contrast enhancement, and presumed diagnosis and confidence. Analysis was performed on a per-lesion and per-patient basis.
Results
30 ICAD, 12 IVas and 12 RCVS patients with 201 lesions (90 ICAD, 64 RCVS and 47 IVas) were included. For both per-lesion and per-patient analyses, there was significant diagnostic accuracy improvement with luminal imaging+IVWI when compared to luminal imaging alone (per-lesion: 88.8% vs. 36.1%, p<.001, per-patient: 96.3% vs. 43.5%, p<.001), respectively. There was substantial inter-rater diagnostic agreement for luminal imaging+IVWI (κ 0.72) and only slight agreement for luminal imaging (κ 0.04). While there was a significant correlation for both luminal and IVWI pattern of wall involvement with diagnosis, there was a stronger correlation for IVWI finding of lesion eccentricity and ICAD diagnosis than for luminal imaging (κ 0.69 vs. 0.18, p<.001).
Conclusion
IVWI can significantly improve the differentiation of non-occlusive intracranial vasculopathies when combined with traditional luminal imaging modalities.
Search terms: Magnetic resonance, intracranial disease, vascular disease, intracranial vessel wall MRI
Introduction
Early diagnosis of intracranial vasculopathies, including intracranial atherosclerotic disease (ICAD), reversible cerebral vasoconstriction syndrome (RCVS) and infectious/inflammatory vasculopathies (IVas) is important, as inappropriate or delayed therapy can lead to worse outcomes1–3. Current diagnostic imaging algorithms rely on luminal imaging for disease differentiation, with catheter angiography (DSA) serving as the imaging gold standard4. DSA, however, shows limited sensitivity/specificity that can be as low as 30% for IVas, and only 25–43% of pathologically-proven primary angiitis have luminal abnormalities on angiography4–8. If there is suspicion for IVas, invasive tests such as brain biopsy may be implemented, which carry a significant risk of morbidity, but also show sensitivities of only 53–80% for vasculitis9, 10. ICAD frequently remodels outwardly, resulting in luminal-based underestimation of disease burden11, and luminal imaging may not detect culprit plaques at all12. DSA also serves as the imaging standard for RCVS, however the imaging appearance of arterial beading is nonspecific and indistinguishable from IVas9, 13.
Intracranial vessel wall MRI (IVWI) has shown promise in its ability to differentiate and characterize intracranial vasculopathies14. The inclusion of IVWI can better differentiate between moyamoya disease and etiologies of moyamoya syndrome compared to luminal imaging alone15. Etiologies of non-occlusive intracranial vasculopathies can be differentiated using a multi-contrast IVWI protocol16. However, it is unknown if IVWI has added benefit in evaluating non-occlusive intracranial arteriopathies over luminal imaging alone. This study compares the diagnostic accuracy of IVWI+luminal imaging compared to luminal imaging alone in non-occlusive vasculopathy differentiation, specifically ICAD, RCVS and IVas.
Materials and Methods
The authors will make data available for study replication upon request.
Patient Selection
After IRB study approval with waiver of consent, consecutive patients with arterial wall imaging from December 2012 through February 2016 were reviewed from a prospectively maintained database. We extracted cases with documented luminal irregularity/narrowing but without occlusion on the clinically acquired CT angiography (CTA), MR angiography (MRA) and/or DSA. Two stroke neurologists (KJB and ADH) reviewed the clinical data and luminal imaging reports, while blinded to patient identifiers, IVWI information and clinical diagnosis, and categorized the vasculopathies as ICAD, RCVS and/or IVas on a per-patient basis. In terms of final diagnosis, cases diagnosed as RCVS were required to show reversibility of stenosis on follow-up luminal imaging within 3 months. For the diagnosis of IVas, there was a requirement for histologic or CSF evidence of infection/inflammation in combination with arterial stenosis and MRI brain findings compatible with IVas. ICAD diagnosis could not have evidence of CNS inflammation or short-term reversibility of arterial lesions. If there was disagreement in the diagnosis, a third stroke neurologist (DLT) arbitrated. The neurologist review served as the diagnostic gold standard. Cases with other diagnoses, multiple diagnoses or a diagnosis could not be agreed upon by the neurology reviewers were excluded.
MRI Protocol
Patients were scanned on a 3T Siemens Trio MR scanner (Siemens Healthcare, Erlangen, Germany) using a standard head coil. The IVWI protocol included high-resolution multi-planar T1 (0.4×0.35 mm in-plane resolution; 2 mm slice thickness; TR/TE, 1000/10 ms; 36 s per slice) pre and post contrast, T2 (0.4×0.4 mm in-plane; 1 mm slice thickness; TR/TE, 3550/72 ms; 9.3 s per slice), and 3D SPACE T2-weighted (0.6×0.6 mm in-plane; 0.6 mm slice thickness; TR/TE, 2400/80 ms; 64 slices; 10:20 minutes) sequences. More detailed imaging parameters can be found in previous publications15, 16.
Image Analysis
A single rater (MM) reviewed luminal imaging of the included cases independent of diagnosis, clinical data and IVWI to determine the arterial segments with luminal irregularity/stenosis. Luminal imaging was used to identify lesions in order to avoid potential artifacts from non-suppression of blood flow or CSF mimicking lesions on IVWI. The abnormal arterial segments were recorded and used to guide the raters to the lesions to evaluate. Two other raters (DKS and DKH) underwent training in IVWI interpretation, which included review of a packet of articles relating to IVWI interpretation, in-person case reviews, didactic lecture and independent test case review with feedback provided. IVWI characteristics and criteria for each vasculopathy evaluated are included in supplemental Table I. The two independent raters blinded to clinical and IVWI data reviewed consecutive luminal imaging studies (CTA, MRA and/or DSA performed for each subject prior to IVWI) for the vasculopathy subjects. The raters evaluated each lesion individually and independent from other lesions but with knowledge of what additional arterial segments were affected by lesions for each subject (to provide an idea for global disease involvement). Lesions were randomized so each lesion could be reviewed based on its imaging characteristics independent of other patient lesions. The raters evaluated luminal imaging for the pattern of involvement (concentric/eccentric), diagnosis (ICAD, IVas, RCVS or unknown if diagnosis could not be narrowed down to a single disease) and confidence in their diagnosis on a 4-point Likert scale: 0=50% confidence, 1=51–75% confidence, 2=76–90% confidence, 3=>90% confidence)15. On luminal imaging, eccentric lesions were defined as those primarily affecting one side of the lumen on 2-dimensional review or ≤3 sides of the lumen on 3-dimensional review, while concentric was defined as lesions with circumferential narrowing of the lumen. After a 6-week washout period, the raters reevaluated the randomized lesions using both luminal imaging+IVWI individually and independently. The raters evaluated the pattern of wall involvement (eccentric/concentric), presence of enhancement (y/n), pattern of enhancement (focal, heterogeneous, and diffuse), diagnosis and confidence in diagnosis (the 4-point scale)15. Concentric lesions were defined as those where the width of the thinnest wall segment was >50% that of the thickest segment, while for eccentric lesions, the thinnest segment had a width <50% that of the thickest segment17. For the enhancement pattern, focal was a punctate or short linear focus of enhancement, heterogeneous was incomplete enhancement and diffuse was complete enhancement15, 16.
Statistical Analysis
Continuous and categorical variables were summarized as mean±SD and count (percentage), respectively. Clinical characteristics of each subject were summarized and compared between the three clinical diagnoses using the Kruskal-Wallis test (continuous variables) and Fisher’s exact test (categorical variables). For most of the analysis, the units of analysis were the lesion or the read of the lesion, where the read is the assessment of a lesion by a single rater. Thus, there were twice as many reads as lesions.
Throughout, analyses were conducted with both rater’s reads pooled together, which produces results that correspond to the average of the two raters and generally lead to an increase in statistical power. Analyses were repeated for each rater separately to determine whether there were any material differences in the rater-specific results. Multiple lesions per subject and multiple raters per lesion were not treated as independent observations, however. Permutation tests with resampling performed by subject (all lesions and reads from the same subject were permutated together, maintaining their dependence)18 or generalized estimating equations (GEE) models19 clustered by subject were used to account for dependence among the lesions and reads from the same subject when conducting hypothesis tests. Confidence intervals were calculated using the percentile method of the non-parametric bootstrap with resampling by subject18.
Inter-rater agreement was summarized as percent agreement (100% × number of lesions where both raters gave the same rating divided by the total number of lesions) and Cohen’s κ. Percent agreement and Cohen’s κ were compared between the luminal imaging only session and the luminal imaging+IVWI session using the non-parametric bootstrap with resampling by subject as described above.
Diagnostic accuracy was assessed on a per-lesion and per-patient basis, computed as the percentage of reads where the rater’s diagnosis matched the final diagnosis. Under the per-lesion analysis, any reads classified as uncertain or equivocal by the rater, without a rater diagnosis, were considered an incorrect diagnosis. Under the per-patient analysis, the per-lesion reads were aggregated to per-patient reads by first summing the confidence scores of each lesion for each vasculopathy diagnosis (ICAD, RCVS, IVas) separately and then selecting the per-patient diagnosis as the one with the highest total confidence score. The per-patient diagnosis was considered uncertain/equivocal if ≥50% of the constituent lesions had uncertain/equivocal diagnoses or multiple vasculopathy diagnoses were tied in total confidence score. An uncertain/equivocal per-patient diagnosis was considered incorrect for calculating diagnostic accuracy, as with the per-lesion analysis. Diagnostic accuracy was compared between the luminal imaging and the luminal imaging+IVWI sessions using a permutation test based on McNemar test, resampled by subject.
Diagnostic confidence, dichotomized as highly confident (rating = 3) versus not highly confident (rating < 3), was compared between the luminal imaging and luminal imaging+IVWI sessions using GEE-based logistic regression models. These comparisons were performed using all reads as well as within the subgroups where the rater diagnosis was concordant with the final diagnosis and where the rater diagnosis was discordant with the final diagnosis. Individual luminal imaging and IVWI findings were compared between the final diagnosis groups using permutation tests based on the χ2 test, resampled by subject. All statistical calculations were conducted with the statistical computing language R (version 3.1.1; R Foundation for Statistical Computing, Vienna, Austria). Throughout, 2-tailed tests were used with statistical significance defined as P<0.05.
Results
Clinical Diagnoses and Luminal Characteristics
Two hundred and twenty consecutive IVWI cases with luminal imaging were reviewed, of which 54 patients were included in this study: 30 ICAD, 12 RCVS and 12 IVas cases. IVas cases consisted of 4 varicella vasculopathies, 3 primary angiitis, 2 bacterial vasculopathies, and 1 each of Behcet associated vasculopathy not otherwise specified, tuberculous and fungal vasculopathies. Patient clinical and demographic information is in Supplemental Table II. There were significant differences for number of vascular risk factors between vasculopathies as 97% of ICAD, 8% of RCVS and 0% of IVas subjects had ≥2 vascular risk factors, respectively (p<.001). Luminal imaging performed in each vasculopathy are also listed in Supplemental Table II. MRA was performed on all patients. There were variations in the frequency of DSA (25–67%, p=0.080) and CTA (57–75%, p=0.41) performance between disease groups, though these differences were not statistically significant. All patients underwent imaging evaluation based on clinical practice and need.
From these 54 patients, 201 lesions (90 ICAD, 64 RCVS and 47 IVas lesions) were assessed by the two raters, for a total of 402 ratings. There were 1–10 lesions per patient (median 3). For a description of arterial segments involved for each disease, please refer to Supplemental Table III. There was a significant overall association between arterial segment involvement and final diagnosis (p=.007), although this was due to a significant difference in intracranial internal carotid artery (ICA) involvement (p=.007) without a significant difference in disease involvement between other arterial segments (p=0.28). ICAD was significantly more likely to involve the ICA (42.2%) than IVas (9.4%, p=.046) and RCVS (25.5%, p=.001).
Vessel Wall MRI Characteristics
There were significant differences in the IVWI characteristics between ICAD, RCVS and IVas (Table 1). Typical findings are shown in Figure 1. On IVWI, ICAD lesions were most commonly eccentric (91.1%) and diffusely or heterogeneously enhancing (86.7%). RCVS typically had concentric lesions (80.5%) that showed no enhancement (53.9%) or diffuse enhancement (32.8%) (Figure 2). IVas most commonly showed concentric (76.6%), diffusely enhancing lesions (81.9%).
Table 1.
Comparison of luminal imaging and IVWI findings between vasculopathy groups.
| Final Diagnosis* | Pairwise p-values† | ||||||
|---|---|---|---|---|---|---|---|
| Variable | ICAD (N=180) | RCVS (N=128) | IVas (N=94) | ICAD vs. RCVS | ICAD vs. IVas | RCVS vs. IVas | |
| Luminal imaging findings | |||||||
| Pattern of involvement‡ | Concentric, n (%) | 51 (28.8) | 59 (46.8) | 45 (48.9) | 0.002 | 0.006 | 0.81 |
| Eccentric | 126 (71.2) | 67 (53.2) | 47 (51.1) | ||||
| IVWI findings | |||||||
| Pattern of involvement | Concentric | 16 (8.9) | 103 (80.5) | 72 (76.6) | <0.001 | <0.001 | 0.65 |
| Eccentric | 164 (91.1) | 25 (19.5) | 22 (23.4) | ||||
| Wall enhancement | Present | 173 (96.1) | 59 (46.1) | 89 (94.7) | <0.001 | 0.66 | <0.001 |
| Absent | 7 (3.9) | 69 (53.9) | 5 (5.3) | ||||
| Pattern of enhancement|| | Focal | 17 (9.8) | 0 (0.0) | 0 (0.0) | 0.010 | <0.001 | 0.065 |
| Diffuse | 76 (43.9) | 42 (71.2) | 77 (86.5) | ||||
| Heterogeneous | 80 (46.2) | 17 (28.8) | 12 (13.5) | ||||
ICAD = intracranial atherosclerotic disease; IVas = inflammatory vasculopathy; IVWI = intracranial vessel wall MRI; RCVS = reversible cerebral vasoconstrictive syndrome; IVWI
Values are no. (%); observations are reads (201 lesions × 2 raters = 402 total);
Test for difference in findings between the given pair of vasculopathy groups;
Excluding 7 lesions where one rater did not determine eccentricity.
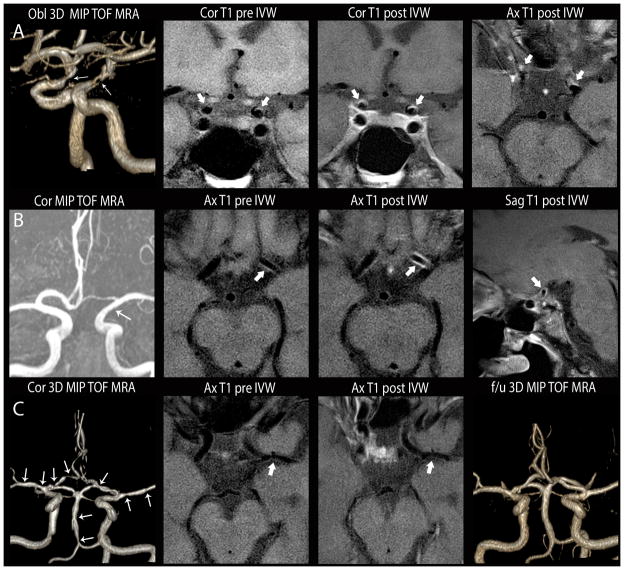
Figure 1.
Comparison of intracranial vasculopathies on luminal imaging and IVWI. A. Atherosclerosis. Oblique 3D MIP 3D TOF MRA (left image) shows irregular narrowing of both supraclinoid ICA (white arrows). Coronal T1 pre-contrast (left middle), coronal (right middle) and axial (right) T1 post-contrast images show irregular, eccentric wall thickening with mild, incomplete lesion enhancement (thick white arrows). B. Inflammatory vasculopathy. Coronal 3D MIP MRA (left) shows subtle diffuse narrowing of the left supraclinoid ICA (white arrow). Axial T1 pre-contrast (left middle), axial (right middle) and sagittal (right) T1 post-contrast IVWI images show circumferential wall thickening with diffuse lesion enhancement (thick white arrows). Subsequent biopsy indicated primary angiitis of the CNS. C. Reversible cerebral vasoconstriction syndrome. Coronal 3D MIP TOF MRA (left) of the circle of willis shows multi-focal narrowing involving all arterial territories (arrows). On axial T1 pre- (left middle) and post- (right middle) contrast, there is minimal wall thickening without enhancement of a left MCA lesion (thick white arrow). On follow-up TOF MRA (left) performed 2 months later, multi-focal stenoses had resolved.
Figure 2.
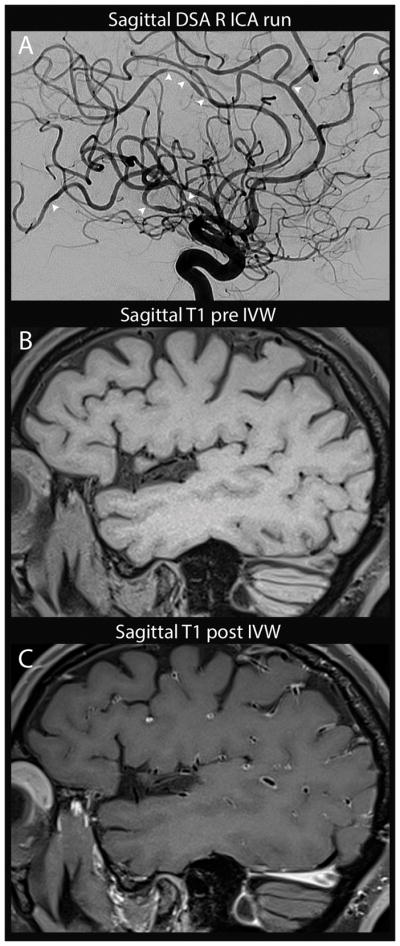
Reversible cerebral vasoconstriction syndrome with associated enhancement. Sagittal DSA run of the right ICA (A) shows multi-focal stenosis throughout the right MCA, ACA and PCA territories (arrowheads) which was seen in all vascular territories (not shown). On sagittal T1 pre (B) and post-(C) contrast IVWI, there is diffuse arterial wall enhancement with minimal wall thickening. Follow-up CTA at 2 months (not shown) and serial inpatient TCD showed improvement in stenoses.
Pattern of involvement by both luminal and IVWI imaging was significantly associated with IVWI disease states (Table 1), specifically ICAD lesions were more likely to be eccentric than RCVS and IVas lesions. However, there was a significantly stronger correlation between final ICAD diagnosis and lesion eccentricity on IVWI than lesion eccentricity detected on luminal imaging alone (κ 0.69 vs. 0.18, p<.001).
Added Benefit of Vessel Wall MRI
On the basis of luminal imaging alone, raters made the correct per-lesion diagnosis in 145 of 402 evaluations (36.1%). When luminal imaging+IVWI were reviewed, rater accuracy significantly increased with 357 of 402 (88.8%) evaluations correctly diagnosed (p<.001) (Table 2). The increase in diagnostic accuracy was significant for each rater individually (rater 1: 26.4% vs. 83.6%, p<.001; rater 2: 45.8% vs. 94.0%, p<.001). Diagnostic accuracy significantly improved with the addition of IVWI for each disease group (Table 2). Of the 257 reads with an incorrect diagnosis on luminal imaging, 221 (86.0%) were correctly reclassified with the addition of IVWI. Of the 145 reads with the correct diagnosis on luminal imaging, 9 (6.2%) were incorrectly reclassified with the addition of IVWI (5 ICAD misclassified as IVas, 2 ICAD to RCVS, 2 IVas to RCVS). Thirty-six reads (8.9% of all 402 reads) had an incorrect diagnosis on both evaluations (50% RCVS, 25% ICAD and 25% IVas).
Table 2.
Change in diagnostic accuracy from luminal imaging (LI) to luminal and IVWI imaging assessments.
| Per-Lesion Analysis | Per-Patient Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Correct Imaging Diagnosis* | Correct Imaging Diagnosis* | |||||||
| Clinical Diagnosis | No.† | LI | LI+IVWI | P-value | No.† | LI | LI+IVWI | P-value |
| All | 402 | 145 (36.1) | 357 (88.8) | <0.001 | 108 | 47 (43.5) | 104 (96.3) | <0.001 |
| ICAD | 180 | 119 (66.1) | 165 (91.7) | <0.001 | 60 | 45 (75.0) | 57 (95.0) | 0.001 |
| RCVS | 128 | 11 (8.6) | 110 (85.9) | <0.001 | 24 | 0 (0.0) | 24 (100.0) | <0.001 |
| IVas | 94 | 15 (16.0) | 82 (87.2) | <0.001 | 24 | 2 (8.3) | 23 (95.8) | <0.001 |
LI = luminal imaging; IVWI = intracranial vessel wall MRI;
Values are no. (%) where the imaging diagnosis matched the final clinical diagnosis (uncertain/equivocal imaging diagnoses were always classified as incorrect);
The denominator of the adjacent percentage columns, corresponding to the number of reads (per-lesion: 201 lesions × 2 raters = 402 reads; per-patient: 54 patients × 2 raters = 108 reads).
The per-patient analysis involved 108 evaluations (54 patients × 2 raters). Using luminal imaging alone, raters made the correct diagnosis at the patient-level in 47 of 108 (43.5%) evaluations (Table 2). Per-patient diagnostic accuracy increased to 104 of 108 (96.3%) when luminal+IVWI were reviewed compared to luminal imaging alone (p<0.001). Similar to the per-lesion analysis, improvement in per-patient diagnostic accuracy was most apparent for RCVS (0.0% to 100.0%, p<0.001) and IVas (8.3% vs. 95.8%, p<0.001) though there was also improvement in diagnostic accuracy of ICAD (75.0% to 95.0%, p=0.001).
Diagnostic Confidence
There was a significant increase in confidence between luminal imaging alone compared to luminal imaging+IVWI (high confidence rating of 3: 17% vs. 44%, p<.001). There was a significant increase in confidence with the inclusion of IVWI when rater diagnosis matched the final diagnosis (34.5% vs. 47.9%, p=.002). However, there was no significant change in confidence ratings when the rater diagnosis did not match the final diagnosis (7.8% vs. 13.3%, p=.15).
Inter-Rater Agreement
Inter-rater agreement on luminal characteristics and diagnosis for luminal and IVWI is summarized in Table 3. There was only slight agreement for pattern of involvement of luminal imaging. Inter-rater agreement for diagnosis on luminal imaging was slight (κ 0.04, 95% CI −0.03–0.12) and substantial for IVWI (κ 0.72, 95% CI 0.64–0.8). This difference in agreement was significant (p<.001).
Table 3.
Inter-rater agreement for LI and IVWI assessments.
| Rater* | Agreement | ||||||
|---|---|---|---|---|---|---|---|
| Variable | R1 | R2 | P-value† | % Agree‡ | Cohen’s κ | (95% CI) | |
| Luminal Imaging | |||||||
| Pattern of involvement§ | Concentric | 108 (55.7) | 45 (23.2) | <0.001 | 47.9% | 0.02 | (−0.07–0.11) |
| Eccentric | 86 (44.3) | 149 (76.8) | |||||
| Diagnosis | ICAD | 69 (34.3) | 152 (75.6) | <0.001 | 35.3% | 0.04 | (−0.03–0.12) |
| RCVS | 13 (6.5) | 5 (2.5) | |||||
| IVas | 25 (12.4) | 22 (10.9) | |||||
| Uncertain/equivocal | 94 (46.8) | 22 (10.9) | |||||
| Vessel Wall Imaging | |||||||
| Diagnosis | ICAD | 81 (40.3) | 93 (46.3) | 0.003 | 81.6% | 0.72 | (0.64–0.80) |
| RCVS | 55 (27.4) | 65 (32.3) | |||||
| IVas | 62 (30.8) | 43 (21.4) | |||||
| Uncertain/equivocal | 3 (1.5) | 0 (0.0) | |||||
ICAD = intracranial atherosclerotic disease; LI = luminal imaging; RCVS = reversible cerebral vasoconstrictive syndrome; IVas = inflammatory vasculopathy; IVWI = vessel wall imaging;
Values are no. (%);
Test for average difference between raters;
Percent of lesions where raters gave the same classification;
Excluding 7 lesions where one rater did not determine eccentricity.
Discussion
We report the first study to assess the added benefit of IVWI over current luminal diagnostic algorithms in the differentiation of non-occlusive vasculopathies, specifically ICAD, IVas and RCVS. This study shows that inclusion of IVWI can significantly improve imaging diagnostic differentiation of these vasculopathies over luminal imaging alone. In previous studies, we established substantial to almost perfect agreement in IVWI characteristics for non-occlusive16 and steno-occlusive15 intracranial vasculopathy differentiation. There was only slight agreement in pattern of involvement on luminal imaging in the current study, indicating that this is a less reliable and reproducible assessment tool. This is especially important considering that luminal imaging is the standard of care for intracranial vasculopathy evaluation and differentiation. In addition, the arterial segment of disease involvement showed no significant correlation with final diagnosis when intracranial internal carotid artery involvement was excluded. The likelihood of a correct diagnosis in the setting of non-occlusive vasculopathy significantly increased when IVWI was evaluated in addition to luminal imaging (per-lesion: 36.1% to 88.8% and per-patient: 43.5% to 96.3%), and this increase was significant overall as well as for ICAD, RCVS and IVas individually. There was substantial inter-rater diagnostic agreement for IVWI+luminal imaging, while only slight agreement for luminal imaging assessment alone.
Historically, angiographic imaging has served as the reference standard for the differentiation and characterization of non-occlusive vasculopathies. Specifically, DSA is considered the imaging gold standard, with differentiating features for ICAD from RCVS and IVas being lesion location and pattern of involvement. In the current study, however, 12% of ICAD lesions involved distal branches, while 41% of RCVS and 32% of IVas involved 1st order branches of the ACA, MCA and PCA, indicating prominent overlap in traditional patterns of disease involvement. In terms of pattern of involvement, there was a significant difference in eccentricity on luminal imaging of ICAD lesions relative to RCVS and IVas, however, 53.2% of RCVS and 51.1% of IVas lesions were rated as eccentric on luminal imaging as compared to 71.2% of ICAD lesions. In addition, there was a stronger correlation between ICAD diagnosis and the described eccentric pattern on IVWI than there was for luminal imaging. The current study, similar to previous studies16, 17, 20, 21, establishes distinctive IVWI patterns for ICAD, RCVS and IVas that can improve diagnostic accuracy over luminal imaging alone. ICAD typically showed eccentric, heterogeneously or diffusely enhancing lesions, IVas showed concentric, diffusely enhancing lesions and RCVS had concentric non-enhancing or diffusely enhancing lesions.
A few previous studies have compared the IVWI appearances of ICAD, RCVS and/or IVas. With a multi-contrast IVWI protocol16, there were significant differences in IVWI appearance of these vasculopathies, with the following typical descriptions for each disease-ICAD: eccentric, diffuse or incompletely, mildly or moderately enhancing lesion with mixed T2 signal intensity; RCVS: concentric, non-enhancing (when enhancement was present it was diffuse and mild) lesion with minimal iso- or hypo-intense wall thickening; IVas: concentric, diffusely and moderately enhancing lesions. Obusez et al21 compared 13 IVas and 13 RCVS cases with IVWI and found 9/13 IVas cases had smooth, concentric, strong (defined as thick-walled enhancement) enhancement, 3/13 showed eccentric, strong involvement and 1 case showed no enhancement. RCVS showed smooth concentric wall thickening in 10/13 cases, four of which showed mild enhancement (defined as thin-walled enhancement), while 3 had no wall abnormality. These findings are similar to our study, though we found a higher occurrence of vessel wall enhancement in RCVS (46.1% as compared to 18.2%16 and 31%21). Other studies have also documented the presence of enhancement in cases of RCVS22. These studies, however, established the intensity of enhancement or the presence of wall thickening, if present, to be a differentiating characteristic for enhancing RCVS (thin-walled/mild enhancement) as compared to IVas (thick-walled/moderate enhancement)16, 20, 21. Mandell et al20 compared 3 cases of RCVS with 4 cases of IVas, and found RCVS to have minimal or absent enhancement, while all IVas cases showed wall enhancement. Swartz et al17 compared 13 ICAD and 3 IVas cases and found that ICAD showed eccentric enhancing lesions while IVas typically had concentric enhancing lesions.
There are several limitations of this study. This was a retrospective imaging review. Luminal imaging modalities performed were heterogeneous; however, best practice guidelines were used for imaging and patient care and the image review approximates clinical practice at many institutions. Histologic confirmation was not available for most cases. The number of RCVS and IVas cases in this study is limited, which are relatively rare conditions. There is the possibility that some patients may have had lesions from more than one vasculopathy, however, we attempted to limit this by excluding patients with more than one suspected diagnosis. This study only evaluates the added value of IVWI in differentiating certain vasculopathies as only a subset of potential vascular diseases is studied. No healthy reference is provided for comparison to vascular pathological states. Lesions were evaluated individually and independently. While this does not match typical clinical review for luminal imaging, each lesion should be reviewed individually on IVWI. To mitigate this discrepancy, the raters were aware of additional lesions per patient during lesion review. We utilized 2D T1 TSE before and after contrast, which requires many planes of scanning and requires more scan time and physician supervision compared to isotropic 3D IVWI acquisitions, which can be reformatted into multiple planes with a single acquisition. This was a single-center study performed on a single MRI platform. For these reasons, a large, multi-center, multi-platform prospective study is needed to confirm these findings.
Summary
The addition of IVWI to the diagnostic algorithm improves diagnostic accuracy and confidence in the differentiation of non-occlusive intracranial vasculopathies, specifically ICAD from RCVS and IVas when compared with luminal imaging alone. This differentiation is important as treatment algorithms for each condition differ significantly. With further confirmation of the added benefit of IVWI in disease differentiation, its inclusion in diagnostic algorithms may improve patient management and outcomes while potentially limiting invasive diagnostic tests.
Supplementary Material
Acknowledgments
Study funding:
NIH R56 NS092207 01
NIH R01 NS092207 01A1
Footnotes
Disclosures:
Mossa-Basha, Shibata, Hallam, deHavenon, Becker, Tirschwell and Balu-no disclosures.
Hippe-grants from GE, Philips and Toshiba Healthcare unrelated to the current work.
Hatsukami and Yuan-grants from Philips Healthcare unrelated to the current work.
References
- 1.Kimmoun A, Baux E, Das V, Terzi N, Talec P, Asfar P, et al. Outcomes of patients admitted to intensive care units for acute manifestation of small-vessel vasculitis: A multicenter, retrospective study. Critical care (London, England) 2016;20:27. doi: 10.1186/s13054-016-1189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komotar RJ, Kellner CP, Raper DM, Strozyk D, Higashida RT, Meyers PM. Update on the natural history of intracranial atherosclerotic disease: A critical review. World journal of radiology. 2010;2:166–171. doi: 10.4329/wjr.v2.i5.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singhal AB, Topcuoglu MA. Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology. 2017;88:228–236. doi: 10.1212/WNL.0000000000003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajj-Ali RA, Singhal AB, Benseler S, Molloy E, Calabrese LH. Primary angiitis of the cns. The Lancet. Neurology. 2011;10:561–572. doi: 10.1016/S1474-4422(11)70081-3. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Archives of neurology. 2009;66:704–709. doi: 10.1001/archneurol.2009.76. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese LH, Furlan AJ, Gragg LA, Ropos TJ. Primary angiitis of the central nervous system: Diagnostic criteria and clinical approach. Cleveland Clinic journal of medicine. 1992;59:293–306. doi: 10.3949/ccjm.59.3.293. [DOI] [PubMed] [Google Scholar]
- 7.Salvarani C, Brown RD, Jr, Calamia KT, Christianson TJ, Weigand SD, Miller DV, et al. Primary central nervous system vasculitis: Analysis of 101 patients. Annals of neurology. 2007;62:442–451. doi: 10.1002/ana.21226. [DOI] [PubMed] [Google Scholar]
- 8.Vollmer TL, Guarnaccia J, Harrington W, Pacia SV, Petroff OA. Idiopathic granulomatous angiitis of the central nervous system. Diagnostic challenges. Archives of neurology. 1993;50:925–930. doi: 10.1001/archneur.1993.00540090032007. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya S, Berkowitz AL. Primary angiitis of the central nervous system: Avoiding misdiagnosis and missed diagnosis of a rare disease. Practical neurology. 2016;16:195–200. doi: 10.1136/practneurol-2015-001332. [DOI] [PubMed] [Google Scholar]
- 10.Torres J, Loomis C, Cucchiara B, Smith M, Messe S. Diagnostic yield and safety of brain biopsy for suspected primary central nervous system angiitis. Stroke. 2016;47:2127–2129. doi: 10.1161/STROKEAHA.116.013874. [DOI] [PubMed] [Google Scholar]
- 11.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–1147. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 12.de Havenon A, Yuan C, Tirschwell D, Hatsukami T, Anzai Y, Becker K, et al. Nonstenotic culprit plaque: The utility of high-resolution vessel wall mri of intracranial vessels after ischemic stroke. Case reports in radiology. 2015;2015:356582. doi: 10.1155/2015/356582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible cerebral vasoconstriction syndrome, part 2: Diagnostic work-up, imaging evaluation, and differential diagnosis. AJNR. American journal of neuroradiology. 2015;36:1580–1588. doi: 10.3174/ajnr.A4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossa-Basha M, Alexander M, Gaddikeri S, Yuan C, Gandhi D. Vessel wall imaging for intracranial vascular disease evaluation. Journal of neurointerventional surgery. 2016;8:1154–1159. doi: 10.1136/neurintsurg-2015-012127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossa-Basha M, de Havenon A, Becker KJ, Hallam DK, Levitt MR, Cohen WA, et al. Added value of vessel wall magnetic resonance imaging in the differentiation of moyamoya vasculopathies in a non-asian cohort. Stroke. 2016;47:1782–1788. doi: 10.1161/STROKEAHA.116.013320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke. 2015;46:1567–1573. doi: 10.1161/STROKEAHA.115.009037. [DOI] [PubMed] [Google Scholar]
- 17.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced mri. Neurology. 2009;72:627–634. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 18.DAaH DV. Bootstrap methods and their application. Cambridge, United Kingdom: Cambridge University Press; 1997. [Google Scholar]
- 19.Diggle PHP, Liang KY, Zeger S. Analysis of longitudinal data. New York City, NY: Oxford University Press; 2002. [Google Scholar]
- 20.Mandell DM, Matouk CC, Farb RI, Krings T, Agid R, terBrugge K, et al. Vessel wall mri to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: Preliminary results. Stroke. 2012;43:860–862. doi: 10.1161/STROKEAHA.111.626184. [DOI] [PubMed] [Google Scholar]
- 21.Obusez EC, Hui F, Hajj-Ali RA, Cerejo R, Calabrese LH, Hammad T, et al. High-resolution mri vessel wall imaging: Spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR. American journal of neuroradiology. 2014;35:1527–1532. doi: 10.3174/ajnr.A3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieleman N, van der Kolk AG, van Veluw SJ, Frijns CJ, Harteveld AA, Luijten PR, et al. Patterns of intracranial vessel wall changes in relation to ischemic infarcts. Neurology. 2014;83:1316–1320. doi: 10.1212/WNL.0000000000000868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.