Abstract
In recent years there have been an increasing number of in vitro and in vivo studies that show positive results regarding antimicrobial photodynamic therapy (aPDT) used in dentistry. These include applications in periodontics, endodontics, and mucosal infections caused by bacteria present as biofilms. Antimicrobial photodynamic therapy is a therapy based on the combination of a non-toxic photosensitizer (PS) and appropriate wavelength visible light, which in the presence of oxygen is activated to produce reactive oxygen species (ROS). ROS induce a series of photochemical and biological events that cause irreversible damage leading to the death of microorganisms. Many light-absorbing dyes have been mentioned as potential PS for aPDT and different wavelengths have been tested. However, there is no consensus on a standard protocol yet. Thus, the goal of this review was to summarize the results of research on aPDT in dentistry using the PubMed database focusing on recent studies of the effectiveness aPDT in decreasing microorganisms and microbial biofilms, and also to describe aPDT effects, mechanisms of action and applications.
Keywords: photochemotherapy, bacteria, biofilms, photosensitizing agents
1. Introduction
1.1. Definition
Photodynamic therapy (PDT) has generally been developed as an alternative approach for cancer treatment, and unlike traditional therapies (surgery, chemotherapy and radiotherapy), PDT does not have severe side effects and can be often repeated. It destroys cells by necrosis or apoptosis, and can be used for localized destruction of living tissue with abnormal growth [1]. Therefore, other diseases such as bacterial, fungal and viral infections, which have in common the characteristic of uncontrolled cell proliferation and the presence of undesirable microbial cells, can be treated by PDT, in this case it is called antimicrobial photodynamic therapy (aPDT) or photo-dynamic inactivation (PDI) [2–4].
The first historical records of PDT began in ancient Greece, Egypt and India, and soon disappeared. New reports occurred in the early twentieth century, in western civilization. Niels Finsen, a Danish physician, was the first to report the successful use of PDT, employing an arc lamp to treat Lupus Vulgaris [1]. Then, over one hundred years ago in 1903, it was observed that the reaction between a visible light source and dyes, associated with oxygen, was named ‘photodynamic action’ [1, 2].
Initially PDT was used for the treatment of human tumors by topically applying a photosensitizer (PS) dye and illuminating with a lamp. Later the PS was injected systemically and a laser was used as a more focused light source at a suitable wavelength to excite the dye [1].
1.2. Mechanism of action of aPDT
Antimicrobial photodynamic therapy activity is based on the combination of a non-toxic PS and an appropriate wavelength of visible light, which in the presence of ambient oxygen is activated and can promote a phototoxic response. The reactive oxygen species (ROS) that are produced can cause damage of biomolecules and cause oxidation of cellular structures leading to the death of microorganisms [5]. Each of these factors (PS, light, oxygen) is harmless by itself, but when combined together can produce lethal cytotoxic ROS that can selectively destroy cells [6]. The aPDT action mechanism is briefly described by the excitation of a nontoxic light absorbing dye (PS) that forms a long-lived excited triplet state, which then transfers energy to the surrounding molecules, generally to molecular oxygen, to form highly reactive and cytotoxic ROS such as hydroxyl radicals and singlet oxygen (figure 1) [7–9]. ROS can modify the plasma membrane structures or even the DNA [10] and also cause cell death through several mechanisms including: lipid per-oxidation, inhibition of enzymatic systems, and agglutination of proteins that are critical to other biological systems [11, 12]. PDT can be highly selective to the microorganisms or diseased tissue, such as cancerous cells [7, 8]. During aPDT, only cells with selective accumulation of the PS that also receive light exposure are killed [9]. Thus, PDT can be repeated several times, since it is a non-invasive procedure, which does not cause cumulative toxicity. Moreover, due to its low risk, it can be used in elderly or severely weakened people [8].
Figure 1.
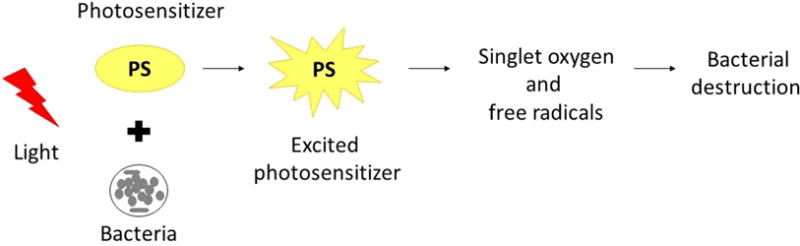
Mechanism of PDT. The photosensitizer is absorbed by the microorganisms and following exposure to light under an appropriate wavelength it becomes activated to an excited state. Then, the photosensitizer transfers energy from light to molecular oxygen to generate singlet oxygen and free radicals that are cytotoxic to cells.
1.3. Interaction microorganism-PS
The effectiveness of aPDT for different microorganisms depends on PS type, its concentration and the class of microorganisms (Gram-positive bacteria, Gram-negative bacteria, fungus or virus), which together determine the site of action. This site of action depends on the physicochemical characteristics of the microorganism-PS interaction. The important parameters for PS interaction include: its relative solubility in water and lipids, ionization constant and other more specific factors, such as, light absorption characteristics and the efficiency of formation of the excited state triplet and singlet oxygen production [14, 15].
The morphology of the microbial cell shows a wide variation between different species as well as between strains, which modulates the interaction of exogenous PS with cellular constituents, affecting the pathways of the photoinactivation process [3, 10]. The cell wall of different groups of microorganisms has a great variability in its complexity, structural architecture, permeability and its binding capacity with external molecules [15].
Therefore, aPDT is more effective in the inactivation of Gram-positive bacteria, since the outer portion of their cell wall (composed of peptidoglycan and lipoteichoic acid) is relatively more porous, allowing PS to reach the cytoplasmic membrane (figure 2) [14]. In contrast, Gram-negative bacteria present a much more complex morphology. The outer portion of their cell wall contains negatively charged lipopolysaccharide, lipoproteins and proteins with a porin function, in addition to peptidoglycan. This structural organization forms a physical and functional barrier that hinders the incorporation of PS [2]. It is extremely important to note that, when these microorganisms are in the biofilm form, the photodynamic activity of PS is generally reduced, because there is a structural difference in the cell membranes of these microorganisms and the presence of other components, such as extracellular polysaccharide matrix and quorum-sensing factors, hamper the PS-microorganism interaction [15]. In periodontics, aPDT has shown positive effects against periodontal bacteria such as, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Capnocytophaga gingivalis, Fusobacterium nucleatum, Prevotella intermedia and Streptococcus sanguis. While in endodontics, aPDT has shown effects against Actinomyces israelli, F. nucleatum, P. gingivalis and P. intermedia [9, 16].
Figure 2.
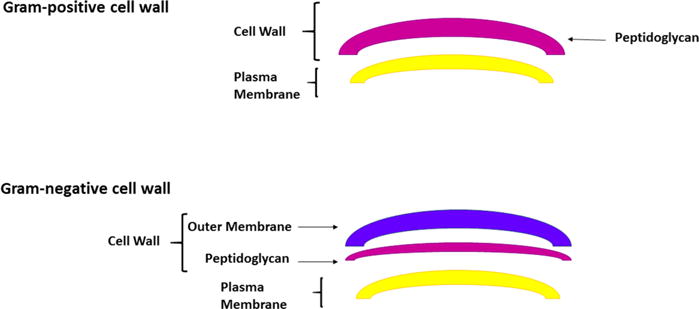
Morphological structure of bacteria: Gram-positive and Gram-negative.
2. Objective
Considering the above-mentioned concepts and the potential application of PDT in infectious diseases, this article aims, through a critical review of the literature, to describe the effects, mechanism of action and application of aPDT in dentistry. As a search strategy, the databases PubMed/Medline, Capes Portal of Journals and Web of Science were used, and articles were retrieved from 1992 until February 2016, using the following key-words: photochemotherapy, photosensitizing agents, biofilms, bacteria and PDT.
3. Photosensitizers
A PS can localize in a specific cell type or tissue type, and its subsequent activation by irradiation with low-energy tissue-penetrating light with an appropriate wavelength characterize the underlying principle of PDT [17]. An ideal PS should be a single pure substance stable at room temperature, have minimal dark toxicity and only be cytotoxic in the presence of light, exhibiting optimal absorption, distribution, metabolism and excretion. Ideally it should absorb light wavelengths between ~600 and 800 nm that penetrate deeply into tissue, should produce singlet oxygen and other ROS, and should be inexpensive and commercially available in order to promote extensive utilization of treatment. It should be selective for specific cells or tissues and should not be mutagenic or carcinogenic [17, 30].
Generally PSs are deelpy-colored aromatic molecules with extended conjugation of molecular orbitals, with a high quantum yield of formation of long lived excited triplet states. In terms of energy absorbed by the aromatic system, the wavelength and efficiency (absorption coefficient) depends on the molecular structure involved: PSs based on furocoumarines absorb relatively high energy ultraviolet light (300–350 nm) while macrocyclic heteroaromatic molecules such as phthalocyanines efficiently absorb lower energy, in the near infrared region (700 nm) [18]. There are many recognized PSs and several of them have been tested, both in the medical and in the dental areas.
PSs are generally classified as porphyrin-based (tetrapyrroles) or non-porphyrin-based. Porphyrin-derived PSs are further classified as first, second or third generation PSs. First generation PSs include hematoporphyrin derivative and Photofrin®. The second generation PSs are chemically pure compounds compared with first generation compounds (mixtures), that absorb light at a longer wavelength and cause significantly less skin photosensitization post-treatment. The third generation PSs are bound to carriers such as antibodies and liposomes selective for tumor tissue [17].
First generation porphyrin derivatives, and second generation chlorins and phthalocyanines, have been used for treating tumors and have been approved for clinical use [13]. Some studies [19] have shown the efficiency of dyes such as toluidine blue O (TBO), and methylene blue (MB). These two are part of the group of phenothiazinium salts and are fully synthetic, and more recently the natural product curcumin has been applied in dentistry [20–22].
TBO dye is used to stain the cervix and the buccal cavity to reveal mucosal abnormalities, to delimit the extent of abnormality before subsequent excision. TBO has also proven very effective in killing bacteria in the oral cavity when photo-activated [13]. Toluidine blue is an acidophilic metachromatic dye which absorbs light at 596 nm and 630 nm and that selectively stains acidic tissue components (sulfates, carboxylates, and phosphate radicals). Toluidine blue has an affinity for nucleic acids, and therefore binds to tissues with a high DNA and RNA content [23].
MB has been used for a long time to detect pre-malignant cells and as a tissue marker in surgery. It is effective against Gram-negative bacteria because of its hydrophilicity capacity, low molecular weight, and its positively charge [13]. The characteristic color of MB is caused by the strong absorption band at 550–700 nm region [24]. MB may induce either the formation of hydroxyl radicals (type I) or singlet oxygen (type II) species, which extends the application of MB in PDT [24]. The mechanism of inactivation of bacteria by MB seems to be a mixture of type I and type II processes, and the relative efficiency of each them depends on the cell type and experimental conditions [24]. The easy availability of MB and the possibility of using non-laser polychromatic light sources makes MB a potential PDT sensitizer that could be used in underserved populations for the treatment of a variety of diseases [24].
The two above-mentioned dyes (TBO and MB) were effective against P. gingivalis, F. nucleatum, Actinobacillus actinomycetemcomitans [25], Streptococcus mutans, Streptococcus sobrinus, Lactobacillus casei, Actinomyces viscosus [28] and also Candida albicans [27]. Rose Bengal and erythrosine are examples of xanthene dyes which are characterized by the absorption of light at wavelengths of 450–600 nm and 500–550 nm, respectively; this absorption is associated with the subsequent photochemical reactions. Rose Bengal staining is used for the diagnosis of eye diseases, and erythrosine is used in dentistry to reveal biofilms. Erythrosine, in particular, is ideal for use in PDT compared with other dyes, because it is approved for use in the oral cavity and does not show direct toxicity to the host tissue. Xanthene dyes have been excited with tungsten filament lamps and other light sources for the reduction of Gram-positive bacteria, Gram negative bacteria and yeasts during aPDT [28, 31].
Curcumin is the major constituent of turmeric powder and has been used for centuries in medicine, as food pigment and as a spice, and more recently in dentistry as a PS for PDT [21].
Curcumin is a yellow pigment, isolated from Curcuma longa rhizome. It is frequently used in cooking as a seasoning [21] and has a wide range of pharmacological effects, such as anti-inflammatory action, anti-carcinogenic and anti-infective activities. It displays absorption peaks ranging from 300 to 500 nm of the visible spectrum, has a low cost, easy handling and is effective against yeasts. In addition, it does show any burning sensation, oral soreness or cause ulcers when used in vivo. However, curcumin has very limited solubility in water and the use of oils and synthetic solvents has been suggested to enable its dissolution [22]. Table 1 shows the main photosenstizers used in dentistry.
Table 1.
Main PSs used in dentistry.
| PS | Properties | Authors |
|---|---|---|
Methylene blue (MB)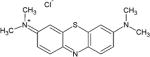
|
Antimicrobial activity against dental biofilm and planktonic cells | Fontana et al [53] |
Toluidine blue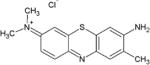
|
Antimicrobial activity by PDT in supragingival biofilms | Qin et al [50] |
Curcumin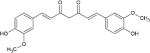
|
Antimicrobial activity by PDT against biofilms and planktonic forms of C. albicans, Candida tropicalis and Candida glabrata | Dovigo et al [21] |
Rose bengal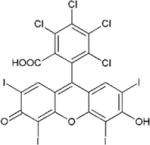
|
Antibiofilm polymeric chitosan nanoparticles with rose bengal. against Enterococcus Faecalis | Shrestha et al [46] |
Chlorin(e6)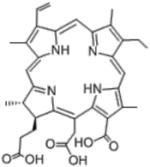
|
Antibacterial effects against periodontal bacteria | Pfitzner et al [9] |
In addition to the requirements outlined above the ideal PS for aPDT must also show high affinity for binding to microorganisms, require a very short drug-light interval, have a broad spectrum of antimicrobial action, low affinity for binding to mammalian cells, show no ability to select for resistant bacterial strains [13, 30–33].
4. Light source
The first light sources used in PDT were polychromatic, non-coherent, lamps designed to emit white light and heat in most cases. With the invention of lasers in the 1960s, laser light used for PDT was monochromatic, coherent radiation, and the treatment could be better defined using the optimum wavelength, a high energy density and light transmission through optical fibers [26, 34, 35].
Laser radiation has gained ground in dentistry in order to reduce the number of microorganisms that cause diseases in the oral cavity [20], mainly bacteria that are involved in tooth decay and periodontal disease [19].
The therapeutic use of lasers, with photobiomodulation action, was suggested in 1965 by Sinclair and Knoll, and Mester in 1968 was the first to use it in clinical medicine, demonstrating that ruby and argon lasers, at low intensity, accelerated healing of chronic ulcers [36].
In dentistry, the laser (light amplification by stimulated emission of radiation) has been used for sterilization of wounds, in cavity preparation; to reduce the bacterial population of endodontic channels and periodontal pockets [37]. A schematic of laser operation is shown in figure 3.
Figure 3.
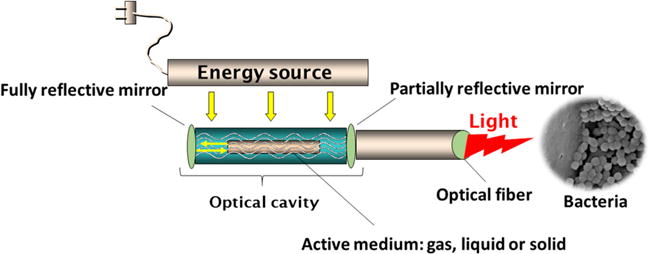
Scheme of laser operation.
High power or surgical lasers have been employed in surgery. Low power lasers, also called photobiomodulation therapy or non-ablative, have been used in procedures for different kinds of clinical therapy.
The high power laser provides the higher power indicated for surgery [20], increases the temperature within the pulp and around the periodontal ligament, which may lead to bone resorption or necrosis of the pulp and is more costly [34].
Low-power lasers can be used in PDT [20]. The low-power laser features power around 30–100 mW, wavelength ranging from 630 to 904 nm, and negligible thermal effects. Its application depends on the amount of light absorbed [20]; its action is to restore the biological balance of the cells; it has analgesic and anti-inflammatory action on tissues [19] and when combined with a PS leads to the death of the microorganisms [34]. The therapeutic laser is employed in tissue biostimulation and promotes hemostasis and stimulation of healing after tooth extractions. Cellular photobiomodulation is determined by photochemical effects within the cells and does not require any photothermal effect [38].
The therapeutic effects of lasers in cell cultures have been investigated for many years. Belkin and Schwartz [39] found that this form of illumination considerably alters the transmembrane transport of various cations, especially calcium. Laser-tissue interaction is photochemical depending on light absorption by a tissue chromophore, such as an enzyme or membrane molecule or other cellular or extracellular constituent. The absorption increases the energy of the chromophore and produces molecular reactions affecting biochemical pathways, consequently, cell metabolism is altered, which affects tissues and organs.
Another light source used is based on the LED (light emitting diode). A LED is a two-lead semiconductor-based light source that uses electricity to excite light emission by recombination of holes and electrons generated in the semi- conductor band-gap without any increase in temperature. LEDs can be designed to emit light in the three colors of visible light (red, blue and green) and also in the near infrared region (>700 nm). LEDs can be applied individually or simultaneously in different combinations. LED light is quasi-monochromatic (often a 30 nm band-pass), and the broad range of colors available has led to widespread use by researchers because of their low cost and narrow band wavelengths [35]. A scheme of LED operation is shown in figure 4.
Figure 4.
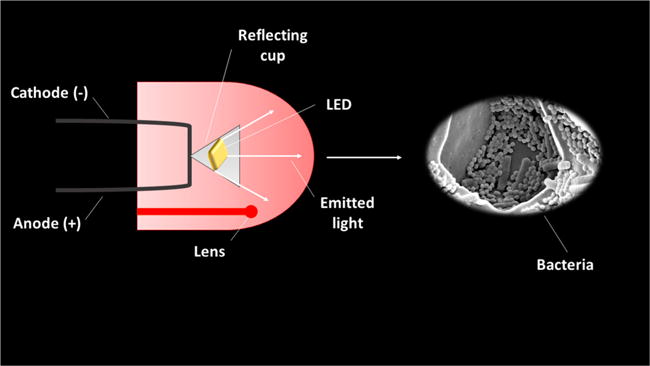
Scheme of LED operation.
5. Application of PDT in dentistry
The table 2 summarizes the studies selected in this work, with authors, description of the studies and the conclusion with the main results.
Table 2.
Antimicrobial photodynamic therapy studies.
| Authors | Study | Conclusion |
|---|---|---|
| Wilson et al [25] | Investigated the bactericidal effect of 27 PSs at concentration 0.005% (wt/vol) for their ability to sensitize Streptococcus sanguinis with 7.3 mW helium/neon (HeNe) laser, during 30 s | Most effective were TBO, MB, aluminum disulphonated phthalocyanine (AlPcS2), crystal violet and dihematoporphyrin esther (DHE). In the absence of light, PSs were not effective |
| Burns et al [26] | Application of PDT against bacteria causing tooth decay (S. mutans, S. sobrinus, L. casei and A. viscosus) with PS aluminium disulphonated phthalocyanine, combined with gallium aluminium arsenide laser with exposure times (30–60 s) | Bacteria were sensitive to laser light combined with a PS, providing a reduction of 108–106 CFU |
| Burns et al [40] | Evaluated PDT mediated by toluidine blue or aluminium disulphonated phthalocyanine combined with helium–neon (876, 1.752 and 3.504 mJ) or gallium–aluminium–arsenide laser (1.188, 2.376 and 4.752 mJ), respectively, in cariogenic bacteria S. mutans | Significant reductions of these cariogenic bacteria S. mutans (107 CFU) when used with 438 and 1.314 mJ of helium–neon laser light and 594 and 1.782 mJ of light from the gallium–aluminium–arsenide laser. Prolonged exposure led to kill of higher concentrations (108–1010 CFU) |
| Pfitzner et al [9] | PDT with chlorine e6, BLC 1010 and BLC 1014 at a concentration of 10 μg was used in periodontal bacteria (P. gingivalis, F. nucleatum, and C. gingivalis) associated with laser (5.3 J cm−2) | The microorganisms were photoinactivated completely with chlorine e6 and BLC 1010 dyes, which were able to induce the inhibition zones of the agar plates |
| Sigusch et al [51] | Investigated PDT efficacy in two periodontal bacterial species (P. gingivalis and F. nucleatum) using PSs chlorine e6 and BLC 1010 associated with 662 nm and 0.5 W diode laser | PDT showed a significant reduction in clinical signs of inflammation and redness in comparison to the control group |
| Meisel and Kocher [52] | This literature review describes the use of PDT under a periodontal perspective | PDT may be an important adjunct therapy to conventional techniques for bacterial control of periodontal diseases |
| Jori et al [10] | Use of PSs positively charged at physiological pH values and characterized by a moderate hydrophobicity to kill microorganisms at 5–10 min and ~50 mW cm−2 | PDT is an efficacious alternative modality for the treatment of localized microbial infections including a variety of oral infections |
| Oliveira et al [53] | Ten patients with aggressive periodontitis were treated with PDT (690 nm; 60 mW cm−2; 10 s) associated with a phenothiazinium PS (10 mg ml−1; 1 min) or scaling and root planing (SRP) with hand instruments | PDT and SRP showed similar clinical outcomes in the non-surgical treatment of aggressive periodontitis. However, PDT presents advantages, such as reducing the treatment time |
| Qin et al [50] | Investigated the parameters for efficient aPDT in supragingival biofilms from 20 volunteers with periodontal disease, using toluidine blue dye and diode laser (635 nm) | The therapeutic effect was improved with the following combination: 1 mg ml−1 of the dye with laser irradiation of 12 J cm−2 with survival of bacteria around 4% |
| Fontana et al [54] | Investigated the effects of the PDT using MB (25 μg ml−1 to planktonic cells and 25 and 50 μg ml−1 for biofilms) on human dental plaque microorganisms using red light at 665 nm, 100 mW cm−2 and 30 J cm−2 | Oral bacteria in biofilms were less affected by PDT (32% killing) than bacteria in planktonic phase (63% killing) |
| Dovigo et al [21] | Evaluated PDT mediated by curcumin against C. albicans, C. tropicalis and C. glabrata. Candida suspensions were treated with curcumin (5, 10 and 20 μM for planktonic forms and 20, 30 and 40 μM for biofilms) using four LEDs (5.28, 18, 25.5 and 37.5 J cm−2, at 595 nm) | The combination of curcumin and light promoted a significant antifungal effect against yeast planktonic forms. The use of 40 mM curcumin reduced the metabolic activity of C. albicans, C. glabrata, C. tropicalis by 85, 85 and 73%, respectively, at 18 J cm−2 |
| Schneider et al [49] | Evaluated the impact of PDT on the viability of S. mutans cells using an artificial model of biofilm and phenothiazinium chloride 1% combined with laser at 660 nm and 100 mW | Laser irradiation was an essential part of aPDT able to reduce the bacteria inside of a 10 μm layer |
| Araujo et al [44] | Susceptibility of S. mutans and Lactobacillus acidophilus to PDT grown as multi-species in the biofilm phase versus in dentine carious lesions was evaluated using curcumin at 0.75, 1.5, 3.0, 4.0 and 5.0 g L−1) combined with blue LED under 5.7 J cm−2 | A significant reduction (p < 0.05) in cell viability of the biofilm phase following photosensitization using all curcumin concentrations was observed. To achieve significant bacterial reduction (p < 0.05) in carious dentine, it was necessary to use 5.0 g L−1 of curcumin in association with blue light |
| Shrestha et al [46] | Evaluated the effect of antibiofilm polymeric chitosan nanoparticles with rose bengal against E. faecalis biofilm | The nanoparticles demonstrated high antibacterial activity by adhesion and lysis of the bacterial cells after photodynamic treatment, reducing the viability of E. faecalis biofilms and leading to disruption of the biofilm structure |
| Bulit et al [47] | Evaluated the effect of PSs (curcumin, eosin Y, and/or rose bengal) on the viability of lactobacilli, the odontoblast like cells, undifferentiated cells of the pulp, and human embryonic stem cells, incubated for 15 min and then irradiated with blue light (240 s) | A significant reduction of viability was found after exposure to different combinations of PSs and light for disinfection |
| Oliveira et al [48] | Antimicrobial effect of PDT using methylene blue (50 μM) and low level laser (660 nm, 100 mW and 9 J) was evaluated against C. albicans, Pseudomonas aeruginosa, E. faecalis and Staphylococcus aureus | Gram-positive bacteria E. faecalis and S. aureus were eliminated more than 90%, showing that despite PDT not reducing the microorganisms completely, the results obtained lead to the conclusion that the treatment was able to promote the reduction of microbial cell viability using the selected parameters |
| Voos et al [45] | Antibacterial efficacy of PDT was compared using safranine O and chlorhexidine (CHX 0.2%) in an ex vivo on planktonic cultures of Streptococcus gordonii, S. mutans, F. nucleatum, P. gingivalis and Aggregatibacter actinomycetemcomitans | PDT promoted bacterial reduction >5 − log10 for both PSs tested in comparison with chlorhexidine (p < 0.05) |
| Melo et al [55] | Investigated the effectiveness of photochemistry as antimicrobial alternative to treat deep caries, using a LED (94 J cm−2; ~630 nm; 150 mW) combined with TBO (100 g ml−1). | Significant reductions on S. mutans, Lactobacillus spp. were demonstrated and this therapy was found to be a promising potential for the treatment of deep caries lesions |
| Nielsen et al [56] | Evaluated the effect of photoactivated disinfection (PAD) using riboflavin (266 μmol l−1) and blue LED light (630 nm) for activation (0.4 W; 37.7 J cm−2; 0.63 W cm−2; 1 min), comparing it to PAD using TBO and red light, for endodontic and periodontal treatment | Limited microbial kills using riboflavin/blue light were found for endodontic and periodontal treatment, which suggests that riboflavin cannot be recommended as PS for PAD |
| Junqueira et al [57] | Utilization of functional polymeric systems composed of poloxamer 407, Carbopol 934P and MB showing a capability for singlet oxygen generation for PDT | They demonstrated the formulation of a system with a gelation temperature and MB release, intended to be applied on the skin and/or mucous membranes |
| Hamblin et al [59] | Describes the perspective of the use of PDT according to the research group of Dr Michael R Hamblin, in the Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School | Regarding application in the field of dentistry, it is expected that nanomaterials will play a great role in restorative dentistry. They demonstrated special interest in the use of self-assembled nano-drug carriers (micelles, liposomes, etc) for PDT approach |
| Souza et al [60] | This review reveals that heterogeneous protocols of aPDT have been used as a strategy for adjunct treatment of aggressive periodontitis | An approach that used 0.25 W cm−2 for 10 s per site in four sessions of aPDT divided over 15 days was found to be the better protocol for this purpose |
| Meerovich et al [65] | Investigated the inactivation capacity in planktonic and biofilm cultures of Gram-negative P. aeruginosa using synthetic bacteriochlorins with four and eight cationic groups | The inactivation was efficient against Gram-negative bacteria in the planktonic and biofilm phases with 0.005 mM and 8 J cm−2, decreasing by 4 (bacteriochlorin-4) and 5 (bacteriochlorin-8) logs for planktonic form. The bacteriochlorin-8 was more efficient than bacteriochlorin-4 |
A study of PDT against oral bacteria was performed by Wilson et al [25]. This study aimed to investigate the bactericidal effect of 27 PSs for their ability to sensitize S. sanguinis with 7.3 mW helium/neon (HeNe) laser. Also, Burns et al [26] began the studies of the application of PDT against bacteria causing tooth decay (S. mutans, S. sobrinus, L. casei and A. viscosus). The results of Wilson et al [25] showed that the most effective PSs were ortho toluidine blue (TBO), MB, AlPcS2, crystal violet and DHE at concentrations of 0.005% (wt/vol), and that in the absence of light, PSs were not effective. While Burns et al [26] which showed that the bacteria were sensitive to laser light (gallium aluminium arsenide laser), exposure times (30–60 s) associated with a PS based on aluminium disulphonated phthalocyanine. During the following year, the same authors [40] treated suspensions of the cariogenic bacteria, S. mutans, with toluidine blue or aluminium disulphonated phthalocyanine and then exposed to light from a helium–neon (876, 1.752 and 3.504 mJ) or gallium—aluminium–arsenide laser (1.188, 2.376, and 4.752 mJ), respectively. They observed significant reductions of these cariogenic bacteria (S. mutans) when used 438 and 1.314 mJ of helium–neon laser light and 594 and 1.782 mJ using gallium–aluminium–arsenide laser.
Recently, PDT has been used as an alternative therapy for treatment of several pathologies, such as skin cancer [41], Cutaneous leishmaniasis [42], candidiasis [43], tooth decay [44] and periodontitis [45].
Pfitzner et al [9] used PDT with chlorin e6, BLC 1010 and BLC 1014 dyes at a concentration of 10 μg to eliminate periodontal bacteria (P. gingivalis, F. nucleatum and C. gingivalis) associated with laser (5.3 J cm−2). Sigusch et al [51] investigated PDT efficacy to reduce inflammatory signs caused by two periodontal bacterial species in Beagle dogs. Two PSs were tested: chlorin e6 and BLC 1010. The animals were infected with P. gingivalis and F. nucleatum in subgingival areas. The signs of inflammation were observed through gingival indexes. PDT was performed using a 662 nm and 0.5 W diode laser with the dyes mentioned above. Pfitzner et al [9] found best results with chlorin e6 and BLC 1010 dyes, which were able to induce the inhibition zones of the agar plates. BLC 1014 dye showed lower photodynamic effect than the others. It was suggested that PDT using dyes such as chlorin e6 and BLC 1010, is effective for suppressing periodontal bacteria. Similar results to those found by Sigusch et al [51] that PDT showed a significant reduction in clinical signs of inflammation and redness in comparison to the control group, with chlorin e6 and BLC 1010 dyes. This study suggested that PDT has advantages for control of periodontal disease.
Meisel and Kocher [52] in a review paper ‘State of the Art in PDT for Periodontal Diseases’, analyzed PDT as seen from a periodontal perspective. Jori et al [10] in a literature review analyzed in vitro and in vivo studies, and concluded that the use of PSs that were positively charged at physiological pH values and were characterized by a moderate hydrophobicity can be used in a micromolar concentration to induce a >4–5 log decrease in the microbial population under following conditions: 5–10 min of pre irradiation time and irradiation intensity under 50 mW cm−2. Both concluded that in experimental models, PDT may be an important adjunct therapy to conventional techniques for bacterial control of periodontal diseases. The application of dyes combined with visible light enables effective killing of periodontopathogens. Additionaly, positively charged PSs are more effective for aPDT due to the presence of pores in the cell wall structures. For oral candidiasis, the review of Jori et al [10] suggests that it could be eradicated, by using red light and topical PSs. In periodontal diseases, which involve mixture of Gram-positive and Gram-negative bacteria, it is suggested that several pathogens can be eradicated using both growth phases, suspension and biofilm, and that toluidine-blue can kill P. gingivalis. The authors Meisel and Kocher [52] and Jori et al [10] describe PDT as an efficacious alternative modality for the treatment of localized microbial infections including chronic ulcers, infected burns, acne vulgaris, and a variety of oral infections, however more clinical studies are needed to confirm the effectiveness of this procedure.
In order to establish a protocol and guidelines for the efficient control of aggressive periodontitis, de Oliveira et al [53] treated ten patients with a clinical diagnosis of aggressive periodontitis using PDT (690 nm; 60 mW cm−2; 10 s) combined with a phenothiazinium PS (10 mg ml−1; 1 min) or scaling and root planning (SRP) with hand instruments. Qin et al [50] investigated the parameters required for efficient aPDT in supragingival biofilms from 20 volunteers presenting periodontal disease. Different concentrations of toluidine blue dye and diode laser (635 nm) were used. In the study of de Oliveira et al [53], the authors concluded that PDT and SRP showed similar clinical outcomes in the non-surgical treatment of aggressive periodontitis, while Qin et al [50] observed improved therapeutic effect with the following combination: 1 mg ml−1 of the dye with laser irradiation of 12 J cm−2, that showed survival of bacteria around 4%. Both studies of de Oliveira et al [53] and Qin et al [50] pointed out some advantages of PDT, such as reducing the treatment time, no need for anesthesia and unlikely development of resistance by the target bacteria must be taken into consideration, and that studies have shown promising results in biofilm control, reducing periodontal bacteria and clinical signs of inflammation and suggesting that PDT could be an adjuvant therapy to conventional mechanical treatment.
In the study by Fontana et al [54], the authors investigated the effects of PDT using MB on human dental plaque microorganisms in planktonic phase and in biofilms. Dental plaque samples were obtained from ten subjects with chronic periodontitis. Suspensions of plaque microorganisms from five subjects were sensitized with MB (25 μg ml−1) for 5 min (pre irradiation time) and exposure to red light under wavelength of 665 nm, power density of 100 mW cm−2 and energy density of 30 J cm−2. Multi-species microbial biofilms developed from the same plaque samples were exposed to MB (25 μg ml−1) and to the same light conditions as the planktonic phase. Also, biofilms were developed with plaque bacteria from five subjects and sensitized with 25 and 50 μg ml−1 MB and exposure to red light. In the planktonic phase, PDT produced approximately 63% killing of bacteria. In biofilms, the effect of PDT resulted in only 32% killing. The authors concluded that oral bacteria in biofilms were less affected by PDT than bacteria in planktonic phase, and suggested that this occurred because the bacteria in planktonic phase are in a free form and lack extracellular material.
In another study, Dovigo et al [21] investigated PDT mediated by curcumin (CUR) against clinical isolates of C. albicans, C. tropicalis and C. glabrata, both in planktonic and in biofilm phases. Candida suspensions were treated with three concentrations of curcumin (5, 10 and 20 μM for planktonic phase and 20, 30 and 40 μM for biofilms) and exposed to four LED fluences (5.28, 18, 25.5 and 37.5 J cm−2 at 520 nm). The protocol that showed the best results for the inactivation of planktonic phase was selected to be evaluated against Candida biofilms, was 20 μM with 5.28 and 18 J cm−2. In addition, two higher concentrations (30 and 40 μM) of curcumin were tested in Candida biofilms. In the study of Araujo et al [44] the susceptibility of S. mutans and L. acidophilus in a multi-species biofilm against tooth decay in dentin was evaluated. They used five different concentrations of curcumin (0.75, 1.5, 3.0, 4.0 and 5.0 g l−1) associated to 5.7 J cm−2 LED. The studies of Dovigo et al [21] and Araujo et al [44] showed that the use of curcumin in combination with light was able to promote a significant antifungal and antibacterial effect against microorganisms.
Dovigo et al [21] observed that when they used 40 μM curcumin, the metabolic activity of C. albicans, C. glabrata, C. tropicalis was reduced by 85, 85 and 73%, respectively, at 18 J cm−2, and that a low concentration of curcumin can be highly effective to inactivate Candida isolates when combined with light excitation. While in studies of Araujo et al [44] the exposure of the biofilm to 0.75; 1.5 and 3.0 g l−1 of curcumin and subsequent illumination with light resulted in 97.5; 95 and 99.9% reduction (p < 0.05) in viable cells, respectively. The use of 4.0 and 5.0 g l−1 of curcumin provided a cell decrease of 100% (p < 0.05). S. mutans and L. acidophilus were sensitive to curcumin in the presence of blue light.
Schneider et al [49] used a laser light source and assessed the impact of aPDT on the viability of S. mutans cells using an artificial model of biofilm. The artificial model of biofilm was induced in chambers, a salivary pellicle layer was formed and S. mutans cells were inoculated in a sterilized culture. The PS used was phenotiazinium chloride (3,7-bis (dimethylamino) phenothiazin-5-ium chloride, MB) at a concentration of 1% buffered, pH 3.5 with an isotonic citrate buffer, and the viscosity was modified with 1% hydroxypropyl methylcellulose combined with laser (660 nm and 100 mW). A reduction in the bacteria culture was observed, whereas without the presence of PS laser irradiation caused no change in the number of fluorescent bacteria. The study showed that the laser irradiation was an essential part of aPDT to reduce the bacteria inside a 10 μm layer. The authors suggested that further studies were needed to assess the maximum thickness of biofilm through which microorganisms can be destroyed.
According to Araujo et al [44] to achieve significant microbial reduction (p < 0.05) in carious dentine it was necessary to use 5.0 g l−1 of curcumin in combination with blue light. The samples treated with curcumin and blue light, the PDT group (L + D+), showed a significantly greater reduction in bacterial numbers (p < 0.05) than any other group of all concentrations of curcumin (0.75; 1.5, 3.0, 4.0 and 5.0 g l−1). The exposure of the biofilm to 0.75; 1.5 and 3.0 g l−1 of curcumin and subsequent illumination with light resulted in 97.5; 95 and 99.9% reduction (p < 0.05) in viable cells, respectively. When the concentration of curcumin was 4.0 and 5.0 g l−1, a decrease of 100% was obtained (p < 0.05). S. mutans and L. acidophilus were sensitive to curcumin in the presence of blue light. It was also noted that without curcumin, light irradiation alone (L + D−) did not affect the viability of the microorganisms. When aPDT was applied in tooth decay in dentin, results showed that bacteria were more resistant to aPDT and a reduction in viable cells was observed with highest concentration of curcumin (5.0 g l−1). The authors suggested that when bacteria was located in a collagen matrix and carious dentin, the effects were less than in suspension, because the penetration of the PS was decreased, giving less binding to bacterial cells or light penetration through the biofilm was attenuated for photoactivating the dye.
Shrestha et al [46] examined the effect of antibiofilm polymeric chitosan nanoparticles containg rose Bengal. Bulit et al [47] evaluated the effect of three PSs on the viability of lactobacilli, odontoblast like cells, undifferentiated cells of the pulp, and human embryonic stem cells, and the bacteria were incubated for 15 min with curcumin, eosin Y, and/or rose Bengal and then irradiated with blue light (240 s) and stained with a LIVE/DEAD Viability kit for bacterial viability assessment. The results of Shresta showed that the nanoparticles were less toxic for fibroblasts and had high antibacterial activity; the photoactivated nanoparticles resulted in reducing the viability of E. faecalis biofilms and disruption of the biofilm structure. These results suggested that the nanoparticles with rose Bengal eliminated the bacteria and improved the chemical stability of the dentin organic matrix, to improve the removal of biofilms and restore the integrity of infected dentin tissue. The results of Bulit et al [47] indicated that curcumin was less effective than eosin Y and rose Bengal. This could be due the differences in electrical charges of the dyes that affect the ability to interact with the bacterial cell wall. This study showed that disinfection mediated by blue light is promising for the development of new treatment strategies for pulp repair that is exposed by decay.
Oliveira et al [48] evaluated, in vitro, the antimicrobial effect of aPDT using MB (50 μM) and low level laser (660 nm, 100 mW and 9 J) against C. albicans, P. aeruginosa, E. faecalis and S. aureus. Laser radiation in the presence of MB eliminated 74.90% for C. albicans, 72.41% for P. aeruginosa, 96.44 and 95.42%, respectively for E. faecalis and S. aureus and showed statistically significant differences among the different groups (p < 0.001). The results indicate that aPDT is effective in reducing the number of viable cells for the studied microorganisms, particularly E. faecalis and S. aureus. This occurred because this microorganisms are Gram-positive bacteria, that are more susceptible to the action of PDT compared to Gram-negative species, due to pores in the membrane formed by a thicker layer of peptidoglycan and lipoteichoic acid allowing greater diffusion of the PS into the cell.
Voos et al [45] compared the antibacterial efficacy of PDT using safranine O as a PS and chlorhexidine (CHX 0.2%) in an ex vivo biofilm model. Firstly, they assessed the antibacterial activity against planktonic cultures of S. gordonii, S. mutans, F. nucleatum, P. gingivalis and Aggregatibacter actinomycetemcomitans. From the plaque and saliva samples of the patients (n = 19) with chronic periodontitis, ex vivo biofilms were established by culture for 24 and 72 h and the colony forming units (CFU ml−1) were determined. They observed that oral pathogens in planktonic suspension could be significantly suppressed by PDT with safranin O, which was a more effective treatment method than 0.2% CHX. Neither of the antibacterial treatments showed any significant effect on biofilms grown for 72 h. The aPDT effect was investigated by the combination of a new broad spectrum of non-coherent visible light and low concentrations of curcumin and toluidine blue for suspensions of S. mutans. PDT promoted bacterial reduction >5 − log10 for both PSs tested in comparison with control groups (p < 0.05). The PS and the light source alone showed no antimicrobial effect and while the combination of a short exposure time to non-coherent light with low concentrations of PSs produced a lethal photoinactivation of S. mutans. This was considered by the authors as an in vitro antimicrobial approach effective in reducing the number of microorganisms involved in the process of dental caries.
Melo et al [55] investigated the effectiveness of photodynamic antimicrobial chemotherapy (PACT) as antimicrobial alternative to treat deep caries, using a LED (94 J cm−2; ~630 nm; 150 mW) combined with TBO (100 g ml−1) in a randomized clinical trial. Samples of dentin were collected before and immediately after treatments for microbiological analysis. The authors observed that no patients reported any sensitivity after the procedures. The results showed statistically significant reductions in S. mutans, Lactobacillus spp. and total viable bacteria. The group treated with aPDT had a reduction in log 1.69 to Lactobacillus and 1.08 to S. mutans, compared with the control group (5.18) in order to increase the antibacterial activity with PACT, alterations in parameters such as irradiation time and the concentration of dye or the pre-irradiation time were suggested as strategies for PACT in vivo. PACT could be a promising alternative for the treatment of deep caries lesions.
In the field of dentistry, PAD has been used as adjunct antimicrobial strategy to mechanical debridement during endodontic and periodontal treatment. In this context, Nielsen et al [56] investigated for the first time, the effect of PAD using riboflavin (266 μmol l−1) as a PS and blue LED light (460 nm) for activation (0.4 W; 37.7 J cm−2; 0.63 W cm−2; 1 min), comparing it to PAD using TBO and red light (630 nm). Limited microbial killing by PAD using riboflavin/blue light was found for Aggregatibacter actinomycetemcomitans, C. albicans, E. faecalis, Escherischia coli, Lactobacillus paracasei, P. gingivalis, P. intermedia and Propionibacterium acnes, which suggests that riboflavin cannot be recommended as PS for PAD of periodontal or endodontic infections, due to irradiation of riboflavin resulting in minor CFU reduction and moreover, lower ROS production. The irradiation time was chosen to be practicable in a dental clinical setting. On the other hand, TBO/red light completely killed the microorganisms.
Recently, in order to improve the uptake of PS by microorganisms during PDT approach, these molecules has been loaded, linked or encapsulated in a drug delivery system for widely different purposes [57, 58].
Junqueira et al [57] demonstrated that the utilization of functional polymeric systems composed of poloxamer 407, Carbopol 934P, and MB have a good capability for singlet oxygen generation in PDT. They demonstrated the formulation of a system with a suitable gelation temperature and good MB release, intended to be applied on the skin and/or oral mucous membranes. The system was tested using the following compositions: 17.5/0.20/0.50, 20/0.15/0.25 and 20/0.15/0.75, poloxamer 407/Carbopol 934P/MB respectively. The results showed that the systems 17.5/0.20/0.50 and 20/0.15/0.25 had good results for singlet oxygen generation. For gelation temperature, the system only needed to stay at the application site at the appropriate concentration, because it does not flow on the skin. Through regression analyses, they observed that poloxamer 407 had the largest effect on gelation temperature and demonstrated that the effect of the transition temperature was fundamental to selecting ideal formulation for application on the skin and mucous membrane. The system recommended by these authors (System 20 P407/0.15 C934P/0.25 MB) did not display any skin permeation and could produce ROS in a satisfactory amount for PDT. The use of this approach as coadjutant for oral infections is promising and should be investigated.
The research group of Dr Michael R Hamblin in the Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School, has demonstrated special interest in the use of self-assembled nano-drug carriers (micelles, liposomes, etc) for PDT approach. This group has emphasized that these new strategies can play an important role in the solubility of the PSs, metal nanoparticles can carry out plasmon resonance enhancement, and fullerenes can act as PSs, themselves. Regarding its application in the field of dentistry, it is expected that these nanomaterials will play an important role in restorative dentistry [59].
According to Souza et al [60], the literature contains a wide variety of protocols used for aPDT as a strategy for adjunctive treatment of aggressive periodontitis. Most of the studies have used MB [61], toluidine blue [62, 63], or phenothiazinium chloride [64] as PSs and diode lasers at wavelengths ranging from 660 to 690 nm as a light source. The number of sessions and the exposure time to aPDT is also variable and depends on the individual study design. Despite this, an approach that used 0.25 W cm−2 for 10 s per site in four sessions of aPDT divided over 15 days was found to be a better protocol for this purpose [64].
Meerovich et al [65], investigated the inactivation of planktonic and biofilm cultures of Gram-negative P. aeruginosa using synthetic bacteriochlorins with four and eight cationic groups. Bacterial biofilms were grown on glass slides for 20 h at 37 °C in wells, washed and exposed to PS in saline for 1 h at 37 °C. The biofilm was washed with fresh saline and irradiated using LPhD-03-Biospec arc lamp source with narrow-band filter (670–840 nm) or LED light source about 761 nm, both with power density of 10–20 mW cm−2. Viable cells were determined by counting of colony-forming unit (CFU). Effectiveness of aPDT was demonstrated by fluorescence microscopy using live/dead dye. The results showed that absorption spectra were similar for both PSs, and the comparison of the absorption and fluorescence spectra showed significant overlap. The authors observed that the intensity of bacterichlorin-8 fluorescence at high concentrations (>0.1 mM) showed a sublinear increase at longer wavelengths, while with bacteriochlorin-4, the sub-linearity started at lower concentrations (above 0.05 mM) suggesting that bacteriochlorin-4 does show some molecular aggregation. Antimicrobial photodynamic therapy was efficient against Gram-negative bacteria in the planktonic and biofilm phases using 0.005 mM and 8 J cm−2, with 4 log decrease (bacteriochlorin-4) and 5 log decrease (bacteriochlorin-8) for planktonic cells. Bacteriochlorin-8 was more efficient than bacteriochlorin-4.
Based on the information highlighted above, we can suggest that aPDT has potential application to combat many oral infections. Antimicrobial photodynamic therapy shows low local toxicity, can accelerate dental treatment, has a low cost, several PSs are available for each type of light source, and the treatment will not cause any harm to the patient. Thus, it is clear that there is a promising future for the use of aPDT to combat the microorganisms causing oral diseases.
6. Conclusion
Recent studies demonstrate that PDT is effective in decreasing cell viability of microbial cells and microbial biofilms and may be an important adjunct therapy to conventional technique for the treatment of several diseases in the dental context.
Acknowledgments
The authors would like to thank CAPES - Coordination for the Improvement of Higher Education Personnel, FAPEAM (Amazon State Research Foundation) and FAPESP (São Paulo Research Foundation, Brazil) for the following research grant (Process 2013/07276-1—CEPOF) and for the financial support for this study. Michael R Hamblin was supported by US NIH grant R01AI050875.
References
- 1.Tabenski L, Buchalla W, Maisch T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front Microbiol. 2014;5:1–17. doi: 10.3389/fmicb.2014.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections—state of the art. Photodiagnosis Photodyn Ther. 2009;6:170–88. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–50. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, Dai T, Hamblin MR. Antimicrobial photodynamic inactivation and photodynamic therapy for infections. Methods Mol Biol. 2010;635:155–73. doi: 10.1007/978-1-60761-697-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tortora GJ, Funke BR, Case CL. Microbiology: an Introduction. San Francisco: Pearson Benjamin Cummings; 2010. [Google Scholar]
- 6.Sharman WM, Allen CM, van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discovery Today. 1999;11:507–17. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 7.Hayek RR, et al. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J Periodontol. 2005;76:1275–81. doi: 10.1902/jop.2005.76.8.1275. [DOI] [PubMed] [Google Scholar]
- 8.Paschoal MA, Tonon CC, Spolidório DMP, Bagnato VS, Giusti JSM, Santos-Pinto L. Photodynamic potencial of curcumin and blue LED against S. mutans in a planktonic culture Photodiagnosis Photodyn. Ther. 2013;10:313–9. doi: 10.1016/j.pdpdt.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Pfitzner A, Sigusch BW, Albrecht V, Glockmann E. Killing of periodontopathogenic bacteria by photodynamic therapy. J Periodontol. 2004;75:1343–9. doi: 10.1902/jop.2004.75.10.1343. [DOI] [PubMed] [Google Scholar]
- 10.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38:468–81. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 11.Andersen R, Loebel N, Hammond D, Wilson M. Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J Clin Dent. 2007;18:34–8. [PubMed] [Google Scholar]
- 12.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39:1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 13.Soukos NS, Goodson JM. Photodynamic therapy in the control of oral biofilms. Periodontology 2000. 2011;55:143–66. doi: 10.1111/j.1600-0757.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- 14.de Melo WC, et al. Photodynamic inactivation of biofilm: taking a lightly colored approach to stubborn infection. Expert Ver Anti Infect Ther. 2013;11:669–93. doi: 10.1586/14787210.2013.811861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M, Hamblin MR. Type I and type II mechanisms of antimicrobial photodynamic therapy: an in vitro study on Gram-negative and Gram-positive bacteria. Lasers Surg Med. 2012;44:490–9. doi: 10.1002/lsm.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan Y, Lai CH. Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med Sci. 2003;18:51–5. doi: 10.1007/s10103-002-0243-5. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor AE, Gallagher WM, Byrne AT. Phorphyrin and nonporphyrin photosensitizers in oconology: preclinical and clinical advanves in photodynamic therapy. Photochem Photobiol. 2009;85:1053–74. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 18.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 19.Zanin ICJ, Gonçalves RB, Junior AB, Hope CK, Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J Antimicrob Chemother. 2005;56:324–30. doi: 10.1093/jac/dki232. [DOI] [PubMed] [Google Scholar]
- 20.Paschoal MA, Lin M, Santos-Pinto L, Duarte S. Photodynamic antimicrobial chemoterapy on Streptococcus mutans using curcumin and toluidine blue activated by a novel LED device. Lasers Med Sci. 2015;30:885–90. doi: 10.1007/s10103-013-1492-1. [DOI] [PubMed] [Google Scholar]
- 21.Dovigo LN, Pavarina AC, Carmello JC, Machado AL, Brunetti IL, Bagnato VS. Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg Med. 2011;43:927–34. doi: 10.1002/lsm.21110. [DOI] [PubMed] [Google Scholar]
- 22.Araujo NC, Fontana CR, Gerbi ME, Bagnato VS. Overall-mouth disinfection by photodynamic therapy using curcumin. Photomed Laser Surg. 2012;30:96–101. doi: 10.1089/pho.2011.3053. [DOI] [PubMed] [Google Scholar]
- 23.Sridharan G, Shankar AA. Toluidine blue: a review of its chemistry and clinical utility. J Oral Maxillofac Pathol. 2012;16:251–5. doi: 10.4103/0973-029X.99081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tardivo JP, Giglio AD, de Oliveira CS, Gabrielli DS, Junqueira HC, Tada DB, Divinomar S, Turchiello RF, Baptista MS. Methylene blue in photodynamic therapy: from basic mechanisms to clinical applications. Photodiagnosis Photodyn Ther. 2005;2:175–91. doi: 10.1016/S1572-1000(05)00097-9. [DOI] [PubMed] [Google Scholar]
- 25.Wilson M, Dobson J, Harvey W. Sensitization of oral bacteria to killing by low-power laser radiation. Curr Microbiol. 1992;25:77–81. doi: 10.1007/BF01570963. [DOI] [PubMed] [Google Scholar]
- 26.Burns T, Wilson M, Pearson GJ. Killing of cariogenic bacteria by light from a gallium aluminium arsenide diode laser. J Dent. 1994;22:273–8. doi: 10.1016/0300-5712(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 27.Souza RC, Junqueira JC, Rossoni RD, Pereira CA, Munin E, Jorge AO. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against C albicans. Lasers Med Sci. 2010;25:385–9. doi: 10.1007/s10103-009-0706-z. [DOI] [PubMed] [Google Scholar]
- 28.Allaker RP, Douglas CW. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents. 2009;33:8–13. doi: 10.1016/j.ijantimicag.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006;57:680–4. doi: 10.1093/jac/dkl021. [DOI] [PubMed] [Google Scholar]
- 30.Fickweiler S, Abels C, Karrer S, Baumler W, Landthaler M, Hofstadter F, Szeimies RM. Photosensitization of human skin cell lines by ATMPn (9-acetoxy-2,7,12,17-tetrakis-(beta-methoxyethyl)-porphycene) in vitro: mechanism of action. J Photochem Photobiol B. 1999;48:27–35. doi: 10.1016/S1011-1344(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 31.Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74:656–69. doi: 10.1562/0031-8655(2001)074<0656:thopap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J Med Chem. 2004;47:3897–915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 33.Karmakova T, et al. Tissue distribution and in vivo photo-sensitizing activity of 13,15-[N-(3-hydroxypropyl)]cycloimide chlorin p6 and 13,15-(N-methoxy)cycloimide chlorin p6 methyl ester. J Photochem Photobiol B. 2006;82:28–36. doi: 10.1016/j.jphotobiol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Nuñez SC, Yoshimura TM, Ribeiro MS, Junqueira HC, Maciel C, Coutinho-Neto MD, Baptista MS. Urea enhances the photodynamic efficiency of methylene blue. J Photochem Photobiol B. 2015;150:31–7. doi: 10.1016/j.jphotobiol.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Alvarenga LH, et al. Aggregatibacter actinomycetemcomitans biofilm can be inactivated by methylene blue-mediated photodynamic therapy. Photodiagnosis Photodyn Ther. 2015;12:131–5. doi: 10.1016/j.pdpdt.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Reddy GK. Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg. 2004;22:141–50. doi: 10.1089/104454704774076208. [DOI] [PubMed] [Google Scholar]
- 37.Puri K, Puri N. Local drug delivery agents as adjuncts to endodontic and periodontal therapy. J Med Life. 2015;6:414–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Castro JFL, Henrique ACG, Lira CC, Santos RNA, Matos JAB, Nascimento SC. Effect of laser therapy on laryngeal carcinoma cell proliferation. Braz Res Pediatr Dent Integ Clinic. 2014;14:275–82. [Google Scholar]
- 39.Belkin M, Schwartz M. New biological phenomena associated with laser radiation. Health Phys. 1989;56:687–90. doi: 10.1097/00004032-198905000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Burns T, Wilson M, Pearson GJ. Effect of dentine and collagen on the lethal photosensitization of. S mutans Caries Res. 1995;29:192–7. doi: 10.1159/000262068. [DOI] [PubMed] [Google Scholar]
- 41.Marrelli M, Menichini G, Provenzano E, Conforti F. Applications of natural compounds in the photodynamic therapy of skin cancer. Curr Med Chem. 2014;21:1371–90. doi: 10.2174/092986732112140319094324. [DOI] [PubMed] [Google Scholar]
- 42.Moreno E, Schwartz J, Fernandez C, Sanmartin C, Nguewa P, Irache JM, Espuelas S. Nanoparticles as multifunctional devices for the topical treatment of C. leishmaniasis. Expert Opin Drug Deliv. 2014;11:579–97. doi: 10.1517/17425247.2014.885500. [DOI] [PubMed] [Google Scholar]
- 43.Alves F, Mima EG, Dovigo LN, Bagnato VS, Jorge JH, Costa CAS, Pavarina AC. The influence of photodynamic therapy parameters on the inactivation of Candida spp: in vitro and in vivo studies. Laser Phys. 2014;24:045601. [Google Scholar]
- 44.Araujo NC, Fontana CR, Bagnato VS, Gerbi ME. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med Sci. 2014;29:629–35. doi: 10.1007/s10103-013-1369-3. [DOI] [PubMed] [Google Scholar]
- 45.Voos AC, Kranz S, Tonndorf-Martini S, Voelpel A, Sigusch H, Staudte H, Albrecht V, Sigusch BW. Photodynamic antimicrobial effect of safranine O on an ex vivo periodontal biofilm. Lasers Surg Med. 2014;46:235–43. doi: 10.1002/lsm.22217. [DOI] [PubMed] [Google Scholar]
- 46.Shrestha A, Hamblin MR, Kishen A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine. 2014;10:491–501. doi: 10.1016/j.nano.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bulit F, et al. Antimicrobial activity and cytotoxicity of 3 photosensitizers activated with blue light. J Endod. 2014;40:427–31. doi: 10.1016/j.joen.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira BPD, Lins CCDSA, Diniz FA, Melo LL, Castro CMMBD. In vitro antimicrobial photoinactivation with methylene blue in different microorganisms. Braz J Oral Sci. 2014;13:53–7. [Google Scholar]
- 49.Schneider M, Kirfel G, Berthold M, Frentzen M, Krause F, Braun A. The impact of antimicrobial photodynamic therapy in an artificial biofilm model. Lasers Med Sci. 2012;27:615–20. doi: 10.1007/s10103-011-0998-7. [DOI] [PubMed] [Google Scholar]
- 50.Qin YL, Luan XL, Bi LJ, Sheng YQ, Zhou CN, Zhang ZG. Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J Periodontal Res. 2008;43:162–7. doi: 10.1111/j.1600-0765.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 51.Sigusch BW, Pfitzner A, Albrecht V, Glockmann E. Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol. 2005;76:1100–5. doi: 10.1902/jop.2005.76.7.1100. [DOI] [PubMed] [Google Scholar]
- 52.Meisel P, Kocher T. Photodynamic therapy for periodontal diseases: state of the art. J Photochem Photobiol B. 2005;79:159–70. doi: 10.1016/j.jphotobiol.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 53.de Oliveira RR, Schwartz-Filho HO, Novaes AB, Jr, Taba M., Jr Antimicrobial photodynamic therapy in the non surgical tretment of aggressive periodontitis: a preliminary randomized controlled clinical study. J Periodontol. 2007;78:965–73. doi: 10.1902/jop.2007.060494. [DOI] [PubMed] [Google Scholar]
- 54.Fontana CR, et al. The antibacterial effect of photodynamic therapy in dental plaque derived biofilms. J Periodontal Res. 2009;44:751–9. doi: 10.1111/j.1600-0765.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melo MAS, Rolim JPML, Passos VF, Lima RA, Zanin ICJ, Codes MB, Rocha SS, Rodrigues LKA. Photodynamic antimicrobial chemotherapy and ultra-conservativecaries removal linked for management of deep caries lesions. Photodiagnosis Photodyn Ther. 2015;12:581–6. doi: 10.1016/j.pdpdt.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen HK, Garcia J, Vaeth M, Schlafer S. Comparision of riboflavin and toluidine blue O as photosensitizers for photoactivated disinfection on endodontic and periodontal pathogens in vitro. PLoS One. 2015;10:1–11. doi: 10.1371/journal.pone.0140720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Junqueira MV, Borghi-Pangoni FB, Ferreira SBS, Rabello BR, Hioka N, Bruschi M. Functional polymeric systems as delivery vehicles for methylene blue in photodynamic therapy. Langmuir. 2016;32:19–27. doi: 10.1021/acs.langmuir.5b02039. [DOI] [PubMed] [Google Scholar]
- 58.Ding Y, Zhou L, Chen X, Wu Q, Song Z, Wei S, Zhou J, Shen J. Mutual sensitization mechanism and self-degradation property of drug delivery system for in vitro photodynamic therapy. Int J Pharm. 2016;498:335–46. doi: 10.1016/j.ijpharm.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 59.Hamblin MR, Chiang LY, Lakshmanan S, Huang YY, Diaz MG, Karimi M, Rastelli ANS, Chandran R. Nanotechnology for photodynamic therapy: a perspective from the laboratory of Dr Michael R Hamblin in the wellman center for photomedicine at Massachusetts general hospital and Harvad medical school. Nanotechnol Rev. 2015;4:359–72. doi: 10.1515/ntrev-2015-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Souza E, Medeiros AC, Gurgel BC, Sarmento C. Antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: a systematic review and meta-analysis. Lasers Med Sci. 2016;31:187–96. doi: 10.1007/s10103-015-1836-0. [DOI] [PubMed] [Google Scholar]
- 61.Garcia FB, Dias AT, Tinoco BEM, Fischeer RG. Evaluation of the efficacy of photodynamic therapy as adjunct to periodontal treatment in patients with aggressive periodontitis. Rev Periodontia. 2011;21:12–9. [Google Scholar]
- 62.Arweiler NB, Pietruska M, Pietruski J, Skurska A, Dolinska E, Heumann C, Auschill TM, Sculean A. Six-month results following treatment of aggressive periodontitis with antimicrobial photodynamic therapy or amoxicillin and metronidazole. Clin Oral Investig. 2014;18:2129–35. doi: 10.1007/s00784-014-1193-6. [DOI] [PubMed] [Google Scholar]
- 63.Chitsazi MT, Shirmohammadi A, Pourabbas R, Abolfazli N, Farhoudi I, Azar D, Farhadi F. Clinical and microbiological effects of photodynamic therapy associated with non-surgical treatment in aggressive periodontitis. J Dent Res Dent Clin Dent Prospects. 2014;8:153–9. doi: 10.5681/joddd.2014.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreira AL, et al. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: a split-mouth randomized controlled trial. J Periodontol. 2015;86:376–86. doi: 10.1902/jop.2014.140392. [DOI] [PubMed] [Google Scholar]
- 65.Meerovich GA, et al. Photodynamic inactivation of bacteria and biofilms using cationic bacteriochlorins. J Phys: Conf Ser. 2016;691:1–7. [Google Scholar]


