Abstract
Ketamine has been used as a pharmacological model for schizophrenia, as sub-anesthetic infusions have been shown to produce temporary schizophrenia-like symptoms in healthy humans. More recently, ketamine has emerged as a potential treatment for multiple psychiatric disorders, including treatment-resistant depression and suicidal ideation. However, the mechanisms underlying both the psychotomimetic and therapeutic effects of ketamine remain poorly understood. This review provides an overview of what is known of the neural mechanisms underlying the effects of ketamine and details what functional magnetic resonance imaging studies have revealed at a systems level focused on brain circuitry. Multiple analytic approaches show that ketamine produces robust and consistent effects at the whole-brain level. These effects are highly conserved across human and nonhuman primates, validating the use of nonhuman primate models for further investigations with ketamine. Regional analysis of brain functional connectivity suggests that the therapeutic potential of ketamine may be derived from a strengthening of executive control circuitry, making it an intriguing candidate for the treatment of drug abuse. There are still important questions about the mechanism of action and therapeutic potential of ketamine that can be addressed using appropriate functional neuroimaging techniques.
Keywords: Ketamine infusion, Pharmacological imaging, Functional connectivity, Dorsolateral prefrontal cortex
Introduction
Ketamine is a non-competitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist with a complex profile of pharmacological effects that has made it an important target in biomedical and neuroscience research. High doses of ketamine have long been used medically to produce anesthesia (Haas and Harper, 1992) and the recreational use of ketamine as a dissociative drug of abuse also has a lengthy history (Lodge and Mercier, 2015). For the past two and a half decades, ketamine has been used as a pharmacological model for schizophrenia, as sub-anesthetic doses have been shown to produce temporary schizophrenia-like symptoms in healthy humans (Krystal et al., 1994; Olney and Farber, 1995). In rodents and nonhuman primates, sub-anesthetic doses of ketamine induce deficits in the startle response and working memory that have translational relevance to schizophrenia (Skoblenick and Everling, 2012; Verma and Moghaddam, 1996; Yang et al., 2010). While ketamine can be administered through several routes, including intramuscular, intranasal and oral, most investigations have utilized intravenous infusions, owing to the precise dosing and ability to adjust rapidly if unwanted side effects occur. The strength of these models has helped lead to new hypotheses of glutamatergic system dysfunction in schizophrenia (Frohlich and Van Horn, 2014).
In addition to its utility for modeling schizophrenia, ketamine has emerged as a potential treatment for multiple psychiatric disorders. Sub-anesthetic doses of ketamine in the same range as those used for modeling schizophrenia have shown efficacy for treating postoperative pain (Schmid et al., 1999), neuropathic pain (Schwartzman et al., 2009), treatment-resistant depression (Berman et al., 2000; Krystal et al., 2013), and suicidal ideation (Ballard et al., 2014; Price and Mathew, 2015). Indeed, the rapid onset of improvement in suicidal ideation induced by ketamine, reported to emerge as quickly as 40 minutes post-infusion (DiazGranados et al., 2010), provides a major advantage for treating this psychiatric emergency, as other effective treatments are slower acting (Reinstatler and Youssef, 2015). Ketamine also exerts rapid antidepressant effects in treatment resistant depression, with peak response reported within 24 hours after a single sub-anesthetic dose (Murrough et al., 2013; Zarate et al., 2006). Additionally, recent studies have begun to investigate the potential use of ketamine as a treatment for drug addiction (Dakwar et al., 2014, 2016).
Neural Mechanisms
The discovery of the remarkable behavioral effects of sub-anesthetic ketamine has led to a great deal of research investigating its underlying neural mechanisms. High doses of ketamine result in general suppression of the central nervous system and produce general anesthesia (Table 1). However, at sub-anesthetic doses that produce psychotomimetic and rapid antidepressant effects (Table 1), ketamine administration leads to enhancement of excitatory glutamatergic transmission (Duncan et al., 1998; Moghaddam et al., 1997). There is growing evidence that sub-anesthetic doses of ketamine predominantly block the NMDA receptors on inhibitory interneurons (Homayoun and Moghaddam, 2007; Wang et al., 2013), resulting in a disinhibition of excitatory projection neurons (Maeng et al., 2008). The reason for ketamine to primarily inhibit interneurons remains unknown. It has been proposed that the tonic firing pattern displayed by many cortical interneurons likely removes the magnesium block from NMDA receptors, allowing ketamine to block the channel. Meanwhile, burst firing pyramidal neurons likely spend more time in magnesium block, reducing the probability that ketamine will block NMDA receptor channels on these excitatory neurons (Wang and Gao, 2009, 2012).
Table 1.
Translational comparison of sub-anesthetic ketamine doses shown to produce psychotomimetic effects, antidepressant effects, and BOLD activation. Typical ketamine doses used for anesthesia are also shown.
| Common active sub-anesthetic ketamine doses (plasma concentration) |
Anesthetic ketamine dose (plasma concentration) |
|
|---|---|---|
|
| ||
| Human | ~0.5 mg/kg I.V. over 40 minutes | ~1–2 mg/kg I.V. |
| (50–250 ng/mL)a,b | (>1,080 ng/mL)d | |
|
| ||
| Rhesus Monkey | ~1 mg/kg I.M. | ~10 mg/kg I.M. |
| (100–200 ng/mL) c | ||
|
| ||
| Rat | ~10 mg/kg I.P. | ~100 mg/kg I.P. |
| (100–300 ng/mL)c | (>2,000 ng/mL)e | |
Ketamine-induced enhancement of glutamatergic function can have significant downstream effects on mesocortical and mesolimbic dopamine pathways (Adams and Moghaddam, 1998; Lorrain et al., 2003; Moghaddam et al., 1997; Vollenweider et al., 2000). Specifically, there is evidence that disinhibition of pyramidal projection neurons in the prefrontal cortex leads to downstream activation of dopaminergic neurons (Carr and Sesack, 2000; Del Arco et al., 2008; Takahata and Moghaddam, 2003). Moreover, sub-anesthetic ketamine infusions increase blood flow and metabolic activity in the prefrontal cortex, striatum and thalamus (Holcomb et al., 2001; Vollenweider et al., 1997) and these effects correlate with the emergence of dissociative and schizophrenia-like symptoms (Driesen et al., 2013a; Holcomb et al., 2001; Vollenweider et al., 1997). Thus, while sub-anesthetic ketamine induces excitation in many brain areas, its effects on prefrontal circuitry may be of particular importance for the induction of psychotomimetic and antidepressant effects (Arnsten et al., 2012; Del Arco and Mora, 2009; Opler et al., 2016). In this regard, the homology of the human prefrontal cortex to that of other primates (Phillips et al., 2014) might add significant translational value to the use of nonhuman primate models for investigating the effects of ketamine. Indeed, even the micro-circuitry within the prefrontal cortex appears to be well conserved across human and nonhuman primates, with NMDA receptors playing an important role in local processing that may not be present in rodents (Wang and Arnsten, 2015). Accordingly, the current review focuses on pharmacological imaging and ketamine effects on brain circuitry with an emphasis on human and nonhuman primate studies.
Pharmacological Imaging Methods
The neuronal signaling changes induced by administration of sub-anesthetic ketamine can be measured using pharmacological magnetic resonance imaging (phMRI). Increases in neuronal signaling lead to increases in metabolic rate as well as cerebral blood flow (CBF) to deliver more oxygen and glucose to meet the increased demand. The increases in CBF and anaerobic glucose metabolism are greater than the increase in oxidative metabolic rate (Fox et al., 1988), leading to a higher blood oxygen concentration and resultant changes in the blood-oxygenation-level dependent (BOLD) signal (Simon and Buxton, 2015). Measurement of the BOLD signal is utilized in phMRI to quantify the effects of drugs on neuronal signaling (Leslie and James, 2000). Previous work has shown that during sub-anesthetic infusion of ketamine, increases in regional CBF (Langsjo et al., 2003) remain coupled with increases in regional glucose metabolic rate (Langsjo et al., 2004). This provides strong evidence that ketamine-induced changes to the BOLD signal accurately reflect underlying changes to neuronal signaling, making phMRI an effective tool for measuring the effects of ketamine on neural networks in the brain. Indeed, phMRI is particularly well-suited for investigating the effects of ketamine at the regional and network level, given its high spatial and temporal resolution compared to other whole-brain imaging modalities such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) (Buxton, 2002). This review will discuss key findings from phMRI studies that have contributed to understanding the effects of ketamine on brain function at the whole-brain, regional, and network levels. All of the phMRI studies reviewed (Table 2) utilized measurements of BOLD signal; however, multiple data analysis methods were employed. Brain activation studies examined regional changes in neuronal firing induced by ketamine, while functional connectivity studies examined changes to functional connections between discrete brain regions.
Table 2.
Dosing procedures for ketamine phMRI studies.
| Study | Sub-anesthetic ketamine dose (plasma concentration, if available) |
Duration of infusion |
Duration of scan |
N Subjects (Condition) |
Analysis Methods |
|---|---|---|---|---|---|
|
| |||||
| (De Simoni et al., 2013) | Low: 0.08 mg/kg + 0.23 mg/kg/hr I.V. | 75 min | 15 min | 10 | BOLD activation |
| (target of 50 ng/mL) | Human | ||||
| High: 0.12 mg/kg bolus + 0.31 mg/kg/hr I.V. | (Healthy Control) | ||||
| (73 ng/mL) | |||||
|
| |||||
| Doyle et al. (2013) | 0.12 mg/kg bolus + 0.31 mg/kg/hr I.V. | 75 min | 15 min | 16 | BOLD activation |
| (63 ng/mL) | Human | ||||
| (Healthy Control) | |||||
|
| |||||
| Deakin et al. (2008) | 0.26 mg/kg bolus + 0.25 mg/kg/hr I.V. | 8 min | 16 min | 33 | BOLD activation |
| Human | |||||
| (Healthy Control) | |||||
|
| |||||
| Downey et al. (2016) | 0.5 mg/kg constant infusion I.V. | 60 min | 45 min | 19 | BOLD activation |
| Human | |||||
| (Major Depressive Disorder) | |||||
|
| |||||
| Driesen et al. (2013a); (Driesen et al., 2013b) | 0.23 mg/kg bolus + 0.58 mg/kg/hr I.V. | 45 min | 2 hr | Human | (a) GBC |
| (162 ng/mL) | (Healthy Control) | (b) Seed-based functional connectivity | |||
|
| |||||
| (Abdallah et al., 2016) | 0.5 mg/kg constant infusion I.V. | 40 min | 4 min (before, and 24 hrs after, infusion) | Human | GBC and Seed-based functional connectivity |
| (N=18 Major Depressive Disorder) | |||||
| (N=25 Healthy Control) | |||||
|
| |||||
| (Anticevic et al., 2015) | 0.23 mg/kg bolus + 0.58 mg/kg/hr I.V. | 1 hr | 4.15 min | 96 | GBC |
| (121 ng/mL) | Human | ||||
| (Healthy Control) | |||||
|
| |||||
| (Joules et al., 2015) | 0.12 mg/kg bolus + 0.31 mg/kg/hr | 75 min | 15 min | 16 | Graph network analysis |
| (63 ng/mL) | (5 min pre-infusion) | Human | |||
| (Healthy Control) | |||||
|
| |||||
| (Dandash et al., 2015) | Bolus + automated I.V. infusion targeting 100 ng/mL | 15 min | 10 min | 19 | Seed-based functional connectivity |
| Human | |||||
| (69 ng/mL) | (Healthy Control) | ||||
|
| |||||
| (Grimm et al., 2015) | Humans: 0.5 mg/kg I.V. | Single bolus | Humans: 10 min | Humans: N=24 | Seed-based functional connectivity |
| (365 ng/mL) | (Healthy Control) | ||||
| Rats: 25 mg/kg S.C. | Rats: N=9 | ||||
| (1700 ng/mL) | Rats: 8.5 min | ||||
|
| |||||
| Gopinath et al. (2016) | 0.345 mg/kg bolus + 0.256 mg/kg/hr I.V. | 53 min | 55 min | 4 | Seed-based functional connectivity |
| (104 ng/mL) | Nonhuman primate | ||||
|
| |||||
| Maltbie et al. (2016) | 0.345 mg/kg bolus + 0.256 mg/kg/hr I.V. | 53 min | 55 min | 4 | BOLD activation |
| (104 ng/mL) | Nonhuman primate | ||||
|
| |||||
| (Chin et al., 2011) | 30 mg/kg I.P. | Single bolus | 40 min | 5 | BOLD activation |
| Rat | |||||
Brain activation
Several studies have used phMRI to characterize regional changes in neuronal activity induced by sub-anesthetic ketamine infusion, by measuring the BOLD activation response. Data acquisition begins prior to the administration of ketamine to establish a baseline signal and continues for at least several minutes beyond the start of ketamine infusion. Various ketamine infusion protocols have been used (Table 2) in human (Deakin et al., 2008), nonhuman primate (Maltbie et al., 2016), and rat (Chin et al., 2011) studies, with each showing a significant ketamine-induced BOLD-response that appears to be proportional to the ketamine dose (De Simoni et al., 2013) and highly robust across species (Maltbie et al., 2016). Sub-anesthetic ketamine induces a reliable pattern of regional increases in BOLD signal indicative of neuronal excitation, which is consistent with the ketamine-induced increases observed in regional glucose metabolism (Duncan et al., 1998; Langsjo et al., 2004). This acute regional BOLD response to sub-anesthetic ketamine may be linked to its psychotomimetic effects.
Functional connectivity
The BOLD signal exhibits spontaneous fluctuations associated with temporal patterns of neuronal network activity during rest. Correlations in these spontaneous signal fluctuations between discrete regions are termed functional connectivity and are thought to underlie communication within brain networks (Fox et al., 2005). Functional connectivity has been used in many different clinical applications (Fox and Greicius, 2010), including studies to evaluate the effects of sub-anesthetic ketamine. Functional connectivity analysis can offer some advantages compared to brain activation studies. Meaningful dynamic alterations in the strength of coupling between discrete regions may be independent from any corresponding increases in overall signal strength (Buckner et al., 2013), and network connectivity patterns within individual subjects are highly consistent across scanning sessions (Braga and Buckner, 2017). Although there are many different ways to perform functional connectivity analysis, the two types of functional connectivity analysis that have been most commonly used to study the effects of ketamine infusion are seed-based analysis and global brain connectivity (GBC).
Seed-based functional connectivity
Seed-based functional connectivity analysis utilizes a region-of-interest approach to calculate functional connectivity between specific regions. This technique compares the average time course of the BOLD signal within a specified seed region with the BOLD time course of every brain voxel outside of the seed region (or within a specified target region), usually by means of the cross-correlation coefficient (CC) between respective time courses (Fox and Raichle, 2007). This is the most common type of functional connectivity analysis used for determining brain networks. Changes to seed-based functional connectivity observed during acute ketamine administration (Dandash et al., 2015; Gopinath et al., 2016) may be related to psychotomimetic effects, while persistent changes observed 24-hours post-infusion (Abdallah et al., 2016; Lv et al., 2016) may be more closely related to antidepressant effects.
Global brain connectivity
GBC is a measure of how connected a given brain area is to every other area in the brain. High values of GBC (at rest) occur in brain areas that are involved in many different brain functions, and hence are connected with multiple brain networks or are strongly connected to most of the other brain regions within one network. GBC is calculated by taking the average correlation between the BOLD time course in a given voxel and the BOLD time course of every other voxel in the brain (Cole et al., 2010). GBC can also be compartmentalized to global connectivity within large brain areas (e.g. frontal cortex) as done by Anticevic et al. (2015). Alterations in GBC have been associated with both schizophrenia (Cole et al., 2011) and acute ketamine administration (Driesen et al., 2013a) and have been investigated in relation to the psychotomimetic effects of ketamine infusion. Further, Abdallah et al. (2016) observed alterations in GBC in subjects with major depressive disorder, which were normalized 24hours after ketamine infusion in responders.
Other functional connectivity analysis methods
Independent component analysis and graph network analysis (Joules et al., 2015; Lv et al., 2016) have also been used to investigate the effects of sub-anesthetic ketamine. These analysis methods use algorithms to automatically segment the brain into intrinsic, functionally connected networks (Braga and Buckner, 2017). This allows for data-driven investigation of functional brain networks, which has both advantages and disadvantages compared to more directly hypothesis driven methods (Buckner et al., 2013). At present, there is smaller body of literature utilizing these methods, making it difficult to correlate the imaging data with the behavioral effects of ketamine.
Pharmacological Imaging and the Behavioral Effects of Ketamine
Psychotomimetic effects
Several phMRI studies have investigated associations between the changes in neuronal signaling and changes in subjective ratings of behavior induced by ketamine. Various subjective ratings scales have been utilized for this purpose. In order to measure psychosis-like symptoms, the Brief Psychiatric Rating Scale (BPRS) (Kopelowicz et al., 2008), Rating Scale for Psychotic Symptoms (RSPS) (Chouinard and Miller, 1999), Psychotomimetic States Inventory (PSI) (Mason et al., 2008), and positive dimension of the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) have been employed. Meanwhile, the negative dimension of PANSS (Kay et al., 1987) has been used to measure the schizophrenia-like blunted affect and social withdrawal, and the Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998) has been used to assess the dissociative symptoms evoked by ketamine.
Brain activation
Deakin et al. (2008) were the first to examine the effects of sub-anesthetic ketamine infusion on BOLD activation (in healthy control subjects). They found an extensive cortical BOLD signal response, with peak signal changes occurring 3–5 minutes after the start of infusion in all regions. This timing corresponds very well with the peak ketamine concentration in the blood, and with the onset of behavioral effects (see below). BOLD activation was quite extensive, with multiple frontal, parietal, temporal, and limbic regions showing increased signal. When subjects were pretreated with the sodium channel blocker lamotrigine to reduce enhancement of glutamate release, there was significant attenuation of ketamine-induced BOLD activation throughout the brain. This provides evidence that enhancement of glutamatergic signaling is involved in the BOLD-response to ketamine, and agrees with previous work showing that the behavioral effects of ketamine can also be attenuated by lamotrigine (Anand et al., 2000).
Deakin et al. (2008) further found several regions in which changes in BOLD were correlated with ratings of dissociative state (CADSS) or psychotic symptoms (BPRS), thus establishing the relevance of BOLD activation to the psychotomimetic effects of ketamine. Deactivation in the subgenual cingulate and medial orbitofrontal cortex (OFC) was correlated with increased CADSS ratings in the subjects, while deactivation in the medial OFC also correlated with increases in both CADSS ratings and BPRS ratings for psychosis. Activation of the posterior cingulate cortex and frontal pole (BA10) were also correlated with increased BPRS ratings, but not with CADSS ratings.
The test-retest reliability of ketamine-induced BOLD activation was later established by De Simoni et al. (2013). They found the BOLD response to ketamine to be very robust, featuring a consistent magnitude and time course across different sessions, and both within and across healthy control subjects. Further, De Simoni et al. (2013) investigated the dose dependence of ketamine-induced BOLD activation and found that a higher dose of ketamine (producing a 75 ng/mL ketamine blood serum concentration compared to 50 ng/mL at the lower dose) corresponded to greater changes in BOLD signal and greater effect sizes. However, a full ketamine dose-response function has yet to be established with BOLD activation. While De Simoni et al. (2013) also collected behavioral ratings for psychotomimetic (PSI) and dissociative states (CADSS), they did not find any correlations with BOLD activation. They inferred that the lack of correlation with behavior may be due to the low doses they were using for the ketamine infusion. Indeed, the higher of their two doses only produced an average plasma concentration of 73 ng/mL, a concentration that is at the low end of what has been used in phMRI studies of ketamine (Table 2). Despite the low dosing, they still found robust BOLD activation, indicating htat phMRI is highly sensitive to the effects of ketamine.
Doyle et al. (2013) were the first to test the interaction of an antipsychotic drug with the ketamine-induced BOLD response. They evaluated pretreatment with risperidone or lamotrigine on ketamine-induced brain activation in healthy control subjects. Clinically, risperidone is one of the most commonly prescribed antipsychotics, featuring similar efficacy and tolerability to other second-generation (“atypical”) antipsychotics used for the treatment of schizophrenia (Komossa et al., 2011). Risperidone is an antagonist at both dopamine D2 and serotonin 5-HT2A receptors, but with no affinity for any glutamate receptor (Muly et al., 2012). Doyle et al. (2013) showed that risperidone attenuated the BOLD response to ketamine globally, blunting signal changes in frontal, insular, striatal, and thalamic regions. Despite this attenuation, ketamine still induced significant BOLD activation compared to saline in each of these areas following risperidone pretreatment. The magnitude of the activation was simply reduced. This indicates that glutamatergic signaling is not the only pathway involved in the BOLD response to ketamine and that dopamine D2 and serotonin 5-HT2A receptors may also be involved. Among these alternatives, the 5-HT2A antagonist effects of risperidone are a more likely mechanism in attenuating the effects of ketamine, given that the dopamine D2 antagonist haloperidol was ineffective in preventing the psychotomimetic effects of ketamine (Krystal et al., 1999). Note that neurons expressing the 5-HT2A receptor in the prefrontal cortex project to the ventral tegmental area and local antagonism of these receptors blocks dopamine overflow in the prefrontal cortex (Bortolozzi et al., 2005). Hence, 5-HT2A receptors are well positioned anatomically to modulate the psychotomimetic effects of ketamine.
Preclinical animal studies have also used phMRI to characterize the effects of ketamine on brain activation. Careful consideration is required for designing phMRI studies in animals (Steward et al., 2005). In particular, drugs used to anesthetize the animals may interact with the compound of interest (Haensel et al., 2015), as seen with both ketamine (Hodkinson et al., 2012) and phenylcyclidine (Gozzi et al., 2008). These complications can make studies in anesthetized animals difficult to interpret. Methods have been developed for performing phMRI in awake rodents (King et al., 2005). Chin et al. (2011) studied ketamine-evoked BOLD activation in awake rats and found extensive activation in the cortex and hippocampus. This result aligns well with observations reported in human subjects. Methods have also been developed that enable rhesus monkeys to undergo MRI scanning without the use of anesthesia and with minimal restraint stress (Murnane and Howell, 2010). A recent study has shown that awake rhesus monkeys display ketamine-induced BOLD activation (Maltbie et al., 2016) that corresponds closely in both magnitude and extent to what has been reported in humans (De Simoni et al., 2013; Deakin et al., 2008; Doyle et al., 2013). Moreover, pretreatment with risperidone attenuated the ketamine-induced changes in BOLD (Maltbie et al., 2016) to a similar extent as reported in humans (Doyle et al., 2013). These data suggest that the pharmacological effects of ketamine are well conserved across species and attest to the validity of using ketamine in animal models of schizophrenia. Indeed, further preclinical phMRI studies could prove invaluable, particularly when conducted in awake subjects.
Global brain connectivity
Functional connectivity has been shown reliably to be disrupted in schizophrenia (Guo et al., 2013; Meyer-Lindenberg et al., 2001, 2005; Rotarska-Jagiela et al., 2010; Woodward et al., 2011). Further, there is evidence that functional connectivity may predict response to treatment with antipsychotics (Sarpal et al., 2016) and that functional connectivity changes are correlated with alleviation of symptoms following successful treatment (Sarpal et al., 2015).
The first paper to use functional connectivity analysis to study ketamine infusion was published by Driesen et al. (2013a). They examined the effects of ketamine on GBC in healthy control subjects. Ketamine infusion increased the GBC of voxels throughout the brain, illustrating a global increase in functional connectivity. This finding is consistent with coherent neural activity across the brain seen during psychosis (Hakami et al., 2009; Wood et al., 2012). Additionally, Driesen et al. (2013a) found many brain regions (they reported several significant clusters including one extending from the OFC to the cerebellum) in which increased GBC correlated with increased positive schizophrenia symptom scores (PANSS), implying that increased connectivity in many brain networks is associated with the psychotomimetic effects of ketamine. In contrast, they found that stable or reduced GBC in the dorsal and medial anterior striatum was correlated with increased negative psychotomimetic symptoms (PANSS).
Anticevic et al. (2015) compared the effects of ketamine in healthy control subjects to baseline measures from schizophrenic patients at different stages of treatment. They utilized a variant of GBC in which only voxels within the prefrontal cortex were considered. The restricted GBC was shown to increase after ketamine administration and this prefrontal-specific GBC was also significantly elevated in patients who were within one-year of the onset of schizophrenia symptoms. This finding may suggest that elevated functional connectivity in the prefrontal cortex could be a biomarker for schizophrenia. However, other than benefitting from decreased processing time, it is unclear why GBC should be restricted to prefrontal regions. Even if prefrontal regions are of primary interest, these areas receive inputs from many other brain areas outside the prefrontal cortex and are part of highly integrative brain circuits (Arnsten et al., 2012; Del Arco and Mora, 2009). Furthermore, Anticevic et al. (2015) performed global signal regression to remove the average brain signal from every voxel in the brain. Given that ketamine increases global signal, as reported previously, the effects of this regression will be different for ketamine than for baseline or vehicle conditions and could confound any between-condition comparisons (Saad et al., 2012). Thus, while the results of this study are intriguing, they are somewhat difficult to interpret.
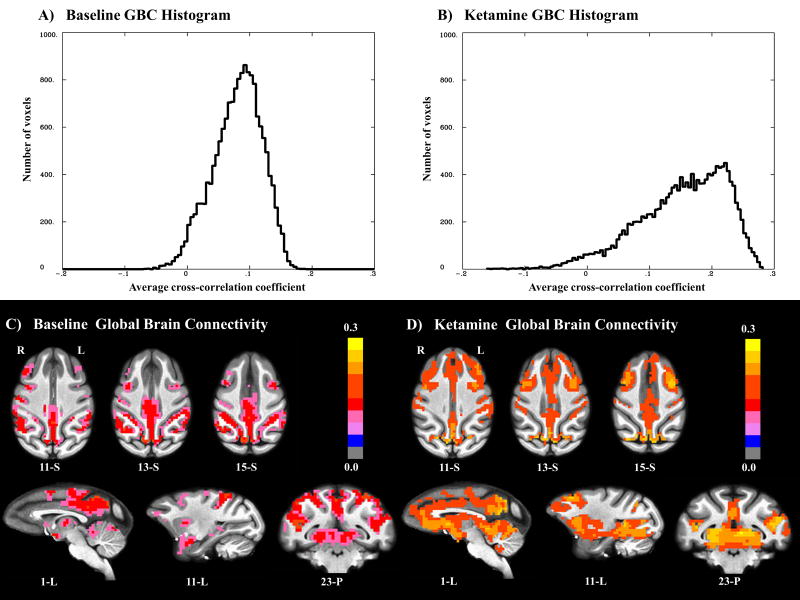
Recent studies in awake rhesus monkeys have demonstrated that ketamine-induced changes in functional connectivity are also well conserved across primate species (Gopinath et al., 2016). GBC analysis (Figure 1; unpublished) shows that ketamine causes global hyperconnectivity in rhesus monkeys that is similar in both magnitude and regional pattern to what Driesen et al. (2013a) observed in human subjects. Thus, there are data from multiple imaging modalities documenting that nonhuman primates provide a highly translational model for evaluating the effects of ketamine on brain function.
Figure 1.
Sub-anesthetic ketamine increases global brain connectivity (GBC) in the awake rhesus brain (N=4). GBC for each voxel is expressed as the effective average cross-correlation (constructed by averaging z-transformed cross-correlations with all other voxel time courses and expressing the result in the form of effective cross-correlation): A) Histogram showing the distribution of GBC in all gray matter voxels during baseline; B) Histogram showing the distribution of GBC in all gray matter voxels during ketamine infusion. The noticeable rightward shift in B compared to A indicates increased connectivity between brain regions during ketamine infusion; C) Voxel-wise GBC maps during baseline. Voxels with GBC > 0.1 are highlighted; D) Voxel-wise GBC maps during ketamine infusion. Voxels with GBC > 0.15 are highlighted. Ketamine-induced increases in GBC are noticeable throughout the brain and appear particularly intense in frontal and subcortical regions.
Joules et al. (2015) investigated the effects of sub-anesthetic ketamine infusion on whole-brain functional connectivity in healthy control subjects using a graph theory analysis. The measures of whole-brain connectedness they considered are similar to GBC but, rather than being calculated on every voxel, they are calculated between anatomical regions within a whole-brain parcellation map. Joules et al. (2015) found a shift in whole-brain functional connectivity with ketamine infusion that is consistent with the reported increase in GBC (Driesen et al., 2013a). They further showed that a pattern recognition algorithm could be used consistently to classify the pattern of functional connectivity induced by ketamine infusion as different from placebo infusion. This finding demonstrates the robustness of the effects of ketamine infusion on whole-brain functional connectivity.
Regional functional connectivity
In addition to their GBC study, Driesen et al. (2013b) conducted an investigation in healthy control subjects of the effects of ketamine on functional connectivity to a seed region in the dorsolateral prefrontal cortex (dlPFC). The dlPFC is a region strongly implicated in schizophrenia because of its important role in working memory (Wang et al., 2013) and the group hypothesized that ketamine-induced changes in connectivity to the dlPFC would be associated with impaired performance on a working memory task. Unfortunately, they used a global signal regression that may have confounded their results for the reasons mentioned previously. Accordingly, while they found that ketamine reduced connectivity to the dlPFC seed, this possibly resulted from a greater impact of global signal regression under the ketamine condition than the control condition.
Dandash et al. (2015) used a regional seed-based analysis with seeds placed in the dorsal and ventral putamen, dorsal caudate, and nucleus accumbens. They reported that functional connectivity to the striatum was enhanced in healthy control subjects. While they did not find differences in connectivity to the putamen, they found increased connectivity from the midbrain and thalamus to the dorsal caudate and from the ventromedial prefrontal cortex to the nucleus accumbens. These results may have been limited by relatively low and highly variable ketamine plasma levels (68.6±43.6 ng/ml) producing less robust drug effects. Nevertheless, they found that increases in connectivity between the medial prefrontal cortex and ventral striatum were correlated with ratings of both psychosis-like behavior (RSPS) and dissociative state (CADSS). Further, they found that increases in connectivity between the midbrain and dorsal caudate were associated with increases in ratings of positive schizophrenia symptoms (BPRS) while the observed increases in connectivity between the ventrolateral thalamus and dorsal caudate were associated with lower ratings of psychosis (RSPS) and dissociative state (CADSS). The latter negative association could be important for differentiating the mechanisms underlying the psychotomimetic effects from the therapeutic effects of ketamine. Their finding of increased connectivity between the medial prefrontal cortex and ventral striatum may also inform the study of ketamine as a potential treatment for drug addiction, a condition in which fronto-striatal connectivity has been found to be impaired (Hu et al., 2015; Murnane et al., 2015).
Another study by Grimm et al. (2015) investigated ketamine-induced changes in functional connectivity specifically between the dlPFC and hippocampus. They found that acute ketamine administration increased dlPFC-hippocampus connectivity in both healthy human subjects and in rats. The generality of their results is limited, given that only a single connection was examined, but abnormal dlPFC-hippocampus connectivity has been observed in schizophrenia (Meyer-Lindenberg et al., 2005) and a similar finding of robust ketamine-induced increases in dlPFC functional connectivity has been observed in awake rhesus monkeys (Gopinath et al., 2016). Overall, these data provide strong evidence that the effects of ketamine on functional connectivity are well conserved across species.
A recent study employed a regional seed-based analysis of changes in functional connectivity induced by ketamine in awake rhesus monkeys (Gopinath et al., 2016). As reported previously in humans (Dandash et al., 2015), ketamine increased connectivity to a seed region in the nucleus accumbens, although the increases observed were considerably more extensive. In addition to the accumbens seed, the analysis also featured seed regions in the amygdala, posterior and subgenual cingulate, orbitofrontal cortex, and dlPFC. Among these seed regions, the greatest ketamine-induced changes in functional connectivity were seen in dlPFC projections. This may be a key finding for explaining both the psychotomimetic and antidepressant effects of ketamine (see Discussion).
Antidepressant effects
In addition to healthy control subjects, two phMRI studies have investigated the neuronal effects of ketamine infusion in patients with major depressive disorder (MDD). These two studies used different clinical ratings scales to quantify changes in symptoms of depression following ketamine infusion. The Beck Depression Inventory (BDI) (Beck et al., 1961) was utilized by Downey et al. (2016), while Abdallah et al. (2016) employed the Montgomery–Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979).
Brain Activation
Downey et al. (2016) used phMRI to investigate the acute effects of ketamine infusion in patients with MDD. They performed the phMRI scans during ketamine treatment and correlated the acute effects of ketamine with the alleviation of depression symptoms (BDI) 24hours post infusion. They found a similar pattern of acute ketamine-evoked BOLD activation in subjects with MDD to what has been shown previously in healthy control subjects (De Simoni et al., 2013; Deakin et al., 2008; Doyle et al., 2013) as well as in nonhuman primates (Maltbie et al., 2016). Further, they found that ketamine-induced BOLD activation in the rostral anterior cingulate cortex (rACC) correlated strongly (r=0.61) with alleviation of depression symptoms (measured by BDI). The latter findings are consistent with task-based MRI studies showing that aberrant processing in the rACC is correlated with poorer ketamine treatment outcomes for the alleviation of depression symptoms (Salvadore et al., 2009; Salvadore et al., 2010).
Global brain connectivity
Abdallah et al. (2016) investigated the prolonged effects of ketamine treatment on GBC in major depression. Prior to treatment, patients with MDD displayed reduced GBC within the prefrontal cortex (responders and non-responders) compared to healthy controls. Following sub-anesthetic ketamine treatment (24 hours post-infusion), responders displayed normalized prefrontal GBC, while non-responders maintained significantly reduced prefrontal GBC. The increase in GBC in lateral PFC and in the caudate correlated with the alleviation of symptoms (MADRS). The data correspond very well with the acute effects of ketamine on GBC observed in healthy control subjects (Driesen et al., 2013a) and awake nonhuman primates (Figure 1), including increased GBC throughout the brain, with the most prominent increases seen in the prefrontal cortex. The study by Abdallah et al. (2016) featured a relatively small sample (N=18 MDD patients, with 10 responders) but the results provide evidence that MDD may feature prefrontal dysconnectivity as measured by GBC, and that the increased GBC reliably induced acutely by ketamine could be related to its antidepressant effects. Further investigation is certainly warranted.
Regional brain connectivity
In the same study that examined GBC after ketamine treatment in major depression, Abdallah et al. (2016) investigated the prolonged effects of ketamine (24 hours after infusion) on seed regions in the dlPFC, subgenual cingulate, and posterior cingulate cortex. These regions play an important role in cortico-limbic networks (Mayberg, 2003) responsible for affective processing and may be important for the antidepressant effects of ketamine (Johansen-Berg et al., 2008). Abdallah et al. (2016) reported that subjects with MDD displayed higher connectivity between prefrontal regions and the dlPFC and subgenual cingulate seeds, but lower connectivity between more distant cortical and subcortical regions. They further reported these regional connectivity differences to be normalized following ketamine treatment. They speculate that in MDD, within-region connectivity over short distances may be increased, while between-region connectivity over longer distances may be disrupted, and that ketamine normalizes connectivity within and between brain regions. While further work is needed to establish better the prolonged effects of ketamine on functional connectivity, these initial results imply that the extensive increases in cortical and subcortical connectivity to dlPFC induced acutely by ketamine (Gopinath et al., 2016) may persist following drug clearance and may contribute to the antidepressant effects of the drug.
Discussion
Whether or not the dissociative and psychotomimetic effects of ketamine are separable from the therapeutic effects remains an open question. Ratings for ketamine-induced CADSS have been correlated with improvement in depression symptoms following treatment (Luckenbaugh et al., 2014). However, the same study found the schizophrenia-like symptoms (BPRS) induced by ketamine were not correlated with treatment outcome. phMRI studies of ketamine indicate that the psychotomimetic effects may not be entirely distinguishable from the therapeutic effects. Abdallah et al. (2016) found that increases in prefrontal and striatal GBC 24 hours post-ketamine treatment were correlated with antidepressant efficacy, while Driesen et al. (2013a) found extensive increases in GBC (including a cluster in OFC) induced acutely by ketamine infusion that correlated with positive psychotomimetic symptoms (PANSS). Thus, these phMRI studies indicate that the same neurocircuitry involved in the acute psychotomimetic effects of ketamine is also important for the therapeutic effects.
The effects of ketamine on specific brain regions, particularly the dlPFC, suggest that similar mechanisms underlie psychotomimetic and therapeutic effects. The dlPFC is a region strongly implicated in schizophrenia because of its important role in working memory (Wang et al., 2013). The hyper-connectivity induced by ketamine could be related to improper processing in the dlPFC, leading to aberrant downstream signaling and resulting psychotomimetic effects, as hypothesized by Driesen et al. (2013b). Further, the dlPFC plays an essential role in the executive control of emotion (Ochsner and Gross, 2005), which has been shown to be dysfunctional in major depression (Fales et al., 2008). Direct activation of the dlPFC using repeated transcranial magnetic stimulation is an effective treatment for depression (Concerto et al., 2015), speculatively because of a resultant strengthening of network connections responsible for executive control of emotion (Fox et al., 2012; Koenigs and Grafman, 2009; Ma, 2015). Thus, the ketamine-induced increases in dlPFC connectivity may be a key indicator of psychotomimetic effects present during ketamine infusion as well as the neuroplastic changes thought to underlie the delayed antidepressant effects that follow ketamine administration.
There is also evidence from phMRI studies to suggest differences in the mechanisms underlying the psychotomimetic and therapeutic effects. While Abdallah et al. (2016) found that ketamine-induced increases in GBC in the caudate were correlated with alleviation of depression symptoms (MADRS) in MDD patients, Dandash et al. (2015) observed that increases in functional connectivity between dorsal caudate and ventrolateral thalamus were associated with lower ratings of psychosis (RSPS) and dissociative state (CADSS) induced by ketamine in healthy control subjects. Driesen et al. (2013a) likewise found that healthy controls exhibiting greater increases to GBC in both dorsal caudate and ventrolateral thalamus displayed fewer increases in negative schizophrenia symptom scores (PANSS). Hence, while the psychotomimetic and therapeutic effects of ketamine may have convergent mechanisms within the prefrontal cortex (and dlPFC in particular) there appear to be differences in effects on striatal processing that influence the behavioral effects.
While these phMRI studies have provided significant insight into the brain circuitry mediating the behavioral effects of ketamine, the extent to which the therapeutic effects of ketamine can be isolated from the psychotomimetic and dissociative effects remains an open question that requires further investigation.
Future Directions
phMRI and ketamine mechanism of action
While ketamine-induced BOLD activation has been a useful first step for studying the whole-brain effects of ketamine, functional connectivity may prove to be more informative for understanding the mechanism of action of ketamine in the brain. It is important to note that when neural activity increases, metabolic activity (and therefore BOLD signal) is primarily enhanced at the synapses and not at the cell bodies (Buxton, 2002). Thus, in the case of localized disinhibition of pyramidal neurons, as presumed with ketamine, the downstream areas receiving projections from the disinhibited region(s) may show the greatest enhancement of BOLD signal. On the other hand, a region that becomes disinhibited may increase its functional coupling to downstream areas and hence may show increased functional connectivity even when it does not show a strong enhancement in BOLD signal (Gusnard et al., 2001). This may explain why the dlPFC shows the most extensive increases in functional connectivity (Gopinath et al., 2016) but only moderate increases in BOLD signal during ketamine infusion (De Simoni et al., 2013; Maltbie et al., 2016).
Future studies should use phMRI to address several additional questions regarding the pharmacology of ketamine. The dose-response relationship for ketamine remains poorly understood. This is true both for the efficacy of ketamine as an antidepressant and for the acute effects of ketamine on brain activity. De Simoni et al. (2013) reported that BOLD activation increased with increasing dose; however no peak dose has been established and no investigation of dose dependency with functional connectivity has been conducted. Further, no phMRI studies have investigated the effects of repeated ketamine treatments, despite the potential relevance of chronic administration to the use of ketamine for the treatment of depression (aan het Rot et al., 2010; Papp et al., 2017).
The independent contributions of the individual pharmacological components of ketamine remain largely unknown. Ketamine is a chiral compound consisting of a pair of (R,S) enantiomers and there is some evidence suggesting that R-ketamine may have greater antidepressant efficacy (Zhang et al., 2014) while also producing fewer psychotomimetic effects (Yang et al., 2015). Further,one report found a specific metabolite of ketamine to be sufficient for producing antidepressant effects (Zanos et al., 2016). phMRI with these independent components of ketamine may lead to important new insights into mechanism of action and therapeutic utilty. Such experiments may also help to determine whether the psychotomimetic effects of ketamine can be truly segregated from the antidepressant effects.
phMRI and translational models
Nonhuman primate models offer translational advantages in behavioral and pharmacological research (Phillips et al., 2014; Preuss, 1995; Wang and Arnsten, 2015). The introduction of techniques for performing phMRI experiments in awake nonhuman primates provides even greater translational relevance. As detailed in this review, phMRI coupled with sub-anesthetic ketamine infusion has been used to create a promising translational pharmacological model of schizophrenia in nonhuman primates (Gopinath et al., 2016; Maltbie et al., 2016). Indeed, awake nonhuman primate phMRI can provide important insights into the therapeutic mechanisms of ketamine.
There are limitations to traditional animal models of major depression (Berton et al., 2012), with treatment-resistant depression proving to be particularly challenging (Willner and Belzung, 2015). While nonhuman primate models have been underutilized in studies of depression (Shively and Willard, 2012), the use of phMRI in conscious animals could be particularly important for investigating the mechanisms underlying the rapid antidepressant effects of ketamine. The only study to investigate the sustained effects of ketamine in nonhuman primates (Lv et al., 2016) reported significant changes in functional connectivity 24 hours post-infusion. However, very different analysis methods were used than in Abdallah et al. (2016) and the use of anesthesia represents a potential confound (Hodkinson et al., 2012; Hudetz, 2012). Replicating this study in awake animals should be informative.
Further investigation of sub-anesthetic ketamine as a treatment for drug addiction in humans could benefit significantly from the use of nonhuman primate models. Nonhuman primate self-administration represents the gold standard for modeling the abuse-related effects of drugs in animals, and phMRI studies provide strong evidence that ketamine induces highly translational effects on BOLD signal and functional connectivity in nonhuman primates. Thus, nonhuman primates may provide a valid animal model for investigating the efficacy of ketamine for reducing drug self-administration, as well as the predictive value of dlPFC functional connectivity as a biomarker for abuse-related behavior.
Ketamine has already shown tremendous therapeutic value in the treatment of major depression, and the results from functional imaging experiments provide important neurocircuitry-level evidence for additional therapeutic uses for ketamine. The finding that ketamine enhances connectivity to the dlPFC (Gopinath et al., 2016), and potentially causes neuroplastic changes that strengthen executive control, has major implications for the potential use of ketamine in treating other psychiatric disorders. Impaired executive control has been associated with multiple disorders and is a particularly common finding in drug addiction (Goldstein and Volkow, 2011; Jentsch and Taylor, 1999; Volkow et al., 2011). Indeed, acute administration of cocaine has been shown to significantly reduce functional connectivity between dlPFC and nucleus accumbens in awake nonhuman primates, and the connectivity between these regions is negatively correlated with cocaine intake during self-administration (Murnane et al., 2015). There is already some evidence to indicate that sub-anesthetic ketamine infusion may be an effective treatment for cocaine abuse (Dakwar et al., 2016; Dakwar et al., 2014). Given the lack of FDA-approved medications for the treatment of psychostimulant abuse, further investigation is certainly warranted despite the abuse liability of ketamine.
Conclusion
Pharmacological imaging has proven extremely useful for studying the effects of ketamine in the brain. Acute administration of sub-anesthetic ketamine produces a robust, global increase in BOLD signal that is correlated with the psychotomimetic effects of ketamine. Functional connectivity also undergoes robust, global increases during acute ketamine administration that correlate with the psychotomimetic effects of ketamine. These effects are very well conserved across primate species and could be used to create a translational pharmacological model of schizophrenia in nonhuman primates. Ketamine shows exciting potential as a therapeutic and the results from phMRI experiments suggest it may strengthen executive control circuits, making it a particularly good candidate for investigation in the treatment of drug abuse. Future phMRI studies may be able to elucidate many of the questions that remain unanswered about the mechanisms mediating the effects of ketamine.
Acknowledgments
Special thanks to the Yerkes Imaging Center, technicians Marisa Olsen and Juliet Brown, and to Christopher Muly, MD, PhD, Naoko Urushino, PhD, and Doty Kempf, DVM.
Funding sources:
This research was supported by P51OD11132 (Yerkes National Primate Research Center), Sunovion Pharmaceuticals, Ltd (LLH) and DA031246 (LLH).
Footnotes
Conflicts of interest:
None declared by any of the authors.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, et al. N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry. 2015;77:569–580. doi: 10.1016/j.biopsych.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–166. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Berton O, Hahn CG, Thase ME. Are we getting closer to valid translational models for major depression? Science. 2012;338:75–79. doi: 10.1126/science.1222940. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Diaz-Mataix L, Scorza MC, Celada P, Artigas F. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem. 2005;95:1597–1607. doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- Braga RM, Buckner RL. Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron. 2017;95:457–471. doi: 10.1016/j.neuron.2017.06.038. e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Introduction to functional magnetic resonance imaging : principles and techniques. Cambridge University Press; Cambridge, UK; New York: 2002. [Google Scholar]
- Carr DB, Sesack SR. Dopamine terminals synapse on callosal projection neurons in the rat prefrontal cortex. J Comp Neurol. 2000;425:275–283. doi: 10.1002/1096-9861(20000918)425:2<275::aid-cne9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Chin CL, Upadhyay J, Marek GJ, Baker SJ, Zhang M, Mezler M, et al. Awake rat pharmacological magnetic resonance imaging as a translational pharmacodynamic biomarker: metabotropic glutamate 2/3 agonist modulation of ketamine-induced blood oxygenation level dependence signals. J Pharmacol Exp Ther. 2011;336:709–715. doi: 10.1124/jpet.110.173880. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Miller R. A rating scale for psychotic symptoms (RSPS) part I: theoretical principles and subscale 1: perception symptoms (illusions and hallucinations) Schizophr Res. 1999;38:101–122. doi: 10.1016/s0920-9964(99)00012-2. [DOI] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concerto C, Lanza G, Cantone M, Ferri R, Pennisi G, Bella R, et al. Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: A six-month clinical follow-up study. Int J Psychiatry Clin Pract. 2015;19:252–258. doi: 10.3109/13651501.2015.1084329. [DOI] [PubMed] [Google Scholar]
- Dakwar E, Levin F, Foltin RW, Nunes EV, Hart CL. The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol Psychiatry. 2014;76:40–46. doi: 10.1016/j.biopsych.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakwar E, Hart CL, Levin FR, Nunes EV, Foltin RW. Cocaine self-administration disrupted by the N-methyl-D-aspartate receptor antagonist ketamine: a randomized, crossover trial. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O, Harrison BJ, Adapa R, Gaillard R, Giorlando F, Wood SJ, et al. Selective augmentation of striatal functional connectivity following NMDA receptor antagonism: implications for psychosis. Neuropsychopharmacology. 2015;40:622–631. doi: 10.1038/npp.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni S, Schwarz AJ, O’Daly OG, Marquand AF, Brittain C, Gonzales C, et al. Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage. 2013;64:75–90. doi: 10.1016/j.neuroimage.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm (Vienna) 2009;116:941–952. doi: 10.1007/s00702-009-0243-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Mora F. Blockade of NMDA receptors in the prefrontal cortex increases dopamine and acetylcholine release in the nucleus accumbens and motor activity. Psychopharmacology (Berl) 2008;201:325–338. doi: 10.1007/s00213-008-1288-3. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Domino SE, Smith RE, Domino LE, Goulet JR, Domino KE, et al. Ketamine kinetics in unmedicated and diazepam-premedicated subjects. Clin Pharmacol Ther. 1984;36:645–653. doi: 10.1038/clpt.1984.235. [DOI] [PubMed] [Google Scholar]
- Downey D, Dutta A, McKie S, Dawson GR, Dourish CT, Craig K, et al. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur Neuropsychopharmacol. 2016;26:994–1003. doi: 10.1016/j.euroneuro.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O’Daly OG, Williams SC, et al. Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. J Pharmacol Exp Ther. 2013;345:151–160. doi: 10.1124/jpet.112.201665. [DOI] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013a;18:1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D’Souza DC, et al. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013b;38:2613–2622. doi: 10.1038/npp.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Knapp DJ, Mueller RA, Breese GR. Metabolic mapping of the rat brain after subanesthetic doses of ketamine: potential relevance to schizophrenia. Brain Res. 1998;787:181–190. doi: 10.1016/s0006-8993(97)01390-5. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J Psychopharmacol. 2014;28:287–302. doi: 10.1177/0269881113512909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K, Maltbie E, Urushino N, Kempf D, Howell L. Ketamine-induced changes in connectivity of functional brain networks in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology (Berl) 2016;233:3673–3684. doi: 10.1007/s00213-016-4401-z. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz A, Crestan V, Bifone A. Drug-anaesthetic interaction in phMRI: the case of the psychotomimetic agent phencyclidine. Magn Reson Imaging. 2008;26:999–1006. doi: 10.1016/j.mri.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Grimm O, Gass N, Weber-Fahr W, Sartorius A, Schenker E, Spedding M, et al. Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology (Berl) 2015;232:4231–4241. doi: 10.1007/s00213-015-4022-y. [DOI] [PubMed] [Google Scholar]
- Guo S, Kendrick KM, Zhang J, Broome M, Yu R, Liu Z, et al. Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression. Neuroimage Clin. 2013;2:818–826. doi: 10.1016/j.nicl.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haas DA, Harper DG. Ketamine: a review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992;39:61–68. [PMC free article] [PubMed] [Google Scholar]
- Haensel JX, Spain A, Martin C. A systematic review of physiological methods in rodent pharmacological MRI studies. Psychopharmacology (Berl) 2015;232:489–499. doi: 10.1007/s00213-014-3855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PloS one. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson DJ, de Groote C, McKie S, Deakin JF, Williams SR. Differential Effects of Anaesthesia on the phMRI Response to Acute Ketamine Challenge. Br J Med Med Res. 2012;2:373–385. doi: 10.9734/bjmmr/2012/1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA. Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology. 2001;25:165–172. doi: 10.1016/S0893-133X(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- Hudetz AG. General anesthesia and human brain connectivity. Brain Connect. 2012;2:291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joules R, Doyle OM, Schwarz AJ, O’Daly OG, Brammer M, Williams SC, et al. Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, et al. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Schwarz S, Schmid F, Hunger H, Kissling W, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD006626.pub2. CD006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelowicz A, Ventura J, Liberman RP, Mintz J. Consistency of Brief Psychiatric Rating Scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41:77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsjo JW, Kaisti KK, Aalto S, Hinkka S, Aantaa R, Oikonen V, et al. Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:614–623. doi: 10.1097/00000542-200309000-00016. [DOI] [PubMed] [Google Scholar]
- Langsjo JW, Salmi E, Kaisti KK, Aalto S, Hinkka S, Aantaa R, et al. Effects of subanesthetic ketamine on regional cerebral glucose metabolism in humans. Anesthesiology. 2004;100:1065–1071. doi: 10.1097/00000542-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Leslie RA, James MF. Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends Pharmacol Sci. 2000;21:314–318. doi: 10.1016/s0165-6147(00)01507-8. [DOI] [PubMed] [Google Scholar]
- Lodge D, Mercier MS. Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacol. 2015;172:4254–4276. doi: 10.1111/bph.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q, Yang L, Li G, Wang Z, Shen Z, Yu W, et al. Large-Scale Persistent Network Reconfiguration Induced by Ketamine in Anesthetized Monkeys: Relevance to Mood Disorders. Biol Psychiatry. 2016;79:765–775. doi: 10.1016/j.biopsych.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry. 2015;20:311–319. doi: 10.1038/mp.2014.24. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Maltbie E, Gopinath K, Urushino N, Kempf D, Howell L. Ketamine-induced brain activation in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology (Berl) 2016;233:961–972. doi: 10.1007/s00213-015-4175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason OJ, Morgan CJ, Stefanovic A, Curran HV. The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res. 2008;103:138–142. doi: 10.1016/j.schres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Muly EC, Votaw JR, Ritchie J, Howell LL. Relationship between dose, drug levels, and D2 receptor occupancy for the atypical antipsychotics risperidone and paliperidone. J Pharmacol Exp Ther. 2012;341:81–89. doi: 10.1124/jpet.111.189076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Howell LL. Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods. 2010;191:11–20. doi: 10.1016/j.jneumeth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL. Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology (Berl) 2015;232:745–754. doi: 10.1007/s00213-014-3709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Opler LA, Opler MG, Arnsten AF. Ameliorating treatment-refractory depression with intranasal ketamine: potential NMDA receptor actions in the pain circuitry representing mental anguish. CNS Spectr. 2016;21:12–22. doi: 10.1017/S1092852914000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Gruca P, Lason-Tyburkiewicz M, Willner P. Antidepressant, anxiolytic and procognitive effects of subacute and chronic ketamine in the chronic mild stress model of depression. Behav Pharmacol. 2017;28:1–8. doi: 10.1097/FBP.0000000000000259. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, et al. Why primate models matter. Am J Primatol. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Price RB, Mathew SJ. Does ketamine have anti-suicidal properties? Current status and future directions. CNS Drugs. 2015;29:181–188. doi: 10.1007/s40263-015-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinstatler L, Youssef NA. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D. 2015;15:37–43. doi: 10.1007/s40268-015-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M, et al. Baseline Striatal Functional Connectivity as a Predictor of Response to Antipsychotic Drug Treatment. Am J Psychiatry. 2016;173:69–77. doi: 10.1176/appi.ajp.2015.14121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82:111–125. doi: 10.1016/S0304-3959(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147:107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Shaffer CL, Osgood SM, Smith DL, Liu J, Trapa PE. Enhancing ketamine translational pharmacology via receptor occupancy normalization. Neuropharmacology. 2014;86:174–180. doi: 10.1016/j.neuropharm.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp Neurol. 2012;233:87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AB, Buxton RB. Understanding the dynamic relationship between cerebral blood flow and the BOLD signal: Implications for quantitative functional MRI. Neuroimage. 2015;116:158–167. doi: 10.1016/j.neuroimage.2015.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoblenick K, Everling S. NMDA antagonist ketamine reduces task selectivity in macaque dorsolateral prefrontal neurons and impairs performance of randomly interleaved prosaccades and antisaccades. J Neurosci. 2012;32:12018–12027. doi: 10.1523/JNEUROSCI.1510-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward CA, Marsden CA, Prior MJ, Morris PG, Shah YB. Methodological considerations in rat brain BOLD contrast pharmacological MRI. Psychopharmacology (Berl) 2005;180:687–704. doi: 10.1007/s00213-005-2213-7. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- Veilleux-Lemieux D, Castel A, Carrier D, Beaudry F, Vachon P. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2013;52:567–570. [PMC free article] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, et al. Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PloS one. 2011;6:e16573. doi: 10.1371/journal.pone.0016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, et al. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG) Eur Neuropsychopharmacol. 1997;7:9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL. Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res. 2000;34:35–43. doi: 10.1016/s0022-3956(99)00031-x. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Prolonged exposure to NMDAR antagonist induces cell-type specific changes of glutamatergic receptors in rat prefrontal cortex. Neuropharmacology. 2012;62:1808–1822. doi: 10.1016/j.neuropharm.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Arnsten AF. Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neurosci Bull. 2015;31:191–197. doi: 10.1007/s12264-014-1504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Belzung C. Treatment-resistant depression: are animal models of depression fit for purpose? Psychopharmacology (Berl) 2015;232:3473–3495. doi: 10.1007/s00213-015-4034-7. [DOI] [PubMed] [Google Scholar]
- Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32:3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Hong CJ, Huang YH, Tsai SJ. The effects of glycine transporter I inhibitor, N-methylglycine (sarcosine), on ketamine-induced alterations in sensorimotor gating and regional brain c-Fos expression in rats. Neurosci Lett. 2010;469:127–130. doi: 10.1016/j.neulet.2009.11.058. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]