Abstract
The CD27–CD70 pathway is known to provide a costimulatory signal with CD70 expressed on antigen-presenting cells while CD27 functioning on T cells. Although CD70 is also expressed on activated T cells, it remains unclear how T cell-derived CD70 affects T cell function. Therefore, we have assessed the role of T cell-derived CD70 using adoptive transfer models including autoimmune inflammatory bowel disease (IBD) and allogeneic graft-versus-host disease (GVHD). Compared with WT T cells, CD70−/− T cells surprisingly caused more severe IBD and GVHD and produced higher levels of inflammatory cytokines. Mechanistic analyses reveal that IFN-γ induces CD70 expression in T cells, and CD70 limits T cell expansion via a regulatory T cell-independent mechanism that involves caspase-dependent T cell apoptosis and upregulation of inhibitory immune checkpoint molecules. Notably, T cell intrinsic CD70 signaling contributes as least partially to the inhibitory checkpoint function. Overall, our findings demonstrate for the first time that T cell-derived CD70 plays a novel immune checkpoint role in inhibiting inflammatory T cell responses. This study suggests that T cell-derived CD70 performs a critical negative feedback function to downregulate inflammatory T cell responses.
Introduction
Costimulation is an essential component to T cell activation and constitutes a multitude of receptor/ligand interactions that play unique roles in T cell response. The most well studied families of costimulation are the immunoglobulin (Ig) superfamily and the tumor necrosis factor receptor (TNFR) family (1). These two families of receptors work in concert to orchestrate T cell activation, expansion and effector function. Among them, CD28 of the Ig superfamily is the prototypical costimulatory receptor on T cells that provides a critical second signal alongside T cell receptor (TCR) ligation for naive T cell activation (2). In addition, other costimulatory receptors including CD27 of the TNFR family play complex and dynamic roles in T cell response (3). On the other hand, immune checkpoint molecules constitute inhibitory pathways that negatively influence T cell responses. CTLA-4 of the Ig superfamily is an archetypical checkpoint receptor constitutively expressed in regulatory T (Treg) cells and also upregulated in conventional T cells upon activation. CTLA-4 inhibits T cell activation by binding CD80 and CD86 ligands with greater affinity thus outcompeting CD28 for its ligands (4). Several additional immune checkpoint receptors have been discovered recently. PD-1 of the Ig superfamily limits the responses of activated T cells by binding to two ligands, PD-L1 and PD-L2, and promoting T cell apoptosis (5–7). LAG-3 is a CD4-related checkpoint receptor that suppresses immune responses by contributing to the suppressive activity of CD4+ Treg cells as well as direct inhibitory effects on CD8+ T cells (8, 9). TIM-3 is identified as another checkpoint receptor in CD4+ and CD8+ T cells that functions by triggering T cell apoptosis upon interaction with galectin-9 or other ligands (10).
CD27–CD70 is known as a costimulatory receptor-ligand pair in the TNFR family, with the CD27 receptor constitutively expressed on naïve and memory T cells (also observed on subsets of activated B cells, NK cells, and hematopoietic progenitor cells) (3). CD27 signaling makes essential contributions to CD4+ and CD8+ T cell function via supporting antigen-specific expansion of naive T cells, promoting survival of activated T cells, complementing CD28 in establishment of the effector T cell pool and generation of T cell memory (11–13). In addition, CD27 signaling has been shown to provide survival signals for Treg cells in the thymus (14), increase the frequency of Treg cells in the periphery (15), promote Th1 development (16), and inhibit Th17 effector cell differentiation and associated autoimmunity (17).
Known as the sole ligand for CD27, CD70 is more tightly regulated and mainly expressed by various types of antigen presenting cells (APCs), including mature hematopoietic APCs (18), intestinal non-hematopoietic APCs (19), a unique subset of lamina propria cells (20), and epithelial and dendritic cells in the thymic medulla (14). Accordingly, CD70-dependent function of these APCs has been implicated in the proliferation and differentiation of antigen-specific T cells including Th17 in the gut mucosa and Treg cell development in the thymus (14, 19, 20).
Interestingly, CD70 is also expressed on T cells after activation (18). However, unlike the well-studied role of T cell-expressed CD27 receptor, the role of T cell-expressed CD70 ligand remains unclear. Therefore, we have assessed the role of T cell intrinsic CD70 using multiple adoptive transfer models including autoimmune inflammatory bowel disease (IBD) and allogeneic graft-versus-host disease (GVHD). Overall, this study reveals for the first time that T cell-derived CD70 plays a novel immune checkpoint role in suppressing inflammatory T cell responses. Our findings strongly suggest that T cell-derived CD70 performs a critical negative feedback function to downregulate inflammatory T cell responses.
Materials and Methods
Mice
CD70−/− mice have been backcrossed for 13 generations to the C57BL/6Ncr strain and were provided by Dr. Jonathan Ashwell at NCI (21, 22). C57BL/6Ncr WT, BALB/c WT and FVB WT mice were purchased from NCI and Charles River–Frederick. C57BL/6 RAG1−/− mice were purchased from The Jackson Laboratory. All mice were maintained in specific pathogen-free conditions. All experiments were conducted in accordance with protocols approved by the animal studies committee at Roswell Park Cancer Institute.
T cell transfer colitis
CD4+CD25− T cells were isolated from the spleens of naive C57BL/6 WT mice or CD70−/− mice using mouse PanT isolation kit II supplemented with biotinylated anti-CD25 and anti-CD8 and LS columns (Miltenyi). With confirmed purify >95%, 1×106 sorted cells were injected via tail veins into gender-matched RAG1−/− mice, which were weighed initially and then weekly after adoptive transfer. Mice were sacrificed when body weight dropped >20% from initial weight along with signs of diarrhea, hunching, and ruffled fur.
Flow cytometry
Cells were stained with antibodies to CD3 (145-2C11), CD4 (RM4–5), CD8 (53-6.7), CD25 (PC61), Foxp3 (FJK-16s), LAG-3 (C9B7W), PD-1 (29F.1A12), TIM-3 (B8.2C12), CTLA-4 (UC10-4B9), and fixable Live/Dead Aqua (Invitrogen) or Zombie UV™ (Biolegend). Active caspase-8 and 3 staining was performed using CaspGLOW™ Fluorescein Active Caspase-8 or 3 staining kit (eBioscience). All samples were run on either a LSRFortessa (BD Biosciences). All data were analyzed with FlowJo (Tree Star).
ef670 dilution
Single cell suspensions of sorted CD4+CD25− T cells were resuspended in 5mL of 37°C PBS. Equal volume of 10µM ef670 in 37°C PBS was added to the T cell suspension and incubated for 10 min at 37°C. After incubation, 5mL of 10% FBS containing RPMI was added and cells were washed. Cells were then washed twice in PBS before injection.
Histopathology scoring
Mice were sacrificed at days 17, 36, and 66, and large and small intestines were formalin-fixed, sectioned, and stained with H&E. Intestine tissues were examined using a previously established scoring system (23–25). Blinded assessments were made for the presence of crypt epithelial cell apoptosis, crypt regeneration, surface erosion, ulceration, lamina propria inflammation, chronic atrophy, chronic crypt branching, chronic endocrine cell excess, chronic Paneth cell excess, epithelioid vacuolization, attenuation, sloughing into the lumen lymphocytic infiltrate and neutrophilic infiltrate.
Luminex assay
Serum was collected by retro-orbital eye bleeding on the indicated days following transplant. Blood was immediately placed on ice until all samples were collected. Once the final sample was collected, all samples were incubated at room temperature for 20 min to allow for clotting. After incubation, vials were centrifuged at 4°C for 10 min at 2000g. Serum was collected and then frozen at −80°C. Mouse Cytokine and Chemokine 11-plex was performed by the Flow and Image Cytometry, Luminex Divison at Roswell Park Cancer Institute as per manufacturer’s instructions.
Allogeneic hematopoietic cell transplant (allo-HCT) and graft-versus-host disease (GVHD)
For bone marrow (BM) only controls, T cell depletion (TCD) was performed using CD90.2 microbeads and LS columns (Miltenyi), resulting in <5% of original T cell composition of BM. Using LS columns for negative sorting of unlabeled cells, CD4+CD25− and CD8+ T cell sorts resulted in >95% purity of desired cell types. On day −1 BALB/c or FVB hosts received 850 cGy from a Cesium-137 source. In the B6 to BM1 HCT, BM1 hosts received 1000cGy on Day −1. Hosts were transplanted on day 0 with purified WT or CD70−/− T cells as described above. Host mice were then weighed twice weekly and considered moribund when body weight reached below 80% of initial weight.
Results
T cell-derived CD70 inhibits inflammatory bowel disease (IBD)
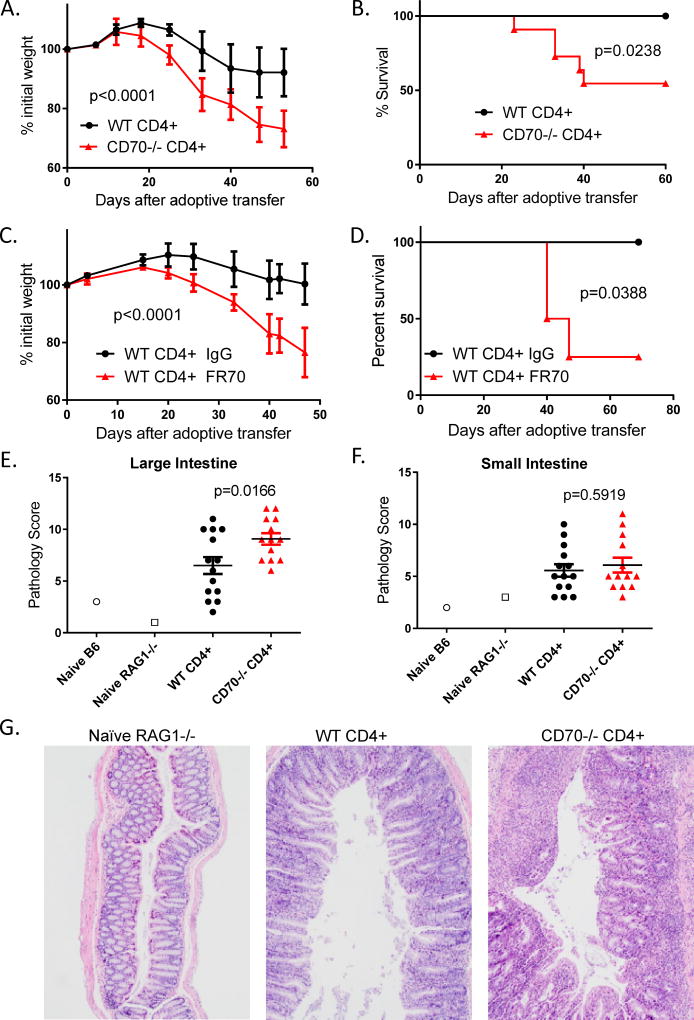
Inflammatory bowel disease (IBD) is an intestinal complication resulting from an inappropriate inflammatory response that is mediated by genetic, immune, and environmental factors (26, 27). IBD is characterized by an aberrant mucosal T cell-mediated inflammation (28). The T cell transfer model of colitis is the most widely used to determine the contribution of T cells in the initiation, induction, and regulation of IBD (29). To study the role of T cell-derived CD70 in IBD, we purified CD4+CD25− T cells from naïve C57BL/6 WT and CD70−/− mice and injected these cells into syngeneic RAG1−/− mice that lack T and B cells. After adoptive transfer, mice that received CD70−/− T cells had significantly more severe weight loss compared to mice that received WT T cells (Figure 1A), with nearly 50% of them lost more than 20% of body weight and succumbed to disease while no lethality occurred for mice that received WT T cells (Figure 1B). To test whether it is an inherent difference in T cells from WT versus CD70−/− mice that causes this phenotype, we used the most commonly used FR70 antibody to block CD70 after adoptive of WT T cells. Blocking CD70 also exacerbated colitis in a fashion similar to CD70 deficiency in T cells (Figure 1C–D), indicating that it is CD70 signaling, but not an inherent difference in T cells from WT versus CD70−/− mice that causes the suppression of disease. To confirm the presence of IBD, host mice were sacrificed on days 17, 36 and 66 (before, during and after disease onset). A blinded pathologist scored large and small intestines based on disease criteria outlined in the Experimental Procedures. Mice that received CD70−/− T cells had a significantly higher disease score in large intestines compared to mice that received WT T cells (Figure 1E and 1G). In contrast, there was no significant difference in disease scores for small intestines between these two groups (Figure 1F). Overall, these results indicate that RAG1−/− mice receiving WT CD4+CD25− T cells had less severe IBD than mice that receiving CD70−/− T cells. Therefore, these findings led us to a surprising conclusion that T cell intrinsic CD70 suppresses IBD.
Figure 1. T cell-derived CD70 inhibits inflammatory bowel disease (IBD).
(A) Naïve RAG1−/− mice in the C57BL/6 strain background were injected with 1×106 CD4+CD25− T cells purified from C57BL/6 WT or CD70−/− mice. Body weight was monitored after adoptive transfer. Representative weight data from 1 out of 3 independent experiments were shown as mean ± SD (n=4 per group), with statistical significance determined by two-way ANNOVA. (B) Kaplan-Meier survival data were summarized by combining 3 independent experiments (n=10–11 per group), with statistical significance determined by Log-rank test. (C-D) Naïve RAG1−/− mice were injected with 1×106 CD4+CD25− T cells purified from C57BL/6 WT mice, and then treated from day 1 with 100ug of either CD70 antibody (FR70) or IgG control twice weekly for the duration of the experiment. (C) Body weight was shown as mean ± SD, with statistical significance determined by two-way ANNOVA. (D) Kaplan-Meier survival curves were shown with statistical significance determined using Log-rank test. (E-F) RAG1−/− host mice were sacrificed on days 17, 36 and 66. A blinded pathologist scored large (E) and small (F) intestines based on disease criteria outline in the Experimental Procedures. Summary scores combined from 3 independent experiments were shown, with statistical significance determined by unpaired student t test. (G) Representative histopathological images were shown for large intestine samples harvested from RAG1−/− host mice at day 36 after adoptive transfer.
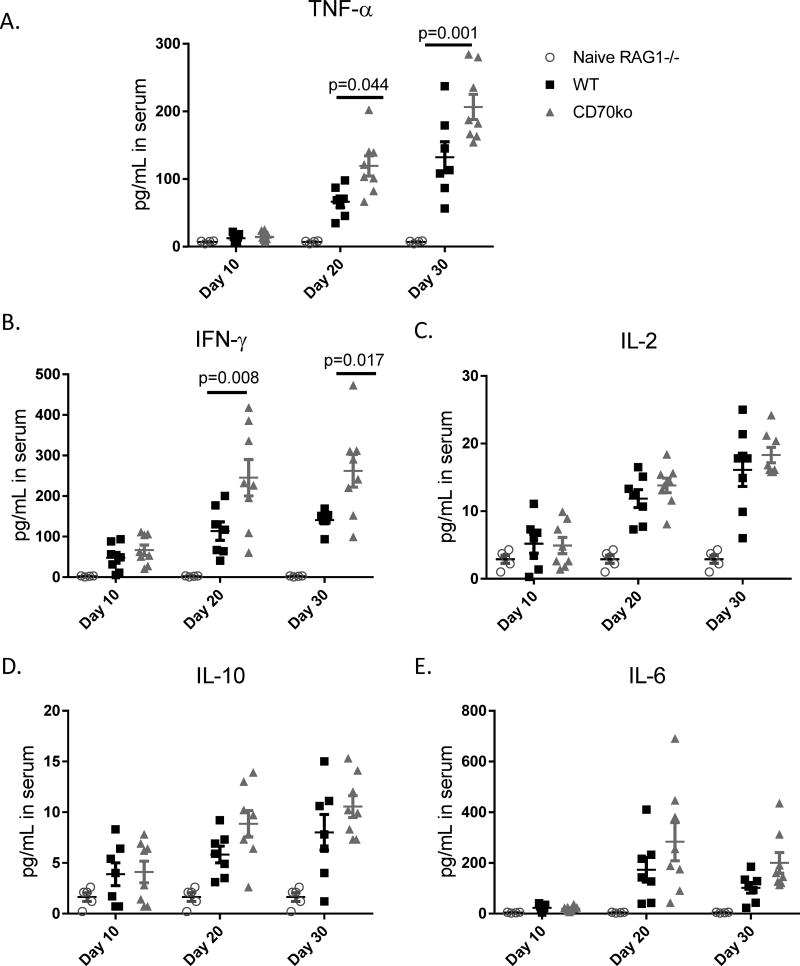
T cell-derived CD70 decreases the levels of inflammatory cytokines
The increased disease severity seen in hosts that received CD70−/− T cells prompted us to examine cytokine levels related to an inflammatory condition. After adoptive transfer, serum samples were harvested via eye bleeding on days 10, 20, and 30, and then analyzed via Luminex assays to determine blood levels of various proinflammatory cytokines. Beginning on Day 20, similar timing to disease onset, mice that received CD70−/− T cells had significantly increased levels of TNF-α and IFN-y (Figure 2A–B). The increased inflammatory cytokine levels in mice receiving CD70−/− T cells persisted to day 30 after adoptive transfer. Yet IL-2, IL-10 and IL-6 were not significantly increased in mice that received CD70−/− T cells (Figure 2C–E). We also measured IL-17A and IL-23, for which most of samples were below detection limits and therefore did not yield conclusive results. Furthermore, we harvested spleen cells from the RAG1−/− hosts and performed ex vivo PMA/ionomycin activation to examine the frequency of cytokine producing T cells and the cytokine expression levels in individual cells. Our results (Supplemental Figure 1) show that T cell-derived CD70 reduces the frequency of cytokine producing T cells but does not directly inhibit cytokine production per cell.
Figure 2. T cell-derived CD70 decreases the levels of inflammatory cytokines.
Naïve RAG1−/− mice in the C57BL/6 strain background were injected with 1×106 CD4+CD25− T cells purified from C57BL/6 WT or CD70−/− mice. Serum samples were harvested via retro-orbital eye bleeding at days 10, 20 and 30 after adoptive transfer. Luminex assays were performed to measure the levels of the indicated cytokines. Each dot on the plots represents a single mouse at the indicated time points, with statistical significance determined by two-way ANNOVA.
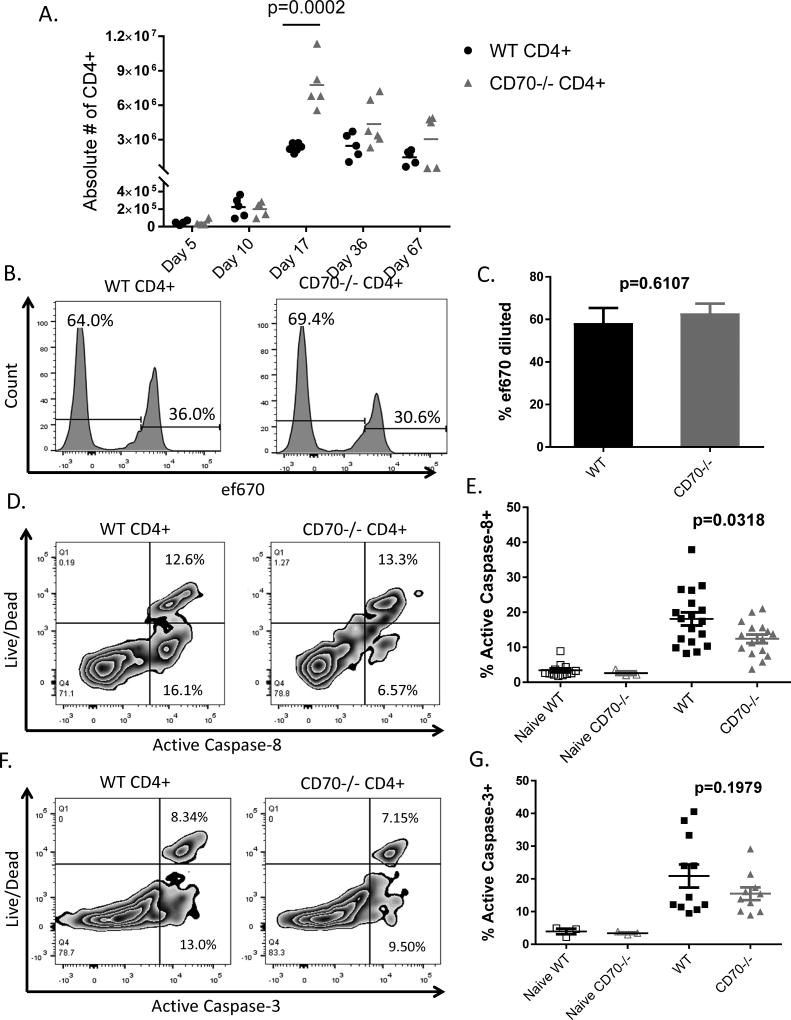
T cell-derived CD70 suppresses T cell expansion by inducing caspase-dependent apoptosis
To determine the mechanism by which T cell-derived CD70 inhibits IBD, we analyzed the proliferation, survival and differentiation of T cells after adoptive transfer. RAG1−/− hosts were sacrificed at Days 5, 10, 17, 30, and 67 in order to analyze T cells at different stages of disease. Beginning day 17, the absolute number of CD4+ T cells was significantly higher in mice that received CD70−/− T cells compared to those that received WT T cells, and the difference persisted until Day 67 (Figure 3A). To determine what caused the difference in CD4+ T cell expansion, we analyzed T cell proliferation by using the cell proliferation dye ef670. WT and CD70−/− CD4+CD25− T cells were stained with ef670 and then injected into RAG1−/− mice, which were then sacrificed at Day 6 after adoptive transfer to analyze T cell proliferation. Gating on Live CD4+ T cells, we determined by df670 dilution that there was no difference in CD4+ T cell proliferation between hosts that received WT and CD70−/− T cells (Figure 3B–C). These results were confirmed at later time points using BrdU and Ki-67 staining (Supplemental Figure 2A).
Figure 3. T cell-derived CD70 suppresses T cell expansion by inducing caspase-dependent apoptosis.
Naïve RAG1−/− mice in the C57BL/6 strain background were injected with 1×106 CD4+CD25− T cells purified from C57BL/6 WT or CD70−/− mice. (A) Host mice were sacrificed at the indicated days after adoptive transfer. Absolute numbers of CD4+ T cells were calculated by multiplying the total numbers of spleen cells by the percentages of TCRβ+CD4+ T cells present in spleen samples determined by flow cytometry. (B–C) WT and CD70−/− CD4+CD25− T cells were stained with the cell proliferation dye ef670 and then injected into RAG1−/− host mice, which were then sacrificed at day 6 after adoptive transfer. TCRβ+CD4+ T cell proliferation was measured by ef670 dilution with data shown as mean ± SD (n=5 per group), with statistical significance determined by unpaired student t test. (D–G) Day 6 after adoptive transfer, host mice were sacrificed and spleen cells were analyzed by flow cytometry. TCRβ+CD4+ T cells were gated to examine a live/dead marker versus active caspase-8 or caspase-3. Cells positive for both live/dead and caspase, considered dead via apoptosis, and cells positive for only caspase, dying via apoptosis, were added to determine the percent of cells that activated Caspase-8 and −3 pathways for apoptosis. Shown are representative flow plot (D) and summary data (E) of caspase-8 activation. Also shown are representative flow plot (F) and summary data (G) of caspase-3 activation. Summary data are combined from 3 independent experiments. Each dot represents a single mouse, with statistical significance determined by unpaired student t test.
We next examined the survival of T cells after adoptive transfer. At Day 6 after adoptive transfer, host mice were sacrificed and spleens removed to analyze active caspase-8 and −3. We gated on CD4+ T cells to examine a live/dead marker versus active caspase-8 or caspase-3 markers. We have observed significantly decreased caspase-8 activation (Figure 3D–E) and a trend towards decreased caspase-3 activation (Figure 3F–G) in CD70−/− T cells compared to WT T cells. Therefore, T cell intrinsic CD70 is associated with higher levels of caspase activation in T cells. These results suggest that the higher expansion of CD70−/− T cells was not due to increased proliferation, but a decrease in caspase-dependent T cell apoptosis.
A previous work showed that CD27 signaling in T cells can induce Fas-mediated activation induced cell death (AICD) in T cells encountering high antigen loads (30). Since our results show that T cell-derived CD70 induces a similar apoptosis phenotype, we analyzed the expression of Fas and Fas Ligand (FasL) on CD4+ T cells after adoptive transfer. However, CD70 deficiency does not seem to affect either Fas or FasL expression in this IBD model (data not shown), suggesting that Fas-independent mechanisms may act downstream of CD70 to induce CD4+ T cell apoptosis.
The CD27–CD70 pathway has been shown to promote Treg and Th1 development and suppress Th17 effector differentiation (14–17). Meanwhile Th1, Th17 and Treg cells have all been shown to play critical roles in the pathogenesis of IBD. Therefore, we examined whether T cell-derived CD70 affects T cell polarization and thereby suppressing IBD. We used flow cytometry to examine the master transcription factors including Tbet, GATA-3, RORγt and Foxp3 that respectively define Th1, Th2, Th17 and Treg lineages after adoptive transfer. Although we observed substantial expression of these transcription factors, there is no significant difference observed between WT and CD70−/− T cells (data not shown), suggesting that T cell-derived CD70 does not skew CD4+ T cell polarization in this IBD model.
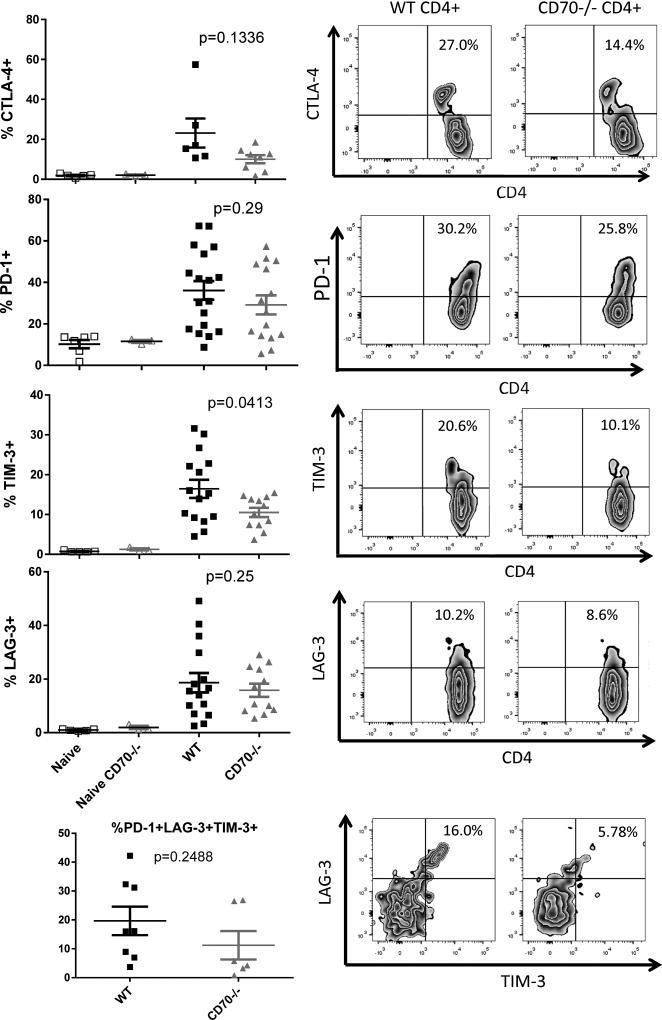
T cell-derived CD70 is associated with higher levels of immune checkpoint signals on T cells
We have shown that T cell-derived CD70 inhibits IBD and associated inflammatory T responses via a mechanism that involves caspase-dependent T cell apoptosis. Together, these phenotypic and mechanistic analyses reveal an immune inhibitory function for T cell intrinsic CD70, which highly resonates with the roles of several known immune checkpoint molecules such as PD-1 and TIM-3 (5–7, 10). This notion prompts us to examine the expression of the known checkpoint signals on T cells. Host mice were sacrificed on Day 6 after adoptive transfer, and spleens cells were analyzed for expression of various checkpoint markers. We have observed significantly increased TIM-3 expression and trends towards increased CTLA-4, PD-1, and LAG-3 expression on WT T cells compared to CD70−/− T cells (Figure 4). These results confirm that T cell-derived CD70 is closely associated with a suppressed state for T cells in this IBD model.
Figure 4. T cell-derived CD70 is associated with higher levels of checkpoint signals on T cells.
Naïve RAG1−/− mice in the C57BL/6 strain background were injected with 1×106 CD4+CD25− T cells purified from C57BL/6 WT or CD70−/− mice. Day 6 after adoptive transfer, host mice were sacrificed and spleens removed to analyze the expression of CTLA-4, PD-1, TIM-3, and LAG-3 by flow cytometry. Also analyzed are the purified CD4+CD25− T cells as pre-transplant controls. TCRβ+CD4+ T cells were gated to examine the percentages of cells that are positive for the indicated checkpoint signals. Statistical significance was determined by unpaired student t test.
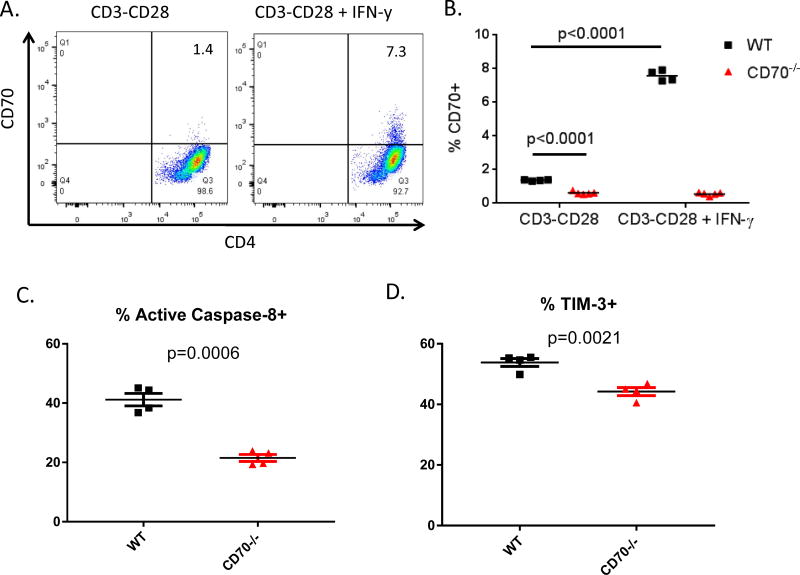
IFN-γ stimulates T cell-derived CD70 expression and function
To determine what signals induce CD70 expression in T cells, we used CD3 and CD28 antibodies to activate purified CD4+CD25− T cells in vitro. Following 48 hours in vitro activation, only minimal CD70 expression was detected by flow cytometry (Figure 5A). We reason that the inflammatory condition in vivo in the IBD model may have provided additional factors to enhance CD70 activation. Based on the in vivo cytokine profiles (Figure 2), we have tested various cytokines including IFN-γ, TNF-α, IL-2, IL-6 and IL-10 in addition to LPS for their ability to upregulate CD70 on T cells. Indeed, IFN-γ treatment significantly upregulates CD70 on T cells (Figure 5A–B) while other factors show no significant effect. Under this condition, WT T cells show significantly higher levels of caspase-8 activation (Fig 5C) and TIM-3 expression (Fig 5D) compared to CD70−/− T cells. In contrast, no significant difference was observed between WT and CD70−/− T cells with regard to the expression of T cell activation markers such as CD25, CD69, 41BB, ICOS, 41BBL and LIGHT (Supplemental Figure 2B). These results suggest that IFN-γ has the ability to stimulate T cell-derived CD70 expression and its immune checkpoint function.
Figure 5. IFN-γ stimulates T cell-derived CD70 expression and function.
CD4+CD25− T cells were isolated from naïve C57BL/6 WT and CD70−/− mice and added with soluble anti-CD28 to a 48-well plate coated with anti-CD3 the day prior. 0.5×106 T cells were added into each well in 0.5ml medium and treated with IFN-γ at 10ng/ml. After 48 hours T cells were harvested and analyzed by flow cytometry for CD70 expression. Shown are representative flow plots (A) and summary data (B) from 3 independent experiments with similar results. Statistical significance was determined by unpaired student t test. (C) TCRβ+CD4+ T cells were gated to examine a live/dead marker versus active caspase-8. Cells positive for both live/dead and caspase-8 were added to show the percent of cells that activated Caspase-8 pathway for apoptosis. (D) TCRβ+CD4+ T were gated to examine expression of TIM-3. Statistical significance was determined by unpaired student t test.
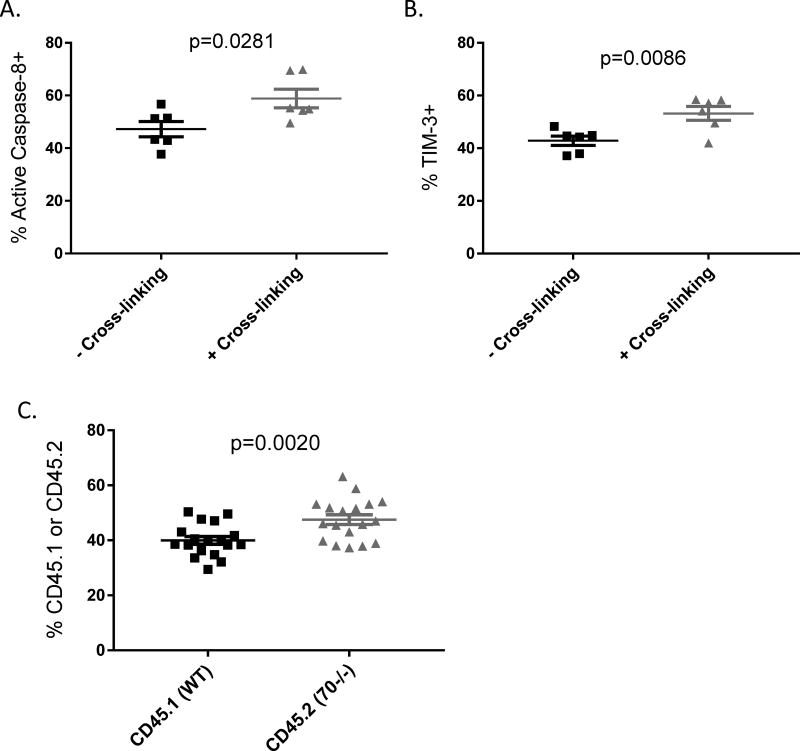
T cell intrinsic CD70 signaling contributes to the inhibitory checkpoint function
To test whether CD70 signals intrinsically in T cells to limit their expansion and upregulate immune checkpoint molecules, we performed in vitro activation of purified T cells with CD3 and CD28 antibodies followed by crosslinking CD70 with the FR70 Rat antibody and a secondary anti-Rat antibody. Under this condition, CD70 cross-linking indeed induces higher levels of caspase-8 activation and TIM-3 upregulation (Figure 6A–B). To test whether T cell intrinsic CD70 signaling occurs in vivo after adoptive transfer, we performed competition assays by mixing CD70+/+ (CD45.1) and CD70−/− (CD45.2) T cells in a 1:1 ratio for adoptive transfer. Our result shows that CD70−/− (CD45.2) T cells exhibit significantly higher expansion than CD70+/+ (CD45.1) T cells (Figure 6C). However, CD70-dependent differential expansion in vivo in the competition assays appears to be merely moderate, raising the question whether T cell-derived CD70 also interacts with CD27 on other cells to limit T cell expansion. To test this notion, we used CD27−/− T cells for adoptive transfer. Interestingly, we have found that CD27−/− T cells also caused more severe IBD (Supplemental Figure 3), suggesting that CD27–CD70 interaction also plays a suppressive role in this model. Altogether, these results demonstrate that T cell intrinsic CD70 signaling at least partially contributes to the observed immune checkpoint function.
Figure 6. T cell intrinsic CD70 signaling contributes to the inhibitory checkpoint function.
CD4+CD25− T cells were isolated from naïve C57BL/6 WT and CD70−/− mice and added with soluble anti-CD28 to a 48-well plate coated with anti-CD3 the day prior. 0.5×106 T cells were added into each well in 0.5ml medium and treated with IFN-γ at 10ng/ml. 22 hours later the Rat anti-CD70 antibody was added at 5ug/ml to “cross-linking” wells and then 2 hours later a secondary anti-Rat antibody was added to all wells and incubated for another 24 hours. Cells were harvested and analyzed by flow cytometry for active caspase-8 (A) and TIM-3 expression (B). (C) Naïve RAG1−/− mice were injected with 0.5×106 CD4+CD25− T cells purified from CD45.1 mice mixed with 0.5×106 CD4+CD25− T cells from CD45.2 CD70−/− mice. Day 17 after adoptive transfer, spleens were harvested to analyze the percentages of CD45.1 versus CD45.2 cells in the gated TCRβ+CD4+ T cells. Statistical significance was determined by unpaired student t test.
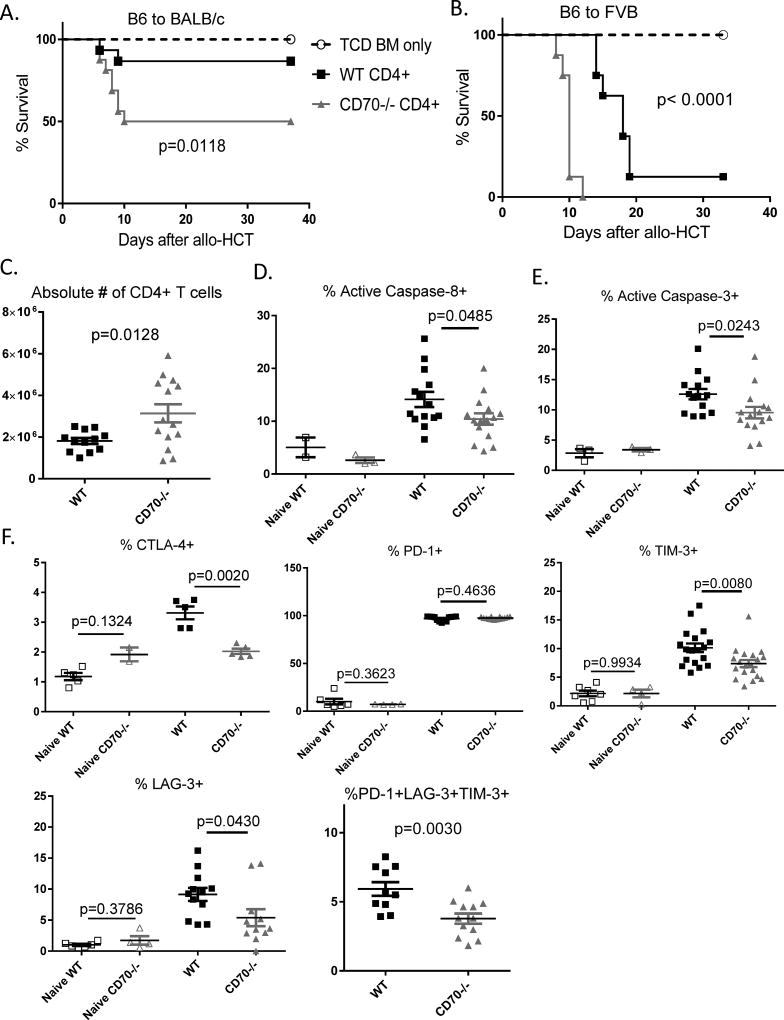
T cell-derived CD70 inhibits allogeneic CD4+ T cell response
Thus far our findings have revealed an unexpected immune inhibitory function for T cell-derived CD70 that is highly similar to and closely associated with defined immune checkpoint molecules. Next we tested whether its immune inhibitory function is limited to autoimmune T cell response in the IBD model. Therefore, we expand this study to examine the role of T cell-derived CD70 in allogeneic T responses. We performed allogeneic hematopoietic cell transplantation (allo-HCT) to study graft-versus-host disease (GVHD), an allogeneic condition that bears marked differences from autoimmune IBD but shares common features of inflammatory T cell responses and intestinal damages. First, we used the commonly used B6 → BALB/c model to test the role of T cell-derived CD70 in GVHD. When we used CD4+CD25− T cells to induce GVHD, CD70−/− T cells caused more severe lethal GVHD than WT T cells (Figure 7A). As the secondary GVHD model, we transplanted CD4+CD25− T cells in a B6 → FVB model, and observed that CD70−/− T cells also caused more severe lethal GVHD than WT T cells (Figure 7B). To further explore the underlying mechanisms, we examined donor T cell expansion, survival and expression of immune checkpoint molecules. Notably, CD70−/− T cells showed significantly higher expansion than WT T cells (Figure 7C), which exhibited significantly increased levels of caspase-8 and caspase-3 activation than CD70−/− T cells (Figure 7D–E). Remarkably, WT T cells also showed higher levels of expression of immune checkpoint molecules than CD70−/− T cells (Figure 7F). Together, these results indicate that in the allo-HCT model, T cell-derived CD70 inhibits GVHD via a mechanism highly similar to that in the IBD model that involves caspase-dependent T cell apoptosis and upregulation of defined immune checkpoint molecules. In addition, to test whether these checkpoint molecules have any biological impact in our system, we performed TIM-3 blockade and PD-1 blockade in our GVHD model. Our data (Supplemental Figure 4) showed that blocking TIM-3 shows a trend of making GVHD worse and blocking PD-1 indeed makes GVHD worse, in a fashion similar to CD70 deficiency in donor T cells. These data suggest that these immune checkpoint molecules do have a biological impact in our models, which are consistent with a few previous publications (31–33).
Figure 7. T cell-derived CD70 inhibits allogeneic CD4+ T cell response.
Lethally irradiated BALB/c or FVB hosts were transplanted with CD4+CD25− T cells purified from naïve C57BL/6 WT or CD70−/− mice. (A) Kaplan-Meier survival curves of BALB/c hosts injected with 2×104 CD4+CD25− T cells and 3×106 TCD BM. Data were summarized by combining 2 independent experiments (n=16 per group). (B) Kaplan-Meier survival curves of FVB hosts injected with 1×106 CD4+CD25− T cells and 3.5×106 TCD BM. Data were summarized by combining 2 independent experiments (n=8 per group). (A–B) Statistical significance was determined by Log-rank test. (C–F) Lethally irradiated BALB/c hosts were injected with 5×105 CD4+CD25− T cells and 3×106 TCD BM and sacrificed on Day 5. (C) Absolute numbers of CD4+ T cells were calculated by multiplying the total numbers of spleen cells by the percentages of TCRβ+CD4+ T cells present in spleen samples determined by flow cytometry. Summary data of caspase-8 activation (D) and caspase-3 activation (E) were combined from 3 independent experiments. (F) Summary data of CTLA-4, PD-1, TIM-3, and LAG-3 expression on TCRβ+CD4+ T cells at Day 5 after allo-HCT. Statistical significance was determined by unpaired student t test.
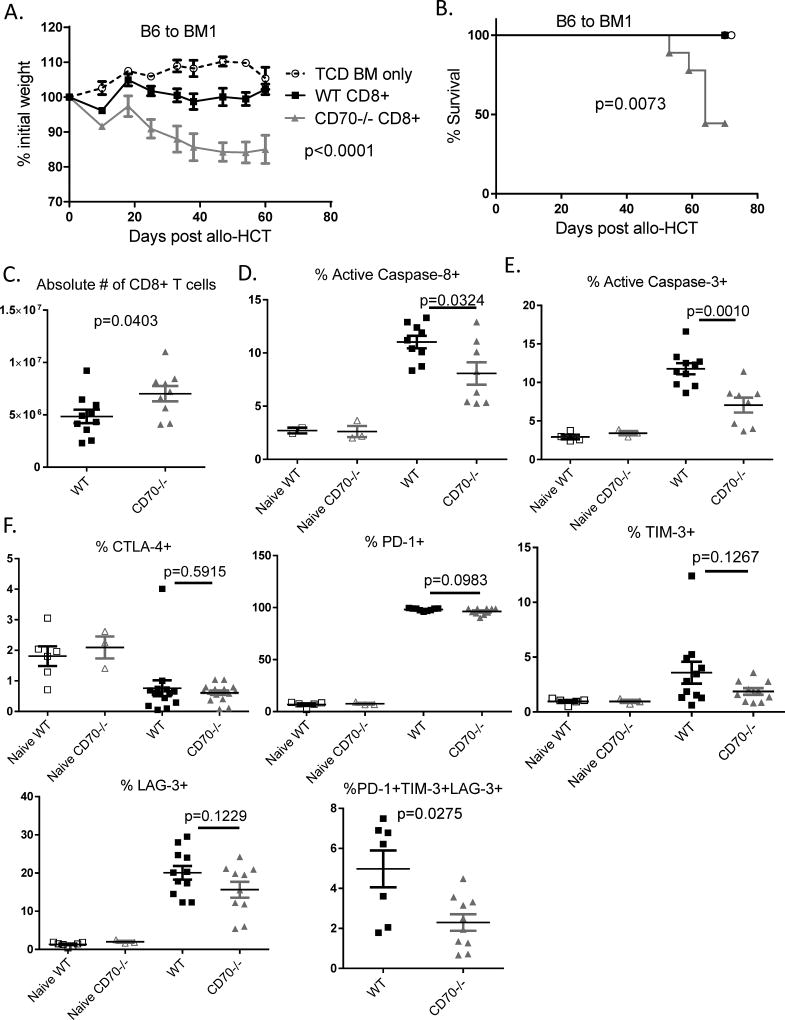
T cell-derived CD70 inhibits allogeneic CD8+ T cell response
The CD27–CD70 pathway is known to promote Treg cell development (14). As a result, Treg cell number is reduced by about 30% in CD70−/− mice. By a means of ruling out skewing the IBD and GVHD models by different number of Treg cells in the CD4+ T cells used for transfer, thus far we have used Treg-depleted CD4+CD25− T cells in this study. However, it remains an important question whether CD70-mediated immune inhibitory function observed with CD4+CD25− T cells also applies to CD8+ T cells. To answer this question, we used BM1 (H-2Kbm1) mice as hosts to perform allo-HCT in order to determine the contribution of CD70 in CD8+ T cell-mediated GVHD. BM1 mice carry an H-2K region b haplotype mutation which can stimulate MHC I-dependent activation of CD8+ T cells from C57BL/6 donors. As expected, CD70−/− CD8+ T cells caused more severe lethal GVHD than WT CD8+ T cells (Figure 8A–B). Next we examined donor CD8+ T cell expansion, survival and expression of immune checkpoint molecules in order to explore the underlying mechanisms. Indeed, CD70−/− CD8+ T cells showed significantly increased expansion than WT CD8+ T cells (Figure 8C), which exhibited significantly increased levels of caspase-8 and caspase-3 activation than CD70−/− CD8+ T cells (Figure 8D–E). Finally, WT CD8+ T cells also showed increased expression of immune checkpoint molecules than CD70−/− CD8+ T cells (Figure 8F), although the pattern of expression of these checkpoint signals varied slightly from that of CD4+ T cells. Overall, these results indicate that in this CD8+ T cell-mediated model, T cell intrinsic CD70 inhibits GVHD via essentially the same mechanism as that observed for CD4+ T cells in the IBD and GVHD models. That is, T cell-derived CD70 inhibits both CD4+ and CD8+ T cell responses via caspase-dependent T cell apoptosis and upregulation of inhibitory immune checkpoint molecules.
Figure 8. T cell-derived CD70 inhibits allogeneic CD8+ T cell response.
Lethally irradiated BM1 or FVB hosts were transplanted with CD8+ T cells purified from naïve C57BL/6 WT or CD70−/− mice. (A) Body weight was monitored after BM1 hosts injected with 2×106 CD8+ T cells and 3.5×106 TCD BM. Representative data from 1 of 2 experiments is shown as mean ± SD (n=5 per group), with statistical significance determined by two-way ANNOVA. (B) Kaplan-Meier survival data of BM1 hosts are combined from 2 independent experiments (n=8–9 per group). Statistical significance was determined by Log-rank test. (C–F) FVB hosts injected with 1×106 CD8+ T cells and 3×106 TCD BM and sacrificed on Day 9 or 12. (C) Absolute numbers of CD8+ T cells were calculated by multiplying the total numbers of spleen cells by the percentages of TCRβ+CD8+ T cells present in spleen samples determined by flow cytometry on day 12. Summary data of caspase-8 activation (D) and caspase-3 activation (E) are combined from 3 independent experiments. (F) Summary data of CTLA-4, PD-1, TIM-3, LAG-3 expression on TCRβ+CD8+ T cells at Day 9 after allo-HCT. Statistical significance was determined by unpaired student t test.
Discussion
The CD27–CD70 pair is typically known as a costimulatory pathway. Although the CD27–CD70 interaction has been previously studied in autoimmune disorders, viral infection and tumor models, earlier studies examined CD70 expression and function on APCs or host tissue cells. In this context, our study reveals a novel immune inhibitory function for T cell-derived CD70. In other words, while APC-expressed CD70 delivers a costimulation signal by binding to CD27 on T cells, T cell-expressed CD70 plays an unexpected immune checkpoint role that directly restrains T cell responses. Our results suggest that T cell-derived CD70 behaves in a similar fashion to the defined immune checkpoint molecules such as PD-1 and TIM-3. For the first time to our knowledge, our findings demonstrate that T cell intrinsic CD70 suppresses inflammatory T cell responses and does so by promoting T cell apoptosis. Remarkably, it is the typical proinflammatory IFN-γ that activates CD70 expression in T cells. Altogether, these findings strongly suggest that T cell-derived CD70 performs a critical negative feedback function to downregulate inflammatory T cell responses.
Our findings bestow an immune inhibitory function to T cell-derived CD70, which appears to be contradictory to a previous report wherein an ani-CD70 antibody was shown to suppress IBD (28). While we speculate that it is likely their ani-CD70 antibody (clone 3B9) may inadvertently agonize CD70 signaling in vivo rather than blocking CD27–CD70 interaction, our experiments with the CD70-knockout T cells provide a cleaner system to define the contribution of T cell-derived CD70. In addition, we used the most commonly used FR70 antibody and showed that blocking CD70 indeed exacerbated colitis in a fashion similar to CD70-knockout T cells. Meanwhile, we have confirmed this immune inhibitory function in both IBD and allogeneic GVHD models. Furthermore our recent publication shows that host-derived CD70 suppresses GVHD by limiting donor T cell expansion and effector function (34). Together our studies have demonstrated that T cell intrinsic CD70 signaling as well as CD27–CD70 interaction in both donor T cells and the host immune cells may contribute to the suppression of inflammatory T cell responses. Although initially unexpected, this inhibitory function fits with several recent studies that also started to reveal inhibitory roles for the CD27–CD70 pathway. For example, CD27 signaling has been shown to promote Treg development and Treg-mediated inhibition of tumor immunity (14, 15), and inhibit Th17 effector cell differentiation and associated autoimmunity (17). Furthermore, even earlier work suggested that when CD27-expressing T cells become activated in the presence of higher load of viral antigens, CD27 signaling could overturn from being costimulatory to being inhibitory via activation of Fas-driven T cell apoptosis (30). This mechanism helps to maintain T cell homeostasis during a viral infection. In this context, our data with the IBD and GVHD models suggest that chronic antigen presentation in the gut environment may contribute to the observed CD70-dependent inhibitory checkpoint function. Interestingly, another study demonstrated that CD70-CD27 ligation between neural stem cells and CD4+ T cells induces FasL expression on CD4+ T cells and subsequently Fas-FasL mediated T cell apoptosis (35). Nevertheless, our analyses found that CD70 deficiency in T cells does not affect either Fas or FasL expression after adoptive transfer, suggesting that Fas-independent mechanisms account for CD70-induced T cell apoptosis. This also raises a critical question on how T cell-derived CD70 back-signals into T cells to activate caspase-dependent apoptosis. Notably, several reports have shown that other TNF family members (CD40L, LIGHT, and 4-1BBL) may function via reverse signaling (36–38). However, identifying the intracellular signaling molecules proximal to these cell surface proteins will require further investigation.
When CD4+ T cells become activated they differentiate into Th1, Th2, Th17 and Treg cells. While terminal differentiation into these lineages play a critical role in both IBD and GVHD, it is also associated with upregulation of immune checkpoint molecules including TIM-3, which engages with its ligand galectin-9 to induce T cell apoptosis (39). Results from our study suggest that when CD4+ and CD8+ T cells become activated in the T cell transfer models of IBD and GVHD, CD70−/− T cells are less able than WT controls to upregulate immune checkpoint molecules including CTLA-4, TIM-3, PD-1, and LAG-3. These CD70−/− T cells also undergo less caspase-dependent apoptosis. Conversely the decreased survival of WT T cells contributes to ameliorate diseases in both IBD and GVHD. Importantly, our findings corroborate well with a recent study of lymphoma patients, which suggests that CD70 upregulation induces exhaustion of effector memory T cells in B-cell non-Hodgkin's lymphoma (40). Specifically, CD70+ effector memory T cells from these patients have an exhausted phenotype and express higher levels of PD-1 and TIM-3 compared with CD70– T cells. Signaling transduction, proliferation and cytokine production are profoundly decreased in the CD70+ T cells, and they are highly susceptible to apoptosis. Clinically, increased numbers of intratumoral CD70+ T cells correlate with an inferior patient outcome (40). Overall, this study of lymphoma patients is consistent with our murine models in defining an inhibitory immune checkpoint role for T cell-derived CD70, and provides clinical relevance to our findings.
In summary, our work demonstrates for the first time that T cell-derived CD70 directly inhibits inflammatory T cell responses via a regulatory T cell-independent mechanism that involves caspase-dependent T cell apoptosis and upregulation of inhibitory immune checkpoint molecules. Our findings indicate that CD70 expressed on T cells has the ability to ameliorate inflammatory diseases. Therefore, activating T cell-derived CD70 may be beneficial in the treatment of inflammatory diseases such as IBD and GVHD.
Supplementary Material
Acknowledgments
We thank Dr. Jonathan Ashwell at NCI for providing the CD70-deficienct mice for our project.
This work was supported by NIH research grant # R01CA184728 (X.C.) and utilized Shared Resources supported by the Roswell Park Cancer Institute’s Comprehensive Cancer Center Support Grant CA016056.
Footnotes
Disclosure of Conflicts of Interest
The authors have no special/competing interests to disclose.
References
- 1.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature reviews. Immunology. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 Costimulation: From Mechanism to Therapy. Immunity. 2016;44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annual review of immunology. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 7.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annual review of immunology. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 8.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. The Journal of clinical investigation. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Current topics in microbiology and immunology. 2011;350:1–15. doi: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- 11.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nature immunology. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Y, Peperzak V, Keller AM, Borst J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J Immunol. 2008;181:1071–1082. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]
- 13.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. The Journal of experimental medicine. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y, Neves JF, Fonseca-Pereira D, Jacobs H, Pennington DJ, Silva-Santos B, Borst J. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. The Journal of experimental medicine. 2013;210:715–728. doi: 10.1084/jem.20112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claus C, Riether C, Schurch C, Matter MS, Hilmenyuk T, Ochsenbein AF. CD27 signaling increases the frequency of regulatory T cells and promotes tumor growth. Cancer research. 2012;72:3664–3676. doi: 10.1158/0008-5472.CAN-11-2791. [DOI] [PubMed] [Google Scholar]
- 16.van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MH, Eldering E, van Lier RA. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. International immunology. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 17.Coquet JM, Middendorp S, van der Horst G, Kind J, Veraar EA, Xiao Y, Jacobs H, Borst J. The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity. Immunity. 2013;38:53–65. doi: 10.1016/j.immuni.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 19.Laouar A, Haridas V, Vargas D, Zhinan X, Chaplin D, van Lier RA, Manjunath N. CD70+ antigen-presenting cells control the proliferation and differentiation of T cells in the intestinal mucosa. Nature immunology. 2005;6:698–706. doi: 10.1038/ni1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 21.Munitic I, Kuka M, Allam A, Scoville JP, Ashwell JD. CD70 deficiency impairs effector CD8 T cell generation and viral clearance but is dispensable for the recall response to lymphocytic choriomeningitis virus. J Immunol. 2013;190:1169–1179. doi: 10.4049/jimmunol.1202353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allam A, Swiecki M, Vermi W, Ashwell JD, Colonna M. Dual function of CD70 in viral infection: modulator of early cytokine responses and activator of adaptive responses. J Immunol. 2014;193:871–878. doi: 10.4049/jimmunol.1302429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian G, Ding X, Leigh ND, Tang Y, Capitano ML, Qiu J, McCarthy PL, Liu H, Cao X. Granzyme B-mediated damage of CD8+ T cells impairs graft-versus-tumor effect. J Immunol. 2013;190:1341–1350. doi: 10.4049/jimmunol.1201554. [DOI] [PubMed] [Google Scholar]
- 24.Ding X, Bian G, Leigh ND, Qiu J, McCarthy PL, Liu H, Aygun-Sunar S, Burdelya LG, Gudkov AV, Cao X. A TLR5 agonist enhances CD8(+) T cell-mediated graft-versus-tumor effect without exacerbating graft-versus-host disease. J Immunol. 2012;189:4719–4727. doi: 10.4049/jimmunol.1201206. [DOI] [PubMed] [Google Scholar]
- 25.Du W, Leigh ND, Bian G, O'Neill RE, Mei L, Qiu J, Chen GL, Hahn T, Liu H, McCarthy PL, Cao X. Granzyme B-Mediated Activation-Induced Death of CD4+ T Cells Inhibits Murine Acute Graft-versus-Host Disease. J Immunol. 2015;195:4514–4523. doi: 10.4049/jimmunol.1500668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizoguchi A. Animal models of inflammatory bowel disease. Progress in molecular biology and translational science. 2012;105:263–320. doi: 10.1016/B978-0-12-394596-9.00009-3. [DOI] [PubMed] [Google Scholar]
- 27.Abraham C, Cho JH. Inflammatory bowel disease. The New England journal of medicine. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manocha M, Rietdijk S, Laouar A, Liao G, Bhan A, Borst J, Terhorst C, Manjunath N. Blocking CD27-CD70 costimulatory pathway suppresses experimental colitis. J Immunol. 2009;183:270–276. doi: 10.4049/jimmunol.0802424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. American journal of physiology. Gastrointestinal and liver physiology. 2009;296:G135–146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wensveen FM, Unger PP, Kragten NA, Derks IA, ten Brinke A, Arens R, van Lier RA, Eldering E, van Gisbergen KP. CD70-driven costimulation induces survival or Fas-mediated apoptosis of T cells depending on antigenic load. J Immunol. 2012;188:4256–4267. doi: 10.4049/jimmunol.1102889. [DOI] [PubMed] [Google Scholar]
- 31.Oikawa T, Kamimura Y, Akiba H, Yagita H, Okumura K, Takahashi H, Zeniya M, Tajiri H, Azuma M. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol. 2006;177:4281–4287. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 32.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 33.Veenstra RG, Taylor PA, Zhou Q, Panoskaltsis-Mortari A, Hirashima M, Flynn R, Liu D, Anderson AC, Strom TB, Kuchroo VK, Blazar BR. Contrasting acute graft-versus-host disease effects of Tim-3/galectin-9 pathway blockade dependent upon the presence of donor regulatory T cells. Blood. 2012;120:682–690. doi: 10.1182/blood-2011-10-387977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leigh ND, O'Neill RE, Du W, Chen C, Qiu J, Ashwell JD, McCarthy PL, Chen GL, Cao X. Host-Derived CD70 Suppresses Murine Graft-versus-Host Disease by Limiting Donor T Cell Expansion and Effector Function. J Immunol. 2017;199:336–347. doi: 10.4049/jimmunol.1502181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee EM, Hurh S, Cho B, Oh KH, Kim SU, Surh CD, Sprent J, Yang J, Kim JY, Ahn C. CD70-CD27 ligation between neural stem cells and CD4+ T cells induces Fas-FasL-mediated T-cell death. Stem cell research & therapy. 2013;4:56. doi: 10.1186/scrt206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner B, Koppenhoefer U, Lepple-Wienhues A, Grassme H, Muller C, Speer CP, Lang F, Gulbins E. The CD40 ligand directly activates T-lymphocytes via tyrosine phosphorylation dependent PKC activation. Biochemical and biophysical research communications. 1997;239:11–17. doi: 10.1006/bbrc.1997.7415. [DOI] [PubMed] [Google Scholar]
- 37.Lim SG, Suk K, Lee WH. Reverse signaling from LIGHT promotes pro-inflammatory responses in the human monocytic leukemia cell line, THP-1. Cellular immunology. 2013;285:10–17. doi: 10.1016/j.cellimm.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, Lee SC, Kwon BS, Mittler RS, Cho HR, Kwon B. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E13–22. doi: 10.1073/pnas.1112256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Chen G, Li Y, Wang R, Wang L, Lin Z, Gao X, Feng J, Ma Y, Shen B, Han G. Involvement of T cell Ig Mucin-3 (Tim-3) in the negative regulation of inflammatory bowel disease. Clin Immunol. 2010;134:169–177. doi: 10.1016/j.clim.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Yang ZZ, Grote DM, Xiu B, Ziesmer SC, Price-Troska TL, Hodge LS, Yates DM, Novak AJ, Ansell SM. TGF-beta upregulates CD70 expression and induces exhaustion of effector memory T cells in B-cell non-Hodgkin's lymphoma. Leukemia. 2014;28:1872–1884. doi: 10.1038/leu.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.