Hypertrophic cardiomyopathy (HCM) is a genetically transmitted cardiac disease, characterized by increased left ventricular (LV) wall thickness in the absence of abnormal loading conditions 1. The estimated prevalence of HCM is approximately 1 per 500 in the general population 2, with a diverse clinical course including heart failure and sudden cardiac arrest (SCA), but also asymptomatic survival to normal life expectancy 3. SCA due to ventricular arrhythmias is a major cause of mortality in young and middle-aged HCM patients 4. With modern management strategies, including implantable cardioverter-defibrillator (ICD) therapy for high-risk individuals, contemporary disease-related mortality has been reported to be around 0.5% annually 4. These estimates are derived from several HCM patient registries, representing a somewhat selected patient population. However, the absence of symptoms causes HCM to remain undetected in a large proportion of patients. Since little data exist on the burden of HCM-related SCA in the community, in the present study we assessed the incidence and characteristics of HCM-related SCA in the young and middle-aged general population, from the ongoing Oregon Sudden Unexpected Death Study (Oregon SUDS). This study was approved by the Institutional Review boards of Cedars-Sinai Medical Center, Oregon Health and Science University, and all participating hospitals.
The Oregon SUDS uses multiple-source ascertainment to prospectively identify all cases of out-of-hospital SCA occurring in the Portland, Oregon, metropolitan area (catchment population approximately one million) 5. All existing medical information, including emergency medical services reports, patient’s lifetime clinical records, death certificates and autopsy reports, were obtained for each case. Subjects included in the present study were 5–59 years of age. Due to the likely low burden of HCM-related SCA in the elderly 3, and increasing difficulties in differentiating HCM from secondary LV hypertrophy, our study did not include patients ≥60 years of age. Diagnosis of HCM was based on echocardiography or autopsy reports. As recommended in the current guidelines 1, echocardiographic left ventricle (LV) wall thickness ≥15mm without a secondary cause such as aortic stenosis or hypertension was used to diagnose HCM. In autopsied patients, significant unexplained LV hypertrophy ≥17mm with histological evidence of HCM, or characteristic asymmetric LV hypertrophy was required for HCM diagnosis. For comparisons between clinical and demographic characteristics of HCM and other SCA cases, independent samples t-test and Pearson’s chi-squared test were used. For calculation of SCA incidence rates, the analysis was limited to data collected from Portland’s Multnomah County (average 5–59-year-old population 547,570). For the subgroup of SCA patients without significant SCA-comorbidities diagnosed prior to arrest, and no autopsy or lifetime echocardiography data available, the incidence of HCM was imputed based on prevalence of HCM detected by echocardiography and autopsy (Figure).
Figure.
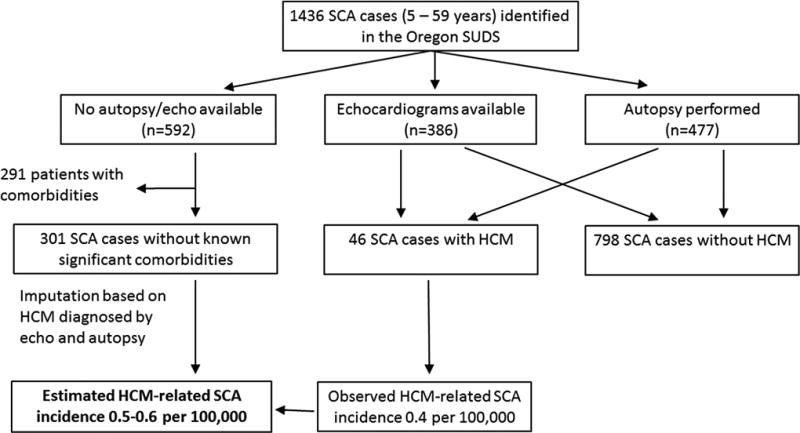
Flowchart for determination of HCM-related SCA and estimation of incidence in the general population. HCM was documented in 3.2% of all SCA cases, and in 5.5% of those who underwent echocardiography or autopsy.
Between 2002–15, 1436 SCAs were identified in subjects aged 5–59 years (mean age 47.8±10.4 years; 75.0% male), including 154 patients (10.7%) who survived to hospital discharge. Echocardiography had been performed on 386 subjects (26.9%) and 477 (33.2%) underwent autopsy. After a detailed review, 46 cases were identified as HCM-related SCA. 89% of HCM cases were male, and compared to other SCA cases, they were >10 years younger (36.9±13.2 vs. 48.1±10.1 years; p<0.001) and had significantly fewer cardiac risk factors and comorbidities. 73% of HCM cases presented with ventricular tachycardia or fibrillation at the time of arrest compared to only 49% of other SCA cases (p=0.009). Among HCM patients, 15 (33%) of the cardiac arrests occurred during or immediately after vigorous physical activity; 20 (43%) occurred during routine daily activities and 11 (24%) at rest or during sleep. For 37 (80%) patients, HCM diagnosis was made only at autopsy, with a mean wall thickness of 24±6 mm. Of HCM patients, 72% had isolated septal hypertrophy, and the remainder presented with concentric, apical or free wall hypertrophy.
Overall, HCM-related SCA comprised 3.2% of all SCAs among the young and middle-aged population (10.2% among 5–34-year-old; 2.2% among 35–59-year-old). Among those who underwent echocardiography or autopsy, HCM-related SCA comprised 5.5% (46/844) of overall SCA. After imputing for SCA cases for whom autopsy or echocardiography was not available, the estimated incidence for HCM-related SCA was 0.5–0.6 per 100,000 inhabitants (Figure). Given the estimated prevalence of HCM in the community (1:500) 2, the annual SCA incidence in this unselected HCM patient population that was mostly undiagnosed during their lifetime, would be 0.2–0.3%.
In summary, HCM was responsible for 1 in 30 SCAs in the young and middle-aged general population, which translated to an annual SCA incidence of 0.2–0.3% among unselected HCM patients in the community. In the large majority of SCA cases, the diagnosis of HCM was missed prior to their cardiac arrest. However, the low estimated rate of SCA in young and middle-aged HCM patients demonstrates the relatively benign course of the disease in the vast majority of affected individuals.
Acknowledgments
Funding: Funded by National Institutes of Health, National Heart Lung and Blood Institute grants R01HL122492 and R01HL126938 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Chair in Cardiac Electrophysiology at Cedars-Sinai, Los Angeles. Dr Aro is funded by grants from Finnish Cultural Foundation, Finnish Foundation for Cardiovascular Research, Paavo Nurmi Foundation, Orion Research Foundation, Biomedicum Helsinki Foundation and Emil Aaltonen Foundation.
Footnotes
Disclosures: None
References
- 1.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice G 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2011;124:2761–2796. [Google Scholar]
- 2.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the Cardia study. Coronary artery risk development in (young) adults. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Rowin EJ, Casey SA, Haas TS, Chan RH, Udelson JE, Garberich RF, Lesser JR, Appelbaum E, Manning WJ, Maron MS. Risk stratification and outcome of patients with hypertrophic cardiomyopathy >=60 years of age. Circulation. 2013;127:585–593. doi: 10.1161/CIRCULATIONAHA.112.136085. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Rowin EJ, Casey SA, Link MS, Lesser JR, Chan RH, Garberich RF, Udelson JE, Maron MS. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol. 2015;65:1915–1928. doi: 10.1016/j.jacc.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large U.S. Community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]


