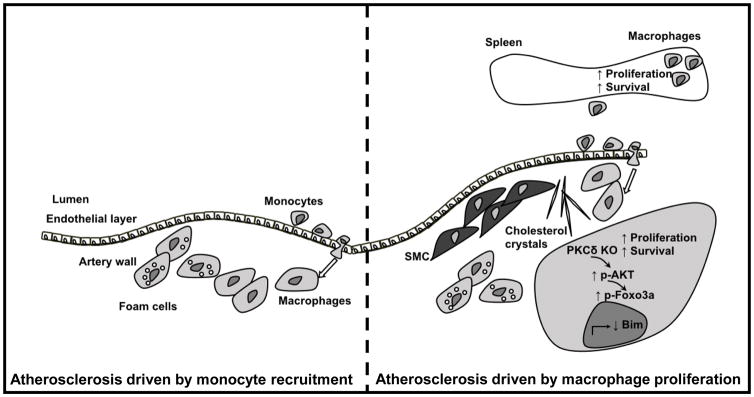
Atherosclerosis, the underlying pathology in most cardiovascular disease, is a chronic inflammatory disease1, characterized by accumulation of macrophages in the sub-endothelial space. Macrophages are protagonists of the disease, both early in the disease as well as during later stages of atherosclerotic lesion progression, with a continuous turnover of the cells2. Blood monocytes enter the artery wall and differentiate into macrophages, contributing to atherosclerotic lesion growth3, but local proliferation and survival of existing artery wall macrophages also contribute to lesional macrophage plaque burden (Figure)2.
Figure 1.
Macrophages play an important role in all phases of atherosclerosis. Monocyte recruitment is critical during early atherosclerotic disease (and perhaps during certain other situations; left part of figure) and thus at this stage of the disease, blood monocyte levels are an important determining factor. Local macrophage proliferation dominates macrophage accumulation in later stages of atherosclerosis (right part of figure). Macrophage PKCδ is induced by modified lipids that can be found in the plaque. Myeloid cell PKCδ primarily impairs macrophage survival and proliferation, via regulation of phosphorylation of Akt and Foxo3a and ultimately the apoptotic regulator Bim. Deletion of PKCδ results in increased proliferation and survival, both in the artery wall and in the spleen resulting in more atherosclerosis and enlargement of the spleen. SMC-smooth muscle cells.
In this issue of Circulation Research, Li and co-workers demonstrate an unexpected role for Protein Kinase C-δ (PKCδ) in monocytes and macrophages in the development of atherosclerosis in a mouse model4. PKCδ is part of a group of PKCs that are typically activated by diacylglycerol, and PKCδ is thought to play an important role in the development of insulin resistance and perhaps its complications5, but its role atherosclerosis is unknown. Some studies6, but not all7, have suggested that PKCδ is involved in foam cell formation, a hallmark of atherosclerosis. Li and co-workers demonstrate that PKCδ is induced in monocytes and macrophages in response to dyslipidemia and inflammatory stimuli associated with metabolic disease and atherosclerosis, but that myeloid cell PKCδ does not appear to play any role in macrophage lipid loading in vivo4.
Since PKCδ was induced in myeloid cells in response to dyslipidemia associated with atherosclerosis, one might have hypothesized that deleting this enzyme in myeloid cells would have had a protective effect. On the contrary, selective deletion of PKCδ in myeloid cells, using LysM-Cre-mediated deletion, dramatically increased atherosclerosis4. The increased atherosclerosis was associated with an increase in lesional macrophage accumulation, but with no evidence of increased recruitment of monocytes into the lesion. Deletion of PKCδ dramatically reduced circulating levels of more or less all blood leukocytes, including monocytes, neutrophils, B cells and T cells in response to a high-fat challenge, without altering bone marrow progenitor cells. The authors explain the reduction in all circulating cells by arguing that the observed splenomegaly is trapping circulating cells, thus resulting in generalized leukopenia. The reduction in these circulating cells, which by many would have been predictive of a reduced propensity for atherogenesis8, 9, did not lead to reduced lesion size in the mice with myeloid cell PKCδ-deficiency. Coincidentally, also in this issue of Circulation Research, Williams and coworkers10 demonstrate a reduction in atherosclerosis in mice maintained in thermoneutrality, which they attribute to a reduction in circulating monocyte numbers in response to thermoneutral ambient temperatures (30°C) 10, further highlighting the surprising and important finding by Li and colleagues. Although the animal models that were used were different in the two studies, it is becoming increasingly clear that reduced levels of blood monocytes are not always associated with an athero-protective effect.
So how does myeloid cell PKCδ-deficiency increase atherosclerosis? Robbins and coworkers demonstrated in a hallmark paper in 2013 that local proliferation dominates macrophage accumulation in more advanced atherosclerotic lesions2. A similar finding was reported by Lindau et al., who demonstrated that reduced circulating monocyte levels only affected early lesions but not more advanced atherosclerotic lesions11. In the present study, Li et al. demonstrated that myeloid cell deficiency in PKCδ indeed increase macrophage proliferation in the atherosclerotic lesion (figure)4. The authors could show increases in both BrdU-incorporation and Ki67-staining in PKCδ-deficient macrophages in the atherosclerotic lesion, both which are markers of proliferation. The authors also found a concomitant reduction in macrophage apoptosis. This increase in proliferation and reduction in apoptosis resulted in larger, more macrophage-rich lesions in mice with PKCδ deletion in myeloid cells. The necrotic cores were not larger in the animals with myeloid cell PKCδ-deficiency, further strengthening the authors’ conclusion, as dying macrophages greatly contribute to the necrotic core formation12. However, one would be curious of what would happen if the lesions were allowed to progress even further, at later time points. Would having increased numbers of surviving, live macrophages aid in the clearance of necrotic debris, thereby counteracting the accelerated atherosclerosis? On a mechanistic level, Li et al. showed, using isolated macrophages, that the lack of PKCδ reduces the cell’s sensitivity to apoptotic stimuli by increasing and/or maintaining phosphorylated Akt levels. The authors then went on to demonstrate the this increase in Akt phosphorylation results in a reduction in the pro-apoptotic regulator Bim, via a Foxo3a-dependet mechanism. Finally, Li and coworkers showed that myeloid cell PKCδ-deficiency results in macrophage proliferation in the spleen resulting in splenomegaly. The splenomegaly is an interesting observation. The contribution of splenomegaly to systemic leukocyte levels and atherogenesis could be further tested using for example splenectomized mice.
In summary, Li and colleagues4 have highlighted the complexity of macrophage biology in atherosclerosis. Not only did deletion of PKCδ not have the anticipated athero-protective effect, but deletion of this kinase in myeloid cells also demonstrated the interplay between recruitment of blood monocytes and local macrophage proliferation and reduced apoptosis. Monocyte recruitment may be a less significant contributor to lesional macrophage accumulation during the later stages of disease, where local macrophage proliferation dominates. However, it is not inconceivable that certain situations are changing the dynamics of monocyte recruitment versus lesional macrophage proliferation and survival, such as diabetes13 or myocardial infarction14, both thought to accelerate atherosclerosis via monocytosis. In both diabetes and myocardial infarction, monocytosis does not appear to be driven by dyslipidemia, but rather by hyperglycemia13 and activation of the sympathetic nervous system14, respectively. So, is the relative contribution of these mechanisms different in different animal models? Furthermore, the relevance and contribution of these pathways in human disease remains to be elucidated. Finally, all monocytes and macrophages are not created equally, and will not behave the same way. If we can find ways to reduce their inflammatory potential and stimulate them to take on a more reparative phenotype, the number of monocytes and macrophages might become less relevant.
Acknowledgments
Source of Funding
Research in the author’s laboratory is funded in part by the American Diabetes Association grant 1-16-IBS-15 and Diabetes Research Center Pilot and Feasibility grant (P30 DK017047).
Footnotes
Conflicts of interest
None
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–72. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Park K, Xia Y, Matsumoto M, Qi W, Fu J, Yokomizo H, Khamaisi M, Wang X, Rask-Madsen C, King GL. Regulation of Macrophage Apoptosis and Atherosclerosis by Lipid Induced PKCdelta Isoform Activation. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.117.311606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezy O, Tran TT, Pihlajamaki J, Suzuki R, Emanuelli B, Winnay J, Mori MA, Haas J, Biddinger SB, Leitges M, Goldfine AB, Patti ME, King GL, Kahn CR. PKCdelta regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J Clin Invest. 2011;121:2504–17. doi: 10.1172/JCI46045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CS, Lin FY, Ho LJ, Tsai CS, Cheng SM, Wu WL, Huang CY, Lian CH, Yang SP, Lai JH. PKCdelta signalling regulates SR-A and CD36 expression and foam cell formation. Cardiovasc Res. 2012;95:346–55. doi: 10.1093/cvr/cvs189. [DOI] [PubMed] [Google Scholar]
- 7.Szilagyi K, Meijer AB, Neele AE, Verkuijlen P, Leitges M, Dabernat S, Forster-Waldl E, Boztug K, Belot A, Kuijpers TW, Kraal G, de Winther MP, van den Berg TK. PKCdelta is dispensible for oxLDL uptake and foam cell formation by human and murine macrophages. Cardiovasc Res. 2014;104:467–76. doi: 10.1093/cvr/cvu213. [DOI] [PubMed] [Google Scholar]
- 8.Dutta P, Nahrendorf M. Regulation and consequences of monocytosis. Immunol Rev. 2014;262:167–78. doi: 10.1111/imr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman MS, Murphy AJ, Woollard KJ. Effects of dyslipidaemia on monocyte production and function in cardiovascular disease. Nat Rev Cardiol. 2017;14:387–400. doi: 10.1038/nrcardio.2017.34. [DOI] [PubMed] [Google Scholar]
- 10.Williams JW, Elvington A, Ivanov S, Kessler S, Luehmann H, Baba O, Saunders BT, Kim KW, Johnson MW, Craft CS, Choi JH, Sorci-Thomas MG, Zinselmeyer BH, Brestoff JR, Liu Y, Randolph GJ. Thermoneutrality but Not UCP1 Deficiency Suppresses Monocyte Mobilization Into Blood. Circ Res. 2017;121:662–76. doi: 10.1161/CIRCRESAHA.117.311519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindau A, Hardtner C, Hergeth SP, Blanz KD, Dufner B, Hoppe N, Anto-Michel N, Kornemann J, Zou J, Gerhardt LM, Heidt T, Willecke F, Geis S, Stachon P, Wolf D, Libby P, Swirski FK, Robbins CS, McPheat W, Hawley S, Braddock M, Gilsbach R, Hein L, von zur Muhlen C, Bode C, Zirlik A, Hilgendorf I. Atheroprotection through SYK inhibition fails in established disease when local macrophage proliferation dominates lesion progression. Basic Res Cardiol. 2016;111:20. doi: 10.1007/s00395-016-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50(Suppl):S382–7. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–9. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]