Abstract
Our previous studies found that mitochondrial uncouplers induced vasodilation. Triclosan, the broad spectrum antibacterial agent, is the active ingredient in soaps and toothpastes. It was reported that triclosan induced mitochondrial uncoupling, so we aim to investigate the effects of triclosan on vascular function of rat mesenteric arteries and aorta. The isometric tension of rat mesenteric artery and thoracic aorta was recorded by multi-wire myograph system. The cytosolic [Ca2+]i, mitochondrial reactive oxygen species (mitoROS), and mitochondrial membrane potential of smooth muscle cells (A10 cells) were measured using laser scanning confocal microscopy. Triclosan treatment relaxed phenylephrine (PE)- and high K+ (KPSS)-induced constriction, and pre-treatment with triclosan inhibited PE- and KPSS-induced constriction of rat mesenteric arteries. In rat thoracic aorta, triclosan also relaxed PE- and KPSS-induced constriction. Triclosan induces vasorelaxation without involving KATP channel activation in smooth muscle cells of arteries. Triclosan treatment increased cytosolic [Ca2+]i, mitochondrial ROS production and depolarized mitochondrial membrane potential in A10 cells. In conclusion, triclosan induces mitochondrial uncoupling in vascular smooth muscle cells and relaxes the constricted rat mesenteric arteries and aorta of rats. The present results suggest that triclosan would indicate vasodilation effect if absorbed excessively in vivo.
KEY WORDS: Triclosan, Mitochondrial uncoupling, Artery, Smooth muscle cells, Vasorelaxation
Graphical abstract
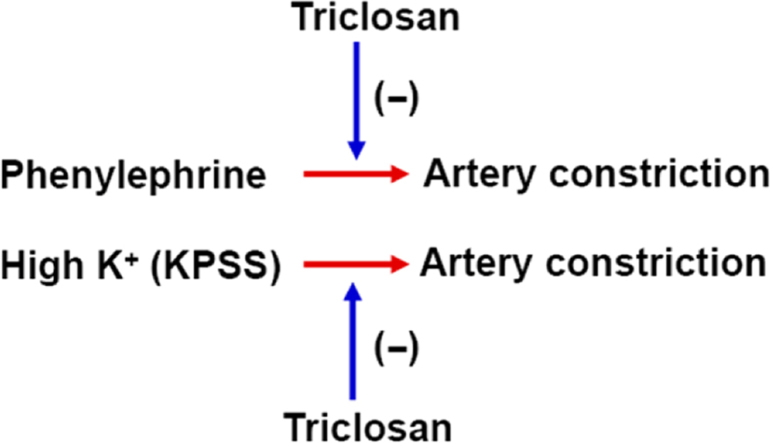
Triclosan induces mitochondrial uncoupling in vascular smooth muscle cells and relaxes the constricted mesenteric arteries and aorta of rats in endothelium-independent manner.
1. Introduction
Triclosan (2,4,4ʹ-trichloro-2ʹ-hydroxydiphenyl), a broad spectrum antimicrobial agent, has been widely used in soaps, mouthwashes, toothpastes and other products in household personal care and hospital applications. In addition to their antimicrobial effects, triclosan has multiple biological functions, including disrupting endocrine function1 and suppressing the function of natural killer cell2 and mast cells3, and was reported inducing the mitochondrial uncoupling4. Because of the ubiquitous use, contamination of triclosan has been detected in different environmental matrices including terrestrial, aquatic and biosolids5, thus the implication of triclosan pollution for human and environmental health has been concerned.
Mitochondrial uncoupling is a process of proton leaking from the intermembrane space into the mitochondrial matrix which prevents the development of a proton electrochemical gradient and reduces ATP production. Mitochondrial uncoupling is generally induced by mitochondrial uncoupling proteins (UCPs) or chemical mitochondrial uncouplers. Our previous studies found that mitochondrial uncouplers carbonyl cyanide m-chlorophenylhydrazone (CCCP) and niclosamide induced vasodilation of constricted arteries6, 7. We put forward an opinion that the chemical mitochondrial uncouplers hold the properties of vasoactivity in common, in other words, chemical mitochondrial uncouplers relax the constricted arteries and prevent stimuli-induced artery constriction. Weatherly et al.4 reported that triclosan induced mitochondrial uncoupling in living rat and human mast cells and in primary human keratinocytes. Based on our opinion, triclosan would induce vasorelaxation. Therefore the aim of the present work is to investigate the effects of triclosan on vascular function of rat mesenteric arteries and aorta.
2. Materials and methods
2.1. Animals and agents
The adult Sprague–Dawley rats (male, body weight 320–350 g, 8–10 weeks) were purchased from Charles River (Charles River Laboratory Animal, Beijing, China). All the experimental procedures were approved by the Institutional Animal Care and Use Committee of Harbin Medical University, China. Phenylephrine (PE) was purchased from Shanghai Harvest Pharmaceutical Co., Ltd., China. Triclosan were purchased from Shanghai Dibai biological technology Co., Ltd. (Shanghai, China). PE and acetylcholine (Ach) were dissolved in distilled water, and others were dissolved in DMSO (Tianjin Fuyu Fine Chemical Co., Ltd.).
2.2. Cell culture
Arterial smooth muscle cells (A10) were purchased from American Type Culture Collection. A10 cells were cultured in DMEM medium (high glucose) containing 15% FBS and 1% penicillin/streptomycin at 37 °C/5% CO2. Cells were used within 8 passages.
2.3. Rat mesenteric artery and thoracic aorta preparation
Adult male Sprague–Dawley rats were sacrificed after anesthetized by sodium pentobarbitone (40 mg/kg, i.p.). The entire mesentery and thoracic aorta was removed quickly, then transferred into cold (4 °C) modified physiological salt solution (PSS) with the following composition (mmol/L): NaCl, 130; KCl, 4.7; MgSO4·7H2O, 1.17; KH2PO4, 1.18; NaHCO3, 14.9; CaCl2, 1.6; D-glucose, 5.5 (pH 7.35–7.45). Fat tissues of mesenteric artery and thoracic aorta were separated. The mesenteric arteries and thoracic aorta were dissected into 2-mm and 3–4-mm rings, respectively.
2.4. Isometric tension recording of mesenteric artery and thoracic aorta
The experiments were carried out according to our previous work6, 7, 8, 9. The mesenteric artery and thoracic aorta rings were randomized for different treatments. Mesenteric arterial ring was mounted between two wires, and fixed in the bath filled with 5 mL PSS and were continuously bubbled with gas (95% O2 + 5% CO2). Thoracic aortic rings were mounted in triangle-shape hook and then suspended in the bath filled with 10 mL PSS and were continuously bubbled with gas (95% O2 + 5% CO2). The isometric contractions of mesenteric arterial rings were measured by using multi wire myograph system (model 620 DMT, Danish Myo Technology, Denmark), and the isometric contractions of thoracic aortic rings were measured by using multi-channel myograph system (BL-420S, Chengdu Taimeng Software Co., Ltd., China). The arterial rings were equilibrated for 60 min before the experiment. The resting tension of mesenteric arterial ring was 0.6 mN and this value of thoracic aorta ring was 1.96 mN, which were the optimal preload for force development of the vessels determined in preliminary studies. Then a wake-up protocol was performed to reactivate the mechanical, function, and signaling properties of the vessels by using high K+ PSS (KPSS) and phenylephrine (PE) stimuli. The KPSS (60 mmol/L K+) solution inducing vasoconstriction was composed of (mmol/L): NaCl, 74.7; KCl, 60; MgSO4·7H2O, 1.17; KH2PO4, 1.18; NaHCO3, 14.9; CaCl2, 1.6; D-glucose, 5.5; EDTA, 0.026. In order to avoid the error induced by natural rundown of the artery tension, we calculated the relaxation ratio of triclosan by subtracting the relaxation ratio of corresponding control (DMSO).
2.5. Measurement of mitochondrial reactive oxygen species
The methods in detail were described as in our previous studies6, 7, 8. Cultured arterial smooth muscle cells (A10) were loaded with MitoSOX (2 µmol/L) for 20 min and Hoechst (1 µg/mL) for 15 min at 37 °C and then the fluorescence was measured by using confocal microscopy. Confocal microscope images were collected by using a Zeiss LSM 700 with the Zeiss LSM software (Zeiss, Oberkochen, Germany). Images of MitoSOX fluorescence were obtained using a 40 × oil objective with an excitation at 555 nm and an emission of 585 nm. Images of Hoechst staining were obtained by using excitation at 405 nm and an emission of 435 nm. The levels of mitochondria ROS were represented by the relative intensity of fluorescence.
2.6. Measurement of cytosolic [Ca2+]i of smooth muscle cells
The methods in detail were described as in our previous studies6, 7, 8. Cultured arterial smooth muscle cells (A10) were loaded with 5 µmol/L fluo-3/AM for 15 min (37 °C) and rinsed four times with warm PBS (37 °C). The fluorescence was measured by using confocal microscopy (Zeiss LSM 700). The excitation and emission wavelength for signal detection was 488 and 518 nm respectively. The levels of cytosolic [Ca2+]i were represented by the relative intensity of fluorescence.
2.7. Measurement of mitochondrial membrane potential by tetramethylrhodamine methyl ester staining (TMRM)
The methods in detail were described as in our previous studies6, 7, 8. Cultured arterial smooth muscle cells (A10) were loaded with TMRM (50 nmol/L) for 45 min and Hoechst (1 µg/mL) for 15 min at 37 °C and after rinsed four times with warm PBS (37 °C) then the fluorescence was measured by using confocal microscopy. Confocal microscope images were collected by using a Zeiss LSM 700 with the Zeiss LSM software. Images of TMRM fluorescence were obtained using a 20× objective lens with an excitation at 535 nm and an emission of 600 nm. Images of Hoechst staining were obtained by using excitation at 405 nm and an emission of 435 nm. The mitochondrial membrane potential was represented by the relative intensity of fluorescence.
2.8. Statistical analysis
Data are presented as mean ± SEM. Significance was determined by using Student׳s t-test for comparison of two groups. All statistical tests were performed using SigmaPlot (12.5 verison). P<0.05 was considered significant.
3. Results
3.1. Triclosan relaxes phenylephrine (PE)- and high K+ (KPSS)-induced constriction of rat mesenteric arteries
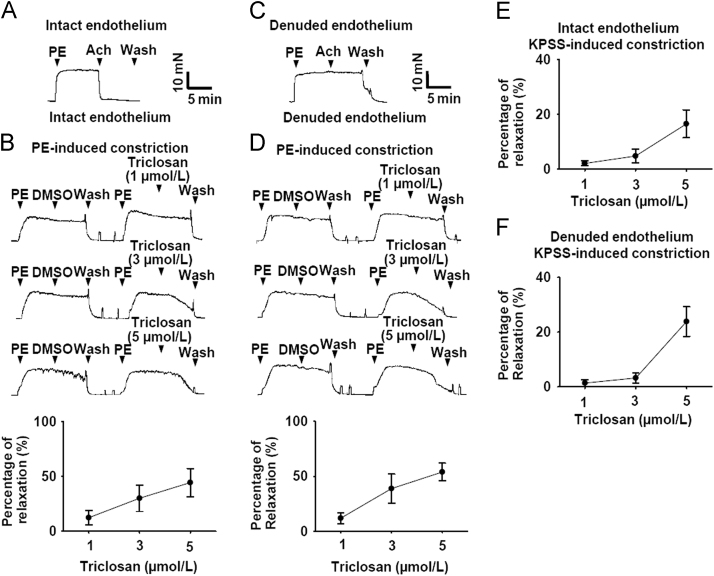
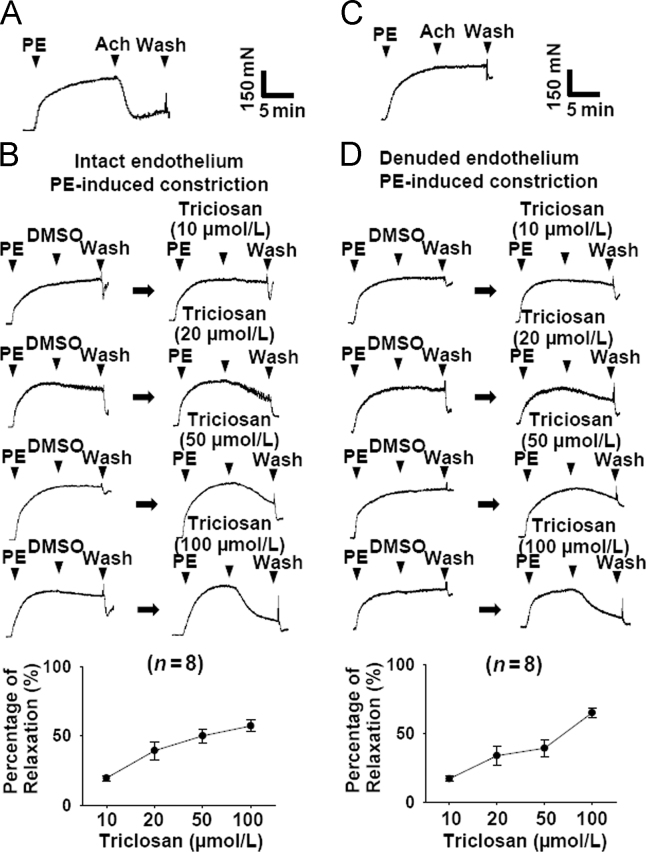
As shown in Fig. 1A, the intact endothelium of mesenteric arteries was confirmed by the presence of Ach-induced (1 µmol/L) relaxation. Treatment with triclosan caused concentration-dependent relaxation of endothelium-intact rat mesenteric arteries pre-contracted with PE (5 µmol/L, Fig. 1B). Furthermore, in rat mesenteric arteries with denuded endothelium (Fig. 1C) treatment with triclosan also induced concentration-dependent relaxation pre-contracted with PE (5 µmol/L, Fig. 1D). In high K+ (KPSS)-induced vasoconstriction model, treatment with triclosan induced vasorelaxation in endothelium-intact and -denuded rat mesenteric arteries (Fig. 1E and F). These results indicated that triclosan-induced vasorelaxation was endothelium-independent. The comparison of Fig. 1B and E, or D and F suggested that PE-induced vasoconstriction was more sensitive to triclosan than KPSS-induced vasoconstriction.
Figure 1.
Triclosan induced vasodilation of rat mesenteric arteries pre-contracted with phenylephrine (PE) and KPSS. (A) Original traces demonstrating mesenteric arteries with intact endothelium. (B) Original traces and summary data showing the vasorelaxation effect of triclosan on endothelium-intact rat mesenteric arteries pre-contracted with PE (5 µmol/L). (C) Original traces demonstrating mesenteric arteries with denuded endothelium. (D) Original traces and summary data showing the vasorelaxation effect of triclosan on endothelium-denuded rat mesenteric arteries pre-contracted with PE (5 µmol/L). PE, phenylephrine. (E) The summary data showing the vasorelaxation effect of triclosan on endothelium-intact rat mesenteric arteries pre-contracted with KPSS. (F) The summary data showing the vasorelaxation effect of triclosan on endothelium-denuded rat mesenteric arteries pre-contracted with KPSS.
3.2. Triclosan pre-treatment inhibits phenylephrine (PE)- and high K+ (KPSS)-induced constriction of rat mesenteric arteries
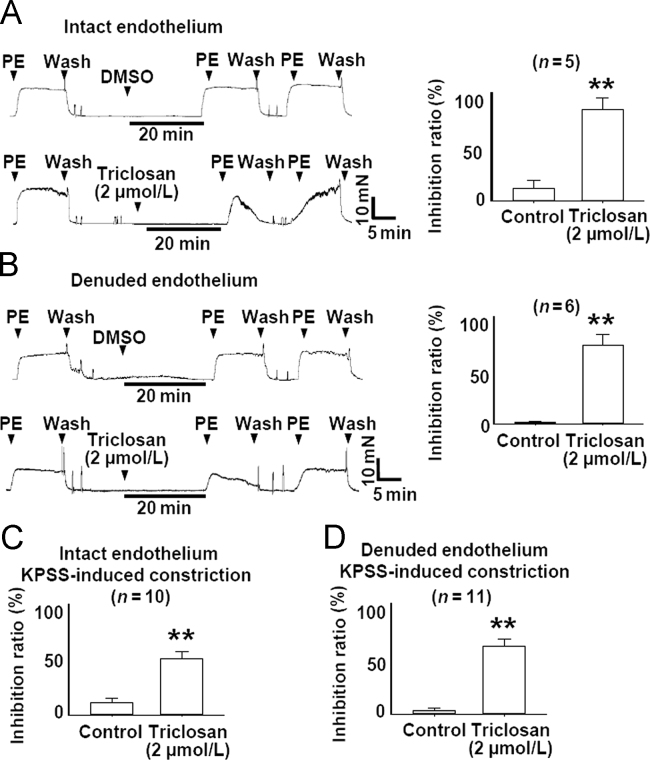
We further examined the effect of pretreatment with triclosan on PE- and KPSS-induced vasoconstriction. As shown in Fig. 2, triclosan (2 µmol/L) pretreatment for 20 min significantly inhibited PE- (5 µmol/L) and KPSS-induced vasoconstriction in endothelium-intact and -denuded rat mesenteric arteries.
Figure 2.
Pretreatment with triclosan prevented PE- and KPSS-induced constriction of rat mesenteric arteries with intact and denuded endothelium. (A) Original traces and summary data showing that pretreatment with triclosan (2 µmol/L) inhibited PE- induced (5 µmol/L) constriction of rat mesenteric arteries with intact endothelium. **P<0.01 vs. control. (B) Original traces and summary data showing that pretreatment with triclosan (2 µmol/L) inhibited PE-induced (5 µmol/L) constriction of rat mesenteric arteries with denuded endothelium. **P<0.01 vs. control. (C) The summary data showing that pretreatment with triclosan (2 µmol/L) inhibited KPSS-induced constriction of rat mesenteric arteries with intact endothelium. **P<0.01 vs. control. (D) The summary data showing that pretreatment with triclosan (2 µmol/L) inhibited KPSS-induced constriction of rat mesenteric arteries with denuded endothelium. **P<0.01 vs. control. PE, phenylephrine.
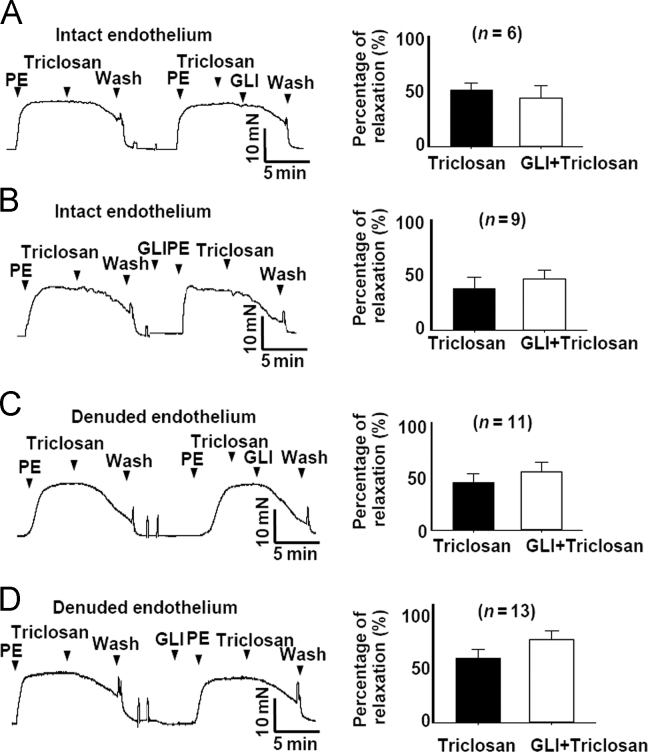
3.3. Triclosan induces vasorelaxation without involving KATP channel activation in rat mesenteric arteries
Mitochondrial uncouplers decrease the ATP production and increase ADP/ATP ratio6, 7, 10, which was reported to be involved in activation of KATP channels11. So we speculated that KATP channel activation might be involved in triclosan-induced vasorelaxation. However, in both endothelium-intact and endothelium-denuded rat mesenteric arteries, the KATP channel blocker glibenclamide showed no effect on triclosan-induced vasorelaxation (Fig. 3). Our previous studies showed that KATP channel activation was not involved in CCCP- and niclosamide-induced vasorelaxation6, 7. Together with the present results, it suggested that mitochondrial uncouplers-induced vasoconstriction was unrelated to KATP channels.
Figure 3.
Triclosan induced vasorelaxation without involving KATP channel. Glibenclamide application whenever before or after triclosan treatment did not affect triclosan-induced vasorelaxation in endothelium-intact and endothelium-denuded rat mesenteric arteries pre-contracted with PE (5 µmol/L). PE, phenylephrine. GLI, glibenclamide.
3.4. Triclosan relaxes phenylephrine (PE)- and high K+ (KPSS)-induced constriction of rat thoracic aorta
In order to test whether triclosan had the similar effect on the large conduit arteries, we examined the effect of triclosan on rat thoracic aorta. As shown in Fig. 4A–D. triclosan treatment relaxed PE-induced vasoconstriction in rat thoracic aorta with intact or denuded endothelium. However, compared with the vasorelaxation effect in mesenteric arteries, the higher concentration of triclosan was required for the vasorelaxation effect of triclosan on aorta.
Figure 4.
Triclosan induced vasodilation of rat thoracic aorta. (A) Original traces demonstrating thoracic aorta with intact endothelium. (B) Triclosan induced dose-dependent relaxation of endothelium-intact thoracic aorta pre-contracted with PE (1 µmol/L). (C) Original traces demonstrating thoracic aorta with denuded endothelium. (D) Triclosan induced dose-dependent relaxation of endothelium-denuded thoracic aorta pre-contracted with PE (1 µmol/L). PE, phenylephrine.
3.5. Triclosan depolarizes mitochondrial membrane potential, increases cytosolic [Ca2+]i and mitochondrial ROS production in vascular smooth muscle cells (A10)
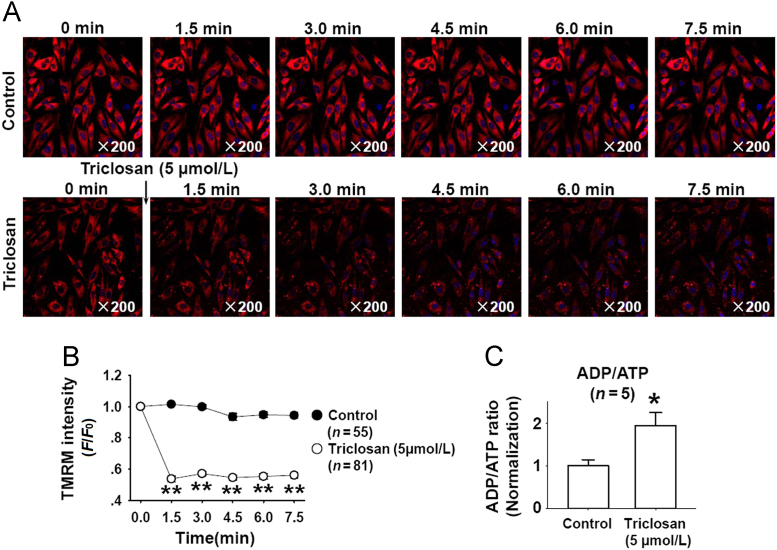
Triclosan induced mitochondrial uncoupling in mast cells and keratinocytes4. Here, we further confirmed the mitochondrial uncoupling effect of triclosan in vascular smooth muscle cells (A10). As shown in Fig. 5A, the tetramethylrhodamine methyl ester (TMRM) staining showed that triclosan treatment significantly decreased mitochondrial membrane potential, and the analyzed data of this dynamic process was shown in Fig. 5B. We further measured the ADP/ATP ration in A10 cells after triclosan treatment and found that triclosan treatment increased ADP/ATP ratio (Fig. 5C). These results indicated that triclosan induced mitochondrial uncoupling in vascular smooth muscle cells.
Figure 5.
Triclosan depolarized mitochondrial membrane potential and increased ADP/ATP ratio in vascular smooth muscle cells (A10). (A) The representative time–lapse images showed that triclosan decreased TMRM fluorescence. (B) The summarized data of triclosan-induced decrease of TMRM intensity. **P<0.01 vs. control. (C) Triclosan treatment (5 µmol/L, 20 min) increased ADP/ATP ratio in A10 cells. *P<0.05 vs. control.
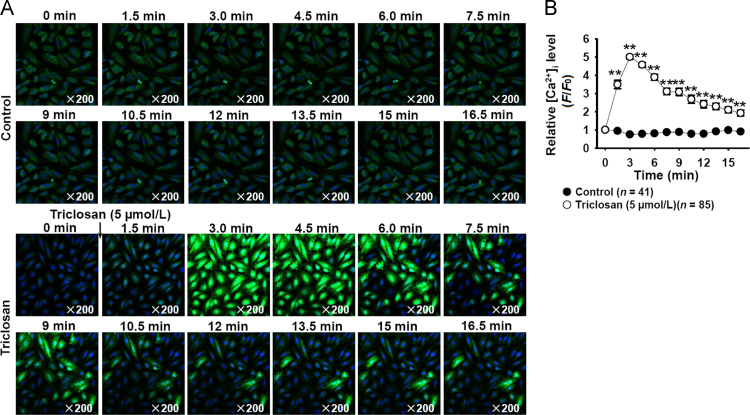
Our previous works showed that mitochondrial uncouplers CCCP and niclosamide relaxed the constricted arteries but increased cytosolic [Ca2+]i in vascular smooth muscle cells6, 7. The present results showed that triclosan treatment also significantly increased cytosolic [Ca2+]i in vascular smooth muscle cells (Fig. 6), indicating that the vasorelaxation effects and the increasing of cytosolic [Ca2+]i in vascular smooth muscle cells might be the common properties of mitochondrial uncouplers.
Figure 6.
Triclosan increased cytosolic [Ca2+]i in vascular smooth muscle cells (A10). (A) The representative time–lapse images showed that triclosan treatment increased cytosolic [Ca2+]i. (B) The summarized data of triclosan-induced increase of cytosolic [Ca2+]i. **P<0.01 vs. control.
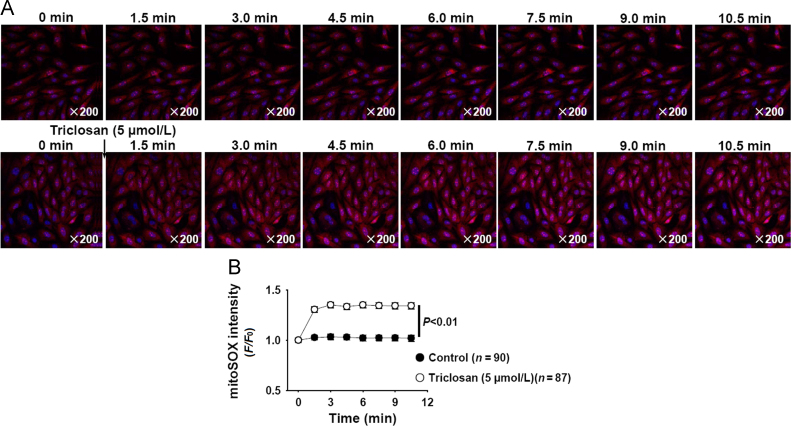
The effects of mitochondrial uncoupler on mitochondrial ROS production were not consistently reported12, 13. We further measured the effects of triclosan on mitochondrial ROS production in vascular smooth muscle cells (A10) by using mitoSOX staining, and found that triclosan treatment slightly increased mitochondrial ROS production (Fig. 7A and B).
Figure 7.
Triclosan increased mitochondrial ROS production in vascular smooth muscle cells (A10). (A) The representative time–lapse images showed that triclosan treatment increased mitochondrial ROS production. (B) The summarized data of triclosan-induced increase of mitochondrial ROS production.
4. Discussion
Triclosan has multiple biological functions, including antimicrobial effects and immunosuppressive effects. Here we reported for the first time that triclosan showed vasorelaxation effects.
The idea of the present study came from the findings of our group that mitochondrial uncouplers, CCCP and niclosamide, were found to induce vasodilation of constricted arteries and the chemical mitochondrial uncouplers hold the properties of vasoactivity in common6, 7. Triclosan was reported to induce mitochondrial uncoupling4 and we also proved that triclosan depolarized mitochondrial membrane potential of vascular smooth muscle cells (Fig. 5A and B). Based on our findings, we hypothesized that triclosan would have vasorelaxation effects. Results showed that triclosan indeed has vasorelaxation effect.
Triclosan is an antimicrobial used widely in hospitals and personal care products, at ~10–75 mmol/L14, 15. Its bioaccumulation after chronic use or inadvertent absorption would induce toxic effects16. Cherednichenko et al.17 have shown that triclosan induced severe cardiovascular impairments in mice in vivo. Our results indicated that triclosan would induce vasodilation effect in vivo.
The effects of triclosan on vascular function and cytosolic [Ca2+]i, mitochondrial ROS and mitochondrial membrane potential of vascular smooth muscle cells (A10 cells) were similar to those of CCCP and niclosamide reported in our previous works6, 7, indicating that the mitochondrial uncouplers share the same pharmacological effects on arteries. Based on this theory, the vascular effects of a new drug could be inferred if it was a mitochondrial uncoupler.
It is very interesting that the mitochondrial uncouplers CCCP, niclosamide and triclosan induced significant vasodilatation, but increased cytosolic [Ca2+]i in vascular smooth muscle cells. The phenomenon has been discussed in our previous study7. We speculated that the increased cytosolic [Ca2+]i tended to constrict the vessel, whereas the other vasorelaxant actions of triclosan (decreased ATP generation and loss of mitochondrial energy) counteracted the vasoconstriction. The mitochondrial uncouplers had the tendency to induce both vasoconstriction and vasorelaxation through different mechanisms, but the net result was vasorelaxation. Therefore, the triclosan-induced vasoactivity and cytosolic [Ca2+]i changes seemed unparalleled. In the present study, we found that triclosan increased cytosolic [Ca2+]i in vascular smooth muscle cells (A10 cells). There are several studies showing that triclosan is able to alter/elevate intracellular free Ca2+ levels in animal and human cell lines3, 18. Cherednichenko et al.17 reported that triclosan depressed excitation–contraction coupling, inhibited L-type Ca2+ currents, and Ca2+ transients in cardiac muscle, indicating that the regulation of triclosan on Ca2+ signals in smooth muscle and cardiac muscle was through distinct mechanism.
The effects of mitochondrial uncoupler on mitochondrial ROS production are not consistent12, 13. In a recent article, González-Pleiter et al.19 reported that triclosan promoted an extensive formation of ROS such as superoxide anion and hydrogen peroxide in exposed Chlamydomonas reinhardtii cells. Our results showed that triclosan at 5 µmol/L increased mitoROS generation in vascular smooth muscle cells.
The mesenteric arteries were more sensitive to triclosan than that of aorta (Fig. 4), thus we hypothesized that triclosan would mainly affect the small resistance vessels in vivo. We did not perform the in vivo experiments; however, previous study had examined the effects of triclosan on cardiovascular function of mice in vivo17. They found that mice receiving triclosan (6.25, 12.5 or 25 mg/kg, i.p.) showed significantly impaired hemodynamic functions in a dose-dependent manner. Cardiovascular impairments included significantly reduced cardiac output, lower left ventricular end-diastolic volume, and reduction in the maximum time-derivative of the left ventricular pressure development, implying that triclosan induced severe cardiovascular impairments. Our findings were consistent with their results.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81373406 and 81421063). The authors declare no conflicts of interest in this work.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Koeppe E.S., Ferguson K.K., Colacino J.A., Meeker J.D. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ. 2013;445-446:299–305. doi: 10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Udoji F., Martin T., Etherton R., Whalen M.M. Immunosuppressive effects of triclosan, nonylphenol, and DDT on human natural killer cells. J Immunotoxicol. 2010;7:205–212. doi: 10.3109/15476911003667470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer R.K., Hutchinson L.M., Burpee B.T., Tupper E.J., Pelletier J.H., Kormendy Z. Antibacterial agent triclosan suppresses RBL-2H3 mast cell function. Toxicol Appl Pharmacol. 2012;258:99–108. doi: 10.1016/j.taap.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Weatherly L.M., Shim J., Hashmi H.N., Kennedy R.H., Hess S.T., Gosse J.A. Antimicrobial agenttriclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J Appl Toxicol. 2016;36:777–789. doi: 10.1002/jat.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhillon G.S., Kaur S., Pulicharla R., Brar S.K., Cledón M., Verma M. Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Inter J Environ Res Public Health. 2015;12:5657–5684. doi: 10.3390/ijerph120505657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S.L., Yan J., Zhang Y.Q., Zhen C.L., Liu M.Y., Jin J. Niclosamide ethanolamine inhibits artery constriction. Pharmacol Res. 2017;115:78–86. doi: 10.1016/j.phrs.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y.Q., Shen X., Xiao X.L., Liu M.Y., Li S.L., Yan J. Mitochondrial uncoupler carbonyl cyanide m-chlorophenylhydrazone induces vasorelaxation without involving KATP channel activation in smooth muscle cells of arteries. Br J Pharmacol. 2016;173:3145–3158. doi: 10.1111/bph.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M.Y., Jin J., Li S.L., Yan J., Zhen C.L., Gao J.L. Mitochondrial fission of smooth muscle cells is involved in artery constriction. Hypertension. 2016;68:1245–1254. doi: 10.1161/HYPERTENSIONAHA.116.07974. [DOI] [PubMed] [Google Scholar]
- 9.Jin J., Shen X., Tai Y., Li S., Liu M., Zhen C. Artery relaxation is coupled to inhibition of mitochondrial fission in arterial smooth muscle cells: comparison of vasorelaxant effects of verapamil and phentolamine. Acta Pharm Sin B. 2017;7:319–325. doi: 10.1016/j.apsb.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao H., Zhang Y., Zeng X., Shulman G.I., Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat Med. 2014;20:1263–1269. doi: 10.1038/nm.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumann P., Poitry S., Roatti A., Baertschi A.J. Plasmalemmal KATP channels shape triggered calcium transients in metabolically impaired rat atrial myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H2296–H2305. doi: 10.1152/ajpheart.00393.2002. [DOI] [PubMed] [Google Scholar]
- 12.Kabir A.M., Clark J.E., Tanno M., Cao X., Hothersall J.S., Dashnyam S. Cardioprotection initiated by reactive oxygen species is dependent on activation of PKCε. Am J Physiol Heart Circ Physiol. 2006;291:H1893–H1899. doi: 10.1152/ajpheart.00798.2005. [DOI] [PubMed] [Google Scholar]
- 13.Park B.K., Gonzales E.L., Yang S.M., Bang M., Choi C.S., Shin C.Y. Effects of triclosan on neural stem cell viability and survival. Biomol Ther. 2016;24:99–107. doi: 10.4062/biomolther.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones R.D., Jampani H.B., Newman J.L., Lee A.S. Triclosan: a review of effectiveness and safety in health care settings. Am J Infect Control. 2000;28:184–196. [PubMed] [Google Scholar]
- 15.Rodricks J.V., Swenberg J.A., Borzelleca J.F., Maronpot R.R., Shipp A.M. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- 16.Olaniyan L.W., Mkwetshana N., Okoh A.I. Triclosan in water, implications for human and environmental health. Springerplus. 2016;5:1639. doi: 10.1186/s40064-016-3287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherednichenko G., Zhang R., Bannister R.A., Timofeyev V., Li N., Fritsch E.B. Triclosan impairs excitation-contraction coupling and Ca2+ dynamics in striated muscle. Proc Natl Acad Sci U S A. 2012;109:14158–14163. doi: 10.1073/pnas.1211314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura I., Saito M., Nishimura Y., Satoh M., Yamamoto H., Oyama Y. Elevation of intracellular Ca2+ level by triclosan in rat thymic lymphocytes: increase in membrane Ca2+ permeability and induction of intracellular Ca2+ release. J Health Sci. 2011;57:540–546. [Google Scholar]
- 19.González-Pleiter M., Rioboo C., Reguera M., Abreu I., Leganés F., Cid Á. Calcium mediates the cellular response of Chlamydomonas reinhardtii to the emerging aquatic pollutant triclosan. Aquat Toxicol. 2017;186:50–66. doi: 10.1016/j.aquatox.2017.02.021. [DOI] [PubMed] [Google Scholar]