Abstract
As a leading cause of respiratory disease, influenza A virus (IAV) presents a pandemic threat in annual seasonal outbreaks. Given the limitation of existing anti-influenza therapies, there remains to be a requirement for new drugs. Compound Yi-Zhi-Hao pellet (CYZH) is a famous traditional Chinese medicine (TCM) used in the clinic, whose formula has been recorded in Complication of National Standard for Traditional Chinese Medicine to treat common cold. In this study, we found that CYZH exhibited a broad-spectrum anti-influenza activity and inhibited the expression of viral RNA and proteins in vitro. Mechanistically, CYZH had no inhibitory activities against viral protein hemagglutinin and IAV RNA-dependent RNA polymerase. Instead, it induced activation of erythroid 2-related factor 2 (Nrf2) and nuclear factor kappa B (NF-κB), which subsequently upregulated heme oxygenase-1 (HO-1) expression. Also, CYZH protected cells from oxidative damage induced by reactive oxygen series. In conclusions, CYZH inhibits IAV replication in vitro, at least partly by activating expression of the Nrf2/HO-1 pathway.
KEY WORDS: TMC, Influenza A virus, Heme oxygenase 1, Hemagglutinin, Nrf2, NF-κB
Graphical abstract
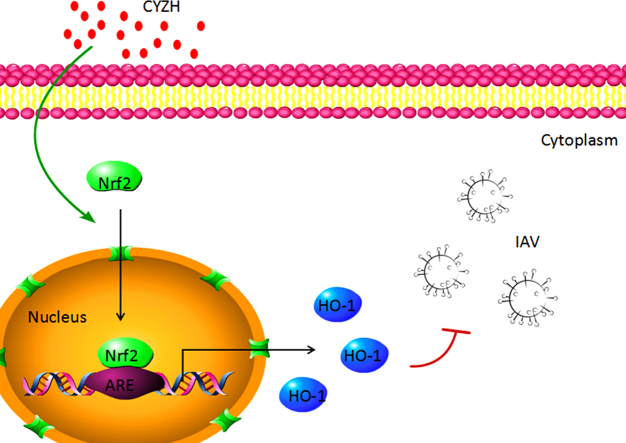
The Chinese herbal medicine, compound Yi-Zhi-Hao pellet had a broad-spectrum anti-influenza activity in vitro, including clinically drug-resistant strains. It protected influenza A virus-infected cells from oxidative damages, at least partly by activating expression of Nrf2/HO-1 pathway.
1. Introduction
Influenza A virus (IAV), a member of the Orthomyxoviridae family, is an enveloped virus with eight segmented, negative-sense, single-stranded RNAs. IAV is the main cause of seasonal or pandemic flu with high worldwide morbidity. During World War I, the 1918 influenza pandemic (H1N1) was unusually severe, resulting in about 50 million deaths worldwide. During the 21st century, the 2009 influenza pandemic (H1N1) exerted a great negative impact on both of public health and social development1.
Although vaccines are a vital strategy for prophylaxis, the lag time between virus identification and vaccine distribution weakens its preventive effects. Over a short time, antiviral therapy is the best option to control the spread of influenza. To date, licensed drugs in the clinic only include M2 ion-channel blockers (amantadine and rimantadine), neuraminidase inhibitors (oseltamivir and peramivir) and RNA-dependent RNA polymerase (RdRp) inhibitor [favipiravir (T705)]2, 3. However, the rapid emergence of drug-resistant viral mutants restricts the utility of these drugs4.
Traditional Chinese medicines (TCMs) may be a supplement to overcome the challenge. These are traced back to ancient times and some are still used as alternative therapy to treat influenza-like illness5. Recently, a number of studies evaluated the potential benefit of TCM in the treatment of influenza-induced pneumonia, and some TCMs have documented anti-influenza activities in vitro6. In line with this, a retrospective analysis showed that TCM therapy was effective for reducing the length of influenza virus (H1N1) shedding in patients with no less than 38.0 °C of body temperature7.
Compound Yi-Zhi-Hao (CYZH) is a TCM formula, which is recorded in Complication of National Standard for Traditional Chinese Medicine8. It is used to treat common cold in the clinic as a listed drug (Xin-Jiang-Xi-Yu Pharmaceutical Co., Ltd., lot number Z20026711). In the previous works from Prof. Jianguo Xing (Xinjiang Institute of Materia Medica, Xinjiang, China), CYZH was prepared as described below mentioned and shown to have anti-IAV effects in mice9. However, the anti-IAV mechanism of CYZH has not been elucidated. Herein, we evaluated its inhibitory activities against a panel of influenza viruses, including drug-resistant strains of influenza A virus and influenza B virus. Furthermore, we explored a possible antiviral mechanism of CYZH against influenza virus.
2. Materials and methods
2.1. Preparation of CYZH pellet
CYZH pellet was provided by Prof. Jianguo Xing at the Xin-Jiang Institute of Materia Medica, Xinjiang, China. It was mainly composed of three dried raw materials: Ban-Lan-Gen (Isatis Tinctoria, Isatidis Radix), Yi-Zhi-Hao (Artemisia Rupestris L., Artemisiae Rupestridis Herba), and Da-Qing-Ye (Isatis Tinctoria, Isatidis Folium). CYZH Pellet was prepared as previously published, containing about 3.5 mg rupestonic acid per 12 g as documented by high performance liquid chromatography9. The Isatidis Radix powder was soaked for 48 h, after which the same amount of Isatidis Folium and 8-fold volume of water were added. The mixture was extracted 3 times in water at room temperature. The same amount as the former two components of Artemisiae Rupestridis Herba was extracted with 8-fold volume of 70% ethanol. Finally, the two extractions were equivalently mixed and evaporated at 40 °C under vacuum. The dried residue was pelletized with microcrystalline cellulose on a 1 to 1.5 ratio and then passed through a 30–40 mesh screen.
2.2. Viral strains, cell lines and reagents
Madin-Darby canine kidney (MDCK) cells (America Type Culture Collection, ATCC, USA) were grown in minimum essential medium (MEM) with 10% fetal bovine serum (FBS), 100 U/mL penicillin G and 100 μg/mL streptomycin.
Influenza strain A/Fort Monmouth/1/1947 (H1N1) was purchased from ATCC. Clinical isolated IAV strains, including A/TianjinJinnan/15/2009 (H1N1, oseltamivir resistant), A/Wuhan/359/1995 (H3N2), A/FujianTongan/196/2009 (H3N2, amantadine resistant) and BV/Shenzhen/155/2005, were kindly provided by Prof. Yuelong Shu, Institute for Viral Disease Control and Prevention, China Centers for Disease Control and Prevention (Beijing, China). IAV strains were prepared by propagating in 10-day-old embryonated chicken eggs for 72 h.
Oseltamivir phosphate (OP, Chinese National Institutes for Food and Drug Control, Beijing, China), amantadine hydrochloride (AH, Sigma--Aldrich, USA), Ribavirin (RBV, Sigma-Aldrich, USA) and favipiravir (T705, provided by Prof. Quanhong Wang, Academy of Military Medical Sciences, China) were used as reference compounds. Stock solutions of CYZH (20 mg/mL) were ground, dissolved in double distilled water and centrifuged at 1000 rpm (Sorvall ST 16 R, Thermo Fisher Scientific, USA) for 5 min to remove insoluble materials. Stock solutions of AH (2 mg/mL) were dissolved in dimethyl sulfoxide (DMSO, Sigm--Aldrich, USA). Stock solutions of T705, OP and RBV (2 mg/mL) were dissolved into double distilled water. These drugs were diluted to the indicated concentration needed in different experiment assays.
2.3. CPE assay of in vitro anti-influenza virus activity
MDCK cells seeded in plates were treated with influenza strain A/Fort Monmouth/1/1947 (H1N1) at 100 TCID50 (50% tissue culture infective dose) for 2 h with or without the tested compounds. Then the unbound viruses were removed by medium with or without the tested compounds. The cells were cultured at 37 °C under 5% CO2. Experiments involving viral infection were performed under bio-safety level 2 (BSL-2) condition. The viable cells were determined by the virus-induced cytopathic effect (CPE) assay10. The 50% inhibitory concentration (IC50) was calculated based on Reed and Muench method and the selectivity index (SI) of compounds was calculated as the ratio of TC50/IC5012.
2.4. MTT assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium reduction colorimetric assay (MTT assay) was used to evaluate the cytotoxicity of compounds13. Briefly, MDCK cells grown in 96-well plate were treated with serial two-fold dilutions of CYZH for 60 h. Then, 10 μL of 5 mg/mL MTT (Promega, Madison, WI, USA) dissolved in phosphate-buffered saline (PBS) was added to each well. After 4 h of incubation at 37 °C, the medium was replaced by 150 μL of DMSO and the plates were shaken for 10 min. Finally, the results were measured by scanning absorbance at 450 nm on Enspire (Perkin Elmer, Waltham, MA, USA). The 50% toxicity concentration (TC50) of CYZH was calculated based on Reed and Muench method11.
2.5. Western blot assay
Total proteins were extracted by ice-cold M-PER mammalian protein extraction reagent containing halt protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA). The protein concentrations were determined by BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Equal amount of samples (20 μg protein) were subjected to SDS-PAGE using a 10% (w/v) running gel, and then electro-transferred to PVDF membrane (Millipore, Billerica, MA, USA) and blocked by 5% (w/v) milk for 1 h at room temperature. The membranes were incubated with mouse antibodies against influenza A M2 (Santa Cruz, USA), actin (Cell Signaling Technology, USA) and HO-1 (Abcam Cambridg, USA) for 2 h at room temperature. Then goat-anti-mouse secondary antibody (Cell Signaling Technology, USA) was incubated for 1 h at room temperature. The signals were detected using ECL detection kit (GE Healthcare Life Sciences, Pittsburgh, PA, USA).
2.6. Quantitative real-time RT-PCR
Total RNA was isolated from MDCK cells using RNeasy Mini Kit (Qiagen, USA). The mRNA of IAV M2, HO-1, GAPDH were amplified by quantitative real-time RT-PCR with specific primers (Table 1). One-step quantitative real-time polymerase chain reaction (qRT-PCR) was amplified by SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen, USA) and carried out on an ABI 7500 Fast real-time PCR advice (Applied Biosystems, USA). The PCR conditions were shown as follows: 50 °C for 3 min, 95 °C for 5 min, 35 cycles of 95 °C for 15 s, 60 °C for 30 s. The relative mRNA levels of IAV M2 and HO-1 were calculated by comparative Ct method after normalizing against the quantity of GAPDH mRNA.
Table 1.
Oligonucleotides used for real-time RT-PCR.
| Oligonucleotide | Sequence (5ʹ–3ʹ) |
|---|---|
| 5ʹM2(INFLUENZA) | GACCRATCCTGTCACCTCTGAC |
| 3ʹM2(INFLUENZA) | GGGCATTYTGGACAAAKCGTCTACG |
| 5ʹHO-1(DOG) | CATGAAGAACTTTCAGAAGGGC |
| 3ʹHO-1(DOG) | GTTGTGCTCGATCTCCTCC |
| 5ʹGAPDH(DOG) | AGTCAAGGCTGAGAACGGGAAACT |
| 3ʹGAPDH(DOG) | TCCACAACATACTCAGCACCAGCA |
2.7. Hemagglutination assay
The hemagglutination assay was used to evaluate the inhibitory effect of compound on viral attachment to cells13. Briefly, 50 µL drug solved by saline was mixed with an equal volume of ice-cold saline containing IAV in a “U” bottom 96-well plate with incubation at 4 °C for 30 min. Then 100 μL of 1.2% chicken erythrocyte suspension was added to each well for 40 min at room temperature.
2.8. IAV RNA-dependent RNA polymerase (IAV-RdRp) assay
In our previous study14, a high-throughput screening (HTS) method was used to monitor IAV RNA transcription and replication by IAV RNA-dependent RNA polymerase (IAV-RdRp) and to evaluate the effect of CYZH on IAV-RdRp. T-705, a strong inhibitor of IAV-RdRp, was chosen as a positive control in this assay15. MDCK cells were co-transfected with pHW181-PB2, pHW182-PB1, pHW183-PA, pHW185-NP (provided by Prof. Shan Cen, Chinese Academy of Medical Sciences & Pekin Union Medical College, Beijing, China) expressing firefly luciferase and pRL-SV40 vector (Promega, USA) expressing Renilla luciferase in white 96-well plate. After the treatment with CYZH for 48 h, the luminescence was detected by Dual-Glo Luciferase Assay System (Promega, USA) on EnSpire (PerkinElmer, Singapore).
2.9. Luciferase assays
MDCK cells were co-transfected with pGL4.37[luc2P/ARE/Hygro] (Promega, USA) or pGL4.37[pAP-1-Luc] or pGL4.37[pNF-κB-Luc] (provided by Prof. Jianping Ye, Louisiana State University Rockefeller University, USA) and pRL-SV40 vector (Promega, USA). After the infection of influenza virus and treatment with CYZH for 24 h, the luminescence was detected by Dual-Glo Luciferase Assay System (Promega, USA) on EnSpire (PerkinElmer, Singapore).
2.10. Measurement of intracellular reactive oxygen species (ROS)
Intracellular ROS was detected by a fluorescent probe 2,7-dichlorofluorescein diacetate (DCFH-DA). DCFH-DA is oxidized by intracellular ROS to DCF (29,79-dichlorofluorescein) which is highly fluorescent at 530 nm. The effect of CYZH on ROS formation was obtained by the Reactive Oxygen Species Assay Kit (Beyotime Institute of Biotechnology, China)16. After infection, MDCK cells grown in 6-well plates were treated with CYZH for 24 h. Then cells were washed three times with PBS and PBS in wells was replaced with medium containing 10 µmol/L DCFH-DA. After incubation of 30 min at 37 °C in the dark, cells were washed three times with PBS. The fluorescence signal in each well was quantified with a multi-detection microplate reader EnSpire (PerkinElmer, Singapore) with excitation at 485 nm and emission at 535 nm.
2.11. Statistical analyses
The data were represented as the mean ± SD from triplicate experiments. The statistical analysis was performed with one-way ANOVA using GraphPad Prism6.0 software. A threshold of P < 0.05 was defined as statistically significant.
3. Results
3.1. Antiviral activities of CYZH against influenza viruses in vitro
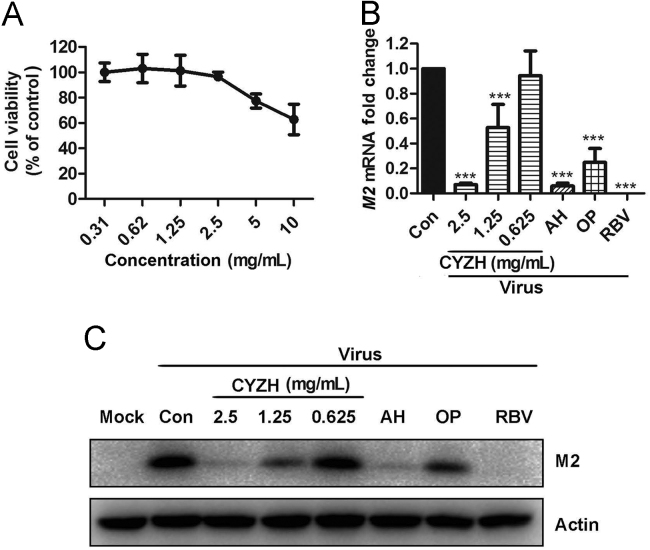
The cytotoxicity of CYZH was first determined in MDCK cells through the MTT assay. The result showed that the maximal nontoxic concentration (TC0) value was 2.5 mg/mL (Fig. 1A). Then antiviral activities were determined based on the CPE assay. TC50 and IC50 values are summarized in Table 2, which shows that CYZH had a broad-spectrum antiviral effect against influenza A and B strains with IC50 values ranging 1–1.5 mg/mL. Moreover, CYZH showed similar inhibitory activities against the oseltamivir-resistant influenza mutant A/TianjinJinnan/15/2009(H1N1)and amantadine-resistant influenza mutant A/FujianTongan/196/2009(H3N2). Consistent with the results above, CYZH decreased the M2 protein expression and M2 RNA replication of IAV A/Fort Monmouth/1/1947(H1N1) in a dose-dependent manner (Fig. 1B and C).
Figure 1.
Antiviral activity of CYZH against influenza viruses in vitro. (A) The effect of CYZHP on viabilities of MDCK cells measured by MTT. (B) and (C) Effects of CYZH and reference drugs on IAV M2 protein and mRNA expression in MDCK cells after 24 h of infection. Mock: normal cells without treatment; virus: cells infected with IAV A/FM1/1947 at an MOI of 0.005. Con: treated with equal amounts of ddH2O as CYZH; CYZH, 2.5, 1.25 or 0.625 mg/mL; AH, 10 μg/mL; OP, 10 μg/mL; RBV, 10 μg/mL. The experiments were performed in triplicate and the data represents mean ± SD. ***P < 0.001 vs. Con.
Table 2.
Inhibitory activities of compound CYZH against five influenza strains.
| Strain | CYZHa | AHb | OPb | RBVb | |
|---|---|---|---|---|---|
| TC50 | 12.99 ± 3.36 | >200 | >200 | >200 | |
| A/Fort Monmouth/1/1947 | IC50 | 1.25 ± 0 | 0.21 ± 0.04 | 2.71 ± 0.29 | 0.66 ± 0.05 |
| SI | 10.39 | >952.38 | >73.8 | >303.08 | |
| A/TianjinJinnan/15/2009 | IC50 | 1.49 ± 0.12 | 1.51 ± 0.37 | – | 0.59 ± 0.05 |
| SI | 8.72 | >132.50 | – | >340.51 | |
| A/FujianTongan/196/2009 | IC50 | 1.15 ± 0.14 | – | 0.63 ± 0.01 | 0.62 ± 0 |
| SI | 11.3 | – | >320 | >318.72 | |
| A/Wuhan/359/1995 | IC50 | 1.12 ± 0.18 | 0.84 | 0.27 ± 0.06 | 1.54 |
| SI | 11.59 | >238.67 | >741.51 | >129.96 | |
| BV/Shenzhen/155/2005 | IC50 | 1.07 ± 0 | – | 2.2 ± 0.35 | 0.91 ± 0.07 |
| SI | 12.14 | – | >88.71 | >219.24 | |
amg/mL; bμg/mL.
IC50: 50% cell inhibitory concentrations; SI: selectivity index, SI=TC50/IC50. n = 3, the data represents mean ± SD. AH, amantadine hydrochloride; OP, oseltamivir phosphate; RBV, ribavirin.
– No antiviral activity at the maximal nontoxic concentration.
3.2. CYZH was not an inhibitor of IAV envelope glycoprotein HA and IAV RdRp
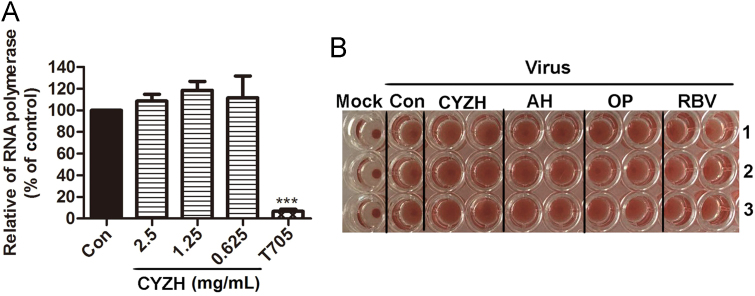
Hemagglutinin (HA) is required for both adsorption and penetration of influenza virus into the host cell17, 18. The hemagglutination inhibition assay was used to determine the effect of CYZH on binding of viral hemagglutinin to sialic acid receptors. The results showed that CYZH was not the inhibitor of IAV envelope glycoprotein HA (Fig. 2A). The effect of CYZH was then examined on IAV RNA-dependent RNA polymerase (RdRp) which is required for IAV RNA replication. CYZH had no effect on RdRP, while T705, a known RdRp inhibitor, showed a significantly inhibitory activity14 (Fig. 2B). Therefore, CYZH was not an inhibitor of IAV envelope glycoprotein HA and IVA RNA polymerase (RdRp).
Figure 2.
CYZH was not an inhibitor of IAV envelope glycoprotein HA and IVA RNA polymerase (RdRp). (A) Inhibitory effects of CYZH on IAV RNA polymerase (RdRp) in MDCK cells. MDCK cells (2.5×105) were co-transfected with the plasmids expressing RdRp and then treated with CYZH and T705. RdRp activities were measured by Dual-Glo Luciferase Assay System. Con: treated with equal amounts of ddH2O as CYZH; CYZH: treated CYZH with the concentration of 2.5, 1.25 and 0.625 mg/mL, respectively; T705: treated with the concentration of 50 μmol/L.The experiments were performed in triplicate, and the data represents mean ± SD. ***P < 0.001 vs. Con. (B) The effect of CYZH on HA inhibition. The maximum concentrations (in row 1 of each plate) of CYZH, AH, OP and RBV were 2.5 mg/mL,10 μg/mL,10 μg/mL and 10 μg/mL, respectively. A serial of 2-fold dilution for all drugs were adopted in rows 2 and 3. The experiments were performed in triplicate.
3.3. CYZH up-regulated the expression of HO-1 mRNA and protein
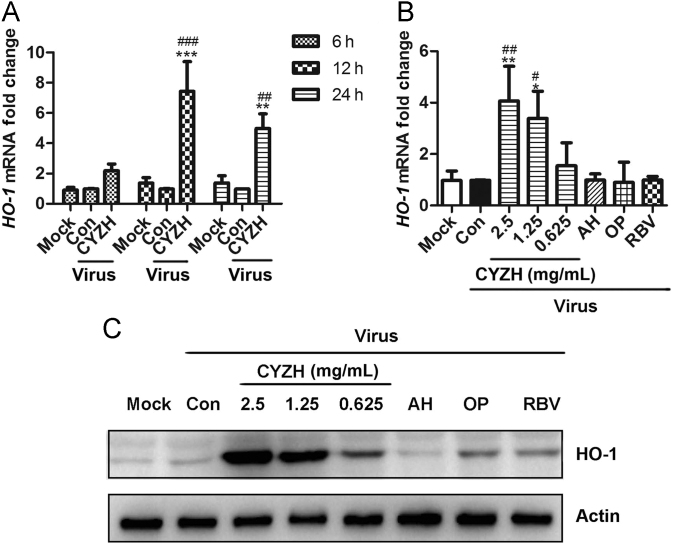
Rupestonic acid, extracted from Artemisiae Rupestridis Herba, has been demonstrated to have antioxidant activities19. Recently, a derivative YZH-106 of rupestonic acid was found inhibit the replication of IAV by up-regulating HO-1 expression20. Given that CYZH contains the ingredients from this herb, we speculated that the anti-IAV mechanism of CYZH might be also associated with induction of HO-1 expression. Firstly, HO-1 mRNA and its protein expression were examined in MDCK cells infected with IAV in the presence or absence of 2.5 mg/mL CYZH. We found that CYZH significantly increased the levels of HO-1 mRNA after 12 and 24 h treatment (Fig. 3A) in dose-dependent manner (Fig. 3B). Consistent with this, CYZH dose-dependently increased the expression of HO-1 protein (Fig. 3C). However, the reference antiviral compounds did not have such an effect on the expression of HO-1 mRNA and the protein.
Figure 3.
CYZH up-regulated the expression of HO-1 mRNA and the protein. (A) MDCK cells were infected with IAV A/FM1/1947 (MOI = 0.005) in the presence or absence of 2.5 mg/mL CYZH for 6, 12 or 24 h. mRNA was collected in the indicated time points and then HO-1 mRNA expression was analyzed by qRT-PCR. (B) and (C) Infected MDCK cells were treated with or without CYZH (0.625, 1.25 or 2.5 mg/mL), AH (10 μg/mL), OP (10 μg/mL) or RBV (10 μg/mL) for 24 h. HO-1 mRNA was analyzed by qRT-PCR (B) and the protein by Western blot (C). The experiments were performed in triplicate, and each value represents mean ± SD. *P < 0.05, **P< 0.01, ***P < 0.001 vs. Mock; #P< 0.05, ##P < 0.01, ###P< 0.001 vs. IAV infected cells treated with equal concentration of solvent (Con).
3.4. CYZH activates Nrf2/ARE pathway
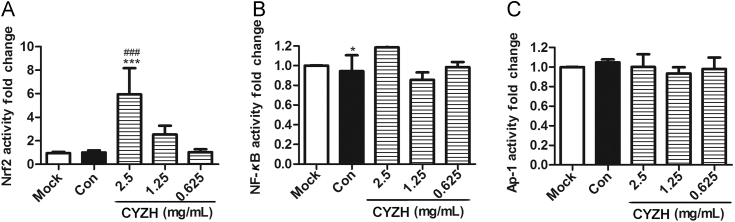
To determine how CYZH upregulated HO-1 expression, we analyzed effects of CYZH promoter activities mediated by the Nrf2, nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), all of which have been known to play an important role in regulating HO-1 expression. Thus, we used the Dual-Luciferase reporter gene system to test promoter activities in the presence of CYZH. We found that CYZH dose-dependently increased Nrf2/ARE-driven luciferase activity (Fig. 4A). However, 2.5 mg/mL CYZH produced a mild increase of NF-κB-driven luciferase activity (Fig. 4B). Also, CYZH had no effects on AP-1-driven activity (Fig. 4C). These results suggest that CYZH-mediated HO-1 up-regulation is associated with activation of the Nrf2–ARE signaling pathway in MDCK cells.
Figure 4.
CYZH dose-dependently increased Nrf2-driven luciferase activity in MDCK cells. Transcription factor DNA binding activities were analyzed in MDCK cells co-transfected with pGL4.37-pAP-1-Luc/[luc2P/ARE/Hygro]/pNF-κB-Luc and pRL-SV40 vector in the presence or absence of 2.5, 1.25 or 0.625 mg/mL CYZH for 12 h (A/B/C). The experiments were performed in triplicate, each value represents mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Mock; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. IAV infected cells treated with equal concentration of solvent (Con).
3.5. CYZH alleviated the oxidative damage induced by IAV
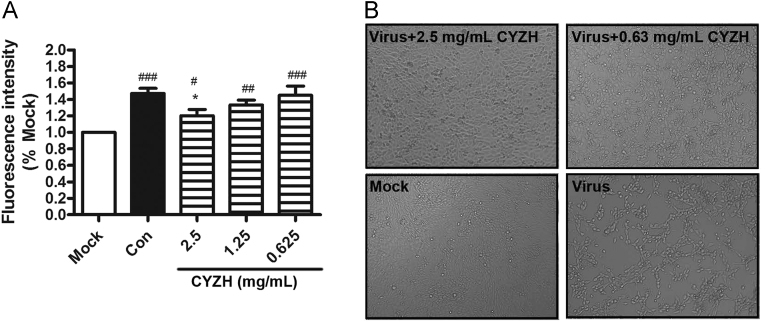
Oxidative stress is implicated in the pathogenesis of several viral infections, including IAV, HCV, and HIV21, 22, 23. Given that CYZH significantly increased the expression of HO-1 (known to play a role in inhibiting oxidative stress) we speculated that the mechanism CYZH anti-IAV was associated with the decreased oxidative stress. Therefore, in the infected MDCK cells, we studied CYZH effects on the generation of ROS. The data showed that CYZH dose-dependently inhibited the production of ROS induced by IAV (Fig. 5A). Furthermore, as presented in Fig. 5B, CYZH dose-dependently inhibited virus-induced CPE in MDCK cells against IVA A/Fort Monmouth/1/1947(H1N1) infection.
Figure 5.
CYZH alleviated ROS production induced by IAV. (A) MDCK cells were infected with IAV A/FM1/1947 (0.005 MOI) and incubated by various concentrations of CYZH and OP. After 24 h, MDCK cells were treated with 10 μmol/L DCFH-DA and then fluorescence intensity was determined with EnSpire. (B) The inhibition of IAV-induced CPE by CYZH. Cells were examined using a microscopy (100 ×). Mock: normal cells without treatment; Virus: cells infected with IAV A/FM1/1947 at an MOI of 0.005; Con: treated with equal amounts of ddH2O as CYZH; CYZH: treated CYZH with the concentration of 2.5, 1.25 or 0.625 mg/mL, respectively; OP: treated OP with the concentration of 10 μg/mL. The experiments were performed in triplicate, each value represents mean ± SD. **P < 0.01 vs. Mock; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. IAV infected cells treated with equal concentration of solvent (Con).
4. Discussion
Chinese people used TCMs to treat infectious diseases thousands of years ago. Now, many TCMs have been shown to exert therapeutic effects in pre-clinical experiments or clinic trials, such as Liu-Wei-Di-Huang Decoction and Xiao-Qing-Long Decoction, which have been ratified in clinic24, 25. CYZH is a TCM used for the treatment of common cold, which is composed of 3 medicinal herbs, which have relative activities. Until now, the studies on the mechanism of CYZH against IAV remain limited. Presently, CYZH was shown to exert a broad-spectrum antiviral activity against influenza A and B strains, as well as against the oseltamivir-resistant and amantadine-resistant influenza viruses. Therefore, CYZH might be a supplement for the available treatment of influenza, especially drug-resistant influenza strains.
Although the therapeutic actions of CYZH against influenza infection have been shown, little was known about its mechanism of action. Each of the herbs contained in CYZH has been reported to have the anti-IAV activity. Isatidis Radix is the root of Isatis tinctoria and Isatidis Folium is the leaf of the herb. Both of them have been used to treat common cold for a long time in China. Several studies showed that crude extract and monomers isolated from Isatis tinctoria inhibited IAV-replication through mechanisms involved in the inhibition of HA or RdRP26, 27, 28, 29, 30. However, in our study, CYZH with the components from Isatis tinctoria had no effect on HA and RdRP. This difference may be the result of different extraction methods, which vary between our study and others mentioned above. Artemisia rupestris L. had been widely utilized in the Xinjiang Uyghur Autonomous Region, China for inflammation and infection. Rupestonic acid isolated from Artemisia rupestris L. was known to be an important active compound, which has a sesquiterpene with multifunctional groups and possesses high activity against influenza virus B31. In addition, several rupestonic acid derivatives were bio-assayed in vitro to determine their activities against influenza A (H1N1, oseltamivir resistant H1N1, H3N2) and B viruses32. Recently, our group found a new rupestonic acid derivative, YZH-106, with strong inhibitory activities against a panel of influenza viruses20. Interestingly, the anti-IAV role of YZH-106 is linked to activation of HO-1-mediated IFN system and anti-oxidation. Therefore, we wanted to determine whether the anti-IAV effect of CYZH is associated with up-regulation of HO-1. We found that CYZH induced the up-regulation of HO-1 mRNA and the protein. Furthermore, we showed that HO-1 up-regulation induced by CYZH was associated with Nrf2/ARE pathway through Dual-Luciferase reporter gene system. Combined with our previous study20, we believe that broad-spectrum inhibitory activities of CYZH against influenza virus are at least partly linked to HO-1 upregulation.
Oxidative stress induced by RNA virus infections can contribute to several aspects of viral disease pathogenesis including apoptosis, loss of immune function, inflammatory response and loss of body weight33. Activation of the Nrf2/ARE pathway induces expression of anti-inflammatory and anti-oxidative genes, such as HO-1, which is known to play a role in alleviating oxidative stress and tissue protection34, 35. For example, many previous studies indicated that Nrf2 protected cells from the cytopathic effects of IAV, most likely by increasing the expression of antioxidant genes in human alveolar epithelial cells and modifies IAV entry and replication in nasal epithelial cells36, 37. In line with this, we found that up-regulation of HO-1 induced by CYZH significantly inhibited ROS formation in the infected IAV MDCK cells. Also, CYZH dose-dependently inhibited virus-induced CPE in MDCK cells.
Conclusively, our present study verified the favorable role of CYZH in preventing the viral replication and damage in IAV-infected MDCK cells. CYZH exerts its antiviral effects, at least partly by inhibiting the production of ROS formation. Furthermore, we showed that decreased ROS is associated with Nrf2-mediated up-regulation of HO-1. Given the multiple components in CYZH, the other mechanisms of anti-IAV are still needed to be considered in the future study.
Acknowledgments
The work was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (2012ZX10004501-004-001) and The Science & Technology project of Urumqi of Xinjiang Uygur Autonomous Region (Y151310010): Study on the mechanism of anti-influenza virus effect of compound Yi-Zhi-Hao Pellets.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Wen Jiang, Email: 523524359@qq.com.
Yuhuan Li, Email: yuhuanlibj@126.com.
References
- 1.Wever P.C., van Bergen L. Death from 1918 pandemic influenza during the First World War: a perspective from personal and anecdotal evidence. Influenza Other Respir Viruses. 2014;8:538–546. doi: 10.1111/irv.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das K. Antivirals targeting influenza A virus. J Med Chem. 2012;55:6263–6277. doi: 10.1021/jm300455c. [DOI] [PubMed] [Google Scholar]
- 3.Takashita E., Ejima M., Ogawa R., Fujisaki S., Neumann G., Furuta Y. Antiviral susceptibility of influenza viruses isolated from patients pre-and post-administration of favipiravir. Antivir Res. 2016;132:170–177. doi: 10.1016/j.antiviral.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Sheu T.G., Fry A.M., Garten R.J., Deyde V.M., Shwe T., Bullion L. Dual resistance to adamantanes and oseltamivir among seasonal influenza A (H1N1) viruses: 2008–2010. J Infect Dis. 2011;203:13–17. doi: 10.1093/infdis/jiq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han J.N. Treatment of influenza: chinese medicine vs. western medicine. J Thorac Dis. 2012;4:10–11. doi: 10.3978/j.issn.2072-1439.2011.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L.L., Ge M., Wang H.Q., Yin J.Q., Jiang J.D., Li Y.H. Antiviral activities of several oral traditional Chinese medicines against influenza viruses. Evid Based Complement Altern Med. 2015;2015:367250. doi: 10.1155/2015/367250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y.G., Jiang M., Wang R.B., Zha Q.L., Zhang S.J., Zhou G.Q. Duration of viral shedding of influenza A (H1N1) virus infection treated with oseltamivir and/or traditional Chinese medicine in China: a retrospective analysis. J Tradit Chin Med. 2012;32:148–155. doi: 10.1016/s0254-6272(13)60004-7. [DOI] [PubMed] [Google Scholar]
- 8.State Food and Drug Administration. Complication of national standard for traditional Chinese medicine: medicine-lung (1) fascicle. 2002.226-9.
- 9.Jiang W., Yao H., Chen Q., Xing J.G. Antivirus effect of compound Yizhihao pellets on influenza virus H1N1 infection in mice. Pharmacol Clin Chin Mater Clin Med. 2015;31:135–137. [Google Scholar]
- 10.Wagaman P.C., Leong M.A., Simmen K.A. Development of a novel influenza A antiviral assay. J Virol Methods. 2002;105:105–114. doi: 10.1016/s0166-0934(02)00088-5. [DOI] [PubMed] [Google Scholar]
- 11.Serkedjieva J., Ivancheva S. Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum L. J Ethnopharmacol. 1999;64:59–68. doi: 10.1016/s0378-8741(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 12.Tolosa L., Donato M.T., Gómez-Lechón M.J. General cytotoxicity assessment by means of the MTT assay. In: Vinken M., Rogiers V., editors. Protocols in vitro hepatocyte research. Springer; New York: 2015. pp. 333–348. [Google Scholar]
- 13.Clarke D.H., Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z., Zhao F., Gao Q., Liu Z.L., Zhang Y.X., Li X.Y. Establishment of a high-throughput assay to monitor influenza a virus RNA transcription and replication. PLoS One. 2015;10:e0133558. doi: 10.1371/journal.pone.0133558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangawa H., Komeno T., Nishikawa H., Yoshida A., Takahashi K., Nomura N. Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase. Antimicrob Agents Chemother. 2013;57:5202–5208. doi: 10.1128/AAC.00649-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J.K., Yang L., Meng G.L., Yuan Z., Fan J., Li D. Protection by salidroside against bone loss via inhibition of oxidative stress and bone-resorbing mediators. PLoS One. 2013;8:e57251. doi: 10.1371/journal.pone.0057251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo G., Torri A., Harte W.E., Danetz S., Cianci C., Tiley L. Molecular mechanism underlying the action of a novel fusion inhibitor of influenza A virus. J Virol. 1997;71:4062–4070. doi: 10.1128/jvi.71.5.4062-4070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yen H.L. Current and novel antiviral strategies for influenza infection. Curr Opin Virol. 2016;18:126–134. doi: 10.1016/j.coviro.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z., Wang S., Zeng K.W., Cui F.X., Jin H.W., Guo X.Y. Rupestonic acids B–G, NO inhibitory sesquiterpenoids from Artemisia rupestris. Bioorg Med Chem Lett. 2014;24:4318–4322. doi: 10.1016/j.bmcl.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Ma L.L., Wang H.Q., Wu P., Hu J., Yin J.Q., Wu S. Rupestonic acid derivative YZH-106 suppresses influenza virus replication by activation of heme oxygenase-1-mediated interferon response. Free Radic Biol Med. 2016;96:347–361. doi: 10.1016/j.freeradbiomed.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Buffinton G.D., Christen S., Peterhans E., Stocker R. Oxidative stress in lungs of mice infected with influenza A virus. Free Radic Res Commun. 1992;16:99–110. doi: 10.3109/10715769209049163. [DOI] [PubMed] [Google Scholar]
- 22.Paracha U.Z., Fatima K., Alqahtani M., Chaudhary A., Abuzenadah A., Damanhouri G. Oxidative stress and hepatitis C virus. Virol J. 2013;10:251. doi: 10.1186/1743-422X-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov A.V., Valuev-Elliston V.T., Ivanova O.N., Kochetkov S.N., Starodubova E.S., Bartosch B. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev. 2016;2016:8910396. doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H.M., Lin S.K., Yeh C.H., Lai J.N. Prescription pattern of Chinese herbal products for adult-onset asthma in Taiwan: a population-based study. Ann Allergy Asthma Immunol. 2014;112:465–470. doi: 10.1016/j.anai.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Dai B., Wu Q., Zeng C., Zhang J., Cao L., Xiao Z. The effect of Liuwei Dihuang decoction on PI3K/Akt signaling pathway in liver of type 2 diabetes mellitus (T2DM) rats with insulin resistance. J Ethnopharmacol. 2016;192:382–389. doi: 10.1016/j.jep.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z., Wang Y., Zhong S., Zhao S., Zeng X., Mo Z. In vitro inhibition of influenza virus infection by a crude extract from Isatis indigotica root resulting in the prevention of viral attachment. Mol Med Rep. 2012;5:793–799. doi: 10.3892/mmr.2011.709. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Zhou B., Li C., Chen Q., Wang Y., Li Z. Lariciresinol-4-O-β-d-glucopyranoside from the root of Isatis indigotica inhibits influenza A virus-induced pro-inflammatory response. J Ethnopharmacol. 2015;174:379–386. doi: 10.1016/j.jep.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z., Wang Y., Zheng Z., Zhao S., Zhao J., Lin Q. Antiviral activity of Isatis indigotica root-derived clemastanin B against human and avian influenza A and B viruses in vitro. Int J Mol Med. 2013;31:867–873. doi: 10.3892/ijmm.2013.1274. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y.F., Chen M.H., Guo Q.L., Lin S., Xu C.B., Jiang Y.P. Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica. J Asian Nat Prod Res. 2015;17:689–704. doi: 10.1080/10286020.2015.1055729. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z., Yang Z.Q., Xiao H. Antiviral activity of the effective monomers from Folium Isatidis against influenza virus in vivo. Virol Sin. 2010;25:445–451. doi: 10.1007/s12250-010-3142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong J., Lu C., Aisa H.A. Advances in studies on the rupestonic acid derivatives as anti-influenza agents. Mini Rev Med Chem. 2013;13:310–315. [PubMed] [Google Scholar]
- 32.He Y.W., Dong C.Z., Zhao J.Y., Ma L.L., Li Y.H., Aisa H.A. 1,2,3-Triazole-containing derivatives of rupestonic acid: click-chemical synthesis and antiviral activities against influenza viruses. Eur J Med Chem. 2014;76:245–255. doi: 10.1016/j.ejmech.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Reshi M.L., Su Y.C., Hong J.R. RNA viruses: ros-mediated cell death. Int J Cell Biol. 2014;2014:467452. doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wegiel B., Nemeth Z., Correa-Costa M., Bulmer A.C., Otterbein L.E. Heme oxygenase-1: a metabolic nike. Antioxid Redox Signal. 2014;20:1709–1722. doi: 10.1089/ars.2013.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosmider B., Messier E.M., Janssen W.J., Nahreini P., Wang J., Hartshorn K.L. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res. 2012;13:43. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kesic M.J., Simmons S.O., Bauer R., Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med. 2011;51:444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]