ABSTRACT
Excessive inflammation during pregnancy alters homeostatic mechanisms of the developing fetus and has been linked to adverse pregnancy outcomes. An anti-inflammatory diet could be a promising avenue to combat the pro-inflammatory state of pregnancy, particularly in obese women, but we lack mechanistic data linking this dietary pattern during pregnancy to inflammation and birth outcomes. In an ethnically diverse cohort of 1057 mother-child pairs, we estimated the relationships between dietary inflammatory potential [measured via the energy-adjusted dietary inflammatory index (E-DII™)] and birth outcomes overall, as well as by offspring sex and maternal pre-pregnancy body mass index (BMI). In a subset of women, we also explored associations between E-DII, circulating cytokines (n = 105), and offspring methylation (n = 338) as potential modulators of these relationships using linear regression. Adjusted regression models revealed that women with pro-inflammatory diets had elevated rates of preterm birth among female offspring [β = −0.22, standard error (SE) = 0.07, P<0.01], but not male offspring (β=0.09, SE = 0.06, P<0.12) (Pinteraction = 0.003). Similarly, we observed pro-inflammatory diets were associated with higher rates of caesarean delivery among obese women (β = 0.17, SE = 0.08, P = 0.03), but not among women with BMI <25 kg/m2 (Pinteraction = 0.02). We observed consistent inverse associations between maternal inflammatory cytokine concentrations (IL-12, IL-17, IL-4, IL-6, and TNFα) and lower methylation at the MEG3 regulatory sequence (P<0.05); however, results did not support the link between maternal E-DII and circulating cytokines. We replicate work by others on the association between maternal inflammatory diet and adverse pregnancy outcomes and provide the first empirical evidence supporting the inverse association between circulating cytokine concentrations and offspring methylation.
KEYWORDS: Birthweight, cytokines, diet, DNA methylation, epidemiology, imprinted genes, inflammation
Abbreviation
- BMI
Body Mass Index
- DII
Dietary Inflammatory Index
- DMR
Differentially Methylated Region
- E-DII
Energy-Adjusted Dietary Inflammatory Index
- FA
Folic Acid
- FFQ
Food Frequency Questionnaire
- LGA
Large for Gestational Age
- LMP
Last Menstrual Period
- NEST
Newborn Epigenetics Study
- SGA
Small for Gestational Age
Introduction
The developmental origin of health and disease hypothesis posits that maternal factors, through an altered in utero environment, have the capacity to contribute substantially to the offspring's health in adulthood.1,2 There is accumulating evidence that prenatal conditions and exposures, including maternal body size, nutrition, and exposure to toxicants, can alter gene expression profiles of offspring. These factors also influence embryonic, placental, and fetal growth, and thereby predispose the offspring to adult chronic conditions, including obesity, cardiovascular disease, type 2 diabetes, and cancer.3,4 Data from animal and human studies suggest that maternal nutrition during pregnancy has long-term health consequences for her offspring.5 These outcomes may be driven by epigenetic changes, i.e., modifications that result in differential levels of gene expression without altering the base nucleotide sequence and thus could be important targets for behavioral and clinical intervention. Because many epigenetic marks, especially those regulating genomically imprinted genes, are established before gastrulation and are maintained in somatic tissues,6 the downstream effects of early perturbations could be extensive.7-9 However, beyond single-nutrient analysis,10-13 the role of maternal dietary patterns and nutrition on the offspring's epigenome and early outcomes remains understudied.
One pathway through which diet could possibly affect the epigenome is inflammation. There is a well-established link between diet and inflammatory biomarkers in the non-pregnant population,14 and though pregnancy itself induces a state of inflammation, evidence suggests the link persists among pregnant mothers.15,16 Chronic inflammation during pregnancy—such as that observed in obese women and the associated release of pro-inflammatory cytokines from adipose tissue—may alter homeostatic mechanisms of the developing fetus and has been linked to adverse pregnancy outcomes including prematurity, intrauterine growth restriction, and preeclampsia.17-21 Recent data show that maternal diet is associated with both inflammation and early-life outcomes in offspring.15,16 In a cohort of approximately 1800 women living in Massachusetts, the dietary inflammatory index (DII), a tool for assessing the inflammatory potential of the diet, was associated with small-for-gestational-age (SGA) infants among mothers with pre-pregnancy obesity.15 Maternal DII also was associated with maternal markers of inflammation [C-reactive protein (CRP)]; however, the mechanistic links between these markers and fetal adiposity remain obscure.22 Mechanistic insights into the association between maternal diet, inflammation, and offspring adiposity could provide a promising avenue to combat the pro-inflammatory state of pregnancy, particularly in obese women.
Given the strong relationship between diet and the epigenome, a promising hypothesis is that a pro-inflammatory dietary pattern produces elevated concentrations of cytokines and other inflammatory molecules that alter the regulation of key genes in the developing fetus, mediated by epigenetic mechanisms. Therefore, in an ethnically diverse population of 1057 mother-child pairs participating in the Newborn Epigenetic Study (NEST), the objectives of this analysis are to: (1) examine the association between maternal DII and offspring birth outcomes; (2) explore the effects of higher cytokine concentrations on the methylation patterns of genomically imprinted genes in offspring at birth; and (3) examine the relationship between maternal DII and circulating cytokines (Fig. 1). We also estimated the direct effect of pro-inflammatory diet on offspring DNA methylation of 9 differentially methylated regions (DMRs) of genomically imprinted genes, including the IGF2 DMR, H19 DMR, MEG3-IG DMR, MEG3 DMR, MEST DMR, NNAT DMR, PEG3 DMR, SGCE/PEG10 DMR, and the PLAGL1 DMR. These DMRs are known to be important for regulating the associated imprinted genes with regard to their critical roles in fetal growth and development,23,24 and associate with birthweight in our study population.12 Given known associations between inflammation and maternal obesity, as well as the sex-specificity of certain epigenetic perturbations,25 we also examined potential modification by maternal pre-pregnancy obesity and infant sex.
Figure 1.
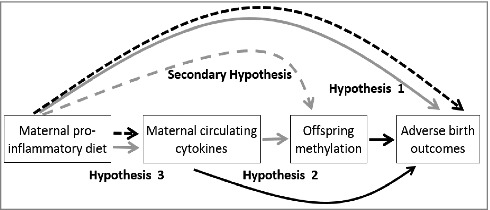
Diagram of previous findings and proposed study aims among 1057 mother-child pairs enrolled in the Newborn Epigenetics STudy cohort.
Solid black line = established in the literature
Dashed black line = suggestive in the literature
Solid gray line = primary aims of current study
Dashed black line = secondary aims of current study
Results
Demographic and clinical characteristics of the 1057 study participants appear in Table 1. The study participants’ characteristics included: a race-ethnicity distribution of 43% African-American, 34% White, and 23% Hispanic; 59% aged less than 30 years; 73% nonsmokers; and 49% with a household income lower than $25,000. The median E-DII score in the study cohort was −1.37 with a range of −5.00 to 4.96. African-American race, higher maternal BMI and smoking were associated with a more pro-inflammatory diet (i.e., higher E-DII score), while older age, higher income and education were associated with a more anti-inflammatory diet (i.e., lower E-DII score). E-DII scores did not differ by other factors, including birth outcomes (mode of delivery, gestational age at delivery, birthweight for gestational age, and infant sex).
Table 1.
Study participant characteristics by dietary inflammatory index (DII) quartiles.
|
more anti-inflammatory ← DII quartile→ more pro-inflammatory |
||||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 1057) | Q1 (n = 264) | Q2 (n = 264) | Q3 (n = 265) | Q4 (n = 264) | ||||
| n | Median (interquartile range*) | |||||||
| DII (overall) | 1057 | −1.37 (2.78) | −3.54 (0.93) | −1.96 (1.31) | −0.68 (0.76) | 1.19 (1.30) | ||
| DII (peri conceptional period) | 599 | −1.70 (2.87) | −3.63 (0.87) | −1.96 (0.60) | −0.75 (0.82) | 1.22 (1.40) | ||
| DII (first trimester) | 123 | −0.99 (2.63) | −3.60 (1.08) | −2.22 (0.71) | −0.50 (0.64) | 1.32 (1.36) | ||
| DII (entire pregnancy) | 260 | −0.48 (2.38) | −3.25 (0.94) | −1.78 (0.50) | −0.58 (0.70) | 1.04 (1.34) | ||
| n | Median (SD) | |||||||
| Birthweight (grams) | 1057 | 3332 (593) | 3367.5 (542) | 3386.0 (588) | 3340.0 (608) | 3210.0 (620) | ||
| Gestational age§ | 1056 | 39 (2.03) | 39 (1.77) | 39 (2.03) | 39 (2.05) | 39 (2.24) | ||
| N (%) | ||||||||
| Age at delivery (years) | 1057 | |||||||
| <20 | 32 (3%) | 5 (2%) | 5 (2%) | 7 (3%) | 15 (6%) | |||
| 20-29 | 587 (56%) | 113 (43%) | 122 (46%) | 166 (62%) | 186 (70%) | |||
| 30-39 | 414 (39%) | 140 (53%) | 130 (49%) | 87 (33%) | 57 (22%) | |||
| 40+ | 24 (2%) | 6 (2%) | 7 (3%) | 5 (2%) | 6 (2%) | |||
| Race/ethnicity | 926 | |||||||
| Black | 394 (43%) | 49 (22%) | 61 (27%) | 103 (44%) | 181 (74%) | |||
| White | 317 (34%) | 102 (46%) | 88 (39%) | 74 (32%) | 53 (22%) | |||
| Hispanic | 215 (23%) | 72 (32%) | 76 (34%) | 55 (24%) | 12 (5%) | |||
| Marital status | 1019 | |||||||
| Married | 452 (44%) | 159 (62%) | 126 (50%) | 105 (41%) | 62 (24%) | |||
| Never married | 272 (27%) | 34 (13%) | 56 (22%) | 70 (28%) | 112 (44%) | |||
| Living with partner | 244 (24%) | 55 (21%) | 64 (25%) | 66 (26%) | 59 (23%) | |||
| Other | 51 (5%) | 9 (4%) | 6 (3%) | 12 (5%) | 24 (9%) | |||
| Household income | 853 | |||||||
| <$25,000 | 412 (49%) | 74 (33%) | 89 (43%) | 113 (56%) | 136 (62%) | |||
| $25,000-$49,999 | 137 (16%) | 32 (15%) | 30 (14%) | 31 (15%) | 44 (20%) | |||
| $50,000-$100,000 | 190 (11%) | 61 (28%) | 57 (27%) | 35 (17%) | 37 (17%) | |||
| >$100,000 | 114 (14%) | 54 (24%) | 34 (16%) | 25 (12%) | 1 (1%) | |||
| Education | 1023 | |||||||
| College graduate | 351 (34%) | 122 (48%) | 100 (40%) | 78 (31%) | 51 (20%) | |||
| High school/GED | 213 (21%) | 38 (15%) | 39 (15%) | 67 (26%) | 69 (27%) | |||
| Less than high school | 282 (28%) | 64 (25%) | 80 (32%) | 67 (26%) | 71 (27%) | |||
| Some college | 177 (17%) | 32 (12%) | 34 (13%) | 43 (17%) | 68 (26%) | |||
| Parity (at enrollment) | 1032 | |||||||
| Multiparous | 656 (64%) | 159 (62%) | 164 (64%) | 161 (62%) | 172 (66%) | |||
| Nulliparous | 376 (36%) | 99 (38%) | 92 (36%) | 97 (38%) | 88 (34%) | |||
| Body mass index (kg/m2)† | 1003 | |||||||
| 18.5-24.99 | 412 (41%) | 117 (46%) | 106 (42%) | 97 (39%) | 92 (37%) | |||
| 25-29.99 | 277 (28%) | 67 (27%) | 76 (30%) | 76 (30%) | 58 (23%) | |||
| >29.99 | 314 (31%) | 67 (27%) | 71 (28%) | 77 (31%) | 99 (40%) | |||
| Gestational weight gain | 1039 | |||||||
| Adequate | 249 (24%) | 65 (25%) | 65 (25%) | 66 (25%) | 53 (20% | |||
| Less than adequate | 246 (24%) | 53 (21%) | 65 (25%) | 61 (24%) | 67 (26%) | |||
| Excessive | 544 (52%) | 140 (54%) | 132 (50%) | 133 (51%) | 139 (54%) | |||
| Smoking status | 1015 | |||||||
| No smoking | 736 (73%) | 217 (86%) | 199 (78%) | 171 (68%) | 149 (58%) | |||
| Smoking prior to pregnancy | 114 (11%) | 25 (10%) | 37 (15%) | 29 (11%) | 23 (9%) | |||
| Smoking during pregnancy | 165 (16%) | 11 (4%) | 18 (7%) | 53 (21%) | 83 (33%) | |||
| Alcohol | 1009 | |||||||
| No alcohol in pregnancy | 995 (99%) | 249 (98%) | 245 (98%) | 250 (99%) | 251 (98%) | |||
| Alcohol in pregnancy | 14 (1%) | 4 (2%) | 4 (2%) | 2 (1%) | 4 (2%) | |||
| Folic acid supplementation | 1005 | |||||||
| Yes | 903 (90%) | 213 (85%) | 230 (90%) | 225 (91%) | 235 (94%) | |||
| No | 102 (10%) | 39 (15%) | 25 (10%) | 22 (9%) | 16 (6%) | |||
| Gestational diabetes | 1041 | |||||||
| No | 977 (94%) | 243 (93%) | 243 (94%) | 249 (95%) | 242 (93%) | |||
| Yes | 64 (6%) | 18 (7%) | 16 (6%) | 13 (5%) | 17 (%) | |||
| Infant sex | 1057 | |||||||
| Male | 560 (53%) | 142 (54%) | 140 (53%) | 137 (52%) | 141 (53%) | |||
| Female | 497 (47%) | 122 (46%) | 124 (47%) | 128 (48%) | 123 (46%) | |||
| Gestational age§ | 1056 | |||||||
| <34 | 28 (3%) | 4 (1%) | 6 (2%) | 9 (3%) | 9 (3%) | |||
| 34-<37 | 62 (6%) | 12 (5%) | 17 (7%) | 12 (5%) | 21 (8%) | |||
| ≥37 | 966 (91%) | 247 (94%) | 241 (91%) | 244 (92%) | 234 (89%) | |||
| Method of delivery | 1055 | |||||||
| Vaginal | 712 (67%) | 178 (68%) | 183 (69%) | 175 (66%) | 176 (67%) | |||
| Cesarean | 343 (33%) | 85 (32%) | 81 (31%) | 90 (34%) | 87 (33%) | |||
| Birthweight(grams) | 1057 | |||||||
| Low, <2500 | 77 (7%) | 13 (5%) | 16 (6%) | 21 (8%) | 27 (10%) | |||
| Normal, 2500-4500 | 964 (91%) | 248 (94%) | 242 (92%) | 239 (90%) | 235 (89%) | |||
| High, >4500 | 16 (2%) | 3 (1%) | 6 (2%) | 5 (2%) | 2 (1%) | |||
| Size for gestational age | 1057 | |||||||
| Small for gestational age | 126 (12%) | 24 (9%) | 22 (8%) | 39 (15%) | 41 (15%) | |||
| Average for gestational age | 855 (81%) | 219 (83%) | 221 (34%) | 207 (78%) | 208 (79%) | |||
| Large for gestational age | 76 (7%) | 21 (8%) | 21 (8%) | 19 (7%) | 15 (6%) | |||
(25th percentage quartile, 75th percentage quartile).
Weeks at delivery.
At last menstrual period.
E-DII and birth outcomes
Birthweight
After adjustment for maternal age at delivery, race/ethnicity, household income and maternal cigarette smoking, we observed no association between infant birthweight and maternal E-DII score, overall, or when stratified by infant sex or maternal pre-pregnancy BMI (Table 2).
Table 2.
Adjusted* regression coefficients for association between maternal dietary inflammatory index (DII, per unit change) and birth outcomes, overall and by infant sex and maternal body mass index.
| β coefficient, standard error, P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infant sex |
Maternal body mass index† |
|||||||||
| Overall (n = 766) | Male (n = 399) | Female (n = 367) | P-value‡ | 18.5-24.99 (n = 291) | 25-29.99 (n = 197) | 30+ (n = 248) | P-value‡ | |||
| Continuous Outcomes | — | |||||||||
| Birthweight (g) | -9.05, 12.68, 0.48 | 9.02, 16.64, 0.59 | -22.35, 19.10, 0.24 | 0.747 | -19.48, 18.83, 0.30 | 20.58, 26.02, 0.43 | -23.31, 23.91, 0.33 | 0.564 | ||
| Gestational age§ | -0.07, 0.04, 0.14 | 0.09, 0.06, 0.12 | -0.22, 0.07, 0.001 | 0.003 | -0.08, 0.06, 0.14 | 0.01, 0.10, 0.93 | -0.08, 0.09, 0.39 | 0.874 | ||
| Binary Outcomes | ||||||||||
| Small for gestational age | 0.01, 0.07, 0.89 | -0.05, 0.10, 0.59 | 0.11, 0.10, 0.31 | 0.186 | 0.05, 0.11, 0.66 | -0.12, 0.17, 0.49 | 0.03, 0.12, 0.81 | 0.555 | ||
| Large for gestational age | -0.06, 0.08, 0.44 | 0.02, 0.12, 0.90 | -0.17, 0.12, 0.16 | 0.187 | -0.04, 0.17, 0.82 | -0.09, 0.15, 0.56 | 0.12, 0.14, 0.38 | 0.211 | ||
| Cesarean delivery | 0.05, 0.05, 0.28 | 0.03, 0.07, 0.66 | 0.08, 0.07, 0.25 | 0.366 | -0.14, 0.09, 0.13 | 0.10, 0.10, 0.33 | 0.17, 0.08, 0.03 | 0.023 | ||
Adjusted for maternal age at delivery, race/ethnicity, household income, and maternal cigarette smoking.
At last menstrual period.
P for interaction (test for homogeneity)
Weeks at delivery
Gestational age at delivery
While maternal E-DII score was not associated with gestational age at delivery overall, we did observe sex-specific effects with a more pro-inflammatory diet associated with lower gestational age at delivery among female offspring (β = −0.22, SE = 0.07, P<0.01), but unrelated to gestational age at delivery in male offspring (β=0.09, SE = 0.06, P<0.12) (P for interaction = 0.003). These associations did not vary by maternal pre-pregnancy BMI (Table 2).
Birthweight for gestational age
There was no association between the E-DII score and birthweight for gestational age [small for gestational age (SGA) or large for gestational age (LGA)] in our study population (Table 2). There was no evidence that associations were modified by infant sex or maternal pre-pregnancy BMI (P for interaction >0.05).
Mode of delivery
The E-DII was not associated with rate of cesarean delivery in our study population overall, or when stratified by infant sex. However, we found a positive association between dietary inflammatory potential and cesarean delivery restricted to women with BMI >30 kg/m2 at LMP (P for interaction = 0.02) (Table 2). Obese women had an approximately 20% increased rate of cesarean delivery per unit increase in maternal E-DII score (β = 0.17, SE = 0.08, P = 0.03).
For all outcomes, upon restricting our study population to women with E-DII scores computed from FFQ1 only, results were not substantially altered (data not shown).
Cytokines and cord blood leukocyte methylation
We investigated the association between maternal circulating cytokines and methylation at 9 differentially methylated regions (DMRs) measured in infant cord blood (Table 3). In multivariate analyses, lower MEG3 methylation was associated with higher levels of IL-12 (β=−0.22), IL-17 (β=−0.16), IL-4 (β = −0.16), IL-6 (β = −0.65), and TNF-α (β = −0.37). We also observed a suggestive association between the MEST DMR and TNFα (β = −0.25, SE = 0.13, P = 0.06). Estimates for other regions were modest and imprecise.
Table 3.
Adjusted* regression coefficients for the association between maternal cytokines (per unit change) and differentially methylated regions (DMRs) in cord blood DNA among term births.
| DMR |
n |
Mean methylation % (SD) |
IFNγ |
IL 12 |
IL 17A |
IL 1β |
IL 4 |
IL 6 |
TNFα |
|---|---|---|---|---|---|---|---|---|---|
| β coefficient, Standard Error, P-value | |||||||||
| IGF2 | 96 | 48.39 (3.80) | −0.003, 0.03, 0.92 | 0.05, 0.07, 0.43 | 0.04, 0.05, 0.39 | 0.09, 0.13, 0.51 | 0.03, 0.07, 0.70 | 0.22, 0.21, 0.29 | 0.08, 0.12, 0.49 |
| H19 | 96 | 51.44 (4.25) | −0.009, 0.03, 0.74 | 0.06, 0.07, 0.40 | 0.01, 0.05, 0.83 | −0.12, 0.14, 0.38 | −0.03, 0.08, 0.71 | 0.16, 0.22, 0.47 | 0.18, 0.13, 0.16 |
| DLK1/MEG3 | 91 | 71.26 (5.82) | −0.07, 0.04, 0.08 | −0.22, 0.11, 0.04 | −0.16, 0.07, 0.02 | −0.33, 0.21, 0.12 | −0.31, 0.11, 0.006 | −0.65, 0.32, 0.04 | −0.37, 0.19, 0.04 |
| DLK1/MEG3 IG | 86 | 50.12 (2.86) | −0.05, 0.03, 0.05 | −0.00, 0.05, 0.99 | −0.06, 0.04, 0.11 | −0.12, 0.12, 0.30 | −0.03, 0.06, 0.59 | −0.21, 0.17, 0.23 | −0.08, 0.10, 0.39 |
| MEST | 85 | 44.05 (4.01) | −0.05, 0.03, 0.12 | −0.13, 0.08, 0.10 | −0.07, 0.05, 0.13 | −0.11, 0.14, 0.41 | −0.07, 0.08, 0.34 | −0.20, 0.22, 0.38 | −0.24, 0.13, 0.06 |
| NNAT | 85 | 55.32 (4.83) | −0.08, 0.04, 0.06 | −0.11, 0.10, 0.27 | −0.06, 0.07, 0.33 | 0.11, 0.19, 0.54 | 0.09, 0.10, 0.37 | −0.09, 0.30, 0.77 | −0.26, 0.17, 0.13 |
| PEG3 | 87 | 35.93 (2.95) | −0.02, 0.02, 0.34 | 0.06, 0.06, 0.35 | −0.04, 0.04, 0.37 | −0.005, 0.12, 0.97 | 0.01, 0.06, 0.85 | 0.04, 0.18, 0.81 | 0.004, 0.11, 0.97 |
| SGCE/PEG10 | 89 | 45.79 (3.72) | −0.02, 0.03, 0.52 | 0.03, 0.07, 0.63 | −0.004, 0.05, 0.94 | 0.08, 0.15, 0.60 | 0.12, 0.09, 0.20 | −0.01, 0.22, 0.95 | −0.07, 0.13, 0.60 |
| PLAGL1 | 105 | 56.38 (5.73) | −0.03, 0.04, 0.44 | 0.04, 0.10, 0.72 | 0.07, 0.07, 0.28 | −0.27, 0.19, 0.15 | 0.08, 0.11, 0.48 | 0.25, 0.31, 0.42 | 0.08, 0.18, 0.64 |
Adjusted for maternal race/ethnicity, household income, body mass index at last menstrual period, and maternal smoking.
E-DII, cord blood methylation, and inflammatory cytokines
We estimated the association between maternal E-DII and methylation at the same 9 DMRs (Table 4). We found no evidence of association, even upon restricting to women with E-DII computed from FFQ 1 (Table S1). Further, with the exception of a weak unstable relationship with IL-1B (r =−0.16), maternal E-DII did not correlate with maternal cytokine circulation (data not shown).
Table 4.
Adjusted* regression coefficients for the association between maternal dietary inflammatory index (DII, per unit change) and differentially methylated regions (DMRs) in cord blood DNA among term births
| |
n |
Mean methylation % (SD) |
β coefficient, SE, P-value |
|---|---|---|---|
| per unit | |||
| IGF2 DMR | 325 | 51.80 (4.18) | -0.08, 0.14, 0.57 |
| H19 DMR | 335 | 47.79 (3.85) | -0.15, 0.13, 0.25 |
| DLK1/MEG3 DMR | 313 | 72.47 (5.46) | -0.02, 0.19, 0.90 |
| DLK1/MEG3 IG DMR | 287 | 49.94 (3.05) | -0.05, 0.11, 0.64 |
| MEST DMR | 293 | 43.71 (4.33) | -0.09, 0.16, 0.58 |
| NNAT DMR | 274 | 55.45 (5.81) | -0.10, 0.22, 0.65 |
| PEG3 DMR | 309 | 36.28 (3.68) | -0.08, 0.13, 0.54 |
| SGCE/PEG10 DMR | 305 | 45.95 (5.52) | 0.06, 0.20, 0.76 |
| PLAGL1 DMR | 338 | 57.81 (6.50) | 0.13, 0.22, 0.56 |
Adjusted for maternal race/ethnicity, body mass index at last menstrual period, and maternal smoking. SD: Standard Deviation SE: Standard Error
Discussion
Our study sought to elucidate the relationships between maternal dietary inflammatory potential and birth outcomes, as well as gain mechanistic insights involving maternal cytokines and offspring methylation. While we found no relationship between E-DII and birth outcomes, overall, we observed differences in the association between E-DII and gestational age by infant sex, with pro-inflammatory maternal diet inversely associated with gestational age among female offspring only, which warrants additional investigation. We also found that obese women with pro-inflammatory diets had a higher rate of caesarean delivery compared with obese women with more anti-inflammatory diets. Despite limited sample size, we observed consistent inverse associations between maternal cytokines and offspring hypomethylation at the MEG3 DMRs. Although many of the biomarkers we used were key in the development of the DII as an assessment tool,26,27 our data do not support the link between maternal E-DII and circulating cytokines (we observed a single weak correlation between E-DII and IL1β).
To our knowledge, Sen et al. are the only other investigators to conduct a comprehensive assessment of maternal inflammatory diet and pregnancy outcomes. They report an increased rate of SGA among obese mothers with similar pro-inflammatory diets.15 We were unable to replicate this association using the same United States national reference.28 A novel positive association reported here between E-DII and cesarean delivery appears among obese women representing approximately 30% of our study population. This result contrasts with that of Sen et al., and may be attributable to the lower percent of obese women in their study (<15%) resulting in lower statistical power that limited ability to detect the association. Notably, the NEST study cohort was younger and had a larger proportion of Black and Hispanic women, which also may influence maternal pre-pregnancy obesity and offspring outcomes.
Given the observed associations with early gestational age at delivery and cesarean delivery in subsets of our study cohort, a secondary goal of this study was to explore potential inflammatory and epigenetic mediators of this association in regulatory regions known to be established in early development and similar in all cell types. Cytokines have consistently been associated with birth outcomes and data show that inflammatory phenotypes (i.e., those that involve increased circulation of cytokines and other inflammatory markers, such as obesity and preeclampsia) are thought to influence birthweight extremes through their effects on inflammatory biomarkers.29-31 For example, McCloskey et al. found a positive association between maternal BMI and newborn adiposity, and upon adjusting for maternal high sensitivity (hs)CRP, the main effect was attenuated by approximately 25%.29 Similarly, data show that women with preeclampsia in pregnancy have increased concentrations of pro-inflammatory markers (CRP, IL's, TNFα, and interferon γ) that are associated with fetal birthweight.30,31 Elevated concentrations of maternal CRP, IL-6, TNF-α, leptin, and homocysteine have each been related to risk of low birthweight,30,32,33 a mechanism which may involve the stimulation of phagocytosis of apoptotic cells by CRPs, and intrauterine growth restriction by IL-6 and TNF-α.30 Maternal IL-6 and TNF-α also have been linked to preterm births,34 which may, in part, drive associations between obesity and low birthweight. Therefore, modulation of circulating cytokines could be one pathway through which an inflammatory diet affects offspring outcomes.
While limited, there is accumulating evidence of an association between maternal inflammatory conditions, such as maternal obesity, and offspring methylation. Nomura et al. showed that maternal obesity was associated with placental global hypermethylation, which was also linked to infant length and head size.35 Maternal BMI also is associated with differential methylation of individual CpG sites involved in lipid metabolism and inflammation.36 The link between inflammatory phenotypes, aberrant methylation, and poor birth outcomes also has been reported in the NEST study population, where maternal obesity was associated with hypermethylation at the PLAGL1 DMR and hypomethylation at the MEG3 DMR.37 These regions were further identified as regulators of placental growth and fetal development.8,38,39
Collectively, these data suggest that both circulating inflammatory biomarkers and aberrant methylation could play an important role in the association between maternal inflammatory conditions and fetal outcomes. Therefore, we hypothesized that an inflammatory diet may similarly alter epigenetic patterns that regulate growth and development through cytokine involvement, as depicted in Fig. 1, although the limited number of women with cytokine data preclude our ability to formally test for mediation. To better understand this potential causal pathway, we examined whether maternal cytokines (IFNγ, interleukins, and TNF-α) could be related to birth outcomes through a pathway involving alterations at genomically imprinted genes. Further, given that the E-DII was developed to reflect the physiologic, inflammatory response to various diets, we hypothesized that maternal E-DII would similarly associate with offspring methylation, and the correlation between maternal E-DII and circulating cytokines would be strong, representing an indirect causal pathway from exposure (maternal E-DII) to outcome (phenotypes at birth).
We observed inverse associations between cytokine concentrations and methylation of MEG3 DMR, as well as a suggestive association between TNFα and methylation at the MEST DMR. Similar to the IGF2/H19 imprinted domain where IGF2 and H19 are reciprocally imprinted, the DLK1 and MEG3 genes (located on chromosome 14q32) also are reciprocally imprinted.40 However, unlike most imprinted genes established during gametogenesis, methylation at the MEG3 DMR is established on the paternally derived allele post-fertilization during global demethylation. MEG3 produces a long noncoding RNA. Aberrant methylation of DMRs in the DLK1/MEG3 domain leads to altered gene expression,41,42 and has been implicated in fetal growth and development,41 tumorigenesis,43 and Rett's Syndrome.44 PEG1/MEST is an imprinted gene located at chromosome 7q32.2 and is expressed from the paternal allele. Deregulated methylation or expression of PEG1/MEST have been associated with growth retardation, defective maternal nurturing and increased pup lethality in mice,45 and invasive breast cancer,46 cervical cancer,47 and uterine leiomyoma48 in humans. Despite finding a novel association between circulating cytokines and offspring methylation, we observed no correlation between maternal E-DII score and maternal blood cytokine concentration. Further, maternal E-DII was not associated with offspring methylation in our study population.
These data may suggest that diet impacts outcomes through alternative pathways. Alternatively, diet may indeed mitigate the pro-inflammatory state of pregnant women, but our study was unable to detect it. Notably, the possible range of scores in the E-DII is much wider than the range captured in our population, so while we did not observe significant associations between E-DII score and either cytokine circulation or DMR methylation, our results do not preclude the potential for more extreme variations in diet to produce these associations. We also cannot exclude the possibility that because cytokines were measured early in pregnancy (∼12 weeks), the timing of FFQ administration was not temporally aligned with blood collection. Although we did not have information on CRP in the NEST population, data from a cohort of women in eastern Massachusetts showed that maternal DII scores in the first and second trimesters were directly associated with CRP in the second trimester—even upon adjustment for maternal BMI.15 In light of our findings, it also is worth considering that other factors may be more important contributors to inflammation than diet in the pregnant population. There are many sources of inflammatory molecules in pregnant women which could make it more difficult to observe the contribution of diet alone. Despite our null findings between maternal E-DII score and both maternal cytokines and offspring methylation, the observed association between E-DII and birth outcomes suggests that diet could still be an important avenue for dietary intervention, irrespective of the underlying mechanism.
Strengths of our study include its large population-based study sample, prospective design, comprehensive assessment of inflammatory diet, and consideration of modifying factors, including maternal BMI. A limitation of our study was that we were unable to analyze all potentially pertinent DMRs associated with imprinted genes as many of the imprint control regions are still unknown.49 However, by focusing only on 9 DMRs for which we had strong evidence of biologic relevance, we were able to increase the power of our study and reduce concerns around multiple comparisons. We recognize that correction for multiple comparisons of cytokine-DMR associations could be important (72 test were performed). However, given our study hypotheses were based on strong biologic rationale and we targeted cytokines known to play a role in perinatal outcomes, we were primarily interested in estimating effects and corresponding CIs without adjusting for multiple comparisons. Drawing inferences from study results should entail broader considerations than P-values based on hypothesis testing, whether or not they are adjusted. We have provided unadjusted P-values, with the goal of letting the reader draw her/his own conclusions and with recognition that additional studies are needed. Another potential limitation of our study is the inherent uncertainty of dietary information. FFQs were completed at multiple time points during the study, which may have diluted the effect of diet at any particular stage of pregnancy. In addition, while the FFQ is a fairly reliable estimate of usual diet in the general population,50 this assessment may be unreliable among pregnant women when usual dietary habits are likely to change. When available, we used FFQ data regarding the periconceptual period, as the methylation regulating genomic imprinting is thought to exhibit some plasticity during the period of post-fertilization reprogramming .51 Almost half of the women in our study (43%) did not have FFQ data from this time period, and information about diet during pregnancy was used instead; however, restricting to FFQ 1 did not change the results of our analyses, and these later windows may be relevant for other outcomes.
Conclusions
This study showed that diet-related inflammation during pregnancy may be associated with an increased risk of adverse birth outcomes. We found evidence that maternal inflammatory molecules may associate with birth outcomes, with the potential for long-term effects, through the modification of methylation patterns regulating imprinted genes. Additional higher-powered studies are needed to determine whether diet is an important contributor to maternal inflammation overall, and elucidate the potential impact of dietary interventions during pregnancy on maternal and offspring health.
Subjects and methods
Study participants
The study participants were enrolled in NEST, a prospective cohort study of pregnant women and the index offspring. Written, informed consent was obtained for all study participants before data collection, and study enrollment methods have been described previously.52 Briefly, between 2009 and 2011, English- or Spanish-speaking pregnant women aged 18 y or older were identified from clinic logs of prenatal clinics in Durham County, NC, USA. Women were excluded from the study if they planned to relinquish custody of the child, did not deliver at one of the participating obstetric facilities, anticipated moving from the area in the subsequent 3 years, or had established HIV infection. Among the 2548 eligible women, 1700 (66.7%) consented and were enrolled. Women enrolled in the study were similar in age to those who declined to participate (P = 0.66), but dissimilar with respect to race (P<0.001). Non-participants were more likely to be Asian or Native American. Among the 1700 consenting women, 115 were excluded due to death of the fetus/infant before, during, or soon after birth. Another 281 participants were excluded because either they were illiterate and could not consent, underage, refused further participation, or could not be located. The 1304 participants represent 76.7% of the study population. Of these remaining women, we restricted our analysis to 1057 mother-infant pairs with singleton deliveries for whom dietary data and birth outcomes were available. The NEST protocol was approved by Duke University, Durham Regional Hospital, North Carolina State University Institutional Review Boards, and the University of Texas MD Anderson Cancer Center.
Data collection
Data collection occurred at 2 major time points throughout the study: (1) at enrollment (median ∼12 weeks gestation), during which participants provided peripheral blood samples and completed a self-administered questionnaire that queried women on sociodemographic, reproductive, and lifestyle (including dietary) characteristics in the 6 months before enrollment; and (2) upon delivery, after which birth outcomes were abstracted from medical records and infant cord blood specimens for methylation analyses were obtained.
Assessment of inflammatory diet via the energy-adjusted DII (E-DII)
Dietary data were collected by trained dieticians over the phone using The University of Texas MD Anderson Cancer Center Nutrition & Lifestyle Core Questionnaire, a food frequency questionnaire (FFQ) created in Spanish and English for the study. Typically, an electronic copy of the FFQ was sent to the participant before the phone call for guidance during the interview. FFQs were administered at 3 different time points: 1) at enrollment with a reference period of up to 6 months before pregnancy (i.e., diet during the periconceptional period); 2) during the second trimester to estimate usual diet in the first trimester; 3) between the time period of 36 weeks gestation through delivery date to estimate diet in the last 2 trimesters of pregnancy; and 4) at delivery, a brief version that queried diet throughout the entire pregnancy was administered to women who did not complete FFQs 1 or 2. To capture dietary exposure during the periconceptional period (between fertilization and implantation), a time window thought to heighten vulnerability of the regulatory regions associated with genomically imprinted genes, we gave highest priority to FFQ data from the first round of administration (n = 599 participants with FFQ 1). The second and third FFQs were prioritized equally (n = 198), and the brief FFQ (which queried women on a subset of foods captured in the full version) was used when no other FFQ data were available (n = 260). There also were 23 participants with duplicate FFQs from the same round of data collection and we averaged the duplicate scores for a single final score.
FFQ-derived dietary data were used to calculate E-DII scores for all participants. The DII is based on publications through 2010 linking diet to inflammation, and involves comparison with a global mean intake database. A complete description of the DII is available elsewhere.27 In previous analyses, the DII, which was calculated from 24-hour dietary recalls and a structured questionnaire similar to an FFQ, was positively related to hs-CRP.26 The literature base for the DII—and, by extension, the E-DII—consists of all qualifying publications between 1950 and 2010 reporting one or more associations between dietary components and these inflammatory markers: IL-1β, IL-4, IL-6, IL-10, TNF-α and C-reactive protein.27 A total of 45 different food parameters were identified as being related to the 6 inflammatory biomarkers in the literature search. Each was assigned a “food parameter-specific inflammatory effect score” through a process of counting the number of studies reporting a pro-inflammatory, anti-inflammatory, and no inflammatory effect on one or more of the 6 inflammatory markers, and weighting the scores by study design and size of the literature for each food parameter/inflammatory marker relationship.
To calculate E-DII scores we link data from a particular participant to a world database derived from 11 countries that provides mean intakes and standard deviations for each food parameter.27 For the participants of this study, the dietary data were first linked to a version of the world database from the same 11 countries that provided estimates of energy-adjusted mean intake and standard deviation for each food parameter. These values then become the multipliers to express an individual's exposure relative to the “energy-adjusted standard global mean” as a z-score. This is achieved by subtracting the “standard global mean” from the amount reported and dividing this value by the standard deviation. To minimize the effect of “right skewing” (a common occurrence with dietary data), this value was then converted to a centered percentile score. The centered percentile score for each food parameter for each individual was then multiplied by the respective food parameter-specific inflammatory effect score, which is derived from the literature review, as described above, to obtain a food parameter-specific E-DII score for an individual. All of the food parameter-specific E-DII scores are then summed to create the overall DII score for every participant in the study.27 Higher E-DII scores indicate a more pro-inflammatory diet. To control for the effect of total energy intake, the E-DII was calculated per 1,000 calories of food consumed. The following 27 food parameters were used for E-DII calculation: carbohydrates, proteins, fats, alcohol, fibers, cholesterol, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, omega 3, omega 6, niacin, thiamin, riboflavin, vitamin B6, vitamin B12, iron, magnesium, zinc, selenium, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β carotene, and caffeine.
Assessment of birth outcomes
Trained personnel abstracted parturition data from medical records after delivery, including mode of delivery, birthweight, gestational age at birth (weeks), and infant sex. We calculated sex-specific percentiles of birthweight for gestational age by using US national reference data.28 We defined small for gestational age (SGA) as birthweight for gestational age and sex below the 10th percentile and large for gestational age (LGA) as above the 90th percentile. Both SGA and LGA were compared with appropriate for gestational age infants. Infant birthweight [grams (g)] and gestational age at delivery (weeks) showed no evidence of departure from normality and were analyzed continuously.
Assessment of covariates and effect measure modifiers
Maternal age at delivery (18–19, 20–29, 30–39, ≥40 years), maternal race/ethnicity (Black, White, Hispanic), parity at enrollment (multiparous, primiparous), body mass index at last menstrual period [BMI at last menstrual period (LMP): 18.5–24.99 kg/m2, 25–29.99 kg/m2, >29.99 kg/m2], gestational weight gain (adequate, less than adequate, excessive), and gestational diabetes (yes, no) were self-reported by participants and subsequently verified with abstracted medical records. Infant sex (male, female) was abstracted from medical records. Household income (<$25,000, $25,000-$49,999, $50,000-$100,000, >$100,000), marital status (married, never married, living with a partner, other), maternal education (less than high school, high school/GED, some college, college graduate), maternal cigarette smoking (no smoking, smoking before pregnancy, smoking during pregnancy), and folic acid (FA) supplementation (yes, no) were self-reported. We considered infant sex and maternal BMI (kg/m2) as potential effect measure modifiers of the association between maternal E-DII score and birth outcomes.
Assessment of inflammatory markers
Procedures for specimen collection and handling have been described previously.53 Briefly, 10 ml of peripheral blood was drawn at enrollment in EDTA vacutainer tubes and processed to obtain plasma from which cytokines were measured and buffy coat from which DNA for methylation analysis was extracted. Plasma was stored in 200 µl aliquots to reduce degradation from subsequent freeze-thaw cycles. Based on the magnitude of their associations with perinatal outcomes such as maternal and childhood obesity or steep growth trajectories, and augmented with a literature search, we prioritized 7 cytokines for analyses: IFNγ, TNFα, IL-1β, IL-6, IL-12p70, IL-4, and IL-17A. To measure these cytokines, we used a custom high-sensitivity human cytokine 8-plex MAP® kit from EMD Millipore. We analyzed 25 μl samples in duplicate according to the manufacturer's recommendations. Plates were read on a Luminex® platform by the Duke University Human Vaccine Institute Core Facility. Data were analyzed using Milliplex Analyst® version 5.1.
Assessment of DNA methylation
Infant genomic DNA (800 ng) was modified by treatment with sodium bisulfite using the Zymo EZ DNA Methylation® kit (Zymo Research; Irvine, CA, USA). Pyrosequencing using Pyromark Q96 MD® pyrosequencers (Qiagen) was performed to measure DNA methylation at 9 imprint regulatory regions known to associate with fetal growth and development in NEST study participants.23,24 These DMRs were as follows: the IGF2 and H19 DMRs regulating the Insulin-like Growth Factor 2 (IGF2) and noncoding H19 imprinted domain, the MEG3 and MEG3-IG DMRs regulating the Delta-like homolog 1 and noncoding Maternally Expressed Gene 3 (DLK1/MEG3) imprinted domain, the Paternally Expressed Gene 3 (PEG3) DMR, the Paternally Expressed Gene 1/Mesoderm-Specific Transcript (MEST) DMR, the Epsilon Sarcoglycan and Paternally Expressed Gene 10 (SGCE/PEG10) DMR, the Neuronatin (NNAT) DMR, and the pleomorphic adenoma gene-like 1 (PLAGL1) DMR.6 Assays were established using the Pyromark Assay Design Software (Qiagen), validated and used to query these DMRs. Polymerase chain reaction (PCR) conditions were optimized to produce a single, robust amplification product by adjusting annealing temperature and magnesium chloride concentrations. The primers, chromosomal location, coordinates, and PCR conditions for all 9 DMRs were previously provided.6 A linear increase in detection of methylation values was identified with defined mixtures of fully methylated and unmethylated control DNAs to assess the linearity in detection of increasing amounts of input DNA methylation (Pearson's r >0.99 for all DMRs). Each DMR was analyzed using the same amount of input bisulfite modified DNA from each specimen (40 ng, assuming complete recovery following bisulfite modification), keeping the thermocycler and pyrosequencer constant. Controls were included for each DMR with every sample run to determine the bisulfite conversion efficiency. The conversion efficiency exceeded 97% for all analyzed data. Percent methylation for each CpG cytosine was determined using Pyro Q-CpG Software (Qiagen) and between 4 and 8 CpG sites per DMR were interrogated: IGF2 = 3, H19 = 4, MEG3-IG = 4, MEG3 = 8, PEG3 = 10, MEST = 4, SGCE/PEG10 = 6, NNAT = 3 and PLAGL1 = 6. There was a high correlation between the values of CpGs within a DMR site (Cronbach's αs for these regions were 0.95–0.99). Thus, the average was used.
Statistical analysis
We compared the distribution of demographic and obstetric characteristics across quartiles of the E-DII.54 Multivariate linear regression was used to estimate the association between maternal E-DII score and 4 birth outcomes [birthweight, gestational age, mode of delivery, and birthweight for gestational age (a priori 2-sided P≤0.05)]54). For all models, we considered adjustment for the aforementioned covariates. Final confounders were selected based on directed acyclic graphs55 and backward elimination, where a variable that produced a greater than 10% change in estimate was kept as a confounder in the final set.56 Our final adjustment sets were as follows: (1) birth outcomes – maternal age at delivery, race/ethnicity, household income, and maternal cigarette smoking; and (2) DMRs – maternal race/ethnicity, BMI at LMP, and maternal smoking. Effect measure modification by infant sex and maternal BMI was assessed by examining stratified models, and interaction terms were added to fully adjusted models to assess statistical interactions.
Among 338 mother-infant pairs with methylation data available for at least one of 9 DMRs and dietary data, we examined associations with maternal E-DII. In a subset of women (n = 105) with both maternal cytokine and E-DII data, we similarly estimated associations with offspring methylation. For cytokine models we also considered adjustment for gestational age at blood draw, but it did not meet our minimum 10% change in estimate threshold. Study participants with DMR data available were similar to those without DMR data with respect to DII distribution and birthweight (P >0.05). Multivariate linear regression analysis was computed to examine the association between maternal E-DII score and offspring methylation, as well as maternal cytokine concentrations and offspring methylation.
For all analyses we performed sensitivity analyses by restricting to women with FFQ1 (diet during the periconceptional period). All statistical analyses were conducted in SAS® v9.3 (SAS Institute, Cary, NC, USA)
Supplementary Material
Funding Statement
This work was supported in part by grants from the National Institutes of Health (Grant no. R01-ES016772), National Institute of Environmental Health Science (P30ES025128), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NR01 HD084487), National Cancer Institute (R25CA057726), and National Institute of Diabetes and Digestive and Kidney Diseases (R44DK103377). Funders had no role in the design, analysis or writing of this article.
Disclosure of potential conflicts of interest
Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI. All remaining authors declare that they have no conflicts of interest.
References
- 1.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract 2010; 19(2):87–98. Epub 2010/02/06; PMID:20134170; https://doi.org/10.1159/000273066 [DOI] [PubMed] [Google Scholar]
- 2.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature 2007; 447(7143):433–40. Epub 2007/05/25; PMID:17522677; https://doi.org/10.1038/nature05919 [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstetrics Gynecol 2006; 49(2):270–83. Epub 2006/05/25; PMID:16721106; https://doi.org/10.1097/00003081-200606000-00009 [DOI] [PubMed] [Google Scholar]
- 4.McCormack VA, dos Santos Silva I, Koupil I, Leon DA, Lithell HO. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer 2005; 115(4):611–7. Epub 2005/02/09; PMID:15700315; https://doi.org/10.1002/ijc.20915 [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet (London, England) 1993; 341(8850):938–41. Epub 1993/04/10; PMID:8096277; https://doi.org/10.1016/0140-6736(93)91224-A [DOI] [PubMed] [Google Scholar]
- 6.Murphy SK, Huang Z, Hoyo C. Differentially methylated regions of imprinted genes in prenatal, perinatal and postnatal human tissues. PloS One 2012; 7(7):e40924. Epub 2012/07/19; PMID:22808284; https://doi.org/10.1371/journal.pone.0040924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, Nichols TD, Marks JR, Berchuck A. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res 2006; 4(4):283–92. Epub 2006/04/11; PMID:16603642; https://doi.org/10.1158/1541-7786.MCR-05-0138 [DOI] [PubMed] [Google Scholar]
- 8.Skaar DA, Li Y, Bernal AJ, Hoyo C, Murphy SK, Jirtle RL. The human imprintome: regulatory mechanisms, methods of ascertainment, and roles in disease susceptibility. ILAR J 2012; 53(3-4):341–58. Epub 2013/06/08; PMID:23744971; https://doi.org/10.1093/ilar.53.3-4.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epigenome Jirtle RL.: the program for human health and disease. Epigenomics 2009; 1(1):13–6. Epub 2009/10/01; PMID:22122631; https://doi.org/10.2217/epi.09.16 [DOI] [PubMed] [Google Scholar]
- 10.Rush EC, Katre P, Yajnik CS. Vitamin B12: one carbon metabolism, fetal growth and programming for chronic disease. Euro J Clin Nutrition 2014; 68(1):2–7. Epub 2013/11/14; PMID:24219896; https://doi.org/10.1038/ejcn.2013.232 [DOI] [PubMed] [Google Scholar]
- 11.McCullough LE, Miller EE, Mendez MA, Murtha AP, Murphy SK, Hoyo C. Maternal B vitamins: effects on offspring weight and DNA methylation at genomically imprinted domains. Clin Epigenetics 2016; 8:8. Epub 2016/01/26; PMID:26807160; https://doi.org/10.1186/s13148-016-0174-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyo C, Daltveit AK, Iversen E, Benjamin-Neelon SE, Fuemmeler B, Schildkraut J, Murtha AP, Overcash F, Vidal AC, Wang F, et al. . Erythrocyte folate concentrations, CpG methylation at genomically imprinted domains, and birthweight in a multiethnic newborn cohort. Epigenetics 2014; 9(8):1120–30. Epub 2014/05/31; PMID:24874916; https://doi.org/10.4161/epi.29332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggarty P, Hoad G, Campbell DM, Horgan GW, Piyathilake C, McNeill G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am J Clin Nutrition 2013; 97(1):94–9. Epub 2012/11/16; PMID:23151531; https://doi.org/10.3945/ajcn.112.042572 [DOI] [PubMed] [Google Scholar]
- 14.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutrition 2006; 84(6):1489–97. Epub 2006/12/13; PMID:17158434 [DOI] [PubMed] [Google Scholar]
- 15.Sen S, Rifas-Shiman SL, Shivappa N, Wirth MD, Hebert JR, Gold DR, Gillman MW, Oken E. Dietary inflammatory potential during pregnancy is associated with lower fetal growth and breastfeeding failure: Results from project viva. J Nutr 2016; 146(4):728–36. Epub 2016/03/05; PMID:26936137; https://doi.org/10.3945/jn.115.225581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury J, Henriksen T, Christophersen B, Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. Am J Obstet Gynecol 2005; 193(4):1292–301. Epub 2005/10/06; PMID:16202717; https://doi.org/10.1016/j.ajog.2005.05.016 [DOI] [PubMed] [Google Scholar]
- 17.Walker JJ. Inflammation and preeclampsia. Pregnancy Hypertens 2011; 1(1):43–7. Epub 2011/01/01; PMID:26104230 [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006; 113(10):1126–33. Epub 2006/07/11; PMID:16827826; https://doi.org/10.1111/j.1471-0528.2006.00989.x [DOI] [PubMed] [Google Scholar]
- 19.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, et al. . Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstetr Gynecol 2006; 194(4):921–31. Epub 2006/04/04 [DOI] [PubMed] [Google Scholar]
- 20.Poston L, Igosheva N, Mistry HD, Seed PT, Shennan AH, Rana S, Karumanchi SA, Chappell LC. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am J Clin Nutrition 2011; 94(6 Suppl):1980s–5s. Epub 2011/05/27; PMID:21613560; https://doi.org/10.3945/ajcn.110.001156 [DOI] [PubMed] [Google Scholar]
- 21.Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol 2010; 42(10):1634–50. Epub 2010/07/06; https://doi.org/10.1016/j.biocel.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 22.Farah N, Hogan AE, O'Connor N, Kennelly MM, O'Shea D, Turner MJ. Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine 2012; 60(1):96–9. Epub 2012/06/26; PMID:22726456; https://doi.org/10.1016/j.cyto.2012.05.024 [DOI] [PubMed] [Google Scholar]
- 23.Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. BioEssays 2014; 36(4):359–71. Epub 2014/01/17; PMID:24431278; https://doi.org/10.1002/bies.201300113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoyo C, Murtha AP, Schildkraut JM, Forman MR, Calingaert B, Demark-Wahnefried W, Kurtzberg J, Jirtle RL, Murphy SK. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health 2011; 11(1):46. Epub 2011/01/25; PMID:21255390; https://doi.org/10.1186/1471-2458-11-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet 2009; 18(21):4046–53. Epub 2009/08/07; PMID:19656776; https://doi.org/10.1093/hmg/ddp353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 2014; 17(8):1825–33. Epub 2013/10/11; PMID:24107546; https://doi.org/10.1017/S1368980013002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014; 17(8):1689–96. Epub 2013/08/15; PMID:23941862; https://doi.org/10.1017/S1368980013002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birthweight for gestational age using a United States national reference. BMC Pediatr 2003; 3:6. Epub 2003/07/10; PMID:12848901; https://doi.org/10.1186/1471-2431-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCloskey K, Ponsonby AL, Collier F, Allen K, Tang ML, Carlin JB, Saffery R, Skilton MR, Cheung M, Ranganathan S, et al. . The association between higher maternal pre-pregnancy body mass index and increased birthweight adiposity and inflammation in the newborn. Pediatr Obes 2016. Epub 2016/10/11; PMID:27723247; https://doi.org/10.1111/ijpo.12187 [DOI] [PubMed] [Google Scholar]
- 30.Guven MA, Coskun A, Ertas IE, Aral M, Zencirci B, Oksuz H. Association of maternal serum CRP, IL-6, TNF-alpha, homocysteine, folic acid and vitamin B12 levels with the severity of preeclampsia and fetal birthweight. Hypertens Pregnancy 2009; 28(2):190–200. Epub 2009/05/14; PMID:19437229; https://doi.org/10.1080/10641950802601179 [DOI] [PubMed] [Google Scholar]
- 31.Cemgil Arikan D, Aral M, Coskun A, Ozer A. Plasma IL-4, IL-8, IL-12, interferon-gamma and CRP levels in pregnant women with preeclampsia, and their relation with severity of disease and fetal birthweight. J Matern Fetal Neonatal Med 2012; 25(9):1569–73. Epub 2011/12/22; https://doi.org/10.3109/14767058.2011.648233 [DOI] [PubMed] [Google Scholar]
- 32.Retnakaran R, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, Hamilton JK. Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birthweight among women without gestational diabetes mellitus. CMAJ 2012; 184(12):1353–60. Epub 2012/05/24; PMID:22619341; https://doi.org/10.1503/cmaj.111154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amarilyo G, Oren A, Mimouni FB, Ochshorn Y, Deutsch V, Mandel D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J Perinatol 2011; 31(1):30–2. Epub 2010/04/23; PMID:20410909; https://doi.org/10.1038/jp.2010.53 [DOI] [PubMed] [Google Scholar]
- 34.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D'Anna K, Argys L, Ross RG, Brandt C, Cole S. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun 2012; 26(4):650–9. Epub 2012/03/20; PMID:22426431; https://doi.org/10.1016/j.bbi.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura Y, Lambertini L, Rialdi A, Lee M, Mystal EY, Grabie M, Manaster I, Huynh N, Finik J, Davey M, et al. . Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod Sci (Thousand Oaks, Calif) 2014; 21(1):131–7. Epub 2013/06/15; PMID:23765376; https://doi.org/10.1177/1933719113492206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Chen Q, Tsai HJ, Wang G, Hong X, Zhou Y, Zhang C, Liu C, Liu R, Wang H, et al. . Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ Mol Mutagen 2014; 55(3):223–30. Epub 2013/11/19; PMID:24243566; https://doi.org/10.1002/em.21827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soubry A, Murphy SK, Wang F, Huang Z, Vidal AC, Fuemmeler BF, Kurtzberg J, Murtha A, Jirtle RL, Schildkraut JM, et al. . Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int J Obesity (2005) 2015; 39(4):650–7. Epub 2013/10/26; PMID:24158121; https://doi.org/10.1038/ijo.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: archive of the prenatal environment. Epigenetics 2009; 4(8):526–31. Epub 2009/11/20; PMID:19923908; https://doi.org/10.4161/epi.4.8.10265 [DOI] [PubMed] [Google Scholar]
- 39.Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, et al. . Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 2002; 417(6892):945–8. Epub 2002/06/28; PMID:12087403; https://doi.org/10.1038/nature00819 [DOI] [PubMed] [Google Scholar]
- 40.Wylie AA, Murphy SK, Orton TC, Jirtle RL. Novel imprinted DLK1/GTL2 domain on human chromosome 14 contains motifs that mimic those implicated in IGF2/H19 regulation. Genome Res 2000; 10(11):1711–8. Epub 2000/11/15; PMID:11076856; https://doi.org/10.1101/gr.161600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kagami M, O'Sullivan MJ, Green AJ, Watabe Y, Arisaka O, Masawa N, Matsuoka K, Fukami M, Matsubara K, Kato F, et al. . The IG-DMR and the MEG3-DMR at human chromosome 14q32.2: hierarchical interaction and distinct functional properties as imprinting control centers. PLoS Genet 2010; 6(6):e1000992. Epub 2010/06/30; PMID:20585555; https://doi.org/10.1371/journal.pgen.1000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y, Cheunsuchon P, Nakayama Y, Lawlor MW, Zhong Y, Rice KA, Zhang L, Zhang X, Gordon FE, Lidov HG, et al. . Activation of paternally expressed genes and perinatal death caused by deletion of the Gtl2 gene. Development (Cambridge, England) 2010; 137(16):2643–52. Epub 2010/07/09; PMID:20610486; https://doi.org/10.1242/dev.045724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer 2011; 129(4):773–9. Epub 2011/03/15; PMID:21400503; https://doi.org/10.1002/ijc.26052 [DOI] [PubMed] [Google Scholar]
- 44.Jordan C, Li HH, Kwan HC, Francke U. Cerebellar gene expression profiles of mouse models for Rett syndrome reveal novel MeCP2 targets. BMC Med Genet 2007; 8:36. Epub 2007/06/23; PMID:17584923; https://doi.org/10.1186/1471-2350-8-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Surani MA. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet 1998; 20(2):163–9. Epub 1998/10/15; PMID:9771709; https://doi.org/10.1038/2464 [DOI] [PubMed] [Google Scholar]
- 46.Pedersen IS, Dervan PA, Broderick D, Harrison M, Miller N, Delany E, O'Shea D, Costello P, McGoldrick A, Keating G, et al. . Frequent loss of imprinting of PEG1/MEST in invasive breast cancer. Cancer Res 1999; 59(21):5449–51. Epub 1999/12/20; PMID:10554015 [PubMed] [Google Scholar]
- 47.Vidal AC, Henry NM, Murphy SK, Oneko O, Nye M, Bartlett JA, Overcash F, Huang Z, Wang F, Mlay P, et al. . PEG1/MEST and IGF2 DNA methylation in CIN and in cervical cancer. Clin Transl Oncol 2014; 16(3):266–72. Epub 2013/06/19; https://doi.org/10.1007/s12094-013-1067-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moon YS, Park SK, Kim HT, Lee TS, Kim JH, Choi YS. Imprinting and expression status of isoforms 1 and 2 of PEG1/MEST gene in uterine leiomyoma. Gynecol Obstet Invest 2010; 70(2):120–5. Epub 2010/03/27; PMID:20339302; https://doi.org/10.1159/000301555 [DOI] [PubMed] [Google Scholar]
- 49.Woodfine K, Huddleston JE, Murrell A. Quantitative analysis of DNA methylation at all human imprinted regions reveals preservation of epigenetic stability in adult somatic tissue. Epigenetics Chromatin 2011; 4(1):1 Epub 2011/02/02; https://doi.org/10.1186/1756-8935-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cade JE, Burley VJ, Warm DL, Thompson RL, Margetts BM. Food-frequency questionnaires: a review of their design, validation and utilisation. Nutr Res Rev 2004; 17(1):5–22. Epub 2004/06/01; PMID:19079912; https://doi.org/10.1079/NRR200370 [DOI] [PubMed] [Google Scholar]
- 51.Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harbor Perspect Biol 2014; 6(2):pii: a018382. Epub 2014/02/05; PMID:24492710; https://doi.org/10.1101/cshperspect.a018382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, Overcash F, Kurtzberg J, Jirtle R, Iversen ES, et al. . Depression in pregnancy, infant birthweight and DNA methylation of imprint regulatory elements. Epigenetics 2012; 7(7):735–46. Epub 2012/06/09; PMID:22677950; https://doi.org/10.4161/epi.20734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Hoyo C, Murphy S, Huang Z, Overcash F, Thompson J, Brown H, Murtha AP. DNA methylation at imprint regulatory regions in preterm birth and infection. Am J Obstet Gynecol 2013; 208(5):395.e1-7. Epub 2013/03/13; PMID:23477525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corder G, Foreman D. Nonparametric statistics for non-statisticians: a step-by-step approach. Hoboken, NY: Wiley; 2009 [Google Scholar]
- 55.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989; 79(3):340–9. Epub 1989/03/01; PMID:2916724; https://doi.org/10.2105/AJPH.79.3.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrell F. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer-Verlag New York, Inc; 2001 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


