Abstract
Neuropsychiatric disorders often derive from environmental influences that occur at important stages of development and interact with genetics. This study examined the effects of stress during adolescence in rats selectively bred for different behavioral responses to stress. The effects of chronic adolescent stress were compared between rats selected for susceptibility to reduced activity following acute stress (Swim-test Susceptible rats) and rats resistant to activity reduction after acute stress (Swim-test Resistant rats). Consistent with lineage, exposure to chronic adolescent stress increased swim-test activity of the Swim-test Resistant rats while tending to reduce activity of the Swim-test Susceptible rats. Consistent with the increased activity demonstrated post-stress in the swim test, chronic adolescent stress increased total activity in the open field for Swim-test Resistant rats. Indicative of anhedonia, chronic adolescent stress exposure decreased sucrose consumption in both male and female Swim-test Resistant rats but only in female Swim-test Susceptible rats. Although chronic stress induced changes in behavior across both breeding lines, the precise manifestation of the behavioral change was dependent on both breeding line and sex. Collectively, these data indicate that selective breeding interacts with chronic stress exposure during adolescence to dictate behavioral outcomes.
Keywords: Forced swim, Stress, Adolescent, Sex, Depressive-like, Selective breeding, Sucrose preference, Stress susceptible, Stress resistant, Open field
Recent advances in the collective understanding of psychopathology indicate that neuropsychiatric disorders are developmental in origin [1], and adolescence in particular is a period of vulnerability to stress and susceptibility to neuropsychiatric disease [2,3]. An adolescent episode of an anxiety or depressive disorder predicts a 2- to 3-fold increased risk for adult affective disorders [4]. Sex also alters the behavioral response to stress. Women experience higher rates of depression and female animal models more frequently develop depressive-like behaviors than males in response to stress exposure [5,6]. Conversely, clinical studies suggest that sex differences in depression are diminished if family history is strong [7]. In an effort to produce better animal models for the study of depression, selective breeding was used to develop rat lines with differences in depression-relevant physiology and behavior [8–11]. To represent different backgrounds for the studies described here, we used two lines of rats selectively bred to be differentially sensitive to the effects of stress – the Swim-test Susceptible (SUS) and Swim-test Resistant (RES) rat lines [10]. This nomenclature reflects different behavioral responses shown by the animals in the forced swim test; the SUS rat, following acute stress, responds with reduced activity (depressive-like behavior) in the swim test whereas the RES rat responds to the same stressor with no decrease in swim test activity. Additional studies have characterized responses to antidepressant treatment, substance abuse behaviors, and neurobiology of adult male rats of the RES and SUS lines [12–14]. In sum, these studies have demonstrated distinctly different depression-relevant behavioral and neurochemical phenotypes in adult males of both lines. However, previous studies have not assessed the behavior of adolescents in these lines, effects of chronic stressors, or specific behavior of females. The current study assessed the hypothesis that lineage predicts behavioral consequences of chronic adolescent stress exposure in male and female rats.
Adolescent male and female Sprague-Dawley rats from the RES and SUS lines were housed on a 14:10 reverse light:dark cycle in a facility controlled for humidity (60%) and temperature (20–23 °C). Rodent diet 5001 chow (Purina Mills, Richmond, IN) and water were available ad libitum throughout the study. All experiments were performed in accordance with the Institutional Animal Care and Use Committee of Emory University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The mixed modality chronic stress paradigm used in this study was previously used to elicit sex-specific behavioral changes in adolescent rats [6,15]. Briefly, adolescent stress was defined as individual housing beginning at post-natal day (PND) 36 and continuing throughout the study combined with randomly alternating daily exposure to social defeat or restraint from PND 37–48. The control groups remained pair-housed with a same sex littermate and are referred to as non-stress groups. The following groups were assessed: (1) RES male non-stress (n = 10), (2) RES male stress (n = 10), (3) RES female non-stress (n = 10), (4) RES female stress (n = 10), (5) SUS male non-stress (n = 10), (6) SUS male stress (n = 10), (7) SUS female non-stress (n = 10), and (8) SUS female stress (n = 10). Social defeat stress (up to 5 min of contact, and an additional 25 min of visual and olfactory stimulation) was performed during the light cycle in the home cage of the resident, a mature Long–Evans rat experienced in territorial fighting. Male residents were adult retired breeders and female residents were ovariectomized adults. Although female rats are commonly viewed as devoid of territorial behavior, Long–Evans female rats housed with a male counterpart will develop territorial behavior and have been shown to defeat adolescent female rats [6], a behavior that persists after ovariectomy [16], For the restraint portion of the mixed-modality stressor, animals were restrained for 60 min in acrylic rat restraints (BrainTree Scientific, Braintree, MA, USA) that prevented head to tail turns but did not compress the rat. Adolescents received six total exposures to social defeat and six total exposures to restraint over twelve days in a randomly alternating pattern. The study was not designed to assess specific effects of individual housing, restraint, or social defeat but used this combination of established stressors to induce chronic stress during adolescence.
Exposure to chronic mixed modality stress reduced body mass gain for all rats irrespective of line (Fig. 1; F1,68 = 7.887; p < 0.05), reflecting physiological impact of chronic stress exposure. Consistent with well-established sex differences, males weighed more than females for both lines of rats (F1,68 = 161.953; p < 0.05). Rat line also influenced body mass (F1,68 = 29.167; p < 0.05) such that SUS rats weighed more than RES rats (p < 0.05). Additionally, a significant interaction existed between sex and chronic stress (F1,68 = 5.829; < 0.05). Although stress reduced weight gain in both males and females (p < 0.05 for both comparisons), stress reduced weight gain among females by only 3.0 ± 1.0% but among males by 7.8 ± 1.0%.
Fig. 1.
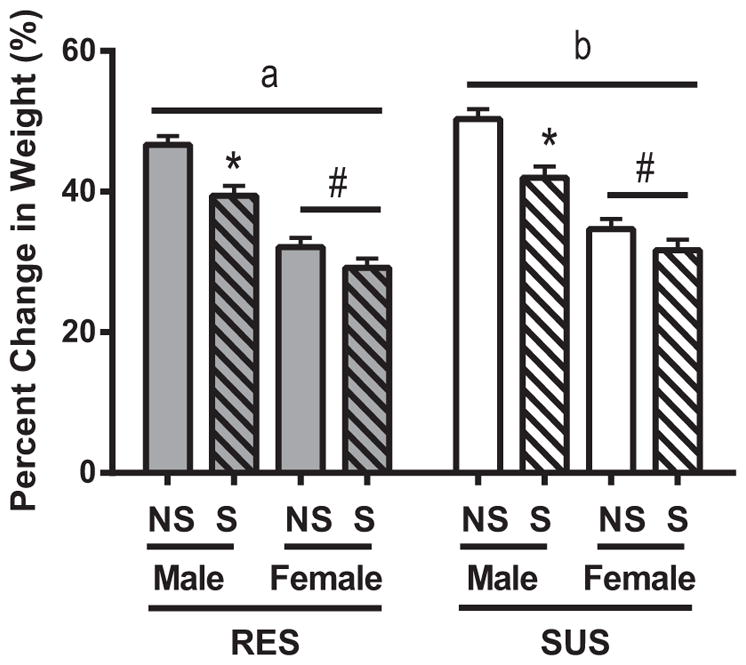
Body mass. Bars represent percent change in body mass over the duration of the study for rats resistant to showing reduced activity in the forced swim test after stress (RES) and rats susceptible to showing reduced activity in the forced swim test after stress (SUS). Body mass was tracked for both rats that were not exposed to stress (NS) and those exposed to chronic mixed modality stress (S) through adolescence. Data are presented as mean ± SEM. Statistics: significant line differences (p < 0.05) are indicated by letters (i.e., “a” and “b”), with different letters indicating significant differences. Also, #p < 0.05 compared to males of same line, and *p < 0.05 as compared to NS of same line.
Behavioral testing commenced 24 h after the final chronic stressor on PND 49, consisting sequentially of a 48-h sucrose consumption test (PND 49–50), open field test (PND 51) and a 10-min Porsolt forced swim test (PND 52). The open field test was conducted 2 h after the onset of the dark cycle and the swim test was conducted during the light cycle. Unlike other studies done with selectively bred lines, this series of experiments did not incorporate the use of an acute stressor immediately prior to testing.
The sucrose consumption test was performed as a measure of hedonic state [17]. Rats were given free access to one bottle of tap water and one bottle of a 0.8% sucrose solution in tap water. Bottles were reversed after 24 h to prevent side bias. Bottles were weighed at the beginning and then after 24 and 48 h to determine sucrose and water consumption. Rats subjected to chronic social defeat typically consume less sucrose than control rats, a behavior described as depressive-like [17]. Chronic adolescent stress decreased sucrose intake compared to non-stressed rats in the second 24 h of exposure (Fig. 2; F1,47 = 4.135; p < 0.05). Rat line also predicted sucrose consumption (F1,47 = 52.380; p < 0.05), as did sex (F1,47 = 11.274; p < 0.05). Both male and female RES rats demonstrated reduced sucrose consumption with chronic stress exposure, but only female SUS rats consumed less sucrose solution following chronic stress (F1,47 = 9.364; p < 0.05). The percent of sucrose solution consumed was also influenced by rat line (F1,47 = 24.996; p < 0.05) and this effect interacted with sex (F1,47 = 4.946; p < 0.05; data not shown), such that female RES rats consumed a greater percentage of sucrose solution than male RES rats (p < 0.05) while no sex difference in the percent of sucrose solution consumed existed among SUS animals.
Fig. 2.
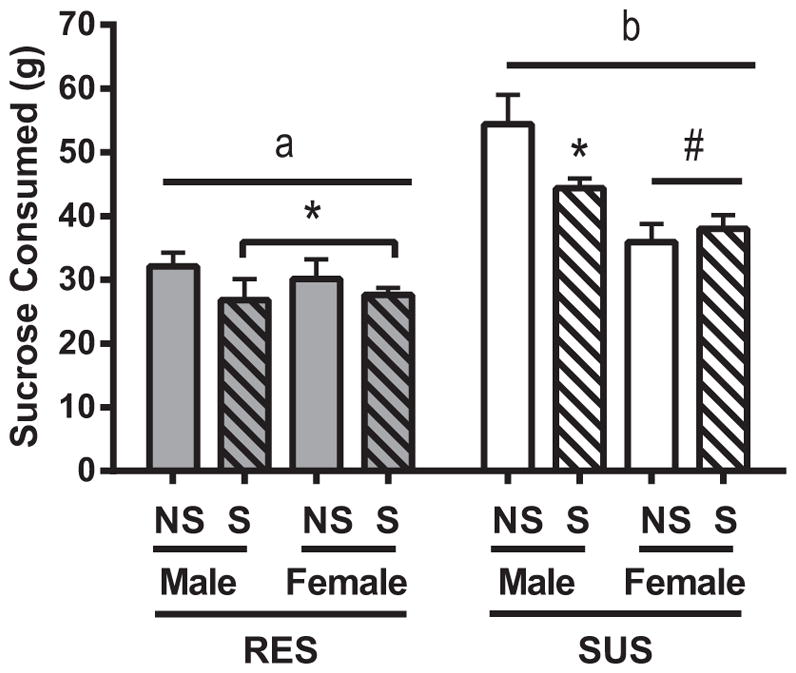
Sucrose intake. Sucrose and water intake for RES and SUS rats for both non-stressed (NS) and stressed (S) animals. Total sucrose solution consumed on the second day of testing is shown. Data are presented as mean ± SEM. Statistics: significant line differences (p < 0.05) are indicated by letters, with different letters indicating significant differences. Also, #p < 0.05 compared to males of same line, and *p < 0.05 as compared to NS of same line.
Rats were tested for their exploratory and anxiety-like behavior in a novel environment [18]. The open field apparatus consisted of a square field (75 cm × 75 cm) surrounded by approximately one-meter high walls. Rats were placed in the corner of the open field and allowed to explore the field freely for 10 min, during which distance traveled was monitored by a video camera connected to automated behavior analysis software (CleverSys, Inc, Reston, VA, USA). RES rats traveled further than SUS rats in the open field both over the total 10-min interval (Fig. 3a; F1,64 = 20.072; p < 0.05) and when activity was assessed in two 5-min intervals (first 5 min: F1,64 = 20.403; p < 0.05; second 5 min: F1,64 = 10.771; p < 0.05; data not shown). Post hoc testing revealed that this greater activity in the RES line was significant within the non-stressed group (NS-RES vs NS-SUS) and within the stressed group (S-RES vs S-SUS) in both the first and second intervals (p < 0.05 for both comparisons). Regardless of rat line, females traveled more in the open field than males when analyzed over the full 10-min interval (F1,64 = 7.190; p < 0.05), but when analysis was broken down over the two intervals, this sex difference in activity was significant only in the first 5 min (F1,64 = 20.551; p < 0.05). Exposure to chronic mixed modality stress during adolescence interacted with sex in the full 10-min interval only (F1,64 = 5.135; p = 0.027) such that chronic stress increased distance traveled for males (p < 0.05) but not for females (p > 0.05). Exposure to chronic mixed modality stress also decreased the latency to enter the center of the open field (F1,58 = 4.268; p < 0.05; data not shown) and interacted with both sex and line (F1,58 = 5.916; p < 0.05). Sex and line also interacted to impact latency to enter the center of the open field (F1,58 = 5.916; p < 0.05); specifically, post hoc testing revealed that female RES rats took longer to enter the center of the open field. When latency to leave the center of the open field after first entry was assessed, analysis revealed main effects indicating that both stressed rats (F1,58 = 10.277; p < 0.05) and SUS rats (F1,58 = 7.786; p < 0.05) showed reduced latency to leave the center.
Fig. 3.
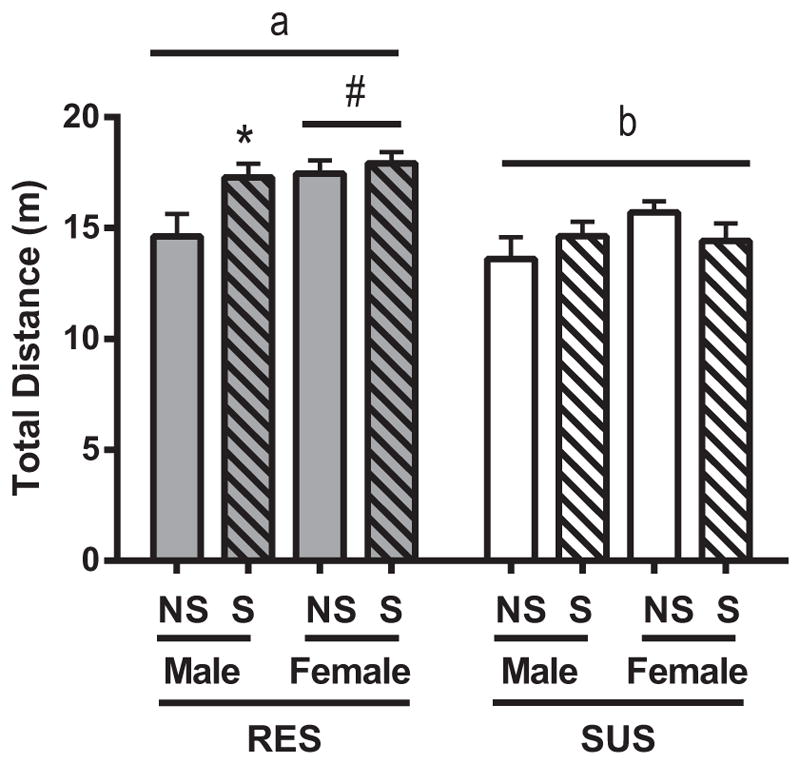
Open field behavior. Distance in the open field for RES and SUS rats for both non-stressed (NS) and stressed (S) animals. Data are presented as mean ± SEM. Statistics: significant line differences (p < 0.05) are indicated by letters, with different letters indicating significant differences. Also, #p < 0.05 compared to males of same line, and *p < 0.05 as compared to NS of same line.
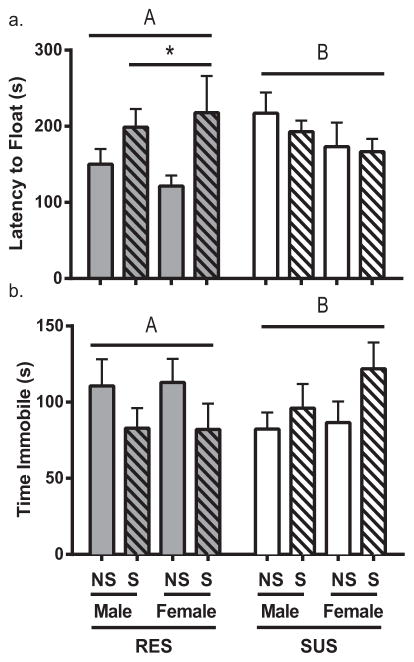
The forced swim test was used to assess motor activity in an inescapable environment and confirm that the adolescent rats showed similar selected behavior to their adult counterparts. This test has been used as a model for depressive-like behavior and antidepressant efficacy such that decreased activity (i.e., decreased struggling and/or increased floating) is typically interpreted as depressive-like [19–21]. Rats were placed in a clear acrylic beaker (40 cm high × 18 cm diameter) filled with room-temperature water for 10 min. Latency to float, time spent struggling (actively swimming), and time spent floating were tabulated by automated behavior analysis software (CleverSys, Inc., Reston, VA, USA). Struggling was defined as the rat breaking the water’s surface with all four limbs in motion. Floating was defined as the rat’s limbs remaining motionless for at least two seconds. Rat line predicted struggling behavior in the forced swim test (F1,68 = 6.756; p < 0.05), but there was no additional effect of chronic stress (p > 0.05). However, chronic adolescent stress interacted with rat line to impact latency to float (Fig. 4a; F1,68 = 5.123; p < 0.05). Consistent with the predicted behavioral phenotype based on lineage, chronic stress increased latency to float among RES rats only (p < 0.05), even though the RES rats had not shown any activity differences between time intervals in the open field in the non-stress condition. This pattern was also evident for total floating time such that chronic adolescent stress interacted with rat line to alter total time floating in the forced swim test (Fig. 4b; F1,67 = 5.911; p < 0.05). Notably, there was no main effect of rat line on immobility (p > 0.05). There was additionally no main effect of rat line on immobility when only the non-stress rats were analyzed by a two-way ANOVA using the factors of sex and line (F1,34 = 3.249; p = 0.080), although there had been a significant effect of greater overall activity for RES rats in the open field.
Fig. 4.
Forced swim behavior. Behavior in the forced swim for RES and SUS rats for both non-stressed (NS) and stressed (S) animals. Shown are (a) latency to begin floating and (b) total time immobile. Data are presented as mean ± SEM. Statistics: Capitalized letters indicate a significant interaction between line and treatment (p < 0.05). Also, #p < 0.05 compared to males of same line, and *p < 0.05 as compared to NS of same line.
Collectively, these data demonstrate that although all rats regardless of lineage or sex demonstrated physical alterations (reduced weight gain) in response to chronic adolescent stress, the behavioral consequences of chronic stress were influenced by both lineage and sex. While both rat lines examined in this study manifested at least one indicator of depressive-like behavior following exposure to adolescent stress, the extent and degree of depressive-like behaviors differed based on lineage. Chronically stressed RES rats, regardless of sex, demonstrated decreased sucrose consumption but increased activity in both the open field and forced swim test. Conversely, chronically stressed male SUS rats also consumed less sucrose but did not exhibit any changes in open field behavior. In addition, stress tended to increase the time both male and female SUS rats spent floating in the forced swim test as expected based on their selective breeding, though this did not reach significance. Importantly, the absence of an acute stressor prior to the forced swim, or the difference in developmental age, could account for this discrepancy to prior reports of the effects of stress on SUS behavior in the forced swim. Together, these results demonstrate that although the physical manifestations of chronic adolescent stress such as reduced weight gain may be similar, behavioral effects of the same chronic stressors can differ depending on the specific behavior assessed depending on lineage and sex.
The forced swim and sucrose preference data described above may at first appear to diverge regarding a depressive-like phenotype of the RES line. The forced swim test and the sucrose consumption test are commonly considered to be tests of depressive-like behaviors [22]. However, these two tests measure different components of depressive-like behaviors, as reduced consumption of sucrose in a two-bottle preference test is generally considered a manifestation of anhedonia [17,23], while decreased activity in the swim test is typically interpreted as behavioral despair [19,20]. On one hand, RES rats showed reduced sucrose consumption, indicative of anhedonia, but on the other hand, showed increased activity in forced swim, indicative of resilience to behavioral despair. Dissociation of findings in the forced swim and sucrose consumption tests is not without precedent [24]. In the case of the RES rat, this divergence perhaps may be explained by previously discovered differences in dopamine physiology that are present in forebrain regions of RES rats relative to SUS rats. Relative to SUS rats, RES rats show considerably higher levels of dopamine (DA) and metabolites in forebrain regions, pointing to increased synthesis of DA, and increased DA release particularly by mesocorticolimbic neurons, both at baseline and in response to stress [10,25]. Insofar as forebrain DA neurotransmission promotes motor activity, this hyperdopaminergic response of RES rats is likely to account for why they are resistant to stress-induced decreased in forced swim activity and also why they show increased motor behavior in the open field after adolescent stress (Fig. 3). Future studies are necessary to test the hypothesis that varying behavioral and sucrose consumption patterns between the lineages are related to differences in DA physiology.
As our previous work has shown, sex differences were evident in the present studies of behavioral effects of chronic adolescent stress. Sex differences in rodent behavior have been well documented both at baseline and in response to stress by many research groups [5]. However, these sex differences were not of the magnitude or direction (namely, females more predominantly affected than males) that our previous work had found for adolescent rats [6]. The diminished magnitude of these effects in females and the presence of effects in males in our current data set suggest that the influence of breeding line may have attenuated typical sex differences in behavior. Other studies of clinical populations have shown that family history exerts an important influence in the context of affective disorders, suggesting that inherited factors are relatively important variables measured in this study [7]. Therefore, the influence of lineage on behavior may be more robust than sex differences in some situations and in relation to certain behavioral outcome measures, and thus may obscure expected sex differences.
Collectively, these data indicate that chronic adolescent stress, selective breeding, and sex interact to dictate behavioral outcome from chronic stress exposure. The use of animal models to study the neurobiology of stress-induced behavioral changes is advantageous in assessing the mediating substrates of neuropsychiatric disorders. A better understanding of the neurobiology of stress-induced behavioral disruptions will ultimately facilitate development of more efficacious treatment strategies.
HIGHLIGHTS.
We examined the effects of chronic adolescent stress in selectively bred rats.
Chronic adolescent stress reduced weight gain in all rats in both lineages and sexes.
Lineage interacted with chronic adolescent stress to dictate activity level.
Stress-induced anhedonia was dependent on lineage.
Sex differences in behavior were attenuated by lineage
Acknowledgments
We gratefully acknowledge support of the Vada and Theodore Stanley Foundation for the Mentally Ill, who supported development of the selectively bred lines of rats used in this research. We also thank Jillybeth Burgado of the Neuroscience and Behavioral Biology Program at Emory University for her tireless technical assistance. Funding was provided by unrestricted funds from the Child and Adolescent Mood Program; the funding source had no role in study design, data collection, analysis and interpretation of data, manuscript preparation, or the decision to submit for publication. All authors declare that they have no conflicts of interests.
References
- 1.Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma, Violence, and Abuse. 2009;10:389–410. doi: 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet. 2012;379:1056–67. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romeo RD. Adolescence: a central event in shaping stress reactivity. Developmental Psychobiology. 2010;52:244–53. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- 4.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 5.Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. Journal of Neuroendocrinology. 2009;21:415–20. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreier A, Hofler M, Wittchen HU, Lieb R. Clinical characteristics of major depressive disorder run in families – a community study of 933 mothers and their children. Journal of Psychiatric Research. 2006;40:283–92. doi: 10.1016/j.jpsychires.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Overstreet DH. Selective breeding for increased cholinergic function: development of a new animal model of depression. Biological Psychiatry. 1986;21:49–58. doi: 10.1016/0006-3223(86)90007-7. [DOI] [PubMed] [Google Scholar]
- 9.Henn FA, Johnson J, Edwards E, Anderson D. Melancholia in rodents: neurobiology and pharmacology. Psychopharmacology Bulletin. 1985;21:443–6. [PubMed] [Google Scholar]
- 10.Scott PA, Cierpial MA, Kilts CD, Weiss JM. Susceptibility and resistance of rats to stress-induced decreases in swim-test activity: a selective breeding study. Brain Research. 1996;725:217–30. doi: 10.1016/0006-8993(96)00093-5. [DOI] [PubMed] [Google Scholar]
- 11.Weiss JM, Cierpial MA, West CH. Selective breeding of rats for high and low motor activity in a swim test: toward a new animal model of depression. Pharmacology Biochemistry and Behavior. 1998;61:49–66. doi: 10.1016/s0091-3057(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 12.Weiss JM, West CH, Emery MS, Bonsall RW, Moore JP, Boss-Williams KA. Rats selectively-bred for behavior related to affective disorders: proclivity for intake of alcohol and drugs of abuse, and measures of brain monoamines. Biochemical Pharmacology. 2008;75:134–59. doi: 10.1016/j.bcp.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 13.West CH, Weiss JM. A selective test for antidepressant treatments using rats bred for stress-induced reduction of motor activity in the swim test. Psychopharmacology. 2005;182:9–23. doi: 10.1007/s00213-005-0048-x. [DOI] [PubMed] [Google Scholar]
- 14.Gutman DA, Coyer MJ, Boss-Williams KA, Owens MJ, Nemeroff CB, Weiss JM. Behavioral effects of the CRF1 receptor antagonist R121919 in rats selectively bred for high and low activity in the swim test. Psychoneuroendocrinology. 2008;33:1093–101. doi: 10.1016/j.psyneuen.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Dobkowska A, Gorski W, Wirski J. Changes in muscle power in elderly subjects induced by electric stimulation. Polski Tygodnik Lekarski. 1978;33:1849–52. [PubMed] [Google Scholar]
- 16.DeBold JF, Miczek KA. Aggression persists after ovariectomy in female rats. Hormones and Behavior. 1984;18:177–90. doi: 10.1016/0018-506x(84)90041-2. [DOI] [PubMed] [Google Scholar]
- 17.Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behavioural Brain Research. 2005;162:127–34. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 19.Detke MJ, Johnson J, Acute Lucki I. chronic antidepressant drug treatment in the rat forced swimming test model of depression. Experimental and Clinical Psychopharmacology. 1997;5:107–12. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- 20.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 21.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 22.Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behavioural Pharmacology. 2002;13:169–88. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 24.Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R, et al. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159:982–92. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 25.West CH, Boss-Williams KA, Weiss JM. Effects of fenfluramine, 8-OH-DPAT, and tryptophan-enriched diet on the high-ethanol intake by rats bred for susceptibility to stress. Alcohol. 2011;45:739–49. doi: 10.1016/j.alcohol.2011.07.007. [DOI] [PubMed] [Google Scholar]