Abstract
We evaluated the correlation between fluoroquinolone use, measured by doses administered and days of therapy, with the emergence of ciprofloxacin and levofloxacin resistance among Gram-negative bacilli infections in children hospitalized at one pediatric center between April 2001 and March 2009. Both metrics and drug resistance were highly correlated.
Keywords: fluoroquinolones, ciprofloxacin, levofloxacin, antimicrobial stewardship, children
Fluoroquinolones were first introduced for use in clinical practice in the 1980s as broad-spectrum antimicrobial agents. By 2002, fluoroquinolones were the most commonly prescribed antibiotic class in adults, accounting for 22 million outpatient visit prescriptions, a 3-fold increase in use since 1995.1 Children <18 years of age accounted for over half a million fluoroquinolone prescriptions written in the same year.2 The overuse of fluoroquinolones has led to a significant reduction in the efficacy of this antibiotic class against a variety of common bacteria.3,4
Global efforts to control the emergence of resistance recommend that hospitals measure and reduce inappropriate antibiotic use.5,6 Yet, the most appropriate metric to measure and correlate antibiotic use and emergence of resistance valid for adult and pediatric patients remains unknown.7 The aim of the study was to determine the correlation between 2 metrics of antibiotic use, doses administered (DA) and days of therapy (DoT), and the emergence of fluoroquinolone-resistant Gram-negative bacilli among hospitalized children.
Methods
The study was conducted at Alfred I. duPont Hospital for Children (AIDHC), Wilmington, DE, between April 1, 2001, and March 31, 2009. AIDHC is a 180-bed tertiary pediatric teaching hospital affiliated with Thomas Jefferson University, Philadelphia, PA.8,9
Microbiology reports retrieved from selected sites demonstrating Gram-negative bacilli were identified by querying the clinical microbiology laboratory database (Misys; Misys Healthcare System, Raleigh, NC). Sites included bloodstream, musculoskeletal, urine (by catheterization only), tracheal aspirate and bronchial lavage, cerebrospinal fluid, peritoneal fluid, deep tissue and pleural fluid. Identification and susceptibility testing of Gram-negative bacilli were performed following the standard procedures at AIDHC microbiology laboratory, using a semiautomated system (MicroScan; Dade Behring, West Sacramento, CA) according to the Clinical Laboratory Standards Institute. The percentage susceptibility for fluoroquinolones was calculated by examining the first isolate per patient, per year following the 2006 Clinical Laboratory Standards Institute recommendations. Repeat isolates of a given bacterial species with the same resistance phenotype obtained from an individual patient were eliminated.10
Patients admitted to the hospital with Gram-negative bacilli tested for ciprofloxacin and levofloxacin susceptibility were eligible for inclusion. Ciprofloxacin and levofloxacin were the only fluoroquinolones approved for use at our institution. Patients whose cultures were obtained in the emergency room or outpatient clinics were excluded. Patient age and gender were retrieved by querying the Nemours Data Warehouse.
Fluoroquinolone DA and DoT were retrieved by querying the electronic administration record of ciprofloxacin and levofloxacin as previously reported.8,9 Annual intravenous and oral fluoroquinolone use was calculated as DA/1000 patient-days and DoT/1000 patient-days. DA accounted for the number of doses of ciprofloxacin or levofloxacin administered, regardless of dosage strength. DoT represented the number of days that a patient received either ciprofloxacin or levofloxacin, regardless of the number of doses administered or dosage strength. Nemours Institutional Review Board reviewed and approved this protocol.
Analysis
Quantitative variables are summarized by median and range. Categorical variables are summarized using frequencies and percentages. Rates of fluoroquinolone-resistant infections were calculated by 1000 admissions per year. A χ2 trend test for proportion was used to test the trend in fluoroquinolone use and susceptibilities applying 2001–2002 data as baseline. The Pearson correlation coefficient was used to evaluate the association between fluoroquinolone use and resistance rates. All tests were 2-tailed using a level of significance of 0.05. Analyses were performed using IBM SPSS software (version 20; IBM Corp., Armonk, NY) and Statistical software R (version 2.10.2).
Results
From 2001 to 2009, a total of 2112 Gram-negative bacilli infections were recorded in 1433 unique patients. Many infections were caused by Escherichia coli (34.5%), Pseudomonas aeruginosa (26.5%), Klebsiella pneumoniae (13.7%) and Enterobacter cloacae (10.6%) (Table 1). The median age for all patients at the time of infection was 4 years (interquartile range 1–11 years). Many infections occurred in women (1262 of 2112, 59.8%). Gram-negative bacilli susceptibility to ciprofloxacin and levofloxacin decreased from 96.1% (173 of 180) and 96.6% (174 of 180) in 2001 to 93.4% (341 of 365; χ2, 71.5; P = 0.003) and 95.9% (350 of 365; χ2, 17.6; P =0.016) in 2009, respectively.
Table 1.
Number and Proportion of Fluoroquinolone-susceptible Gram-negative bacilli Infections Among Hospitalized Children at Alfred I. duPont Hospital for Children, 2001-2009
| Organism | All Isolates N = 2112 (%) | Fluoroquinolone Susceptible Isolates | |
|---|---|---|---|
|
| |||
| Ciprofloxacin N = 1997 (%) | Levofloxacin N = 2012 (%) | ||
| E. coli | 729 (34.5) | 672 (92) | 672 (92) |
| P. aeruginosa | 559 (26.5) | 533 (95) | 534 (95.5) |
| K. pneumoniae | 290 (13.7) | 287 (99) | 287 (99) |
| E. cloacae | 223 (10.6) | 222 (99.5) | 222 (99.5) |
| Serratia marcescens | 119 (5.6) | 118 (99) | 118 (99) |
| Proteus mirabilis | 84 (4) | 83 (99) | 83 (99) |
| S. maltophilia* | 57 (2.7) | 32 (56) | 55 (97) |
| Acinetobacter spp. | 51 (2.4) | 50 (98) | 50 (98) |
S. maltophilia is intrinsically more resistant to ciprofloxacin than levofloxacin.
Many resistant isolates were recovered from tracheal aspirates (24 of 378; 6.4%) and bronchoalveolar lavage (17 of 164; 10.4%), followed by urinary tract infections (61 of 1088; 5.6%). Two patients developed bacteremia with fluoroquinolone-resistant Gram-negative bacilli. One event was associated with E. coli, and the second was due to Acinetobacter spp. All 17 Stenotrophomonas maltophilia isolates associated with bloodstream infection were susceptible to levofloxacin. Rates of hospitalized children with fluoroquinolone-resistant Gram-negative bacilli are depicted in Figure 1.
Figure 1.
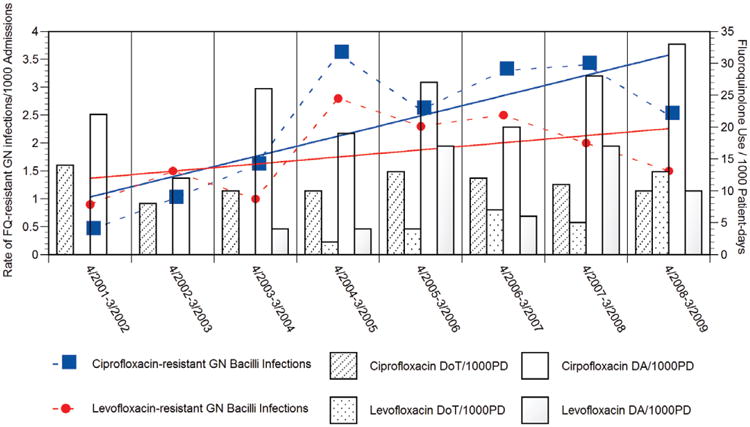
Trends in ciprofloxacin-resistant (blue squares) and levofloxacin-resistant (red dots) Gram-negative bacilli infections among hospitalized children per 1000 admissions, and ciprofloxacin and levofloxacin use expressed as doses administered and days of therapy per 1000 patient-days, Alfred I. duPont Hospital for Children 2001–2009. Sloping of ciprofloxacin resistance (blue line) and levofloxacin resistance (red line) overtime are shown
During the study period, the use of fluoroquinolones increased from 22 DA/1000 patient-days/year in 2001–2002 to 40 DA/1000 patient-days/year in 2008–2009 (χ2, 12.29; P = 0.005). The same trend was seen when fluoroquinolone use was measured as DoT, increasing from 14 DoT/1000 patient-days/year in 2001 to 23 DoT/1000 patient-days/year in 2009 (χ2, 8.28; P = 0.004).
Increased fluoroquinolone use was associated with the addition of levofloxacin to the hospital formulary in 2004. Levofloxacin use increased from 4 DA/1000 patient-days/year and 2 DoT/1000 patient-days/year in 2004–2005 to 10 DA/1000 patient-days/year (χ2, 30.15; P < 0.001) and 13 DoT/1000 patient-days/year (χ2, 33.92; P < 0.001) in 2008–2009. We found a statistically significant correlation between aggregated fluoroquinolone use measured by DA/1000 patient-days/year and DoT/1000 patient-days/year (r = 0.977; P < 0.001). During the study period, the use of ciprofloxacin remained stable measured either as DA (χ2, 1.82; P = 0.18) or as DoT (χ2, 0.0099; P = 0.92). A stronger correlation was noted between DA/1000 patient-days/year and DoT/1000 patient-days/ year for ciprofloxacin (r = 0.955; P < 0.001) than for levofloxacin (r = 0.848; P < 0.001).
Rates of hospitalized children with ciprofloxacin- and levofloxacin-resistant Gram-negative bacilli infections showed a strong correlation between the aggregated use of fluoroquinolone use measured by DA/1000 patient-days/year (ciprofloxacin resistance, r = 0.879; P < 0.001 and levofloxacin resistance, r = 0.874; P < 0.001) and DoT/1000 patient-days/year (ciprofloxacin resistance, r = 0.938; P < 0.001 and levofloxacin resistance, r = 0.945; P < 0.001). Table 2 summarizes the correlation between rates of ciprofloxacin- and levofloxacin-resistant Gram-negative bacilli infections among hospitalized children and aggregate fluoroquinolone, ciprofloxacin and levofloxacin use expressed as DoT and DA/1000 patient-days.
Table 2.
Correlation Between Fluoroquinolone Use and Emergence of Fluoroquinolone Resistance Among Gram-Negative Bacilli Recovered From Hospitalized Pediatric Patients, Alfred I. duPont Hospital for Children, 2001–2009
| Fluoroquinolone Use* | Ciprofloxacin Use* | Levofloxacin Use* | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||
| DA/1000 PD/Y | DoT/1000 PD/Y | DA/1000 PD/Y | DoT/1000 PD/Y | DA/1000 PD/Y | DoT/1000 PD/Y | ||||||||||
|
|
|
|
|
|
|
||||||||||
| Coefficient (r)† | P | Coefficient (r)† | P | Coefficient (r)† | P | Coefficient (r)† | P | Coefficient (r)† | P | Coefficient (r)† | P | ||||
| Ciprofloxacin resistance | 0.879 | 0.001 | 0.938 | 0.001 | 0.846 | 0.001 | 0.937 | 0.001 | 0.930 | 0.001 | 0.951 | 0.001 | |||
| Levofloxacin resistance | 0.874 | 0.001 | 0.945 | 0.001 | 0.811 | 0.001 | 0.941 | 0.001 | 0.928 | 0.001 | 0.969 | 0.001 | |||
Expressed as doses administered per 1000 patient-days per year (DA/1000 PD/Y) and days of therapy per 1000 patient-days per year (DoT/1000 PD/Y).
Pearson correlation coefficient.
Discussion
In our experience, the increased use of fluoroquinolones in children was found to be associated with a reduced efficacy of ciprofloxacin and levofloxacin against common Gram-negative bacilli infections. Nonetheless, ciprofloxacin and levofloxacin susceptibilities remained above 90% throughout the years of the study. Measurement of fluoroquinolone use in children by DA and DoT was concordant, despite different dosage schedules of levofloxacin for children younger than 5 years. When comparing fluoroquinolone use and emergence of resistance, we found strong correlations with both metrics.
Pediatric hospitals that strive to implement antimicrobial stewardship programs focus on 2 main objectives: optimization of clinical outcomes by promoting appropriate antimicrobial usage and reducing the emergence of resistance.5 Nonetheless, the process for measuring and prioritizing these has not been systematic.11 In pediatrics, these predicaments remain more elusive as few children's hospitals have vested interest in these initiatives.12 Despite being the core of antimicrobial stewardship efforts, the best metric to correlate antibiotic use and emergence of resistance in children remains the first and foremost unsolved dilemma.7 The World Health Organization's recommendations to measure antibiotic use by the number of “defined daily doses,” shown to correlate with emergence of resistance in adult patients, cannot be applied in pediatric hospitals because of the wide range of antibiotic doses used in children.11 In 2007, Polk et al7 proposed the use of DoT and cautioned about the risk of overestimating antimicrobials that are dosed multiple times per day. DoT is a practical metric for benchmarking of antimicrobial utilization across institutions but may not be the most accurate measure of antimicrobial exposure in children. In children, dosing frequency of many antimicrobials is dependent on patient's age and clinical condition. Therefore, DoT in pediatrics does not equate to same quantity of antimicrobial exposure when comparing the data across all age range. Hypothetically, DA may be a more sensitive metric for measuring exposure to antimicrobials in children compared with DoT. In contrary, DoT is an acceptable measure of antibiotic exposure among adults because dosing frequencies are standardized across this patient population. The use of DA accounts for every dose of antimicrobials administered per patient, independent of dose adjustments based on age, body weight, condition treated and/or renal or hepatic impairment.
One of the main limitations of studies addressing antibiotic use in hospitalized patients and emergence of resistance is accounting for the possibility of nosocomial transmission and the confounding impact of antibiotic use and horizontal acquisition of multidrug-resistant organisms in the community. Studies designed to control for these potential biases would be very difficult and expensive to conduct. The design of our study does not eliminate these potential confounders. Our findings might not be extrapolated to other antibiotic classes requiring different dosing schedule based on age, weight and/or condition been treated. As an example, linezolid dosing in children younger than 12 years requires doses up to 600 mg 3 times a day, higher than the maximum daily dose of 1200 mg used for older children and adults. The impact of the age differences in pharmacokinetic parameters on antimicrobial resistance has not been studied. Retrieving DAs can be more challenging than DoTs for children hospitals without computerized medication administration records.
As health care organizations continue to support national initiatives to control the emergence of resistance by monitoring and reducing unnecessary antimicrobial use, an optimal metric to effectively measure antimicrobial use in children and adults must be identified. It should be determined whether a metric is selected for benchmarking antibiotic use across hospitals or for adequately tailoring antibiotic use to reduce emergence of resistance. While Antimicrobial Stewardship Program quality and benchmarking metrics have been evaluated, metrics addressing emergence of resistance that could be applied to pediatric patients have not been assessed beyond days of therapy.11,13 Public health and health care organizations are actively supporting national initiatives to control the emergence of resistance by monitoring and reducing unnecessary antimicrobial use. For example, Center for Disease Control and Prevention's Antimicrobial Use and Resistance module of the National Healthcare Safety Network is enrolling facilities to analyze antimicrobial use as a part of local or regional efforts to reduce antimicrobial-resistant infections.6 Although the primary antimicrobial usage metric reported to this surveillance system is DoT, the best metric for benchmarking antibiotic use across hospitals or for adequately tailoring antibiotic use to reduce emergence of resistance is yet to be determined, particularly among children. As we move forward with the implementation of antimicrobial stewardship across children's hospitals, the scrutiny of metrics and outcome indicators to guide the future implementation of meaningful interventions remains the cornerstone for the long-term success of these programs. In our experience, DAs and DoT showed a strong correlation with emergence of fluoroquinolone resistance among Gram-negative bacilli in hospitalized children.
Footnotes
The authors have no funding or conflicts of interest to disclose.
This work was presented in part at the 49th Annual Meeting of the Infectious Diseases Society of America; October 2011; Boston, MA. Abstract 30251, Poster Number: 912.
References
- 1.Linder JA, Huang ES, Steinman MA, et al. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med. 2005;118:259–268. doi: 10.1016/j.amjmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Infectious Diseases. The use of systemic fluoroquinolones. Pediatrics. 2006;118:1287–1292. doi: 10.1542/peds.2006-1722. [DOI] [PubMed] [Google Scholar]
- 3.Meier S, Weber R, Zbinden R, et al. Extended-spectrum β-lactamase-producing Gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39:333–340. doi: 10.1007/s15010-011-0132-6. [DOI] [PubMed] [Google Scholar]
- 4.Livermore DM, Hope R, Brick G, et al. Non-susceptibility trends among Enterobacteriaceae from bacteraemias in the UK and Ireland, 2001–06. J Antimicrob Chemother. 2008;62(suppl 2):ii41–ii54. doi: 10.1093/jac/dkn351. [DOI] [PubMed] [Google Scholar]
- 5.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 6.Fridkin SK, Srinivasan A. Implementing a strategy for monitoring inpatient antimicrobial use among hospitals in the United States. Clin Infect Dis. 2014;58:401–406. doi: 10.1093/cid/cit710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664–670. doi: 10.1086/511640. [DOI] [PubMed] [Google Scholar]
- 8.Di Pentima MC, Chan S. Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29:707–711. doi: 10.1097/INF.0b013e3181d683f8. [DOI] [PubMed] [Google Scholar]
- 9.Di Pentima MC, Chan S, Hossain J. Benefits of a pediatric antimicrobial stewardship program at a children's hospital. Pediatrics. 2011;128:1062–1070. doi: 10.1542/peds.2010-3589. [DOI] [PubMed] [Google Scholar]
- 10.Hindler JF, Stelling J. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis. 2007;44:867–873. doi: 10.1086/511864. [DOI] [PubMed] [Google Scholar]
- 11.Morris AM, Brener S, Dresser L, et al. Use of a structured panel process to define quality metrics for antimicrobial stewardship programs. Infect Control Hosp Epidemiol. 2012;33:500–506. doi: 10.1086/665324. [DOI] [PubMed] [Google Scholar]
- 12.Hersh AL, Beekmann SE, Polgreen PM, et al. Antimicrobial stewardship programs in pediatrics. Infect Control Hosp Epidemiol. 2009;30:1211–1217. doi: 10.1086/648088. [DOI] [PubMed] [Google Scholar]
- 13.Newland JG, Stach LM, De Lurgio SA, et al. Impact of a Prospective-Audit-With-Feedback Antimicrobial Stewardship Program at a Children's Hospital. J Pediatric Infect Dis Soc. 2012;1:179–186. doi: 10.1093/jpids/pis054. [DOI] [PubMed] [Google Scholar]


