Abstract
Objective
To describe the methodology used to develop new classification criteria for adult and juvenile idiopathic inflammatory myopathies (IIMs) and their major subgroups.
Methods
An international, multidisciplinary group of myositis experts produced a set of 93 potentially relevant variables to be tested for inclusion in the criteria. Rheumatology, dermatology, neurology and paediatric clinics worldwide collected data on 976 IIM cases (74% adults, 26% children) and 624 non-IIM comparator cases with mimicking conditions (82% adults, 18% children). The participating clinicians classified each case as IIM or non-IIM. Generally, the classification of any given patient was based on few variables, leaving remaining variables unmeasured. We investigated the strength of the association between all variables and between these and the disease status as determined by the physician. We considered three approaches: (1) a probability-score approach, (2) a sum-of-items approach criteria and (3) a classification-tree approach.
Results
The approaches yielded several candidate models that were scrutinised with respect to statistical performance and clinical relevance. The probability-score approach showed superior statistical performance and clinical practicability and was therefore preferred over the others. We developed a classification tree for subclassification of patients with IIM. A calculator for electronic devices, such as computers and smartphones, facilitates the use of the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria.
Conclusions
The new EULAR/ACR classification criteria provide a patient’s probability of having IIM for use in clinical and research settings. The probability is based on a score obtained by summing the weights associated with a set of criteria items.
Keywords: dermatomyositis, polymyositis, autoimmune diseases
Key messages.
What is already known about this subject?
Currently, the most often used criteria for idiopathic inflammatory myopathies stem from 1975, are based on expert opinion, hence not data driven, and are used interchangeably as both diagnostic and classification criteria.
What does this study add?
This report describes a new methodology to develop classification criteria based on patient data. The individual variables have been given different weights and different scores. The method is based on calculations of probability to define a case as having myositis or non-myositis, which gives a flexibility of the number of variables needed to be tested.
A web-based calculator has been developed that facilitates defining a case as myositis and also defining myositis subgroup.
How might this impact on clinical practice?
This study provides information on a new methodology to develop classification criteria combined for adult and children with myositis and to be used for clinical studies, research and trials.
Introduction
The first European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria for idiopathic inflammatory myopathies (IIMs), collectively known as myositis, and the major subgroups of IIM have been developed.1 This report describes the methodological approach used for the development of the criteria. Detailed information about the classification criteria is given in the companion article.1
IIMs are a group of rare and heterogeneous diseases, involving different medical specialties in patient treatment and care, as well as research. Currently, the most often used criteria for IIM were defined based on expert opinion more than 40 years ago. An international and multidisciplinary collaboration, the International Myositis Classification Criteria Project (IMCCP), was established comprising rheumatologists, paediatric rheumatologists, neurologists, dermatologists, epidemiologists and biostatisticians. The group addressed both childhood-onset and adult-onset IIMs, following the ACR and EULAR recommendations for development of classification criteria.2 3 A large group of experts accepted the task, and a steering committee was appointed. A wider group of investigators formed a working committee that contributed clinical data and clinical expertise to the study. Major professional societies and interest groups (listed in the Methods section) in the field of myositis research and clinical care provided endorsement and support, and participated in the project. This led to the development of the EULAR/ACR classification criteria for IIM and their major subgroups.
Methods
The ACR, EULAR, American Academy of Neurology, the Childhood Arthritis and Rheumatology Research Alliance, the European Neuromuscular Centre (ENMC), the International Myositis Assessment and Clinical Studies Group (IMACS), the Muscle Study Group, the Rheumatologic Dermatology Society, the Pediatric Rheumatology European Society network for JDM and the Pediatric Rheumatology International Trials Organization contributed to this project.
Selection of criteria items
The steering committee had a face-to-face meeting to detail the study design. It was decided what IIM diagnoses would be included in the study along with comparator cases having conditions that were commonly confused with IIM.1 The committee generated candidate criteria based on previously published myositis criteria,4–16 and new items that had been described since the previous criteria were developed and considered relevant characteristics of IIM. A pilot feasibility study was conducted to assess the practicality and the likelihood that these variables could be collected from existing medical records. The list of items was modified and presented again for discussion in a face-to-face meeting, with experts of the working committee achieving consensus on items for inclusion in the study, through a nominal group technique17–20 to ensure face and content validity of the items.2 3 Members of the working committee were asked to choose the 10 items on the list they considered most important. Consensus items were defined and distributed to a large network of experts through the IMACS membership list for further comments and input via a Delphi email method. These were discussed by the steering committee, and a final list was agreed on. Each variable was defined using the ACR nomenclature list.21 22
Data collection
A web-based questionnaire was created on SurveyMonkey (www.surveymonkey.com) (online supplementary table S1). Members of the IMCCP working committee and the IMACS network were asked to contribute IIM cases and comparators. An initial power calculation was based on an expected enrolment of 150 patients in each of the following subgroups: polymyositis (PM), dermatomyositis (DM), juvenile dermatomyositis (JDM), inclusion body myositis (IBM), other IIM cases and 500 comparators. Clinical data were abstracted from patients’ records.
rmdopen-2017-000507supp001.pdf (146.4KB, pdf)
Inclusion criteria for cases and comparators were (1) diagnosis for at least 6 months prior to study inclusion; (2) physician certainty of diagnosis, either known IIM or, as comparators, known non-IIM cases where myositis was considered in the initial differential diagnosis; and (3) patients with the most recent and complete data were prioritised to acquire the most complete data in a consistent manner.
To ensure a balanced number of cases across sites, a maximum of 40 cases and an equal number of comparators were collected from each centre. Cases were chosen based on the certainty of diagnosis and completeness of the variables being collected. In paediatric centres, a minimum of five cases and five comparators was required. The study was approved or exempted from approval by ethics committees at each participating clinic.
Data processing
Before proceeding with the analyses, the available data were checked for accuracy and quality. In addition, univariate and bivariate frequency distributions were compared with those produced directly by the SurveyMonkey server. All variables were grouped into categories: muscle, skin, laboratory, biopsies, MRI, electromyogram (EMG) and others. Non-informative responses and data errors were coded as missing (online supplementary table S2).
Most variables were dichotomous. Age was grouped into three categories (0–17, 18–39, 40+ years) in the analyses. The cut-off values were selected because they corresponded to clinically accepted age categories. In addition, they maximised the goodness of fit of the probability-score model described in the section titled ’Methods to develop new classification criteria.' The association of each variable with the diagnosis (IIM, non-IIM) was assessed by ORs and tested with the Fisher’s exact test. The associations among all predictors were also evaluated. Strong associations indicated that different predictors provided near equivalent information.
The participating clinicians specialising in myositis often diagnosed patients based on only a few variables in the data entry form, leaving the other variables blank. Blanks were considered missing observations in the data analyses. Because it was the doctor’s decision what variables to measure and what to leave blank, the missing observations were assumed to be generated by a missing-at-random mechanism.23 We carefully inspected all the patterns of missing values across all the patients in the dataset. As sensitivity analysis, we explored the possibility of coding all missing values as a separate category. This, however, did not improve the performance of the resulting classification criteria.
Stata statistical software (StatCorp, College Station, Texas, USA) was used for data management and statistical analyses. R statistical software was used for part of the analyses of classification trees and random forests.
Methods to develop new classification criteria
We used three methods to develop the criteria: (1) a probability score, (2) a sum of items and (3) a classification tree. Each approach was developed independently of the others. The variables identified by one approach as potentially relevant predictors were compared with those selected from the other approaches in an iterative process. The items that finally emerged as potentially relevant for the prospective criteria were closely examined for statistical performance, clinical relevance and practicability by the steering committee. Statistical performance was measured by classification accuracy and area under the receiver operating characteristic curve. Internationally, there is wide variation in whether muscle biopsy is felt mandatory, possible or routinely done or available in suspected myositis. Recognising the realities of practice, the committee felt that standardisation and transparency would be best served by having one set of criteria when biopsies are available and another when they are not.
Probability-score approach
A probability-score model consists of items (eg, signs, symptoms, laboratory measures) that can be observed in a patient. A score value is assigned to each item. The total score for a patient is obtained by summing the score values associated with the items observed. The total score is converted to a probability by a formula or by inspection from a graph. A small subset of items generally permits classifying a patient as an IIM case or a non-case. The set of relevant items, however, differs between subgroups of the IIM disease.
The score values of the candidate items were estimated by multivariable logistic regression. Initially, all variables were included one at a time and those having the largest numbers of observations and strongest associations with the diagnosis were identified. In addition to each individual item, new variables from each category (muscle, skin, other signs and symptoms, biopsy, laboratory data and EMG) were assigned a 1 if present and a value of zero if absent. These indicator variables and individual items were entered into a multivariable model one at a time. The statistical significance of the resulting increase in the goodness of fit of the model was assessed by Wald tests. The predictive ability was measured by their classification accuracy, specificity and sensitivity, and area under the receiver operating characteristic curve. When the estimated regression coefficients associated with predictors from the same group were similar, but the predictors were not measured jointly, we defined a new item as present if any of these predictors were present. An example of this type of items is represented by second item in the laboratory section of the EULAR/ACR classification criteria reported in table 1 (CK, LDH, ASAT/AST/SGOT or ALAT/ALT/SGPT). Only models with valid observations on at least 800 subjects, half of the sample, were considered (online supplementary table S2). When the analyses were done in children (<18 years) and adults (18+ years) separately, the score points did not change much, with correlation coefficients ranging between 0.98 and 1.00.
Table 1.
Score points for the European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies to be used when no better explanation for the symptoms or signs exists1
| Variable | Score points | |
| No biopsy | Biopsy | |
| Age of onset of first related symptoms | ||
| 18–40 | 1.3 | 1.5 |
| ≥40 | 2.1 | 2.2 |
| Muscle weakness | ||
| Objective symmetric weakness, usually progressive, of proximal upper extremities | 0.7 | 0.7 |
| Objective symmetric weakness, usually progressive, of proximal lower extremities | 0.8 | 0.5 |
| Neck flexors are relatively weaker than neck extensors | 1.9 | 1.6 |
| In the legs, proximal muscles are relatively weaker than distal muscles | 0.9 | 1.2 |
| Skin manifestations | ||
| Heliotrope rash | 3.1 | 3.2 |
| Gottron’s papules | 2.1 | 2.7 |
| Gottron’s sign | 3.3 | 3.7 |
| Other clinical manifestations | ||
| Dysphagia or esophageal dysmotility | 0.7 | 0.6 |
| Laboratory measurements | ||
| Anti-Jo-1 (anti-histidyl-tRNA synthetase) autoantibody positivity | 3.9 | 3.8 |
| Elevated serum levels of creatine kinase (CK)* or lactate dehydrogenase (LDH)* or aspartate aminotransferase (ASAT/AST/SGOT)* or alanine aminotransferase (ALAT/ALT/SGPT)* | 1.3 | 1.4 |
| Muscle biopsy features | ||
| Endomysial infiltration of mononuclear cells surrounding, but not invading, myofibres | 1.7 | |
| Perimysial and/or perivascular infiltration of mononuclear cells | 1.2 | |
| Perifascicular atrophy | 1.9 | |
| Rimmed vacuoles | 3.1 | |
*Serum levels above upper limit of normal.
The overall aim was to derive an accurate, parsimonious and easy-to-use model based on variables commonly observed. Hence, the content validity and clinical feasibility of each variable was considered before entering it into the model. Some aspects of the statistical performance of the classification criteria were validated internally and externally on independent datasets, as described below.
Sum-of-item approach
This approach has been used by most of the previously published criteria.7–11 14 15 A patient is classified as case if the patient has a certain number of items out of a prespecified set of items. The sum-of-item approach is a special case of the more general probability-score approach described above in which all the constituent items are assigned a score value of 1. When the score values are all equal to 1, summing the score values associated with the items that are present is equivalent to simply counting them. Counting the items that are present may be slightly simpler than summing their score values. However, the resulting classification criteria become substantially less flexible. While the clinical feasibility of the sum-of-item approach was comparable with that of the probability-score approach, the statistical performance of the former was considerably poorer than that of the latter. The sum-of-score approach was therefore abandoned.
Classification-tree approach
A classification tree starts with a specific item and branches into two possible paths depending on whether or not the item is observed in the patient. A binary split is applied to multicategory variables. This is repeated until the tree ends in a leaf that is labelled either IIM or non-IIM.
Classification trees and random forests were estimated by using standard machine-learning algorithms.24 The first tree was obtained by selecting variables from the pool of all the variables available. Missing data were imputed with random forest algorithms beforehand. The random forests provided information on what variables could represent important predictors. Subsequently, trees were obtained from subsets of all the available variables. Only those that showed strong univariate associations, or used in published criteria,7–11 14 15 or appeared most informative in the random forest analysis, and had the largest number of valid observations were used.
The classification-tree approach was clinically feasible, and a number of different trees had similar statistical performance.
Validation
The probability score was validated internally and externally on independent datasets. For the internal validation, bootstrap resampling techniques were used to estimate prediction error and discrimination ability of the candidate classification criteria.25 The external validation of the classification criteria was based on two large external datasets. These datasets contained IIM cases only, and only the sensitivity of the criteria could be assessed. Details about the external datasets and the results of the validation process are given in the companion article.1
Subgroups of IIM defined by classification tree
A classification tree was developed to distinguish subgroups of patients classified with IIM according to the new criteria. The tree was based on the variables in the new classification criteria and on expert opinion. More details on this are provided in the companion article.1
Online calculator
To facilitate the use of the EULAR/ACR classification criteria, an informational and instructional webpage and online calculator was developed (http://www.imm.ki.se/biostatistics/calculators/iim). The calculator computes a range for the probability of IIM. It is free to download and requires no internet connection to use. When sufficient information is entered, the subclassification is displayed. It can be used on any device that supports Java scripts, including most web browsers. A Microsoft Excel spreadsheet can also be downloaded. The website is maintained by the Unit of Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Face and construct validation
All project participants were invited to a face-to-face meeting to provide input on the different approaches, classification criteria and results. In addition, three web-based meetings for all project participants were held as part of the face and construct validation.
Results
Assembly of subjects with IIM and comparators
Data from 976 IIM (74% adults; 26% children) and 624 comparators (82% adults; 18% children) were collected from 17 North American, 1 South American, 23 European and 6 Asian clinics, between 2008 and 2011. The IIM cases comprised all the included subgroups. The non-IIM cases represented a broad spectrum of mimicking conditions (defined in Lundberg et al 1).
Data analysis and development of classification criteria
A final list of 93 candidate classification criteria items to be collected in the questionnaire was defined. The list included demographic data, clinical muscle variables, skin variables, other clinical variables of importance, laboratory variables comprising muscle enzyme measurements, inflammation marker measurements and autoantibody tests, EMG and MRI characteristics of muscle and muscle biopsy features determined by conventional histopathological examination, immunohistochemical staining and electron microscopy (EM) (online supplementary table S1).
The variables corresponding to muscle weakness patterns and skin manifestations had the highest numbers of valid observations (online supplementary table S2). The mean numbers of observations across all variables in the muscle category and in the skin category were 1394 and 1506, respectively. The variables relating to EMG and MRI measurements, immunohistochemical staining of muscle and EM examination of muscle biopsy had the fewest observations (mean number of observations equal to 602, 465, 277 and 121, respectively). The variables with highest ORs associated with IIM were skin manifestations, and in particular heliotrope rash (OR 32.4), Gottron’s papules (OR 26.8) and Gottron’s sign (OR 30.5). The associations of predictors showed strong and significant associations between variables within the same categories, such as muscle weakness pattern and skin manifestations as two clinical variables, but not across different categories.
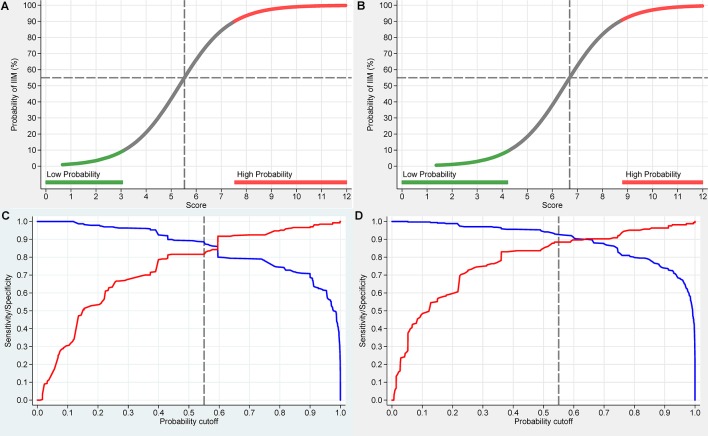
The probability-score approach was used to derive two sets of criteria: one when muscle biopsies are available and the other when they are not. Table 1 shows the score points for the calculation of the probability associated with each of the items contained in the two sets. Both sets include age, patterns of muscle weakness, skin manifestations, dysphagia, elevated muscle enzyme levels in sera and anti-Jo-1 autoantibodies in sera. One of the two sets also includes muscle biopsy features. The total score is obtained by adding the score points corresponding to all items present in the patient, as shown in table 1. The score can be converted to a probability of IIM visually on figure 1A and B or by using the following formulas:
Figure 1.
European League Against Rheumatism/American College of Rheumatology classification criteria probability of having idiopathic inflammatory myopathies (IIMs) over total score values. The total score is obtained from adding up the score values in table 1. Panel A corresponds to total score without muscle biopsy data and panel B with muscle biopsy data. Each score and probability of disease display a unique set of sensitivity (blue line) and specificity (red line) measurement for the classification criteria not including muscle biopsy data (C) or including muscle biopsy data (D). The optimal point of accuracy should be stated in publications and appropriate to the intended purpose, with the recommendation of using a minimum of 55% probability (score of 5.5 without biopsies; 6.7 with biopsies) for classifying a case as IIM (‘probable IIM’) (dotted line). ‘Definite IIM’ corresponds to a probability of at least 90% (score of 7.5 without biopsies; 8.7 with biopsies).
The first formula is to be used when muscle biopsies are available; the second when they are not.
The score points shown in table 1 are calculated as the estimated logistic regression coefficients rounded to the first decimal digit. A constant term was included in the model, but it is not shown in table 1. Its value is subtracted from the score instead, as indicated in the formulas displayed above. The web calculator does not round the value of the logistic regression coefficients. This may result in small differences in the probability as calculated by the web calculator or by hand from table 1.
The score points reported in table 1 can be used to obtain a range for the probability of IIM as follows: when no items are observed, the probability of IIM ranges from zero to one. When some, but not all, items are observed, the probability ranges from smallest to largest possible value. The smallest possible value is calculated by replacing all the unobserved items with ‘absent’. The largest possible value is obtained by replacing all the unobserved values with ‘present’. This is conveniently implemented in the web calculator. When all items are observed, the smallest and the largest possible probability values coincide. However, the probability range quickly narrows as soon as a few items are observed and quickly moves away from the 50% probability towards either 0% or 100%.
A patient is classified as an IIM case if the patient’s probability is above a specified cut-off value. The best balance between sensitivity and specificity (figure 1) is obtained for a cut-off of 55%–60% for the criteria not including muscle biopsy data (panel C) and 55%–75% for when muscle biopsy data are included (panel D). Based on these results, a cut-off of 55% (a total aggregate score of 5.5 without biopsies; 6.7 with biopsies) was decided by the steering committee as the minimal level to classify a case as IIM. The IMCCP steering committee recommends that ‘high probability’ of IIM should be defined as 90% or greater. Patients with a probability of ≥55% to <90% are designated ‘probable IIM’. A probability of ≥90%, or a total aggregate score of ≥7.5 without muscle biopsy and ≥8.7 with muscle biopsy, corresponds to ‘definite IIM’, and a probability ≥50% to <55% is termed ‘possible IIM’.
Subgroup classification tree
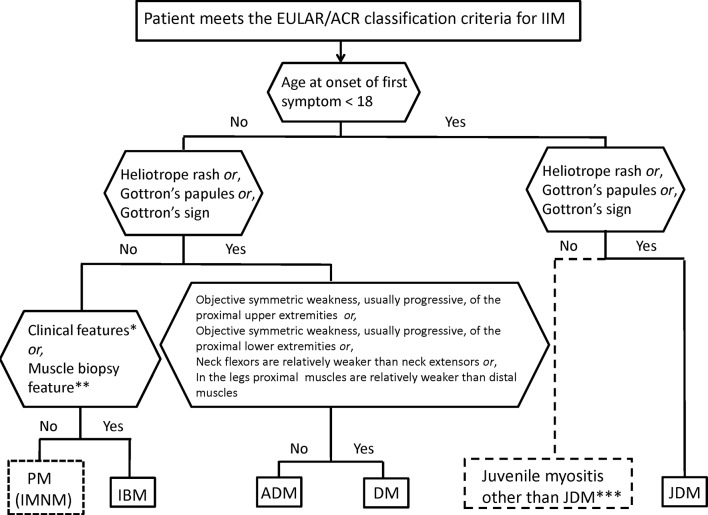
This section also appears in the companion paper.1 A patient classified with IIM by the EULAR/ACR classification criteria probability score can be further subclassified applying a classification tree (figure 2). Age at onset of first symptom distinguishes adult from juvenile IIM. Thereafter, clinical findings and muscle biopsy features subclassify adult patients with IIM into PM, IBM, ADM or DM. Based on our dataset, juvenile patients with skin rash can be classified into JDM. Three subgroups cannot be further separated because of small sample sizes: juvenile PM, immune-mediated necrotising myopathy (IMNM) and hypomyopathic DM.
Figure 2.
Classification tree for subgroups of idiopathic inflammatory myopathies (IIMs). A patient must first be classified as having IIM using the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria. The patient can then be subclassified using the classification tree. The mixed (dotted outlined box) subgroup of patients with PM includes patients with IMNM. For IBM diagnosis, one of the following, *Finger flexor weakness and response to treatment: not improved or **Muscle biopsy: rimmed vacuoles, is required for diagnosis. ***Juvenile myositis other than JDM was developed based on expert opinion and extrapolation from adults. IMNM and hypomyopathic DM were too few to allow subclassification. ADM, amyopathic dermatomyositis; DM, dermatomyositis; IBM, inclusion body myositis; IMNM, immune-mediated necrotising myopathy; JDM, juvenile dermatomyositis; PM, polymyositis.
Among patients with IIM by the EULAR/ACR classification criteria, and with sufficient data to allow subclassification (n=703), the number of cases in the subgroups as defined according to the classification tree was enumerated (online supplementary table S3). The agreement between the classification tree subgroups and the physician-diagnosed subgroups in the dataset was high (92.6% agreement, kappa=0.90, P<0.00001). The agreement proportions, with a probability of 55%, were 1.00 for JDM, 0.89 for DM, 0.94 for ADM, 0.92 for IBM and 0.93 for PM. Raising the probability cut-off of IIM to 90% yielded 94.9% agreement, kappa=0.93, P>0.00001. With a probability cut-off of 90%, the agreement proportions were 1.00 for JDM, 0.96 for DM, 0.95 for ADM, 0.93 for IBM and 0.88 for PM.
Face and construct validation
Twenty-three project participants attended a face-to-face meeting and provided input on project process and results. They also gave suggestions towards facilitating the face and construct validity process among myositis experts. In total, 26 project participants took part in web-based seminars arranged where data collection, data analysis, the classification criteria, their performance and real case demonstrations were vetted. The myositis experts expressed ‘satisfaction’ with respect to both credibility and comprehensiveness of the classification criteria.
Discussion
The EULAR/ACR classification criteria for IIM were developed by a large international and multidisciplinary group of 100 myositis experts.1 These criteria provide a probability of IIM and can be used with adults and children. A patient is classified as an IIM case when this probability is above 55%. The development of the criteria was based on empirical data, guided by clinical and statistical expertise. The EULAR/ACR IIM classification criteria showed greater sensitivity and specificity than the often-used Bohan and Peter’s criteria,7 8 and at least as high as other published myositis classification criteria and a higher sensitivity than the newer ENMC criteria.9–11 14 15 The sensitivity of the EULAR/ACR IIM classification criteria was externally validated in independent cohorts of patients with IIM and effectively captures all myositis subgroups included in the IMCCP study.
Myositis is a rare disease with multiple heterogeneous subgroups. Patients may be examined by physicians from various medical disciplines. Developing new classification criteria therefore called for adequately sized multicentre studies and involvement of representatives from all relevant medical disciplines. The IMCCP is the largest clinical myositis study to date. Experts from five different medical and methodological disciplines from all over the world collaborated to ensure its successful completion.
Unlike most previously published myositis diagnostic and classification criteria, the EULAR/ACR IIM classification criteria are based on clinical information collected from adult and juvenile patients with myositis and comparators from Europe, North and South America, and Asia. While previous criteria assign equal weight to their criteria items, the EULAR/ACR IIM classification criteria assign different weights, each reflecting the relative importance and predictive capacity of the corresponding variable. Although previous criteria do not provide a probability of having IIM, many use qualifiers such as possible, probable or definite IIM. By providing a probability, the EULAR/ACR IIM classification criteria permit setting the classification cut-off to specific values to optimise sensitivity or specificity to meet the specific needs of any given clinical or epidemiological setting. The IMCCP steering committee recommends that ‘high probability’ of IIM should be defined as 90% or greater.
Unlike previous myositis criteria, the EULAR/ACR IIM classification criteria include amyopathic DM patients as part of the spectrum of IIM. The patients with amyopathic DM included in the study routinely received a skin biopsy showing interface dermatitis. Only few patients with IMNM were included in the study due to the fact that this subgroup only became better recognised once the study was underway.15 These patients could not be separated from patients with PM in the subclassification tree. Studies including more patients with IMNM could enable revising these criteria in the future to separate IMNM from the other subgroups. Although interstitial lung disease (ILD) is a common extramuscular manifestation in adult IIM and yielded a strong association with having IIM in the dataset, it is not a common manifestation in juvenile IIM. Dysphagia displayed equally strong weight as ILD and was judged more clinically relevant to include in classification criteria by the myositis experts.
The EULAR/ACR IIM classification criteria offer advantages over previously published myositis criteria. First, they contain symptoms and measurements that are easily clinically accessible and do not require extra effort or costs of the clinic. The only invasive procedure in the new criteria involves muscle biopsies, but an alternative set of criteria was optimised for use when muscle biopsy data are unavailable. The IMCCP steering committee, however, recommends that muscle biopsy data be included when none of the skin manifestations is present. Similarly, especially when no muscle disease exists, it is recommended that a skin biopsy be performed. The new criteria offer flexibility that other criteria may lack. Not all items in the EULAR/ACR IIM classification criteria need to be evaluated. Clinicians may stop measuring items as soon as a sufficiently high or low probability is obtained. All the items in the EULAR/ACR IIM classification criteria are defined unambiguously. This is important, as subjective and inconsistent interpretations have marred accuracy and practicability of previous criteria. In addition, face and content validity was secured by ensuring the continued involvement of an extended panel of myositis experts throughout the entire process. The cases and comparators were random samples of the respective populations, and the prevalence of IIM in our data was 61% (976 cases and 624 comparators). Therefore, the probability of IIM provided by the EULAR/ACR IIM classification criteria is most accurate when the IIM prevalence in the population of patients in which the criteria are applied is about 60%.
The web calculator may prove a practical aid in classifying patients in clinical and research settings alike. When sufficient information is entered, the subclassification is also displayed. It is available for any electronic devices, such as computers and smartphones. A Microsoft Excel spreadsheet is also available for download.
Conclusions
The new EULAR/ACR classification criteria for IIM provide the probability of having IIM for any given patient, based on easily measurable characteristics. In internal-data and external-data validations, the new criteria demonstrated great accuracy in predicting IIM as well as all of the IIM subgroups and were approved by an international panel of myositis experts.
Acknowledgments
We are grateful for contribution of clinical data from investigators and for participants contributing with valuable input at IMCCP meetings (online supplementary appendix). We thank Elin Forslund for assistance with data registration.
Footnotes
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual content. All authors approved the final version to be published and had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: MB, AT, VPW, CP, MdeV, LA, AAA, RJB, MHL, JAS, KD, BMF, HK, PAL, BAL, FWM, LGR, IEL. Acquisition of data: MB, AT, VPW, CP, MdeV, LA, AAA, RJB, MHL, JAS, RA, SA, HC, RGC, KD, MMD, BMF, IG-DLT, PG, TH, JDK, HK, PAL, BAL, YL, CVO, MO, AMR, LR-S, HS, AS-OC, YWS, JV, SRY, FWM, LGR, IEL, The International Myositis Classification Criteria Consortium, working committee members. Analysis and interpretation of data: MB, AT, GS, VPW, CP, MdeV, LA, AAA, RJB, MHL, JAS, RA, BMF, IG-DLT, PG, HK, PAL, BAL, YL, FWM, LGR, IEL.
Funding: This study was financially supported by ACR, EULAR, The Myositis Association (TMA), the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences and the European Science Foundation for the Euromyositis Register. CARRA, Inc. is funded by NIH-NIAMS, Friends of CARRA and the Arthritis Foundation.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government, or the NHS, the National Institute for Health Research or the Department of Health (UK).
Competing interests: JAS has received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Regeneron, Merz, Bioiberica, Crealta and Allergan. JAS serves as the principal investigator for an investigator-initiated study funded by Horizon pharmaceuticals through a grant to DINORA, Inc., a 501 (c)(3) entity. JAS is a member of the executive of OMERACT, an organisation that develops outcome measures in rheumatology and receives arms-length funding from 36 companies; a member of the American College of Rheumatology’s (ACR) Annual Meeting Planning Committee (AMPC); Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee. HC and RGC’s work in myositis is partly funded by grants from Arthritis Research UK (18474) and the Medical Research Council (MR/N003322/1). JV’s work in myositis is supported by the Project (Ministry of Health, Czech Republic) for Conceptual Development of Research Organization 00023728.
Ethics approval: The study was approved by ethics committees at each participating site.
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: Maria Amoruso (Children’s Hospital of Chicago and Northwestern University Feinberg School of Medicine, Chicago, USA), Helena Andersson (Section of Rheumatology, Oslo University Hospital–Rikshospitalet, Oslo, Norway), Nastaran Bayat (Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health, US Department of Health and Human Services, Bethesda, USA), Kavish J Bhansing (Department of Neurology, Radboud University Medical Center, Nijmegen, The Netherlands), Sara Bucher (Department of Neurology, Örebro University, Örebro, Sweden), Richard Champbell (King’s College Hospital, London, UK), Christina Charles-Schoeman (Division of Rheumatology, Department of Medicine, University of California Los Angeles, Los Angeles, USA), Vinay Chaudhry (Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, USA), Lisa Christopher-Stine (Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, USA), Lorinda Chung (Department of Dermatology, Stanford University School of Medicine, Stanford, USA; Division of Rheumatology, Palo Alto Veterans Affairs Health Care System, Palo Alto, USA; Department of Medicine, Division of Rheumatology, Stanford University School of Medicine, Stanford, USA), Mary Cronin (Rheumatology Division, Medical College of Wisconsin, Milwaukee, USA), Theresa Curry (The Myositis Association), Kathe Dahlbom (Department of Neurology, Örebro University, Örebro, Sweden), Oliver Distler (Division of Rheumatology, University Hospital Zurich, Zurich, Switzerland), Petros Efthimiou (New York Methodist Hospital, Brooklyn, NY & Weill Cornell Medical College, New York, USA), Baziel GM van Engelen (Department of Neurology, Radboud University Medical Center, Nijmegen, The Netherlands), Abdullah Faiq (Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health, US Department of Health and Human Services, Bethesda, USA), Payam Noroozi Farhadi (Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health, US Department of Health and Human Services, Bethesda, USA), David Fiorentino (Division of Immunology and Rheumatology, Stanford University School of Medicine, Stanford, USA), Gerald Hengstman (Department of Neurology, Catharina Hospital, Eindhoven, the Netherlands), Jessica Hoogendijk (Rudolf Magnus Institute for Neuroscience, Department of Neurology, University Medical Center Utrecht, Netherlands), Adam Huber (IWK Health Centre and Dalhousie University, Halifax, Canada), Hiroshi Kataoka (Department of Medicine II, Hokkaido University Graduate School of Medicine, Hokkaido, Japan), Yasuhiro Katsumata (Institute of Rheumatology, Tokyo Women’s Medical University, Tokyo, Japan), Susan Kim (Boston Children’s Hospital, Pediatric Rheumatology, Boston, USA), Michelle Kong-Rosario (North shore-LIJ Health System, USA), Apostolos Kontzias (Cleveland Clinic, Cleveland, USA), Petra Krol (Department of Pediatrics and Adolescent Medicine, Charles University, 1st Medical School, Prague, Czech Republic), Takashi Kurita (Department of Medicine II, Hokkaido University Graduate School of Medicine, Hokkaido, Japan), Zhan-Guo Li (Department of Rheumatology and Immunology, People’s Hospital of Beijing University, China), Björn Lindvall (Department of Neurology, Örebro University, Örebro, Sweden), Helen Linklater (Department of Rheumatology, King`s College Hospital NHS Foundation Trust, London, UK), Sue Maillard (Department of Rheumatology, Great Ormond Street Hospital for Children NHS Trust, London, UK), Gulnara Mamyrova (Myositis Center, Division of Rheumatology, Department of Medicine, George Washington University, Washington DC, USA), Renato Mantegazza (Neurology IV, Neuroimmunology and Neuromuscular Diseases Unit, Fondazione Istituto Neurologico Carlo Besta, Milan, Italy), Galina S Marder (North shore-LIJ Health System, USA), Suely Kazue Nagahashi Marie (Division of Neurology, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brasil), Pernille Mathiesen (Paediatric Department, Copenhagen University Hospital Holbaek, Holbaek, Denmark), Clio P Mavragani (Department of Pathophysiology, School of Medicine, University of Athens, Athens, Greece), Neil J McHugh (Royal National Hospital for Rheumatic Diseases, Bath, UK), Mimi Michaels (Department of Neurology, University of Kansas Medical Center, Kansas City, USA), Reem Mohammed (Division of Rheumatology, Department of Paediatrics, University of Toronto and The Hospital for Sick Children, Toronto, Canada), Gabrielle Morgan (Children’s Hospital of Chicago and Northwestern University Feinberg School of Medicine, Chicago, USA), David W Moser (Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA), Haralampos M Moutsopoulos (Department of Pathophysiology, School of Medicine, University of Athens, Athens, Greece).
Contributor Information
The International Myositis Classification Criteria Project consortium, the Euromyositis register and the Juvenile Dermatomyositis Cohort Biomarker Study and Repository (JDRG) (UK and Ireland):
Maria Amoruso, Helena Andersson, Nastaran Bayat, Kavish J Bhansing, Sara Bucher, Richard Champbell, Christina Charles-schoeman, Vinay Chaudhry, Lisa Christopher-stine, Lorinda Chung, Mary Cronin, Theresa Curry, Kathe Dahlbom, Oliver Distler, Petros Efthimiou, Baziel Gm Van engelen, Abdullah Faiq, Payam Noroozi Farhadi, David Fiorentino, Gerald Hengstman, Jessica Hoogendijk, Adam Huber, Hiroshi Kataoka, Yasuhiro Katsumata, Susan Kim, Michelle Kong-rosario, Apostolos Kontzias, Petra Krol, Takashi Kurita, Zhan-guo Li, Björn Lindvall, Helen Linklater, Sue Maillard, Gulnara Mamyrova, Renato Mantegazza, Galina S Marder, Suely Kazue Nagahashi marie, Pernille Mathiesen, Clio P Mavragani, Neil J Mchugh, Mimi Michaels, Reem Mohammed, Gabrielle Morgan, David W Moser, and Haralampos M Moutsopoulos
References
- 1.Lundberg IE, Tjärnlund A, Bottai M, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis and Arthritis Rheum. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Solomon DH, Dougados M, et al. Development of classification and response criteria for rheumatic diseases. Arthritis Rheum 2006;55:348–52. 10.1002/art.22003 [DOI] [PubMed] [Google Scholar]
- 3.Dougados M, Gossec L. Classification criteria for rheumatic diseases: why and how? Arthritis Rheum 2007;57:1112–5. 10.1002/art.23015 [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC. Polymyositis, dermatomyositis, and inclusion-body myositis. N Engl J Med Overseas Ed 1991;325:1487–98. 10.1056/NEJM199111213252107 [DOI] [PubMed] [Google Scholar]
- 5.Medsger TA, Dawson WN, Masi AT. The epidemiology of polymyositis. Am J Med 1970;48:715–23. 10.1016/S0002-9343(70)80006-7 [DOI] [PubMed] [Google Scholar]
- 6.DeVere R, Bradley WG. Polymyositis: its presentation, morbidity and mortality. Brain 1975;98:637–66. 10.1093/brain/98.4.637 [DOI] [PubMed] [Google Scholar]
- 7.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. 10.1056/NEJM197502132920706 [DOI] [PubMed] [Google Scholar]
- 8.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–7. 10.1056/NEJM197502202920807 [DOI] [PubMed] [Google Scholar]
- 9.Griggs RC, Askanas V, DiMauro S, et al. Inclusion body myositis and myopathies. Ann Neurol 1995;38:705–13. 10.1002/ana.410380504 [DOI] [PubMed] [Google Scholar]
- 10.Tanimoto K, Nakano K, Kano S, et al. Classification criteria for polymyositis and dermatomyositis. J Rheumatol 1995;22:668–74. [PubMed] [Google Scholar]
- 11.Targoff IN, Miller FW, Medsger TA, et al. Classification criteria for the idiopathic inflammatory myopathies. Curr Opin Rheumatol 1997;9:527–35. 10.1097/00002281-199711000-00008 [DOI] [PubMed] [Google Scholar]
- 12.Mastaglia FL, Phillips BA. Idiopathic inflammatory myopathies: epidemiology, classification, and diagnostic criteria. Rheum Dis Clin North Am 2002;28:723–41. 10.1016/S0889-857X(02)00021-2 [DOI] [PubMed] [Google Scholar]
- 13.van der Meulen MF, Bronner IM, Hoogendijk JE, et al. Polymyositis: an overdiagnosed entity. Neurology 2003;61:316–21. [DOI] [PubMed] [Google Scholar]
- 14.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971–82. 10.1016/S0140-6736(03)14368-1 [DOI] [PubMed] [Google Scholar]
- 15.Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord 2004;14:337–45. 10.1016/j.nmd.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Troyanov Y, Targoff IN, Tremblay JL, et al. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine 2005;84:231–49. [DOI] [PubMed] [Google Scholar]
- 17.Van de AH, Delbecq AL. The effectiveness of nominal, Delphi, and interacting group decision making processes. Acad Manage J 1974;17:605–21. 10.2307/255641 [DOI] [Google Scholar]
- 18.Fink A, Kosecoff J, Chassin M, et al. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74:979–83. 10.2105/AJPH.74.9.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruperto N, Meiorin S, Iusan SM, et al. Consensus procedures and their role in pediatric rheumatology. Curr Rheumatol Rep 2008;10:142–6. 10.1007/s11926-008-0025-6 [DOI] [PubMed] [Google Scholar]
- 20.Totikidis V. Applying the nominal group technique (NGT) in community based action research for health promotion and disease prevention. Aust Community Psychol 2010;22:18–29. [Google Scholar]
- 21.ARAGlossary Committee. Dictionary of the rheumatic diseases 1985; vol II: diagnostic testing [monograph]. New York, NY: Contact Associates International Ltd, 1985. [Google Scholar]
- 22.ARAGlossary Committee. Dictionary of the rheumatic diseases 1982; vol i: signs and symptoms [monograph]. NewYork, NY: Contact Associates International Ltd, 1982. [Google Scholar]
- 23.Rubin DB. Inference and missing data. Biometrika 1976;63:581–92. 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 24.Breiman L. Random forests. Machine Learning 2001;45:5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 25.Steyerberg EW, Harrell FE, Borsboom GJ, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis J Clin Epidemiol 2001;54:774–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2017-000507supp001.pdf (146.4KB, pdf)