Abstract
Objective
Oxidative stress plays an important role in the pathological processes of various neurodegenerative diseases. Bacopa monnieri (BM) has a potent antioxidant property. Therefore, the purpose of this study was to evaluate the neuroprotective potential of BM against SH-SY5Y neuroblastoma cell death induced by the pro-oxidant insult, tert-Butyl hydroperoxide (TBHP), and to identify possible mechanisms related to its neuroprotective action.
Methods
The neuroprotective effect of BM was evaluated by the degree of protection against TBHP-induced cell death in human SH-SY5Y cells that was measured by calcein-AM assay. ERK1/2 and Akt phosphorylation was evaluated by immunoblotting.
Results
We found that BM exhibited protection against TBHP-mediated cytotoxicity. The neuroprotective effect of BM was abolished in the presence of either ERK1/2 or PI3K inhibitors. In addition, western blotting with anti-phospho-ERK1/2 and anti-phospho-Akt antibodies showed that BM increased both ERK1/2 and Akt phosphorylation.
Conclusion
These results suggest that BM by activation of ERK/MAPK and PI3K/Akt signaling pathways protects SH-SY5Y cells from TBHP-induced cell death.
Keywords: Bacopa monnieri, tert-Butyl Hydroperoxide, SH-SY5Y cells, ERK1/2, PI3K
Introduction
Oxidative stress has long been implicated both in the physiological process of aging and in a variety of neurodegenerative diseases, including Alzheimer’s disease1, 2. Reactive oxygen species (ROS) can cause apoptotic cell death through DNA damage, oxidation of proteins, and peroxidation of lipids3. Therefore, therapeutic strategies to prevent ROS-induced cell death might be a useful strategy for the treatment of neurodegenerative diseases associated with oxidative stress.
BM, family Scrophulariaceae, is a small perennial, creeping herb with numerous branches, and grows naturally in wet, shallow water, and marshy areas within tropical regions of the world4. It has been used in Ayurvedic medicine as a nerve tonic for promoting mental health and improving memory and other related brain functions5. Among the specific components believed to contribute to the activity of BM are five saponin glycosides that include bacoside A3, bacopaside II, bacopasaponin X, bacopasaponin C and bacopaside 16–8. Mechanistically, BM has been reported to have anti-inflammatory, anti-depressant and antioxidant effects9–12. Previous studies have suggested that the antioxidant properties of BM are modulated by metal chelation, free radical scavenging, and lipid peroxidation inhibitory activities as well as through enhancement of antioxidant enzymes13, 14. It has been shown to provide neuroprotective effect against oxidative stress inducer, aluminium15, 16. However, it is not clear whether other cellular mechanisms, such as cell signaling events, may be relevant to BM’s protective effects. Therefore, the purpose of this study was to evaluate the neuroprotective potential of BM against SH-SY5Y neuroblastoma cell death induced by the pro-oxidant insult, TBHP, and to identify possible mechanisms related to its neuroprotective action.
Materials and methods
BM extract preparation
BM was collected from the Petchaburi province, Thailand, and was identified by Associate Professor Wongsatit Chuakul, Faculty of Pharmacy, Mahidol University, Thailand. The voucher specimen (Phrompittayarat 001) was kept at the Pharmaceutical Botany Mahidol Herbarium, Mahidol University, Thailand. The aerial part of the Brahmi plant was cut, dried and then roughly powdered. The dried powder was soaked in water for 24 h. The water was then squeezed out and percolated with 95% ethanol. The plant material was extracted again under the same condition. The extracts yielded from the sequential processing were combined and subsequently dried under reduced pressure. The percent yield obtained was 10% of the starting dried material. The extract contained 6.25% (w/w) of total saponins comprising 0.87% bacoside A3, 1.82% bacopaside II, 0.80% bacopasaponin X, 1.73% bacopasaponin C and 1.03% bacopaside I. The total saponin content was determined using high pressure liquid chromatography as previously reported6, 8. The extract was stored at −20°C in an amber bottle until used.
SH-SY5Y neuroblastoma cell culture
The human neuroblastoma, SH-SY5Y cells were obtained from the American Type Culture Collection (ATCC). Cells were grown in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F12 (DMEM/F12; Invitrogen Life Technologies) supplemented with 15% heat-inactivated fetal bovine serum (Atlanta biological), 1% penicillin-streptomycin solution (Cellgro) and cultured at 37°C in humidified incubator with 5% CO2. Cells were plated at a density of 1 × 104 cells per well in 96-well plates, and maintained for 24 h before differentiated with 10 μM retinoic acid (Sigma Aldrich) for 7 days.
Treatment protocol
In order to induce oxidative stress, TBHP was freshly prepared prior to each experiment. BM was prepared in DMSO/ethanol. Treatments were conducted such that the final concentration of either DMSO or ethanol was less than 0.5% which did not affect the cells viability (data not shown). In order to monitor the protective effects of BM, some cells were co-treated with TBHP together with BM. To see the possible pathways related to its protection, cells were treated with BM and TBHP in the presence of ERK 1/2 or PI3K inhibitors. The survival of neurons was determined 24 h after treatment by calcein AM assay.
Cell viability assay
Cell survival was determined by using the calcein AM assay kit (Molecular Probes). Cells were incubated with calcein AM reagents with the concentration at 4 μM for 30 min in CO2 incubator at 37°C. Live cells were estimated by the assessment of calcein fluorescence (a product of cleavage of the calcein-AM ester by ubiquitous intracellular esterases), measured using a plate reader with the fluorescence at 485nm excitation, 535 nm emission wavelengths.
Immunoblotting
Cells were collected and washed with PBS. Cells were lysed with ice-cold lysis buffer containing 50 mM of Tris-HCl pH 7.4, 150 mM of NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, and a protease inhibitor cocktail (Sigma Aldrich). The lysates were incubated on ice for 30 min. and centrifuged at 12,000g for 20 min. Supernatants were collected and followed by protein concentration determination using Bradford Assay. An equal amount of total protein (50–125 μg) were applied to 10% SDS-PAGE, and subsequently transferred onto a polyvinylidene difluoride membrane. The membrane was incubated in blocking buffer (Tris–buffered saline, pH 7.4, 0.2% Tween20 and 5% skim milk) for 4h at 4°C. This was followed by incubation with primary antibodies (Cell Signaling Tech): anti-phospho-ERK1/2 (1:1000), anti-phospho-Akt (1:1000) and anti GAPDH (1:1000) antibodies for overnight at 4°C. The membrane was washed three times for 5 min each using TBST (TBS and 0.05% Tween 20). After that, it was incubated with goat anti-rabbit IgG secondary antibody conjugated to horseradish peroxidase (1:5000, Thermo Scientific) for 2 h at room temperature. Immunoreactivity was detected using enzyme-linked chemiluminescence, and then exposed on Hyperfilm ECL (GE Healthcare). Quantitative assay of antigen expression was based on the density measurements of protein bands, using the Scion Image program.
Statistical analysis
Results are presented as bar graphs depicting the mean ± standard error of the mean (S.E.M.) of at least six independent experiments, using GraphPad Prism software (San Diego, CA). The data were analyzed using a one-way analysis of variance (ANOVA). Individual group differences were assessed using Bonferroni correction for post-hoc analyses. Differences were considered to be significant when p values were less than 0.05.
Results
TBHP-induced cytotoxicity in SH-SY5Y cells
In order to establish an effective concentration of TBHP to subsequently evaluate the protective effects of BM, we first conducted a concentration-response analysis for the effects of TBHP on the viability of neuroblastoma SH-SY5Y cells. Cells were exposed with TBHP (50–200 μM) for 24 h and cell viability was assessed using the calcein AM assay. TBHP decreased cell viability in a concentration-dependent manner (Figure 1A). Exposure of 100 and 200 μM of TBHP significantly reduced the survival to 66.61 ± 4.82% and 15.86 ± 1.76% of control, respectively (p less than 0.001). Based on these results, we used the 100 μM concentration as a mild-to-moderate insult (approximately 30–40% of dead cells) to assess the protective effects of BM in subsequent studies.
Figure 1.
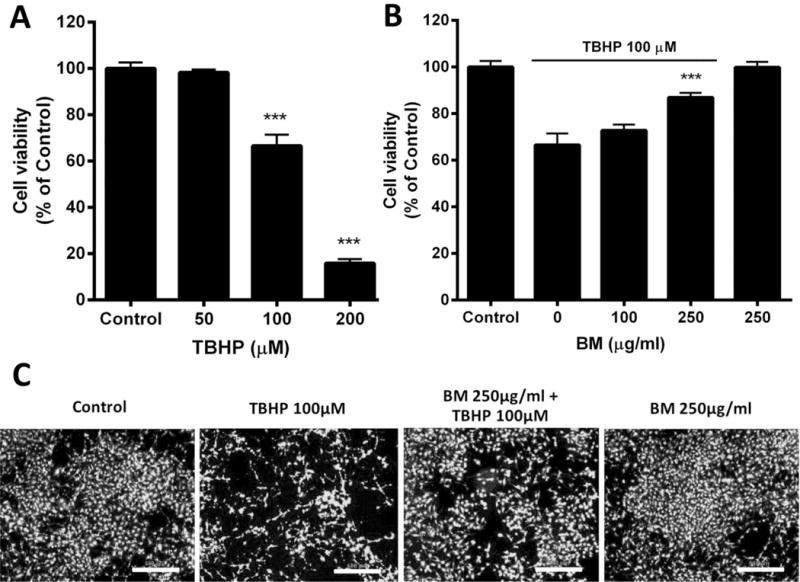
Neuroprotective effects of BM against TBHP toxicity in SH-SY5Y cells. (A) Concentration-dependent effect of TBHP on cell survival in SH-SY5Y cells. Cells were exposed to different concentrations of TBHP for 24 h. Cell viability was assessed using the calcein AM assay. Vehicle treated cells served as the control. *** p < 0.001 as compared to Control; n=7. (B) Neuroprotective effects of BM on TBHP-induced cytotoxicity in SH-SY5Y cells. Cells were treated with 100 μM TBHP for 24 h. Some cells were treated with 100 and 250 μg/ml BM together with 100 μM TBHP. *** p < 0.001 as compared to TBHP alone; n=7. (C) Microscope-assisted visualization of SH-SY5Y cell viability. Bright fluorescent signal indicated cell survival. Scale bar = 500 μm
BM rescued SH-SY5Y cells from TBHP-induced cell death
In order to assess the protective effects of BM, we co-applied increasing concentrations of BM of 100 and 250 μg/ml along with TBHP. As illustrated in Figure 1B, TBHP (100 μM for 24 h) treatment decreased cell survival to 66.61±4.82%. Co-application with BM (250μg/ml) significantly prevented cell death, restoring cell survival to 86.96±1.93% (p less than 0.001). BM by itself, at the highest concentration tested (250 μg/ml) did not cause any apparent neurotoxicity. Microscopy-assisted evaluation of cell viability confirmed that BM (250 μg/ml) was protective against TBHP (Figure 1C). Based on these data, we selected the 250 μg/ml concentrations of BM to test in subsequent experiments.
BM-mediated protective action involves extracellular signal regulated kinase 1 and 2 (ERK1/2) activation
In the nervous system, the ERK/MAPK signaling pathway is critical for neuronal differentiation, plasticity and survival17–21. U0126, an inhibitor of the ERK1/2 signaling pathway, was used to determine whether the protective effect of BM was mediated by this signaling pathway. Treatment with U0126 (10μM) abolished BM-mediated protection against TBHP-induced cell death (Figure 2). Cell viability was significantly decreased from 76.51±2.25% to 58.79±2.66% with 10 μM of U0126 (p less than 0.001). In contrast, U0124 (10μM), the inactive analog of U0126, had no effect on the BM-induced protection against TBHP-induced neurotoxicity. Moreover, U0126 by itself did not cause any apparent neurotoxicity.
Figure 2.
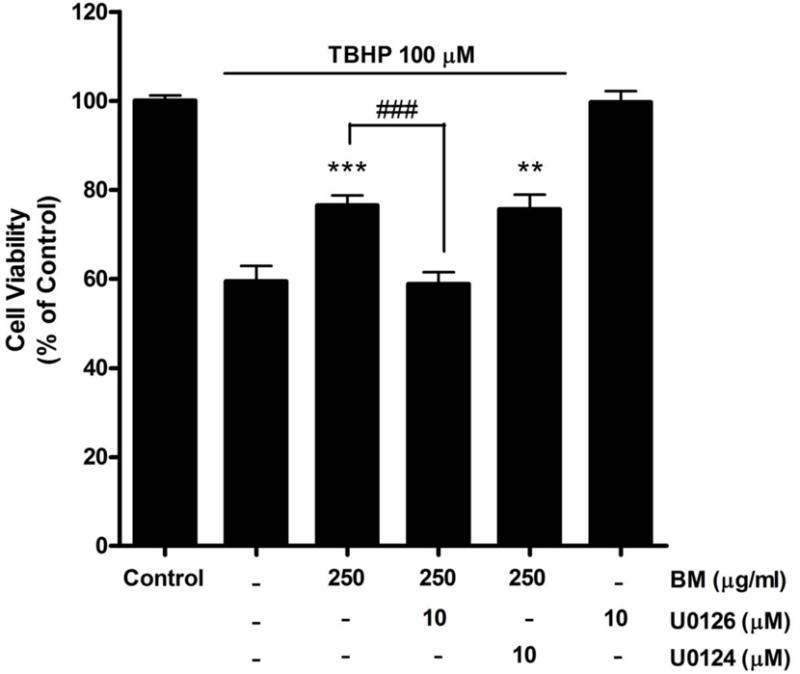
Effect of the ERK 1/2 inhibitor, U0126, on the neuroprotective effect of BM in SH-SY5Y cells. Application of U0126 attenuated the neuroprotective effect of BM. U0124, the inactive analog of U0126 and serving as a negative control, had no effect on the neuroprotection. Cell viability was assessed using the calcein AM assay. ** p < 0.01, *** p < 0.001 as compared to TBHP alone. ### p < 0.001 as compared to TBHP + BM; n=8.
The protective effects of BM are dependent on the Phosphatidylinositol-3 kinase (PI3K) pathway
The PI3K/Akt signaling pathway has also been reported to play an important role in the promotion of cell survival and the suppression apoptosis17, 22–24. We used the PI3K inhibitor, LY294002, to determine whether the PI3K pathway was involved in the effect of BM. Treatment with LY294002 abolished BM-mediated protection against TBHP-induced cell death (Figure 3). Cell viability was significantly decreased from 78.27±1.60% to 64.74±2.95% with 15 μM of LY294002 (p less than 0.001). In addition, LY294002 by itself did not cause any apparent neurotoxicity.
Figure 3.
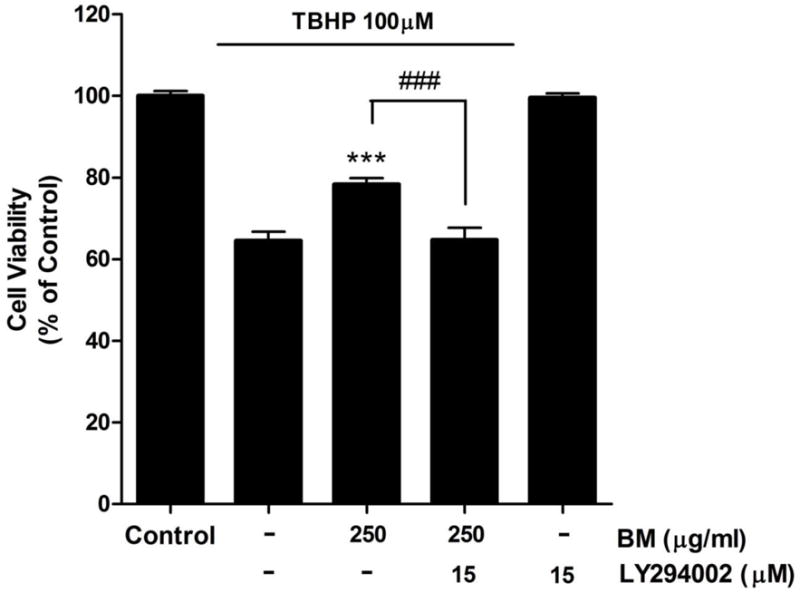
Effect of the PI3K inhibitor, LY294002, on the neuroprotective effect of BM in SH-SY5Y cells. Application of LY294002 attenuated the neuroprotective effect of BM. Cell viability was assessed using the calcein AM assay. *** p < 0.001 as compared to TBHP alone. ### p < 0.001 as compared to TBHP + BM; n=7.
BM increased the amount of both ERK1/2 and Akt phosphorylation
From our results, we found that protective effects of BM might be dependent on mechanisms of ERK1/2 and PI3K activation. In order to confirm that mechanisms are related to its protective action, we evaluated ERK1/2 and PI3K activities during BM treatment by western blotting. We used a phospho-ERK1/2(Thr202/Tyr204) antibody to monitor ERK1/2 activity. In addition, we used a phospho-Akt (Ser473) antibody to monitor PI3K activity because phosphorylation of Akt at Ser473 is required for its full activation. Figure 4 shows that application of BM (250 μg/ml) significantly increased phosphorylation of both ERK1/2 and Akt after treatment for 30 min. These results are consistent with the notion that BM-mediated protective action involves ERK1/2 and PI3K/Akt activation.
Figure 4.
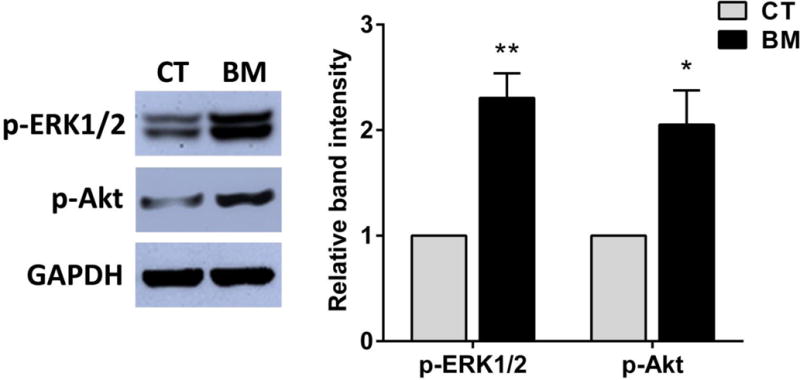
Effects of BM on the activation of ERK1/2 and Akt phosphorylation in SH-SY5Y cells. Cells were treated with 250 μg/ml of BM for 30 min. Protein expressions were analyzed by immunoblotting with antibodies specific to phospho-ERK1/2(p-ERK1/2), phospho-Akt (p-Akt) and GAPDH. The relative band intensities for p-ERK1/2 and p-Akt were determined by normalizing against GAPDH. Results were presented as mean±SEM of three determinations in a bar chart. * p < 0.05, ** p < 0.01 as compared to control. CT=control group, BM=BM treated group.
Discussion
Since the brain is very metabolically active, its high level of oxygen consumption and unique composition of membranes, which contain a large amount of oxidant-sensitive polyunsaturated fatty acids, make it particularly susceptible to free-radical damage25. Oxidative stress is a major cause of cellular injuries in a variety of neurodegenerative disorders1, 2. Therefore, several studies have been conducted in search for natural products with antioxidant and thus, neuroprotective potential. Because BM has received much attention based on its reported anti-oxidant properties in brain7, 15, 16, the present study was designed to investigate the potential for the BM extract as a neuroprotectant. Specifically, the studies conducted addressed whether an extract from BM plant can protect SH-SY5Y neuroblastoma cells from TBHP neurotoxicity and further, to determine the potential mechanism underlying its effects. The human neuroblastoma SH-SY5Y cell line is widely used as model cell system for studying neuronal cell death induced by oxidative stress26–28. In this study, we found that treatment of SH-SY5Y cells with BM at the concentration of 250 μg/ml protected these cells against TBHP-induced cytotoxicity. At this concentration, some evidences had supported that the extract concentrations higher than 150 μg/ml were almost completely prohibited the generation of lipid peroxide products in primary cortical cells7. Several findings suggest that the neuroprotective activities of BM may be attributed partially to the antioxidant effect of the bacoside A, an active ingradient29. BM could exert a neuroprotective effect that relieves neuronal oxidative stress, which might in turn contribute to neuronal apoptosis.
We explored whether alternative mechanisms may also be recruited in the protective effect of BM against TBHP-induced cell death. In this study, we found that treatment with U0126, an inhibitor of the ERK1/2, abolished the protective effect of BM indicating that ERK1/2 pathway is involved in BM-mediated protection of SH-SY5Y cells against TBHP-induced neurotoxicity. The hypothesis was confirmed by the observation that ERK1/2 phosphorylation was increased after application of BM. ERK1/2 has been known to be involved in cell survival17, 19–21. The survival activities mediated by ERK1/2 include the capacity to induce the activation of transcription factors that, in turn, stimulate the expression of various anti-apoptotic proteins. Furthermore, ERK1/2 can also directly affect several cell death/survival regulators20. For instance, the ERK signaling pathway can inhibit the pro-apoptotic protein BAD in addition to inducing the expression of pro-survival genes30. While our data certainly supports the involvement of the ERK1/2 pathway, we must exercise some caution since U0126 has been reported to inhibit not only ERK1/2 but also ERK5 signaling pathway as well31, 32. Both ERK1/2 and ERK5 belong to the family of mitogen-activated protein kinases (MAPKs). Indeed, ERK5 signaling has been associated with the promotion of cell survival33–35. However, there are important differences in the potential role of these two signaling pathways, as it relates to regulating cellular mechanisms associated with cell viability. For example, ERK1/2 and ERK5 signaling pathways have been reported to regulate the transcription of brain-derived neurotrophic factor (BDNF) differentially. ERK1/2 signaling can induce BDNF expression, whereas the ERK5 pathway appears to be an inhibitory regulator of BDNF gene expression32. Since BDNF, like the other neurotrophins, plays an important role in the development, differentiation, and survival of neuronal and non-neuronal cells36–39, it is possible that BM exerts its protective effects via the regulation of BDNF and its associated signaling pathways as well.
Another important signaling pathway linked to the promotion of cell viability is the PI3K/Akt signaling pathway. PI3K is serine/threonine protein kinases that play critical roles in neuronal growth, differentiation and survival24. In general, activation of the PI3K/Akt signaling pathway suppresses apoptosis and promotes cell survival in cultured neurons from the peripheral23 and central nervous systems22. The activation of PI3K leads to phosphorylation and activation of Akt which promotes cell survival by enhancing the expression of anti-apoptotic proteins and inhibiting the activity of pro-apoptotic proteins. Phosphorylated Akt directly inhibits the apoptotic machinery at sites both upstream, Bcl-2 family member BAD40 and downstream, caspase-941 of mitochondrial cytochrome c release. Moreover, an activated Akt also phosphorylates and inactivates FKHRL1, a member of the family of Forkhead transcriptional regulators, which when inactivated, is unable to induce the expression of death genes42. Our study found that treatment of SH-SY5Y cells with LY294002, the inhibitor of PI3K, attenuated the protective effects of BM against TBHP neurotoxicity and Akt phosphorylation was increased after BM application implicating the PI3K/Akt pathway in the protective effects of BM.
Collectively, our data support the neuroprotective effects of BM against TBHP-induced cell death in differentiated SH-SY5Y cells. We determined that mechanisms, such as the recruitment of the ERK/MAPK and the PI3K signaling pathways, may be involved in neuroprotective effect of BM. Importantly, BM by itself was not cytotoxic at any of the concentrations tested, suggesting that consumption of BM may be safe. These studies support the potential of BM as a therapeutic to prevent neuronal dysfunction and/or death associated with age or age-associated diseases such as Alzheimer’s disease wherein oxidative stress plays an important role.
Acknowledgments
This research was supported by grants from the Commission on Higher Education, Thailand under the program Strategic Scholarships for Frontier Research Network for the Join Ph.D. Program Thai Doctoral degree, and grants from the National Institutes of Health (AG022550 and AG027956), USA.
Footnotes
Conflict-of-Interest Notification: There is no conflict of interests regarding the publication of this paper.
References
- 1.Simonian NA, Coyle JT. Oxidative Stress in Neurodegenerative Diseases. Annu Rev Pharmacol Toxicol. 1996 Apr;36(1):83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 2.Gorman AM, McGowan A, O’Neill C, Cotter T. Oxidative stress and apoptosis in neurodegeneration. J Neurol Sci. 1996 Aug;139(Supplement 0):45–52. doi: 10.1016/0022-510x(96)00097-4. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species Its mechanism and measurement in mammalian systems. FEBS Lett. 1991 Apr;281(1–2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 4.Chopra RN, Nayar SL, Chopra IC, Asolkar LV, Kakkar KK, Chakre OJ, Varma BS. Glossary of Indian medicinal plants. New Delhi: Council of Scientific & Industrial Research; 1956. [Google Scholar]
- 5.Singh HK, Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi) Indian J Pharmacol. 1997;29(5):359–65. [Google Scholar]
- 6.Phrompittayarat W, Putalun W, Tanaka H, Wittaya-Areekul S, Jetiyanon K, Ingkaninan K. An enzyme-linked immunosorbant assay using polyclonal antibodies against bacopaside I. Anal Chim Acta. 2007 Feb;584(1):1–6. doi: 10.1016/j.aca.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Limpeanchob N, Jaipan S, Rattanakaruna S, Phrompittayarat W, Ingkaninan K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008 Oct;120(1):112–7. doi: 10.1016/j.jep.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 8.Phrompittayarat W, Putalun W, Tanaka H, Jetiyanon K, Wittaya-Areekul S, Ingkaninan K. Determination of pseudojujubogenin glycosides from Brahmi based on immunoassay using a monoclonal antibody against bacopaside I. Phytochem Analysis. 2007 Sep;18(5):411–8. doi: 10.1002/pca.996. [DOI] [PubMed] [Google Scholar]
- 9.Sairam K, Dorababu M, Goel RK, Bhattacharya SK. Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine. 2002;9(3):207–11. doi: 10.1078/0944-7113-00116. [DOI] [PubMed] [Google Scholar]
- 10.Russo A, Borrelli F, Campisi A, Acquaviva R, Raciti G, Vanella A. Nitric oxide-related toxicity in cultured astrocytes: effect of Bacopa monniera. Life Sci. 2003 Aug;73(12):1517–26. doi: 10.1016/s0024-3205(03)00476-4. [DOI] [PubMed] [Google Scholar]
- 11.Russo A, Izzo AA, Borrelli F, Renis M, Vanella A. Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother Res. 2003 Sep;17(8):870–5. doi: 10.1002/ptr.1061. [DOI] [PubMed] [Google Scholar]
- 12.Channa S, Dar A, Anjum S, Yaqoob M, Atta ur R. Anti-inflammatory activity of Bacopa monniera in rodents. J Ethnopharmacol. 2006 Mar;104(1–2):286–9. doi: 10.1016/j.jep.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000 May;14(3):174–9. doi: 10.1002/(sici)1099-1573(200005)14:3<174::aid-ptr624>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Dhanasekaran M, Tharakan B, Holcomb LA, Hitt AR, Young KA, Manyam BV. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother Res. 2007 Oct;21(10):965–9. doi: 10.1002/ptr.2195. [DOI] [PubMed] [Google Scholar]
- 15.Jyoti A, Sethi P, Sharma D. Bacopa monniera prevents from aluminium neurotoxicity in the cerebral cortex of rat brain. J Ethnopharmacol. 2007 Apr;111(1):56–62. doi: 10.1016/j.jep.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 16.Jyoti A, Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. NeuroToxicology. 2006 Jul;27(4):451–7. doi: 10.1016/j.neuro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Hetman M, Xia Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp. 2000;60(4):531–45. doi: 10.55782/ane-2000-1374. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Carballo G, Moreno L, Masia S, Perez P, Barettino D. Activation of the Phosphatidylinositol 3-Kinase/Akt Signaling Pathway by Retinoic Acid Is Required for Neural Differentiation of SH-SY5Y Human Neuroblastoma Cells. J Biol Chem. 2002 Jul;277(28):25297–304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- 19.Hetman M, Hsuan S-L, Habas A, Higgins MJ, Xia Z. ERK1/2 Antagonizes Glycogen Synthase Kinase-3beta-induced Apoptosis in Cortical Neurons. J Biol Chem. 2002 Dec;277(51):49577–84. doi: 10.1074/jbc.M111227200. [DOI] [PubMed] [Google Scholar]
- 20.Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004 Jun;271(11):2050–5. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- 21.Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003 Jul;5(7):647–54. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995 Jul;15(1):89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 23.Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998 Apr;18(8):2933–43. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999 Nov;13(22):2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Gutteridge JMC. Oxygen radicals and the nervous system. Trends Neurosci. 1985;8(0):22–6. [Google Scholar]
- 26.Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007 Jun;564(1–3):18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 27.Chetsawang B, Putthaprasart C, Phansuwan-Pujito P, Govitrapong P. Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: involvement of nuclear factor kappa B, Bax and Bcl-2. J Pineal Res. 2006 Sep;41(2):116–23. doi: 10.1111/j.1600-079X.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 28.Kang SS, Lee JY, Choi YK, Kim GS, Han BH. Neuroprotective effects of flavones on hydrogen peroxide-induced apoptosis in SH-SY5Y neuroblostoma cells. Bioorg Med Chem Lett. 2004 May;14(9):2261–4. doi: 10.1016/j.bmcl.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Anbarasi K, Vani G, Balakrishna K, Devi CSS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006 Feb;78(12):1378–84. doi: 10.1016/j.lfs.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999 Nov;286(5443):1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 31.Suzaki Y, Yoshizumi M, Kagami S, Nishiyama A, Ozawa Y, Kyaw M, Izawa Y, Kanematsu Y, Tsuchiya K, Tamaki T. BMK1 is activated in glomeruli of diabetic rats and in mesangial cells by high glucose conditions. Kidney Int. 2004 May;65(5):1749–60. doi: 10.1111/j.1523-1755.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 32.Su C, Underwood W, Rybalchenko N, Singh M. ERK1/2 and ERK5 have distinct roles in the regulation of brain-derived neurotrophic factor expression. J Neurosci Res. 2011 Oct;89(10):1542–50. doi: 10.1002/jnr.22683. [DOI] [PubMed] [Google Scholar]
- 33.Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001 Oct;4(10):981–8. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 34.Cavanaugh JE. Role of extracellular signal regulated kinase 5 in neuronal survival. Eur J Biochem. 2004 Jun;271(11):2056–9. doi: 10.1111/j.1432-1033.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- 35.Finegan KG, Wang X, Lee E-J, Robinson AC, Tournier C. Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ. 2009 May;16(5):674–83. doi: 10.1038/cdd.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conover JC, Yancopoulos GD. Neurotrophin regulation of the developing nervous system: analyses of knockout mice. Rev Neurosci. 1997 Jan-Mar;8(1):13–27. doi: 10.1515/revneuro.1997.8.1.13. [DOI] [PubMed] [Google Scholar]
- 37.Li X-C, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc Natl Acad Sci. 2000 Jul;97(15):8584–9. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001 Mar;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008 Nov;59(1):201–20. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell. 1997 Oct;91(2):231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 41.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of Cell Death Protease Caspase-9 by Phosphorylation. Science. 1998 Nov;282(5392):1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 42.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell. 1999 Mar;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]


