Abstract
Background
Prematurity is often complicated by respiratory support, including invasive mechanical ventilation (IMV) and non-invasive support (NIS). Compared to IMV, NIS reduces injury to the lung and brain. Prematurity may also disrupt glomerular architecture. Whether NIS differentially affects glomerular architecture is incompletely understood. We hypothesized that IMV would lead to greater disruption of glomerular architecture than NIS.
Methods
This is a secondary analysis of kidneys from moderately preterm lambs delivered at ∼131d gestation (term ∼150d) that had antenatal steroid exposure and surfactant treatment before resuscitation by IMV. At ∼3h of age, half of the lambs were switched to NIS. Support was for 3d or 21d. Structural indices of glomerular architecture were quantified.
Results
The number of glomerular generations was unaffected by moderate preterm birth and respiratory support, either IMV or NIS. At 3d and 21d of IMV or NIS, glomerular capillary surface density was not different. Glomerular capillary surface density was significantly lower in the inner and outer cortex compared to unventilated gestation age-matched or postnatal age-matched reference lambs.
Conclusion
Moderate preterm birth and invasive or non-invasive respiratory support decreases glomerular capillarisation in the lamb kidney. This adverse effect on glomerular development may contribute to increased risk for adult-onset hypertension and renal dysfunction.
Introduction
Although the absolute number of nephrons appears to be in place by around 36 weeks gestation in the kidney of the human fetus, maturation of nephrons is only about 60% complete 1-4. After preterm birth, the immaturity of the kidneys makes them vulnerable to perinatal and postnatal stresses that may disrupt kidney development. Increasing evidence of prematurity as a risk factor for impaired renal function and hypertension with onset early in childhood 5,6 suggests that altered postnatal renal maturation has a long-term impact on renal health.
Management of premature infants is often complicated by the need for either invasive or non-invasive respiratory support for respiratory distress 7. Invasive support entails endotracheal intubation with mechanical ventilation (IMV), whereas non-invasive support provides respiratory gases via the nose/pharynx of spontaneously breathing neonates. Acute lung injury during IMV in adults is associated with acute kidney injury, and various animal and human studies provide evidence of the interplay between IMV and renal function8,9. The effects of IMV on the immature kidney are incompletely understood. An autopsy study of premature human infants showed ongoing, but altered postnatal glomerulogenesis and glomerular maturation10. However, the causes of illness and death varied, not all of the preterm infants had respiratory failure, and others had intrauterine growth restriction. Therefore, the impact of IMV per se could not be determined. Analysis of the kidney of premature baboons supported by IMV for up to 21 days (d) revealed that glomerulogenesis continued postnatally, but postnatal glomerular development was disrupted11. Both studies examined subjects born before the completion of glomerulogenesis and may demonstrate effects that are different in preterm subjects born around or after the time when all glomeruli are formed. Both studies also reveal that vulnerability of glomeruli is greater in the outer cortex than the inner cortex, a finding that matches the centrifugal wave of glomerulogenesis12.
Non-invasive respiratory support (NIS) is widely used for preterm infants. Together with other measures such as antenatal steroid exposure, surfactant replacement therapy, and caffeine administration, NIS is associated with less bronchopulmonary dysplasia (BPD) compared to IMV13. Evidence from preterm lambs and preterm baboons shows that NIS, compared to IMV, not only reduces injury to the lung, but also the brain14-16. Whether IMV and NIS have differential effects on glomerular development is not known.
The aim of this study is to identify differential effects of IMV versus NIS on glomerular architecture in the kidneys of preterm lambs. Our study is a secondary analysis of fixed kidneys from moderately preterm lambs (∼131d gestation; term ∼150d) that were supported by either IMV or NIS for 3d or 21d for the purpose of identifying effects of mode of respiratory support on alveolarization in the immature lung 17,18. For the present study, we quantified glomerular generations, glomerular capillary surface density (Svgc), and glomerular surface area. We hypothesized that IMV would lead to greater disruption of glomerular architecture than NIS. Our results suggest that glomerular development is vulnerable to disruption by either invasive or non-invasive respiratory support.
Results
Demographic characteristics of preterm lamb 3d and 21d groups
Gestational age at delivery was ∼131d for the 3d groups of preterm lambs (Table 1). Males made up to half of the lamb groups. Birth weight and ending weight were not different among the groups.
Table 1. Demographic characteristics of preterm lambs for 3 d ventilation studies, compared with unventilated fetal reference lambs at matched gestational ages.
| Fetal reference | Preterm 3 d | Fetal reference | ||
|---|---|---|---|---|
|
|
|
|
||
| F130 | IMV | NIS | F136 | |
| Sex (Male:Female) | 2 : 6 | 4 : 4 | 3 : 5 | 4 : 4 |
| Gestational age (d) | 131 ± 1 | 131 ± 1 | 133 ± 2 | 136 ± 1 |
| Weight at delivery (kg) | 3.1 ± 0.8 | 3.7 ± 0.8 | 4.8 ± 1.1 | 4.0 ± 1.4 |
| Weight at study end (kg) | 3.1 ± 0.8 | 3.3 ± 0.9 | 4.3 ± 1.1 | 4.0 ± 1.4 |
(mean ± SD; n = 8/group). F130 = fetal age 130 d. F136 = fetal age 136 d. Fetal ages of reference lambs match gestational ages of preterm ventilated lambs at beginning and end of the study, respectively,
IMV, invasive mechanical ventilation; NIS, noninvasive support.
For the 21d preterm lambs, gestational age at delivery was ∼132d (Table 2). Males made up one-third of the 21d group. Birth weight was not different among groups. Ending weight was not different between the preterm lamb groups, although the change in weight from birth to the end of 21d was 0 Kg for the IMV group and +0.8 Kg for the NIS group. As expected, the T21d reference group weighed more than the preterm lambs.
Table 2. Demographic characteristics of preterm lambs for 21 d ventilation studies, compared with unventilated fetal and term reference lambs at matched gestational and chronological age.
| Fetal reference | Preterm 21 d | Term Reference (∼150 d) | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| F130 | IMV | NIS | T | T21d | |
| Sex (Male : Female) | 2 : 6 | 2 : 6 | 2 : 6 | 5 : 3 | 5 : 3 |
| Gestational age (d) | 131 ± 1 | 132 ± 2 | 132 ± 1 | ||
| Weight at delivery (kg) | 3.1 ± 0.8 | 4.1 ± 0.8 | 4.0 ± 0.8 | 5.3 ± 1.7 | 4.8 ± 0.4 |
| Weight at study end (kg) | 3.1 ± 0.8 | 4.4 ± 0.6 | 4.8 ± 1.2 | 5.3 ± 1.7 | 14.5 ± 3.8* |
(mean ± SD; n = 8/group).
F130 = fetal age 130 d, matching gestational age of preterm ventilated lambs at beginning of the study. T = unventilated term born lamb on day 1 of life, matching gestational age at end of preterm ventilation study. T21d = unventilated term born lamb on day 21 of life, matching chronological age at end of preterm ventilation study.
IMV, invasive mechanical ventilation; NIS, noninvasive support; T, term.
P < 0.05compared with matched preterm ventilated lambs.
Physiological parameters for 3d and 21d preterm lamb groups
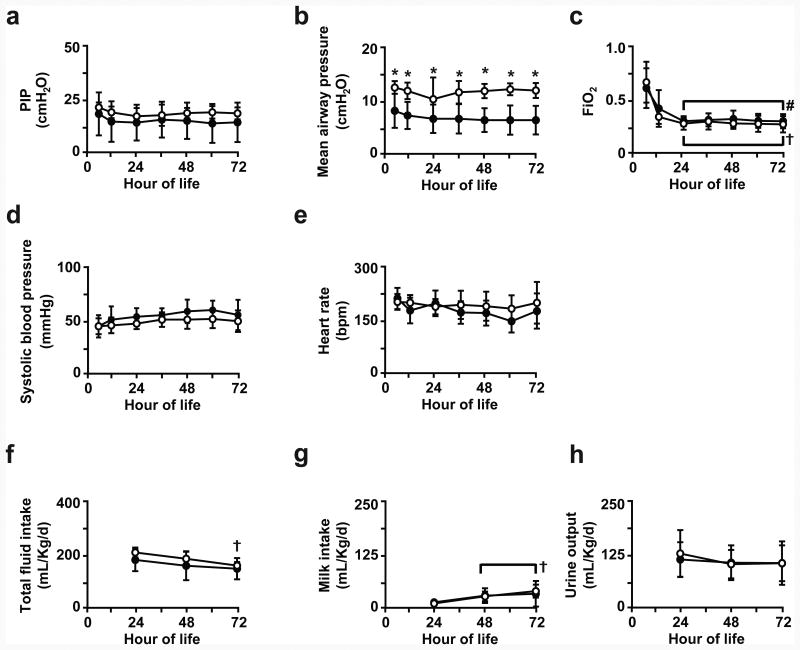
For the 3d preterm groups (Figure 1), PIP setting at the ventilator was similar for the IMV and NIS groups. Mean airway pressure setting at the ventilator was significantly higher for the IMV group compared to the NIS group. PEEP was kept constant at 8 cmH2O for both groups (shown in Supplemental Material). FiO2 setting at the ventilator was not different between the two groups and decreased during the first 24h of life. These ventilator settings kept arterial blood pH, PaCO2, and PaO2 within target range (shown in Supplemental Material). Systolic blood pressure and heart rate were not different between the two groups. Total fluid intake (intravenous plus enteral) and enteral milk intake also were not different between the two groups. Urine output, and plasma sodium and glucose levels (Supplemental Material) also were not different between the IMV and NIS groups.
Figure 1. Physiological parameters for 3d preterm lamb groups.
Parameters measured at 12h intervals for (a) peak inspiratory pressure (PIP), (b) mean airway pressure, (c) fractional inspired oxygen (FiO2), (d) systolic blood pressure, (e) heart rate, (f) total fluid intake (intravenous plus enteral), (g) enteral milk intake, and (h) urine output for preterm lambs that were supported by invasive mechanical ventilation (IMV; white circles) or non-invasive support (NIS; black circles) over the first 72h of life. Between-groups comparisons at indicated hour-of-life by unpaired t-test (*p<0.05 compared to NIS group, and within-group by ANOVA and Dunnett's post-hoc test († p<0.05 compared to d1 IMV; # p<0.05 compared to d1 NIS).
We did not measure creatinine, BUN, or glomerular filtration rate because this study was secondary to respiratory gas exchange and alveolarization assessments.
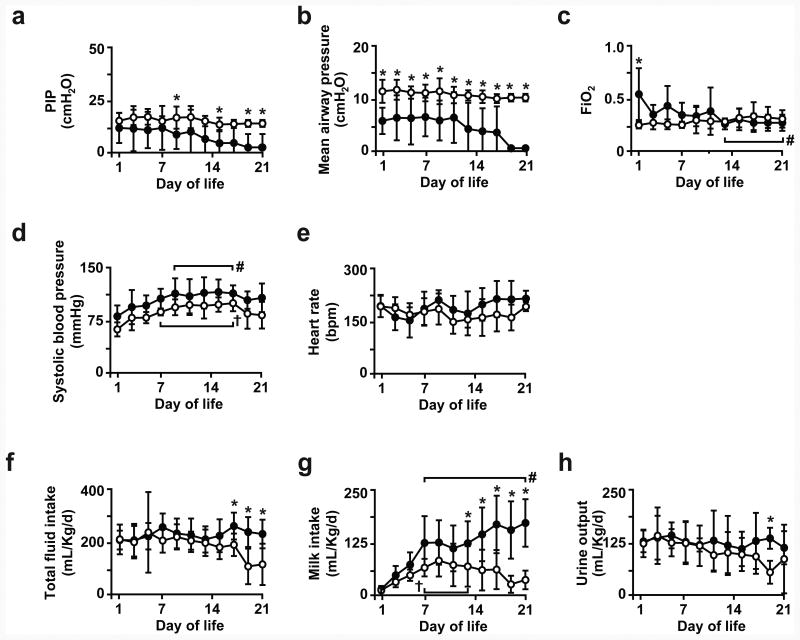
For the 21d preterm groups (Figure 2), PIP and mean airway pressure settings at the ventilator remained constant for the IMV group, whereas PIP and mean airway pressure settings at the ventilator progressively declined for the NIS group. PEEP was kept constant at 8 cmH2O for both groups (Supplemental Material). FiO2 setting at the ventilator was significantly lower for the IMV group on the first day of life compared to the NIS group. Thereafter, FiO2 setting at the ventilator was not different between both groups. Arterial blood gas values (pH, PaO2, PaCO2) were not different and within target range for both groups (Supplemental Material). No differences were detected for systolic blood pressure and heart rate between the two groups. Systolic blood pressure peaked during the second week of life for both groups. Total fluid intake (intravenous plus enteral) and enteral milk intake progressively increased for the NIS group, whereas both intakes decreased for the IMV group. Total fluid intake and enteral milk intake were constant and not different during the first 14d of life for both groups. Both intakes decreased significantly during the third week of life for the IMV group, whereas both intakes increased during the same period for the NIS group. Urine output was not different between the two groups, with an exception of lower urine output on day of life 19 for the IMV group (p<0.05). Plasma sodium and glucose levels were not different between the IMV and NIS groups during the 21d study period (Supplemental Material).
Figure 2. Physiological parameters for 21d preterm lamb groups.
Parameters measured at 24h intervals for (a) peak inspiratory pressure (PIP), (b) mean airway pressure, (c) fractional inspired oxygen (FiO2), (d) systolic blood pressure, (e) heart rate, (f) total fluid intake (intravenous plus enteral), (g) enteral milk intake, and (h) urine output for preterm lambs that were supported by invasive mechanical ventilation (IMV; white circles) or non-invasive support (NIS; black circles) over 21d of life. Between-groups comparisons at indicated day-of-life by unpaired t-test (*p<0.05 compared to NIS group), and within-group by ANOVA and Dunnett's post-hoc test († p<0.05 compared to d1 IMV; # p<0.05 compared to d1 NIS).
Indices of glomerular number and maturation for 3d and 21d preterm lamb groups
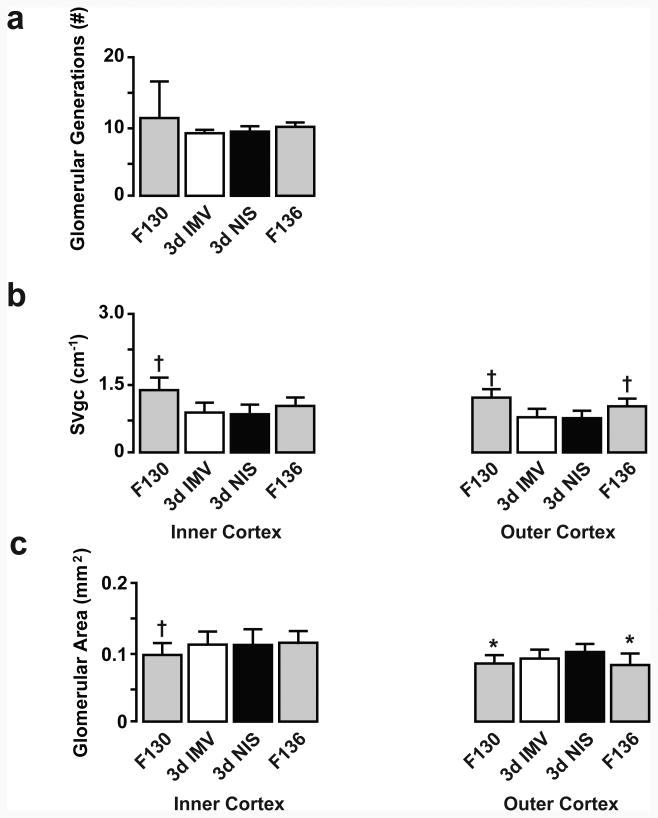
Preterm lambs that were supported by IMV or NIS for 3d did not have differences in the number of glomerular generations (Figure 3). We saw no histological evidence of immature or shrunken glomeruli in either the inner or outer cortex. Immunostaining of glomerular capillaries is shown in Figure 4. We used the immunostained sections for glomerular capillary surface density (SVgc) measurements. SVgc and glomerular surface area were not different between the IMV and NIS groups, regardless of cortical region.
Figure 3. Morphometric results for glomerular architecture for 3d preterm lamb groups.
Glomerular generations (a), glomerular capillary surface density (b), and glomerular surface area (c) were not different between the invasive mechanical ventilation (IMV; white bar) and non-invasive support (NIS; black bar) groups. For normal developmental reference, unventilated, untreated fetal (F) lambs bracket the delivery age in days (F130; gray bar) and ending age (F136; gray bar) of the preterm lambs. Between-groups comparisons by ANOVA and Fishers PLSD post-hoc test († p<0.05 compared to IMV and NIS; * p<0.05 compared to NIS).
Figure 4. Immunohistochemical localization of CD-31 to identify glomerular capillaries.
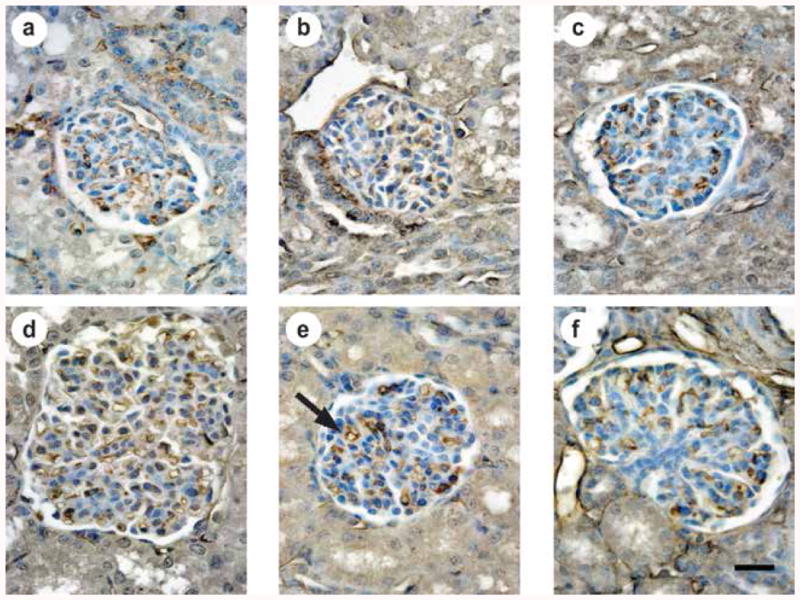
Anti-CD-31 antibody provided uniform immumolocalization of glomerular capillary endothelial cells (brown color; black arrow in e). Images are representative of the morphometric results shown in Figures 3 and 5. (a) Unventilated, untreated fetal lamb at 136d gestation (F136), (b) preterm lamb at 3d IMV, (c) preterm lamb at 3d NIS, (d) unventilated, untreated term 21d-old lamb (T21d), (e) preterm lamb at 21d IMV, (f) preterm lamb at 21d NIS. The scale bar is 20 micrometers in length.
Figure 3 includes results for normal development for contrast with the results for the preterm lamb groups. Glomerular generations were not different among the F130 and F136 reference groups and preterm lamb groups. SVgc was significantly greater for glomeruli located in the inner cortex of the F130 reference group compared to both preterm lamb groups. SVgc was significantly greater for the outer cortex of the F130 and F136 reference groups compared to both preterm lamb groups. Glomerular surface area was significantly less for glomeruli located in the inner cortex of the F130 reference group compared to preterm lambs supported by IMV and NIS. Glomerular surface area was significantly less for the outer cortex of the F130 and F136 reference groups compared to the NIS preterm lamb group.
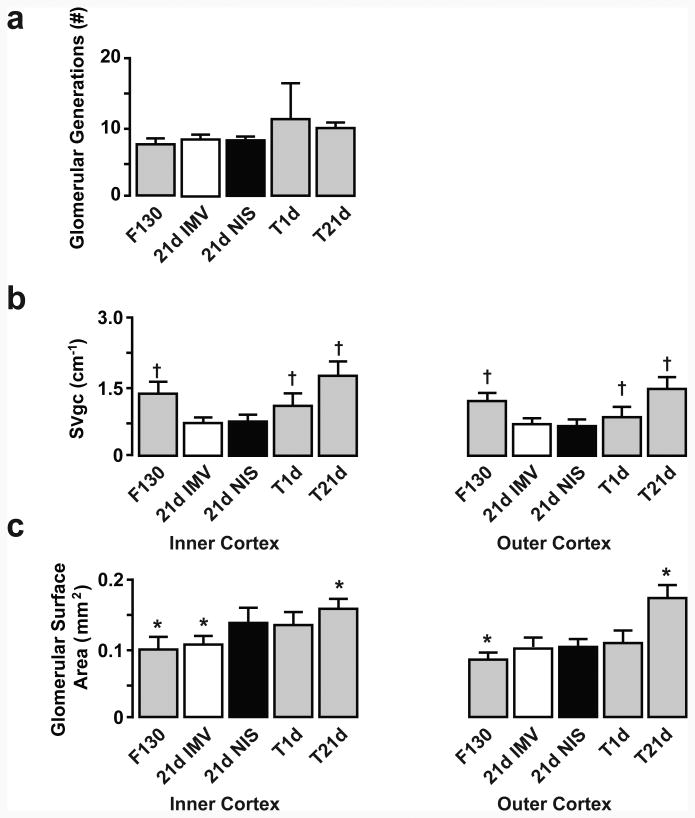
Preterm lambs that were supported by IMV or NIS for 21d also did not have differences in the number of glomerular generations (Figures 4 and 5). SVgc was not different between the IMV and NIS groups, regardless of cortical region. Glomerular surface area was significantly less for glomeruli in the inner cortex of the IMV group compared to the NIS group. For the outer cortex, glomerular surface density was not different between the IMV and NIS groups.
Figure 5. Morphometric results for glomerulogenesis and glomerular architecture for 21d preterm lambs.
Glomerular generations (a), glomerular capillary surface density (b), and glomerular surface area (c) were not different between the invasive mechanical ventilation (IMV; white bar) and non-invasive support (NIS; black bar) groups. For normal developmental reference, unventilated, untreated fetal lambs match gestational age at delivery (F130) and unventilated, untreated term lambs match gestational age that preterm lambs would have been if not delivered prematurely (T1d) and postnatal age of the preterm lambs (T21d)) .Data assessed between-groups by ANOVA and Fishers PLSD post-hoc test († p<0.05 compared to IMV and NIS; * p<0.05 compared to NIS).
The panels in Figure 5 likewise provide contrasting results for normal development. Glomerular generations were not different among the reference groups and preterm lamb groups. SVgc was significantly greater for glomeruli located in the inner cortex among the F130, T1d and T21d groups compared to preterm lambs supported by IMV or NIS. Glomerular surface area was significantly less for glomeruli located in the inner cortex within the F130 reference group compared to the NIS group of preterm lambs. The 21d reference group had significantly greater glomerular surface area than the NIS group of preterm lambs. Glomerular surface area for the outer cortex was significantly less for the F130 reference group compared to both NIS preterm lamb group. The 21d reference group had significantly greater glomerular surface area than the NIS group of preterm lambs.
Discussion
The principal finding of our study is that moderate preterm birth and 3d or 21d of respiratory support, either invasive mechanical ventilation (IMV) or non-invasive support (NIS), impairs postnatal development of glomerular capillary surface density and glomerular surface area. Glomerular generations were not affected, as expected because the preterm lambs were delivered after glomerulogenesis is completed in sheep. Contrary to our hypothesis, early initiation of NIS after premature birth does not minimize the glomerular impairments compared to IMV. Thus, we conclude that moderate preterm birth and respiratory support, whether invasive or non-invasive, impairs glomerular architecture in preterm lambs. This adverse effect on glomerular maturation may contribute to increased risk for adult-onset hypertension and renal dysfunction.
The rationale for the present study is based on comparison of the mode of respiratory support on lung outcomes for preterm lambs14,15. Briefly, non-invasive respiratory support of preterm lambs improves respiratory gas exchange and alveolarisation in the lung compared to IMV. Better physiological and morphological outcomes for the lung during NIS is also evident in the brain of preterm lambs16 and baboons19. Those findings led to this secondary analysis of the kidneys. The detrimental effect of prematurity accompanied by IMV specifically on glomerular architecture development after completion of glomerulogenesis in this study is consistent with reduced total renal filtration surface and reduced glomerular capillary length during IMV in moderately preterm lambs20. Our study adds the insight that NIS also decreases glomerular architectural development equally in the kidney of moderately premature lambs.
Abnormally shaped glomeruli, fewer glomerular generations, and glomerular hypertrophy are abnormal structural findings in autopsy studies of preterm human infants who died with BPD and necropsy studies of mechanically ventilated preterm baboons 10,11. Our study did not detect abnormally shaped glomeruli or fewer glomerular generations. The species-specific relation between gestational age at delivery and the gestational age at which glomerulogenesis is complete may account for the differences between studies. Glomerulogenesis in humans is completed by ∼36 weeks' gestation 1,10,21 so preterm birth earlier makes glomerulogenesis vulnerable to disruption. Likewise, glomerulogenesis in baboons finishes by ∼175 days' gestation, whereas the preterm baboon model uses delivery ∼50d earlier 22. We deliver preterm lambs ∼10d after glomerulogenesis is completed in sheep (∼120d gestation) 23. Lambs delivered at gestational age earlier than 120d are unlikely to survive despite respiratory and other support, based on our experience24
Our study shows that Svgc is greater in the kidney of normal term lambs that are 3 weeks' old compared to fetal lambs and term lambs that are 1 d old (Figure 5b). Postnatal growth of glomerular capillaries may reflect the rise in functional demand after birth. Vascular resistance falls and renal blood flow increases postnatally with concomitantly greater glomerular filtration rate in the first 24 hours of postnatal life in sheep25 and further increase in glomerular filtration rate during the first weeks and months of postnatal life in lambs26 and humans27. The increased perfusion of the kidneys may trigger lengthening and branching of glomerular capillaries, reflected in increased filtration area. Further studies will be necessary to determine the time-course of normal postnatal glomerular capillary lengthening and branching.
IMV may alter kidney function in adults by reducing renal blood flow. We did not measure renal blood flow, owing to the secondary nature of our study, so it does not provide mechanistic insight. Interestingly, the 21d IMV group of preterm lambs had lower systolic blood pressure (not statistically significant) than the NIS group, which might reduce renal blood flow. Another caveat is that blood pressure is not necessarily proportional to blood flow in preterm subjects28.
Our study was not designed to test independent effects of other factors that may influence glomerular architecture the preterm period, either fetal or postnatal. These include exposure to antenatal steroids, respiratory support with oxygen-rich gas, systemic hypotension, exposure to aminoglycosides, sedatives, intrauterine and extrauterine growth restriction, and fluid and dietary intakes. Antenatal steroid exposure prior to the completion of glomerulogenesis in sheep (∼120d gestation) decreases nephron endowment 29-31. Whether antenatal steroid exposure after 120d gestation in sheep affects glomerular architectural maturation remains to be determined. Oxygen-exposure of preterm neonates, including the preterm lambs reported here, was higher postnatally than in utero and therefore could inhibit vascular endothelial growth factor expression, leading to reduced glomerular capillary development32-34. Systemic hypotension did not occur in the preterm lambs. Also, systolic blood pressure did not differ between the IMV and NIS groups. Our study design minimized potential nephrotoxicity among the 21d ventilated groups of preterm lambs by stopping Gentamicin at 10d of treatment, as is done clinically. All of the preterm lambs, regardless of mode of ventilation, were exposed to antenatal steroid and postnatal aminoglycoside antibiotic, and thus were treated uniformly. This uniform design emulates the neonatal intensive care setting for the majority of preterm human infants.
A potential confounding element of study design is the IMV group which received more sedative (pentobarbital) compared to the NIS group35 to prevent discomfort or pain from the endotracheal tube, and to minimize breathing over the ventilator. Larger amounts of sedative may decrease blood pressure, renal blood flow, and renal vascular regulation36,37, which could affect glomerular architecture. However, glomerular architecture was altered the same in the IMV and NIS groups. Thus, differences in the amount of sedative between IMV and NIS did not affect glomerular architectural.
Total fluid intake (IV fluids plus enteral milk) and enteral milk intake alone were lower in the IMV group of preterm lambs only in the last week of the 21d study compared to the NIS group. This difference was due to feeding intolerance of the IMV group, evident as residual milk in the stomach. Because enteral milk intake diminished over the last 7d, IV fluid administration was increased to sustain total fluid intake (targeted at ∼160 mL/Kg/d). Interestingly, despite greater total intake of fluids for the NIS group during the same period, Svgc was not different compared to the IMV group. Therefore, total fluid intake or enteral milk intake does not appear to influence glomerular architecture in preterm lambs at the end of 21d of respiratory support. This contrasts with findings in human adults that show larger intravenous volume abrogates haemodynamic effects on the kidney.8,9
Our study design included untreated, unventilated fetal lambs and term lambs for normal developmental reference from preterm birth at ∼131d gestation to term-plus-21d postnatal age. To provide this context, none of the reference lambs were exposed to antenatal steroid or postnatal aminoglycoside antibiotic. Consequently, our study did not assess the independent effect of either treatment on normal glomerular architecture.
Our study has methodological limitations because of its secondary design. Measurements of kidney weight or volume were not assessed during the original studies. Also, we used morphometric methods, rather than stereological methods38, to quantify glomerular architecture. We also used paraffin-embedded tissue blocks for the morphometric analyses for economy of time and cost for preparing tissue blocks from multiple organs from the same lambs. Nonetheless, we used random, systematic, and uniform sampling approaches and a cycloid grid39,40. We used the same approaches for all lamb groups, so any effects of analytical approach are common among the groups. Nonetheless, our findings of significantly reduced glomerular capillary surface density represents an important starting point for future stereological assessments of glomerular architecture in ventilated preterm lambs.
We also did not measure renal function, such as creatinine, BUN, or glomerular filtration rate. This limitation is the consequence of secondary assessment after analysis of lung alveolarisation14,15. Without measures of renal function, uncertainty remains about evidence for renal dysfunction in the preterm lamb groups. Indirectly, Figures 1 and 2 show that urine output was normal (typically 1-3 mL/Kg/h) and equivalent between the IMV and NIS groups, as were plasma sodium and glucose levels (in supplemental material). We currently measure serum creatinine and BUN, and urinary microalbumin, with attention to the postnatal adaptive changes of renal function in newborn and preterm ventilated subjects25-27.
We consider prematurity and respiratory support (IMV and NIS) to be two influential factors on glomerular architecture. A new report by our group focused on prematurity as an influential factor by comparing glomerular stereological parameters between preterm and term lambs20. That study provides the insight that regardless of being preterm or term, lambs supported by IMV for 3d have significantly decreased glomerular capillary length and surface area, and reduced total renal filtration surface area. The present study provides a different insight; namely, that the mode of respiratory support, either IMV or NIS for 3d or 21d of preterm lambs does not influence glomerular architecture in the kidney. Together, the two studies are complementary and suggest that the immature kidney is vulnerable to prolonged respiratory support. Mechanisms remain to be identified.
In summary, moderate preterm birth after completion of glomerulogenesis and prolonged respiratory support (invasive or non-invasive) impair glomerular architectural development. These adverse effect during the early period of postnatal life in preterm neonates may increase risk for systemic hypertension and renal dysfunction later in life.
Methods
The protocols adhered to the American Physiological Society/National Institutes for Health guidelines for humane use of animals for research, and were approved by the Institutional Animal Care and Use committee at the University of Utah, Health Sciences Center. Kidney results reported herein are from chronically ventilated preterm lambs and unventilated reference lambs that we reported for evolving neonatal chronic lung disease14,15.
Preterm lamb groups for respiratory support for 3d or 21d
This is a secondary analysis of kidneys from moderately preterm lambs delivered at ∼131d gestation that we studied for lung outcomes17. At the time of the lung study, indices of renal function were not measured, other than urine output. The methods for chronically ventilated preterm lambs are reported14,15. Briefly, time-pregnant ewes at -131d of gestation (term ∼150d) were used. Glomerulogenesis finishes at -120d of gestation in sheep41; therefore, our model of moderately preterm ventilated lambs examined glomerular development after glomeruli are formed. Preterm lambs were exposed to antenatal betamethasone (6 mg/Kg; American Regent Inc., Shirley, NY), perinatal surfactant (240 mg; Curosurf®, Chiesi, Parma, Italy), and postnatal caffeine citrate (loading dose of 15 mg/Kg; Paddock Laboratories Inc., Minneapolis, MN; and daily maintenance dose of 5 mg/Kg). Before, delivery, fetuses were prospectively assigned to one of two modes of respiratory support: (1) invasive mechanical ventilation (IMV) or (2) non-invasive support (NIS). After operative delivery, preterm lambs were supported by IMV (Bird VIP ventilator, Palm Springs, CA), with warmed and humidified gases. Target respiratory gas parameters were PaO2 of 60-80 mmHg, PaCO2 of 45-60 mmHg, and pH of 7.25 to 7.35.
Preterm lambs assigned to NIS were weaned from IMV at 2-3h of postnatal life14,15. An uncuffed Murphy tube (ID 3.0-4.0 mm) was inserted through one nostril, after application of topical lidocaine (2%; Hospira Inc., Lake Forest, IL) to minimize pain and discomfort. When the lambs breathed spontaneously, the endotracheal tube was removed before the nasal tube was attached to a pulsatile Flow Ventilator® (VDR4; Percussionaire Corp, Sandpoint, ID) equipped with a humidifier.
Preterm lambs were ventilated for 3d or 21d. Lambs in the IMV group were sedated with pentobarbital sodium (1-3 mg/Kg per dose, iv; Abbott Laboratories, North Chicago, IL) as needed to minimize discomfort associated with the endotracheal tube. However, midway into the study, injectable pentobarbital sodium became unavailable. Consequently, the protocol was changed to Fentanyl (5 μg/Kg/dose, Hospira Inc., Lake Forest, IL) as needed. Additional sedation was buprenorphine (3 μg/Kg every 12h, iv; Buprenex, Reckitt Benckiser Healthcare, Richmond, VA). Lambs in the NIS group were minimally sedated to permit effective spontaneous breathing (pentobarbital sodium, <1 mg/Kg per dose; Fentanyl <1 μg/Kg/dose). All preterm lambs had dextrose and saline solutions administered (iv). Antibiotics were given daily (100,000 U/Kg potassium penicillin; Pfizer, Groton, CT, and 2.5 mg/Kg gentamicin; Hospira, Lake Forest IL, for the first 10d of the 21d study). Enteral (orogastric) feeding of sheep colostrum began at ∼4h of postnatal life. The volume was advanced as tolerated to 60 Kcal/Kg/day of total energy substrate. For the 21d groups, orogastric feeding was switched to sheep milk on postnatal day of life 4 and continued for 21d. The volume of milk was advanced as tolerated. Target for total energy substrate was 150 Kcal/Kg/day.
Monitoring
During respiratory support, we measured heart rate, blood pressure, fractional inspired oxygen (FiO2), partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide (PaCO2), pH, peak inspiratory pressure (PIP), positive end-expiratory pressure (PEEP), mean airway pressure, oxygen saturation, hematocrit, plasma sodium, and plasma glucose. Daily measurements included weight, enteral colostrum/milk and total (enteral plus intravenous) fluid intake, urine and stool output, rectal temperature, and total and differential white blood cell counts. Because the present assessment of renal development was a secondary analysis, relevant biochemical parameters for renal function (creatinine, BUN, glomerular filtration rate) were not measured and could not be measured retrospectively.
Lamb groups for normal developmental reference
Fetal lambs were delivered at gestational ages of 130d (F130) and 136d (F136), bracketing the gestational age at delivery (131-132d) and age at the end of 3d respiratory support (134-135d), respectively for preterm lambs. None of the ewes for the fetal lambs received prepartum dexamethasone. Fetal lambs were not allowed to breathe before tissue collection. Two groups of term lambs were spontaneously born and left with their mothers for 1d after birth (T1d) or 21d after birth (T21d). The T1d group provided the gestation age-matched reference for preterm lambs that were supported for 21d because preterm lambs were delivered 21d early. The T21d group provided the postnatal age-matched reference for the 21d preterm lamb groups that were supported for 21d. The T1d and T21d groups were fed ad libitum by their mothers.
None of the preterm lambs had, or required, urinary bladder catheters. None of the preterm lambs had structural evidence of urinary tract anomalies or obstruction at necropsy.
Postmortem analyses
Lambs were given 1,000 U heparin sodium (iv; APP Pharmaceuticals, Schaumberg, IL) followed by pentobarbital sodium (60 mg/Kg; Beuthanasia solution, Ovation Pharmaceuticals, Inc., Deerfield, IL). The abdomen was opened and both kidneys were removed. One kidney was cut into uniform slices that were immersed in 10% neutral buffered formalin at 4°C for 24h. Systematic, uniform, random methods were used for unbiased sampling11,42 to obtain paraffin-embedded sections (5 μm thickness).
Immunohistochemistry
We used a polyclonal rabbit anti-sheep CD-31 antibody (optimal dilution 1:500) made in our laboratory as a capillary endothelial cell marker18,43. Glomerular capillaries (as well as other capillaries) were uniformly immunostained brown for measurement of Svgc. lmmunohistochemical staining controls were substitution of the primary antibody with an irrelevant primary antibody (monoclonal mouse anti-actin alpha-smooth muscle antibody, Cat A5225, Sigma-Aldrich, St Louis, MO), omission of the primary antibody (replaced with blocking buffer), and omission of the secondary antibody (replaced with blocking buffer).
Quantitative histologic analyses
Glomerular generations and indices of glomerular architecture were measured by computer-assisted image analysis (Bioquant True-Color Windows, R&M Biometrics Inc., Nashville, TN). For glomerular generations, five medullary rays were cut longitudinally to count all glomeruli along one side of each medullary ray44. For glomerular capillarisation, we quantified glomerular capillary surface density (Svgc). The cortex was visually stratified into three layers of equal depth: inner, middle, and outer cortex. From a randomly chosen starting point, 25 sequential glomeruli each were photographed within the inner and outer stratum at 100× magnification. A cycloid grid was placed over each digital image. Intersections and endpoints were counted as either crossing immunostained capillaries or lying over tissue, respectively, within a glomerulus. The ratio of counts of capillary intersections over tissue points was calculated for Svgc 39 We also measured glomerular surface area44.
Data analysis
Results are reported as mean ±SD. Data were normally distributed (Shapiro-Wilk test); therefore, for physiological results, between-group comparison was by unpaired t-test, and within-group comparison was by ANOVA followed by Dunnett's post hoc test, using day of life 1 as the comparison age. Likewise for morphometric results, we used ANOVA followed by Fishers PLSD post hoc test. We used StatView 5.0 (StatView Inc., Nasbit, MS, USA) and assessed statistical significance at p<0.05.
Supplementary Material
Acknowledgments
We thank Dr. Angela Presson, Division of Epidemiology, University of Utah and Dr. Lukas Staub, Institute of Social and Preventive Medicine, University of Bern, Switzerland, for statistical guidance.
Statement of Financial Support: Supported by National Institutes of Health (NIH) grants HL110002 and HL062875 (KHA), the Division of Neonatology, and the University of Utah Undergraduate Research Opportunities Program. Eveline Staub, MD was supported by a Fellowship from the Gottfried and Julia Bangerter-Rhyner Foundation, Basel (Switzerland).
Footnotes
Statement of Conflict of Interest: The authors have no financial ties to products used in this study.
References
- 1.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777–84. [PubMed] [Google Scholar]
- 2.Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. 2004;7:17–25. doi: 10.1007/s10024-003-3029-2. [DOI] [PubMed] [Google Scholar]
- 3.Hughson M, Farris AB, 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–22. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 4.Bacchetta J, Harambat J, Dubourg L, et al. Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int. 2009;76:445–52. doi: 10.1038/ki.2009.201. [DOI] [PubMed] [Google Scholar]
- 5.Duncan AF, Heyne RJ, Morgan JS, Ahmad N, Rosenfeld CR. Elevated systolic blood pressure in preterm very-low-birth-weight infants </=3 years of life. Pediatric nephrology. 2011;26:1115–21. doi: 10.1007/s00467-011-1833-x. [DOI] [PubMed] [Google Scholar]
- 6.Frankfurt JA, Duncan AF, Heyne RJ, Rosenfeld CR. Renal function and systolic blood pressure in very-low-birth-weight infants 1-3 years of age. Pediatric nephrology. 2012;27:2285–91. doi: 10.1007/s00467-012-2265-y. [DOI] [PubMed] [Google Scholar]
- 7.Van Marter LJ, Allred EN, Pagano M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network Pediatrics. 2000;105:1194–201. doi: 10.1542/peds.105.6.1194. [DOI] [PubMed] [Google Scholar]
- 8.Koyner JL, Murray PT. Mechanical ventilation and the kidney. Blood purification. 2010;29:52–68. doi: 10.1159/000259585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pannu N, Mehta RL. Effect of mechanical ventilation on the kidney. Best practice & research Clinical anaesthesiology. 2004;18:189–203. doi: 10.1016/j.bpa.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland MR, Gubhaju L, Moore L, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011;22:1365–74. doi: 10.1681/ASN.2010121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Ren Physiol. 2009;297:F1668–F77. doi: 10.1152/ajprenal.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxel L. Organogenesis of the kidney. Cambrige, CA: Cambrige University Press; 1987. [Google Scholar]
- 13.Schmolzer GM, Kumar M, Pichler G, Aziz K, O'Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. Bmj. 2013;347:f5980. doi: 10.1136/bmj.f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyburn B, Li M, Metcalfe DB, et al. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am J Respir Crit Care Med. 2008;178:407–18. doi: 10.1164/rccm.200802-359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Null DM, Alvord J, Leavitt W, et al. High-frequency nasal ventilation for 21 d maintains gas exchange with lower respiratory pressures and promotes alveolarization in preterm lambs. Pediatr Res. 2014;75:507–16. doi: 10.1038/pr.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albertine KH. Brain injury in chronically ventilated preterm neonates: collateral damage related to ventilation strategy. Clin Perinatol. 2012;39:727–40. doi: 10.1016/j.clp.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Null D, Alvord J, Leavitt W, et al. High Frequency Nasal Ventialation for 21 Days Maintains Gas Exchange with Lower Respiratory Pressures and Promotes Alveolarization in Preterm Lambs. Ped Res. 2014 doi: 10.1038/pr.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyburn B, Li M, Metcalfe DB, et al. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. American journal of respiratory and critical care medicine. 2008;178:407–18. doi: 10.1164/rccm.200802-359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeliger M, Inder T, Cain S, et al. Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics. 2006;118:1640–53. doi: 10.1542/peds.2006-0653. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland MR, Ryan D, Dahl MJ, Albertine KH, Black MJ. Effects of preterm birth and ventilation on glomerular capillary growth in the neonatal lamb kidney. Hypertension. 2016;34(10):1988–1997. doi: 10.1097/HJH.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takano K, Kawasaki Y, Imaizumi T, et al. Development of glomerular endothelial cells, podocytes and mesangial cells in the human fetus and infant. Tohoku J Exp Med. 2007;212:81–90. doi: 10.1620/tjem.212.81. [DOI] [PubMed] [Google Scholar]
- 22.Gubhaju L, Black MJ. The baboon as a good model for studies of human kidney development. Pediatr Res. 2005;58:505–9. doi: 10.1203/01.PDR.0000179397.20862.73. [DOI] [PubMed] [Google Scholar]
- 23.Gimonet V, Bussieres L, Medjebeur AA, Gasser B, Lelongt B, Laborde K. Nephrogenesis and angiotensin II receptor subtypes gene expression in the fetal lamb. Am J Physiol Regul Integr Comp Physiol. 1998;274:F1062–F9. doi: 10.1152/ajprenal.1998.274.6.F1062. [DOI] [PubMed] [Google Scholar]
- 24.Albertine KH, Jones GP, Starcher BC, et al. Chronic lung injury in preterm lambs. Disordered respiratory tract development. American journal of respiratory and critical care medicine. 1999;159:945–58. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura KT, Matherne GP, McWeeny OJ, Smith BA, Robillard JE. Renal hemodynamics and functional changes during the transition from fetal to newborn life in sheep. Pediatr Res. 1987;21:229–34. doi: 10.1203/00006450-198703000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Aperia A, Broberger O, Herin P. Maturational changes in glomerular perfusion rate and glomerular filtration rate in lambs. Pediatr Res. 1974;8:758–65. doi: 10.1203/00006450-197408000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Saint-Faust M, Boubred F, Simeoni U. Renal development and neonatal adaptation. Am J Perinatol. 2014;31:773–80. doi: 10.1055/s-0033-1361831. [DOI] [PubMed] [Google Scholar]
- 28.Evans N. Assessment and support of the preterm circulation. Early human development. 2006;82:803–10. doi: 10.1016/j.earlhumdev.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatric research. 2005;58:510–5. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reproductive sciences. 2010;17:186–95. doi: 10.1177/1933719109351098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moritz KM, De Matteo R, Dodic M, et al. Prenatal glucocorticoid exposure in the sheep alters renal development in utero: implications for adult renal function and blood pressure control. American journal of physiology Regulatory, integrative and comparative physiology. 2011;301:R500–9. doi: 10.1152/ajpregu.00818.2010. [DOI] [PubMed] [Google Scholar]
- 32.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–5. [PubMed] [Google Scholar]
- 33.Tufro-McReddie A, Norwood VF, Aylor KW, Botkin SJ, Carey RM, Gomez RA. Oxygen regulates vascular endothelial growth factor-mediated vasculogenesis and tubulogenesis. Dev Biol. 1997;183:139–49. doi: 10.1006/dbio.1997.8513. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland MR, Bertagnolli M, Lukaszewski MA, et al. Preterm birth and hypertension risk: the oxidative stress paradigm. Hypertension. 2014;63:12–8. doi: 10.1161/HYPERTENSIONAHA.113.01276. [DOI] [PubMed] [Google Scholar]
- 35.Joss-Moore LA, Hagen-Lillevik SJ, Yost C, et al. Alveolar formation is dysregulated by restricted nutrition but not excess sedation in preterm lambs supported by non-invasive ventilation. Ped Res. 2016 Jun; doi: 10.1038/pr.2016.143. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linas SL, Berl T, Aisenbrey GA, Better OS, Anderson RJ. The effect of anesthesia on hemodynamics and renal function in the rat. Pflugers Archiv : European Journal of Physiology. 1980;384:135–41. doi: 10.1007/BF00584429. [DOI] [PubMed] [Google Scholar]
- 37.Priano LL. Effects of high-dose fentanyl on renal haemodynamics in conscious dogs. Canadian Anaesthetists' Society journal. 1983;30:10–18. doi: 10.1007/BF03007710. [DOI] [PubMed] [Google Scholar]
- 38.Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. Journal of anatomy. 2005;207:813–21. doi: 10.1111/j.1469-7580.2005.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyengaard JR. Stereologic methods and their application in kidney research. Journal of the American Society of Nephrology : JASN. 1999;10:1100–23. doi: 10.1681/ASN.V1051100. [DOI] [PubMed] [Google Scholar]
- 40.Hsia CC, Hyde DM, Ochs M, Weibel ER, Structure AEJTFoQAoL An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. American journal of respiratory and critical care medicine. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gimonet V, Bussieres L, Medjebeur AA, Gasser B, Lelongt B, Laborde K. Nephrogenesis and angiotensin II receptor subtypes gene expression in the fetal lamb. The American journal of physiology. 1998;274:F1062–9. doi: 10.1152/ajprenal.1998.274.6.F1062. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland MR, Gubhaju L, Black MJ. Stereological assessment of renal development in a baboon model of preterm birth. Am J Nephrol. 2011;33(Suppl 1):25–33. doi: 10.1159/000327073. [DOI] [PubMed] [Google Scholar]
- 43.Albertine KH, Dahl MJ, Gonzales LW, et al. Chronic lung disease in preterm lambs: effect of daily vitamin A treatment on alveolarization. American journal of physiology Lung cellular and molecular physiology. 2010;299:L59–72. doi: 10.1152/ajplung.00380.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland MR, Gubhaju L, Yoder BA, Stahlman MT, Black MJ. The effects of postnatal retinoic acid administration on nephron endowment in the preterm baboon kidney. Pediatr Res. 2009;65:397–402. doi: 10.1203/PDR.0b013e3181975f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.