Abstract
Multiple cancers arise from sites of infection, chronic irritation, and inflammation. It has been widely accepted that pancreatic cancer is an inflammation-driven cancer. In this study, we investigated the application value of systemic inflammatory markers, neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR), in the prediction of chemotherapy response and prognosis in patients with late pancreatic cancer. 122 patients with inoperable pancreatic cancers were included and separated into two groups according to median values of NLR or PLR (NLR low:<3.81 or NLR high:≥3.81, and PLR low:<142.14 or PLR high≥142.14, respectively). Baseline NLR and PLR levels were significantly higher in pancreatic cancer patients compared with the healthy subjects. Neither of the baseline NLR or PLR levels could predict outcomes. Patients with low baseline level of NLR, but not PLR, had better responses to chemotherapy. Changes in NLR, but not PLR levels, were associated with the therapeutic efficacy. Patients who stayed in or dropped into the low NLR level subgroup after first-line chemotherapy had better responses, comparing to those stayed in or jumped into the high NLR level group. No similar results could be observed when the PLR level was investigated. Therefore, NLR is a more sensitive biomarker than PLR in the prediction of chemotherapy response of patients.
Keywords: pancreatic cancer, systemic inflammatory response (SIR), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR)
INTRODUCTION
Pancreatic cancer is an extremely malignant solid cancer with a collective 1-year survival of just 26%, and 5-year survival less than 5%. Almost all of the patients have lost the chance of surgery at the time of diagnosis. Even for those who received surgery, they might finally suffer from recurrence or metastasis. Chemotherapy is still one of the major treatments for pancreatic cancer [1] [2] [3].
Systemic inflammatory response (SIR) is reported to be closely related to outcomes in many cancers, including pancreatic cancer [4]. It has been proved that neutrophil, lymphocyte and platelet levels in peripheral venous blood could be affected by tumor-induced SIR [5]. Therefore, the quantifications of these hematological parameters have been analyzed as markers of SIR in various malignant tumors, including pancreatic cancer [6, 7].
Researches have found that both platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR) have predictive value in early diagnosis, and can reflect prognosis to some extend. It is reported that elevated levels of NLR usually suggested poor prognosis in patients with colorectal cancer who underwent primary resection or hepatectomy for liver metastases [6, 8]. PLR is regarded as a biomarker combining the apparent pre-inflammatory and prophylactic status of cancer with endogenous residual cancer resistance [9].
NLR and PLR have been proved to be both reliable and cost-effective [10], giving them promising prospects in the diagnosis and treatment of cancers. However, the sensitivities of NLR and PLR have rarely been compared in pancreatic cancer. In our study, we tried to evaluate the value of PLR and NLR in chemotherapy response and outcomes of patients with advanced pancreatic cancer.
RESULTS
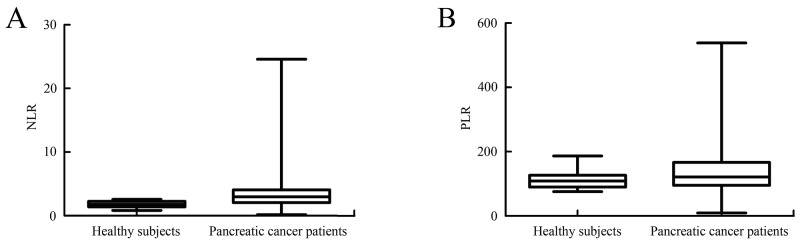
Baseline levels of NL\R and PLR were higher in patients with pancreatic cancer comparing with the controls
The mean baseline NLR level of pancreatic cancer patients was 3.81±3.65, and the corresponding level of healthy subjects was 1.80±0.48. Patients with pancreatic cancer had a significant higher baseline level of NLR than the controls (P<0.001) (Figure 1A). The mean baseline PLR level of pancreatic cancer patients was 142.14±86.76, while the corresponding level of healthy subjects was 112.34±25.52. Pancreatic cancer patients also had a higher baseline PLR level comparing with the controls (P<0.001) (Figure 1B). Increased baseline NLR and PLR values in pancreatic cancer group suggested that these biomarkers could be used to differentiate pancreatic cancer patients from healthy controls and might be used in the early diagnosis of pancreatic cancer.
Figure 1. Comparation of baseline NLR and PLR values in pancreatic cancer group with the ones in healthy subjects.
(A) Comparation of baseline NLR values in pancreatic cancer group with the ones in healthy subjects. Baseline NLR level was significantly higher in pancreatic cancer patients than in the healthy subjects (P<0.001). (B) Comparation of baseline PLR values in pancreatic cancer group with the ones in healthy subjects. Baseline PLR level was significantly higher in pancreatic cancer patients comparing with the healthy subjects (P<0.001).
As presented in Table 1, high baseline PLR and NLR levels both correlated with smoking history. High baseline level of PLR was related to liver metastasis as well.
Table 1. Relationship between baseline NLR, PLR level and clinicopathological features.
| Clinicopathologic features | n | NLR | PLR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (n) | High (n) | χ2 | P value | Low (n) | High (n) | χ2 | P value | ||
| Gender | |||||||||
| Men | 74 | 40 | 34 | 1.788 | 0.181 | 34 | 40 | 0.787 | 0.375 |
| Women | 48 | 20 | 28 | 26 | 22 | ||||
| Age (years) | |||||||||
| <65 | 58 | 26 | 32 | 0.838 | 0.360 | 28 | 30 | 0.036 | 0.849 |
| ≥65 | 64 | 34 | 30 | 32 | 32 | ||||
| BMI (Body mass index) | |||||||||
| <25 | 60 | 28 | 32 | 0.298 | 0.585 | 29 | 31 | 0.034 | 0.854 |
| ≥25 | 62 | 32 | 30 | 31 | 31 | ||||
| Smoke | |||||||||
| No | 53 | 35 | 18 | 10.655 | 0.001** | 32 | 21 | 4.701 | 0.030* |
| Yes | 69 | 25 | 44 | 28 | 41 | ||||
| Liver metastasis | |||||||||
| No | 43 | 29 | 14 | 8.860 | 0.003** | 20 | 23 | 0.189 | 0.664 |
| Yes | 79 | 31 | 48 | 40 | 39 | ||||
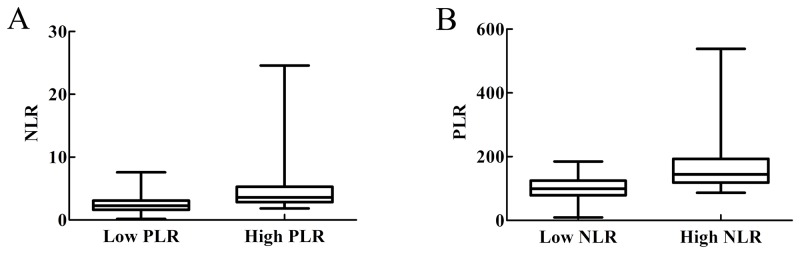
Relationship between baseline NLR level and baseline PLR level
To investigate whether baseline NLR level and baseline PLR level were associated, patients were divided in to low and high PLR or NLR goups, according to baseline PLR or NLR levels respectively. As shown in Figure 2A, NLR levels of patients in high PLR group were higher than those in low PLR group (P<0.001). Likewise, PLR levels of patients in high NLR group were higher than those in low NLR group (P<0.001, Figure 2B). Therefore, baseline NLR level and baseline PLR level correlated with each other.
Figure 2. Relationship between baseline NLR level and baseline PLR level.
(A) Patients were divided into low and high PLR groups. NLR level of patients in high PLR group were higher than that of subjects in low PLR group (P<0.001). (B) Patients were divided into low and high NLR groups. PLR level of patients in high NLR group were higher than that of subjects in low NLR group (P<0.001).
Baseline NLR level predicted chemotherapeutic efficacy
The relationships between baseline PLR or NLR levels and chemotherapeutic efficacy were presented in Table 2 and Table 3. The value of baseline NLR correlated with chemotherapeutic efficacy. However, baseline PLR level was not associated with chemotherapeutic efficacy.
Table 2. Relationship between NLR baseline levels and chemotherapeutic efficacy.
| NLR levels | PR+SD (n=76) | PD (n=46) | χ2 | P value |
|---|---|---|---|---|
| Low (n=60) | 47 | 13 | 12.929 | <0.001 |
| High (n=62) | 29 | 33 |
PR: partial response; SD: stable disease; PD: progressive disease.
Table 3. Relationship between PLR baseline levels and chemotherapeutic efficacy.
| PLR levels | PR+SD (n=76) | PD (n=46) | χ2 | P value |
|---|---|---|---|---|
| Low (n=60) | 33 | 27 | 2.675 | 0.102 |
| High (n=62) | 43 | 19 |
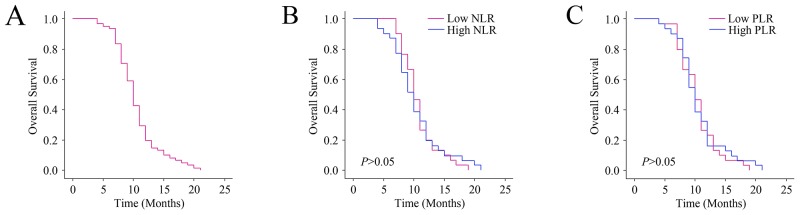
Baseline PLR and NLR levels were not related to outcomes
The median OS of all patients was 10 months (9.73-10.27 months) (Figure 3A). Survivors were followed for 25 months. We used the Kaplan-Meier diagrams to evaluate the impact of the PLR and NLR states on OS (Figure 3B and 3C). The median OS was 10 months (9.52-10.48) for high NLR group, and 10 months (9.68-10.32) for the low NLR group respectively (P>0.05). The median OS of the high PLR group was 10 months (9.65-10.35), and the low PLR group was 10 months (9.65-10.35) (P>0.05). Therefore, baseline PLR and NLR levels were not correlated with outcomes.
Figure 3. Relationship between baseline NLR and PLR levels and the outcomes.
(A) predicted probability of overall survival (OS). (B) The OS according to NLR. (C) The OS according to PLR.
Changes in NLR level after chemotherapy were related to chemotherapeutic efficacy
To identify the relationship between changes of PLR or NLR levels and chemotherapeutic efficacy, blood samples were obtained and computed tomography (CT) scan assessments were performed at the same time subsequent to first-line chemotherapy. After chemotherapy, 42 patients with low baseline NLR levels retained (Table 4), while 18 patients changed to high NLR group. 46 patients with high baseline NLR levels reserved in this group. In contrast, 16 patients with high baseline NLR levels changed to low NLR group. Patients with low NLR levels after first-line chemotherapy (no matter remained or transferred to) had improved responses comparing with those retained or were transferred to high NLR group.
Table 4. Relationship between changes in NLR level and chemotherapeutic efficacy.
| Pre-chemotherapy | Post-chemotherapy | PR+SD (n=76) | PD (n=46) | χ2 | P value |
|---|---|---|---|---|---|
| Low (n=60) | Low (n=42) | 36 | 6 | 4.494 | 0.034 |
| High (n=18) | 11 | 7 | |||
| High (n=62) | Low (n=16) | 11 | 5 | 4.183 | 0.041 |
| High (n=46) | 18 | 28 |
After first-line chemotherapy, 30 patients were still reserved in low baseline PLR level group (Table 5), while 30 patients were transferred to high PLR group. Meanwhile, 34 patients in high baseline PLR level group maintained in this group, and 28 patients from this group changed to low PLR level group. Patients with low PLR levels after first-line chemotherapy (no matter remained or transferred to) did not exhibit improved responses comparing with those remained or transferred to high PLR level group (Table 5).
Table 5. Relationship between changes in PLR level and chemotherapeutic efficacy.
| Pre-chemotherapy | Post-chemotherapy | PR+SD (n=76) | PD (n=46) | χ2 | P value |
|---|---|---|---|---|---|
| Low (n=60) | Low (n=30) | 18 | 12 | 0.606 | 0.436 |
| High (n=30) | 15 | 15 | |||
| High (n=62) | Low (n=28) | 21 | 7 | 0.766 | 0.382 |
| High (n=34) | 22 | 12 |
Therefore, changes in NLR level, but not PLR level, after chemotherapy associated with chemotherapeutic efficacy.
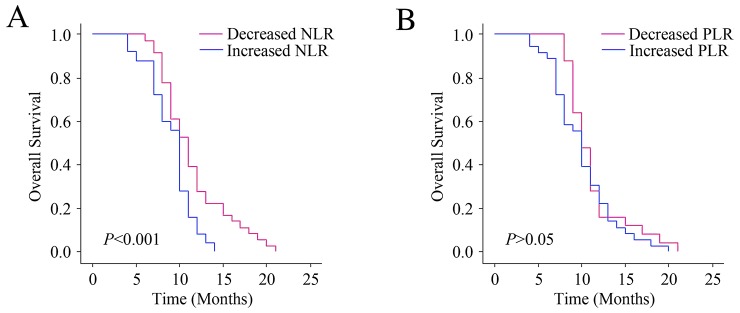
Changes in NLR level predicted outcomes
The Kaplan-Meier diagrams were performed to evaluate the impact of changes in the PLR or NLR levels on OS (Figure 4). The median OS was 10 (9.77-10.23) months for patients with elevated NLR levels after first-line chemotherapy, comparing with 11 months (10.48-11.52) for NLR-decreased patients (P<0.001). The median OS of PLR-increased group was 10 months (9.65-10.35), and the PLR-decreased group was 10 months (9.61-10.39) (P>0.05). As a result, the survival rates of patients with elevated NLR levels after chemotherapy is reduced. But the changes in PLR level did not show prognostic value.
Figure 4. Relationship between changes in NLR and PLR levels after chemotherapy and the outcomes.
(A) The OS according to changes in NLR. (B) The OS according to changes in PLR.
To evaluate the factors which were related to OS, univariate and multivariate analyses were further performed. By using the post-/pre-chemotherapy PLR or NLR ratios, patients were separated into two groups respectively. Post-/pre-chemotherapy ratios <1 represented that the values of NLR or PLR were decreased after chemotherapy, and ≥1 implied that PLR or NLR values were not decreased. As shown in Table 6, univariate and multivariate analysis confirmed that post/pre-chemotherapy NLR ratio is a prognostic factor affecting OS. Therefore, changes in NLR level, but not PLR level, after chemotherapy predicted outcomes.
Table 6. Univariate and multivariate analysis of risk factors for the overall survival.
| Risk factors | Overall survival (OS) | |||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| OR (95% CI) | P value | OR (95% CI) | P value | |
|
Gender (Women or Men) |
0.770 (0.528-1.124) | 0.175 | - | - |
|
Age (>65 years or ≤65years) |
0.773 (0.537-1.110) | 0.163 | - | - |
|
PLR (high or low) |
0.922 (0.641-1.325) | 0.660 | - | - |
|
NLR (high or low) |
1.007 (0.701-1.448) | 0.969 | - | - |
|
Post/pre-chemotherapy PLR ratio (>1 or ≤1) |
1.272 (0.881-1.837) | 0.199 | - | - |
|
Post/pre-chemotherapy NLR ratio (>1 or ≤1) |
1.902 (1.290-2.803) | 0.001** | 1.902 (1.290-2.803) | 0.001** |
DISCUSSION
Inflammation, as a major feature of tumors [11], has been proved to be a crucial and essential process in the progression of malignancies [12] [13], including proliferation, angiogenesis, metastasis and chemotherapy-resistance [11]. Pancreatic cancer has a rather unique and complex microenvironment which is rich in inflammatory cytokines, growth factors and proteinases that are favorable for proliferation, invasion and metastasis of pancreatic cancer cells [14] [15]. Increased serum levels of inflammatory cytokines are also related to clinical features of pancreatic cancer, such as poor performance status, cachexia and OS [16, 17]. Therefore, cancer-associated inflammation is a key molecular feature in pancreatic cancer.
Systemic inflammation is considered to be able to predict poor outcomes among several types of malignances, including pancreatic cancer [18]. So far, lots of systemic inflammatory markers have been found to have predictive value for prognosis [19, 20]. For example, as a SIR marker, incorporating C-reactive protein (CRP), together with serum albumin, has a close relationship with outcomes in cancer patients [9]. Besides, serum lactate dehydrogenase (LDH) levels are also associated with the systemic inflammatory response and serves as a significant prognostic predictor in pancreatic cancer [21]. Systemic inflammation can also induce measurable neutrophilia and relative lymphocytopenia in the peripheral blood [19].
Neutrophils play an active role in both systemic and local inflammatory response. It is believed that up-regulation of neutrophils can reflect an aggressive feature of cancer cells since it is primarily stimulated by hematopoietic cytokines derived from tumor cells [22]. Neutrophils can produce arginase, nitric oxide (NO), reactive oxygen species (ROS), and inhibit the function of cytotoxic lymphocytes [23, 24]. In addition, preclinical researches have showed that neutrophils functioned as tumor-promoting factors via the signaling pathway triggered by transforming growth factor β (TGF-β) [25].
Lymphocytes, on the other hand, possess the anti-cancer activities through repressing tumor growth or metastasis [26]. Lymphocyte counts can reflect endogenous cancer resistance of the immune system [27, 28]. It has been proved that higher number of lymphocytes predicts better outcomes [27, 28]. Elevated numbers of lymphocytes might be related to a tumor shrink after neoadjuvant chemoradiotherapy in locally advanced rectal cancer [29].
By the combination of neutrophils and lymphocytes, high NLR level has been shown to be a promising indicator of poor prognosis for patients with resectable colorectal cancer [6]. Elevated NLR level is suggested as a marker for estimating benefits stage II colon cancer patients obtained from adjuvant treatment, and could identify risks of those patients [30]. Baseline NLR measurements and changes in NLR levels after therapy also have predictive values in outcomes of patients with unresectable gastric cancer [31]. Besides, NLR was also useful in determining prognosis in patients with non-small cell lung cancer cases [32]. Similar results have been found in patients bearing pancreatic cancer. Previous investigations have proved that elevated NLR level correlated with unfavorable prognosis in patients who received curative resection [33] [34] [35] or bypass surgery [36]. The pretreatment NLR could predict survival for patients with advanced pancreatic cancer who received systematic chemotherapy as well [37]. In our present study, we found the elevated NLR level in newly diagnosed pancreatic cancer patients than in healthy subjects. Moreover, patients whose NLR values increased after chemotherapy, predicted worse outcomes. Univariate and multivariate analysis also confirmed that post/pre-chemotherapy NLR ratio is a prognostic marker affecting OS in pancreatic cancer, suggesting the application of NLR not only possesses diagnostic value but also be able to provide prognostic information in pancreatic cancers.
Platelets also play a crucial role in the development of tumors [23]. Researches have proved that platelets can promote angiogenesis, extracellular matrix degradation, release of growth factors and adhesion molecules, all of which are the basic parts for the proliferation and metastasis of tumors. Thus platelets are able to accelerate cancer development [10]. In the blood stream, covering with platelets helps circulating tumor cells (CTCs) to overcome the pressure in the blood, involving the attack from immune system and physical factors [38]. Besides the effect of platelet on the natural course of cancer, number and function of platelets could also be influenced by cancer. Increasing numbers of studies suggested that the proinflammatory cytokines released by tumors, like interleukin-1 (IL-1), interleukin-3 (IL-3), and interleukin-6 (IL-6), can promote megakaryocyte proliferation, and lead to thrombocytosis gradually [10]. Therefore, an alliance of platelet and cancer provides a positive feedback on the progression of malignancies. In view of this, the platelet derived biomarkers present special potential.
By the combination of platelets and lymphocytes, PLR is thought to represent endogenous anticancer proinflammation and precoagulation in malignant tumors [9]. Besides, PLR is also assumed to be both a sensitive marker and a prognostic factor for breast cancers, ovarian cancers and colorectal cancers [39]. However, comparing with the value of NLR, many studies suggest that PLR levels are not qualified to be an independent prognostic factor for survival [10]. Our present findings indicated that, newly diagnosed pancreatic cancer patients have significantly higher PLR values comparing with the healthy controls, suggesting PLR could be a promising and easily available biomarker for the diagnosis of pancreatic cancer. It has been proved that cigarette smoke is a strong stimulator of pulmonary inflammation [40] [41]. In fact, elevated NLR and PLR levels have been observed in chronic obstructive pulmonary disease [42] [43] [44]. Therefore, the up-regulation of NLR and PLR could be correlated with both smoking history and cancer status, both of which are inflammation-related. Since both NLR and PLR are biomarkers reflecting systemic inflammation level, there is no surprise that patients with higher baseline NLR levels also possessed higher baseline PLR levels and vice versa. However, only NLR, but not PLR, associated with liver metastasis. Moreover, changes in PLR values after chemotherapy did not correlate with the response to treatment and overall survival, suggesting PLR is not as sensitive as NLR for the prognostic evaluation of pancreatic cancer, in consisting with a previous study [45].
Our study suggested that both NLR and PLR could be used in the diagnosis of pancreatic cancer. However, only NLR can be used to predict chemotherapy response and follow-up. In view of economic conditions in some areas of the world, this convenient, safe and lower cost biomarker may be beneficial for the patients with pancreatic cancer.
MATERIALS AND METHODS
Subjects and inclusion criteria
The study was conducted as a retrospective investigation of pancreatic cancer patients that had been referred to the First Affiliated Hospital of Soochow University (Jiangsu, China) between Jan 2007 and Mar 2015. Approval for the study was granted by the Medical Ethics Committees of the First Affiliated Hospital of Soochow University. Clinical and pathological records of all the patients participating in the study were reviewed periodically.
In total, 122 pancreatic cancer patients were recruited in this study. Patients had histologic or cytologic evidence of locally advanced or metastatic adenocarcinoma of the pancreas. Patient characteristics are detailed in Table 1. The mean age of the 122 patients was 65 years (range, 32–80 years), and 74 patients were male and 48 were female. The inclusion criteria were as follows: (a) those with histologically or cytologically confirmed recurrent or metastatic gastric cancer; (b) age more than 18 years; (c) Karnofsky performance status (KPS) score of≥70; (d) those with a predicted survival of ≥3 months; (e) either naive to anti-tumor treatment or the post-operative adjuvant chemotherapy was done at least six months after the last dose of chemotherapy; (f) in case of patients who were scheduled for radiotherapy on the target lesion, radiotherapy must have been finished for at least three months; (g) those with at least one measurable lesion (minimum 10mm×10mm on computed tomography (CT) or magnetic resonance imaging (MRI)); and (h) those who met the following laboratory criteria: white blood cells (WBC) ≥4.0×109/L; absolute neutrophil count (ANC) ≥1.5×109/L; platelet (PLT) ≥100×109/L; Serum bilirubin ≤ upper limit of normal (ULN); alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) ≤ ULN×2.5 (if without liver metastasis) or ≤ ULN×5 (if with liver metastasis); urea nitrogen ≤ ULN×1.25; and creatinine ≤ ULN×1.25. The staging of cancer was made according to tumor–nodulus-metastases (TNM) classification and classified through the American Joint Committee on Cancer (AJCC) recommendations, 7th edition [46]. Patients were followed regularly for 60 months. The prognostic analyses were performed regarding progression-free survival (PFS) and overall survival (OS). 30 age–sexes matched healthy subjects were also included into this study.
Blood samples
Peripheral venous blood (5-7 ml) was collected into a sterile ethylenediaminetetraacetic acid (EDTA) tube. All blood samples were fasted and obtained between 6:30 and 7:30 a.m. in order to standardize the known impact of circulating hormones (circadian rhythm) on the number and subtype distribution of the various white blood cell indices. Hematological parameters were analyzed within 30 min after collection using a hematology analyser (Sysmex XE-2100; Sysmex, Kobe, Japan). Neutrophil (103/μl), lymphocyte (103/μl) and platelet (103/μl) counts and mean platelet volume (MPV) levels were recorded. NLR and PLR were calculated as the ratio of the neutrophils and platelets to lymphocytes, respectively. Mean value was used for NLR and PLR as normal distribution was absent. The patients were divided into two groups according to the mean value of NLR or PLR [(NLR low, <3.81 or NLR high, ≥3.81; and PLR low, <142.14 or PLR high, ≥142.14, respectively]. The post-/pre-operative ratios were defined as the rate of pre-operative PLR or NLR values and the corresponding ones obtained one month after operation.
Chemotherapy and evaluation
In this study, most of the patients (98.4 %) underwent gemcitabine-based chemotherapy and a minority of the patients (1.6 %) underwent fluorine pyrimidines (fluorouracil, capecitabine, or S1 [tegafur, 5-chloro-2,4-dihydroxypyridine and oxonic acid]) treatment according to the newest edition of the National Comprehensive Cancer Network (NCCN) Guideline at the time. The regimen of gemcitabine alone was 1,000 mg/m2 (3 times per week followed by a 1-week rest for 6 cycles). Computed tomography (CT) scan was performed for the assessment of response every 2 months and evaluated according to the criteria of Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [47]. The responses to chemoradiotherapy including complete remission, regression, stable disease and disease progression.
Follow-up
We recorded responses to chemoradiotherapy including complete remission, regression, stable disease, and disease progression, and overall survival (OS). After first line chemotherapy, disease progression after chemoradiotherapy was defined as lack of response to chemoradiotherapy. In contrast, stable disease, complete response or disease regression after chemoradiotherapy was defined as response to chemoradiotherapy. Patients were regularly followed for 25 months. Survival time was measured from the date of chemoradiotherapy until death or last clinical evaluation. The prognostic analyses were performed regarding overall survival (OS). OS was defined as the time from the diagnosed date to death from any cause.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 software (Chicago, USA). The associations between NLR or PLR levels and clinicopathologic features or chemotherapeutic efficacy were explored and assessed by the χ2 tests. For analysis of survival data, Kaplan–Meier curves were constructed, and statistical analysis was carried out using the log-rank test. Multivariate Cox regression was performed for each outcome parameter, using a backwards elimination technique to derive a potentially suitable set of predictors. All values of P < 0.05 were considered statistically significant.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This work was supported by grants from the National Natural Science Foundation of China (grant nos. 81472296, 81602091, 81402176, 81402093, 81272542, and 81200369), the Six Major Talent Peak Project of Jiangsu Province (grant no. 2015-WSN-022), The Project of Invigorating Health Care through Science, Technology and Education, Jiangsu Provincial Medical Youth Talent (grant no. QNRC2016709), the Science and Education for Health Foundation of Suzhou for Youth (grant no. kjxw2015003), the Science and Technology Project Foundation of Suzhou (grant nos. SYS201464 and SYS201504), and the Science and Technology Foundation of Suzhou Xiangcheng (grant nos. SZXC2012-70, XJ201538 and XJ201451).
REFERENCES
- 1.Tao M, Liu L, Shen M, Zhi Q, Gong FR, Zhou BP, Wu Y, Liu H, Chen K, Shen B, Wu MY, Shou LM, Li W. Inflammatory stimuli promote growth and invasion of pancreatic cancer cells through NF-kappaB pathway dependent repression of PP2Ac. Cell Cycle. 2016;15:381–393. doi: 10.1080/15384101.2015.1127468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu MD, Liu SL, Feng YZ, Liu Q, Shen M, Zhi Q, Liu Z, Gu DM, Yu J, Shou LM, Gong FR, Zhu Q, Duan W, et al. Genomic characteristics of pancreatic squamous cell carcinoma, an investigation by using high throughput sequencing after in-solution hybrid capture. Oncotarget. 2017;8:14620–14635. doi: 10.18632/oncotarget.14678. https://doi.org/10.18632/oncotarget.14678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong FR, Wu MY, Shen M, Zhi Q, Xu ZK, Wang R, Wang WJ, Zong Y, Li ZL, Wu Y, Zhou BP, Chen K, Tao M, Li W. PP2A inhibitors arrest G2/M transition through JNK/Sp1- dependent down-regulation of CDK1 and autophagy-dependent up-regulation of p21. Oncotarget. 2015;6:18469–18483. doi: 10.18632/oncotarget.4063. https://doi.org/10.18632/oncotarget.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 6.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 7.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 8.Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, Lodge JP, Toogood GJ. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–814. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- 9.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Seretis C, Seretis F, Lagoudianakis E, Politou M, Gemenetzis G, Salemis NS. Enhancing the accuracy of platelet to lymphocyte ratio after adjustment for large platelet count: a pilot study in breast cancer patients. Int J Surg Oncol. 2012;2012:653608. doi: 10.1155/2012/653608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, Kim SG, Kim SH, Jang JS, Kim MC, Kim KH, Han JY, Kim HJ. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer. 2009;9:155. doi: 10.1186/1471-2407-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilincalp S, Ekiz F, Basar O, Ayte MR, Coban S, Yilmaz B, Altinbas A, Basar N, Aktas B, Tuna Y, Erbis H, Ucar E, Erarslan E, Yuksel O. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25:592–594. doi: 10.3109/09537104.2013.783689. [DOI] [PubMed] [Google Scholar]
- 14.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tassi E, Gavazzi F, Albarello L, Senyukov V, Longhi R, Dellabona P, Doglioni C, Braga M, Di Carlo V, Protti MP. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol. 2008;181:6595–6603. doi: 10.4049/jimmunol.181.9.6595. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 17.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metabol. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 19.Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Friesenbichler J, Trajanoski S, Stojakovic T, Eberhard K, Leithner A, Pichler M. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer. 2014;135:362–370. doi: 10.1002/ijc.28677. [DOI] [PubMed] [Google Scholar]
- 21.Yu SL, Xu LT, Qi Q, Geng YW, Chen H, Meng ZQ, Wang P, Chen Z. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep. 2017;7:45194. doi: 10.1038/srep45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y, Kim SH, Han JY, Kim HT, Yun T, Lee JS. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol. 2012;138:2009–2016. doi: 10.1007/s00432-012-1281-4. [DOI] [PubMed] [Google Scholar]
- 23.Kemal Y, Yucel I, Ekiz K, Demirag G, Yilmaz B, Teker F, Ozdemir M. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pac J Cancer Prev. 2014;15:2651–2654. doi: 10.7314/apjcp.2014.15.6.2651. [DOI] [PubMed] [Google Scholar]
- 24.De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Kachala SS, Kadota K, Shen R, Mo Q, Beer DG, Rusch VW, Travis WD, Adusumilli PS. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res. 2011;17:5247–5256. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi R, Takahashi K, Miura K, Ishiwata T, Sakuraba S, Fukuchi Y. Prognostic factors in patients with inoperable non-small cell lung cancer--an analysis of long-term survival patients. Gan To Kagaku Ryoho. 2006;33:1595–1602. [PubMed] [Google Scholar]
- 27.Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, Gilks CB, Clarke B, Kobel M, Nelson BH. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33. doi: 10.1186/1479-5876-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi N, Usui S, Kikuchi S, Goto Y, Sakai M, Onizuka M, Sato Y. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer. 2012;75:223–227. doi: 10.1016/j.lungcan.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5:47. doi: 10.1186/1748-717X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH, Wan DS, Pan ZZ. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427–1433. doi: 10.1007/s00384-010-1052-0. [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Liu ZY, Xia YY, Zhou C, Shen XM, Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR, Tao M, Lian L, Li W. Changes in neutrophil/lymphocyte and platelet/lymphocyte ratios after chemotherapy correlate with chemotherapy response and prediction of prognosis in patients with unresectable gastric cancer. Oncol Lett. 2015;10:3411–3418. doi: 10.3892/ol.2015.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akinci Ozyurek B, Sahin Ozdemirel T, Buyukyaylaci Ozden S, Erdogan Y, Kaplan B, Kaplan T. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18:1417–1421. doi: 10.22034/APJCP.2017.18.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868–872. doi: 10.1007/s00268-011-0984-z. [DOI] [PubMed] [Google Scholar]
- 34.Stevens L, Pathak S, Nunes QM, Pandanaboyana S, Macutkiewicz C, Smart N, Smith AM. Prognostic significance of pre-operative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: a systematic review. HPB (Oxford) 2015;17:285–291. doi: 10.1111/hpb.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng H, Luo G, Lu Y, Jin K, Guo M, Xu J, Long J, Liu L, Yu X, Liu C. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology. 2016;16:1080–1084. doi: 10.1016/j.pan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Okamura Y. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of survival after gastroenterostomy in patients with advanced pancreatic adenocarcinoma. Ann Surg Oncol. 2013;20:4330–4337. doi: 10.1245/s10434-013-3227-8. [DOI] [PubMed] [Google Scholar]
- 37.Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, Liu L, Liu C, Xu J, Ni Q, Yu X. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–676. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 38.Lian L, Li W, Li ZY, Mao YX, Zhang YT, Zhao YM, Chen K, Duan WM, Tao M. Inhibition of MCF-7 breast cancer cell-induced platelet aggregation using a combination of antiplatelet drugs. Oncol Lett. 2013;5:675–680. doi: 10.3892/ol.2012.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yavuzcan A, Caglar M, Ustun Y, Dilbaz S, Ozdemir I, Yildiz E, Ozkara A, Kumru S. Evaluation of mean platelet volume, neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in advanced stage endometriosis with endometrioma. J Turk Ger Gynecol Assoc. 2013;14:210–215. doi: 10.5152/jtgga.2013.55452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 42.Rahimirad S, Ghaffary MR, Rahimirad MH, Rashidi F. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. Tuberk Toraks. 2017;65:25–31. [PubMed] [Google Scholar]
- 43.Lee H, Um SJ, Kim YS, Kim DK, Jang AS, Choi HS, Kim YH, Kim TE, Yoo KH, Jung KS. Association of the neutrophil-to-lymphocyte ratio with lung function and exacerbations in patients with chronic obstructive pulmonary disease. PLoS One. 2016;11:e0156511. doi: 10.1371/journal.pone.0156511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G, Wu C, Luo Z, Teng Y, Mao S. Platelet-lymphocyte ratios: a potential marker for pulmonary tuberculosis diagnosis in COPD patients. Int J Chron Obstruct Pulmon Dis. 2016;11:2737–2740. doi: 10.2147/COPD.S111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, Li YH, Xu RH. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 46.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 47.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]