Abstract
During intervertebral disc ageing, chondrocyte-like cells (CLCs) replace notochordal cells (NCs). NCs have been shown to induce regenerative effects in CLCs. Since vesicles released by NCs may be responsible for these effects, we characterized NC-derived extracellular vesicles (EVs) and determined their effect on CLCs.
EVs were purified from porcine NC-conditioned medium (NCCM) through size exclusion chromatography, ultracentrifugation or density gradient centrifugation. Additionally, the EVs were quantitatively analyzed by high-resolution flow cytometry. The effect of NCCM-derived EVs was studied on canine and human CLC micro-aggregates in vitro and compared with NCCM-derived proteins and unfractionated NCCM.
Porcine NCCM contained a considerable amount of EVs. NCCM-derived EVs induced GAG deposition in canine CLCs to a comparable level as NCCM-derived proteins and unfractionated NCCM, and increased the DNA and glycosaminoglycan (GAG) content of human micro-aggregates, although to a lesser extent than unfractionated NCCM. The biological EV effects were not considerably influenced by ultracentrifugation compared with size exclusion-based purification. Upon ultracentrifugation, interfering GAGs, but not collagens, were lost. Nonetheless, collagen type I or II supplemented to CLCs in a concentration as present in NCCM induced no anabolic effects.
Porcine NCCM-derived EVs exerted anabolic effects comparable to NCCM-derived proteins, while unfractionated NCCM was more potent in human CLCs. GAGs and collagens appeared not to mediate the regenerative EV effects. Thus, NC-derived EVs have regenerative potential, and their effects may be influenced by the proteins present in NCCM. The optimal combination of NC-secreted factors needs to be determined to fully exploit the regenerative potential of NC-based technology.
Keywords: extracellular vesicles, intervertebral disc, notochordal cell, regeneration, nucleus pulposus
INTRODUCTION
Low back pain, affecting up to 85% of the population and resulting in considerable socioeconomic consequences [1, 2], has been associated with intervertebral disc (IVD) degeneration [3]. Since dogs experience back pain and IVD degeneration with similar characteristics as humans, they are considered a suitable animal model for human IVD degeneration [4, 5]. The healthy IVD provides stability and flexibility to the spine and consists of a hydrated nucleus pulposus (NP) surrounded by the annulus fibrosus (AF). The NP, composed of glycosaminoglycan (GAG) and collagen type II, is derived from the notochord [6]. GAGs indirectly attract water, and in this way the IVD provides a shock absorption function for the spine. During maturation, notochordal cells (NCs) are replaced by chondrocyte-like cells (CLCs) in the NP. When the IVD degenerates, the CLCs are not able to maintain healthy tissue anymore. The CLCs become depleted, the GAG and water content decreases and collagen type II is replaced by collagen type I, resulting in a more fibrous tissue. The avascular IVD shows inadequate repair, and a vicious circle develops in which the IVD experiences increased vulnerability to damage by physiologic loading [7].
Current treatments for IVD disease aim at relieving symptoms, but do not address the underlying degeneration. Therefore, regenerative strategies have gained increased attention [8–10]. Successful treatment strategies can be developed by mimicking developmental biology. In this respect, NCs have attracted increasing interest because of their potential regenerative capacity [11]. Large, vacuolated NCs are only present in the NP of young human individuals and disappear around 10 years of age. The replacement of NCs by CLCs precedes the onset of IVD degeneration, implying that NCs may play a role in maintaining IVD health. The regenerative effect of NC-conditioned medium (NCCM) has already been demonstrated on CLCs [12–14], mesenchymal stromal cells (MSCs) [15–17], and NP tissue explants [18] in vitro, and on rat IVDs in vivo [19]. NCCM may exert its effects in several ways: through extracellular matrix (ECM) components such as GAGs [20] and/or through growth factors. Factors that were already found are connective tissue growth factor (CTGF) [19, 21], transforming growth factor-β1 (TGF-β1), Wnt-induced soluble protein 2, insulin-like growth factor binding protein 7, and angiopoietin-like 7 [19] in canine NCCM, and CTGF [22], alpha-2-macroglobulin, clusterin, and tenascin [16] in porcine NCCM.
Recently, extracellular vesicles (EVs) have gained increased attention. EVs are small, membrane-enclosed particles released by cells that play a role in intercellular signaling [23], and are involved in tissue regeneration [24, 25]. We previously proposed that EVs may be responsible for regenerative NCCM effects [26]. The anabolic effect of pelletable (theoretically containing EVs and protein aggregates) NCCM factors was, however, less pronounced than that of soluble (peptides, proteins) NCCM factors [26]. This observation could be attributed to the ultracentrifugation (UC) procedure that may negatively affect the biological EV properties [27] or to the interfering protein aggregates present in the pelletable fraction [28]. Therefore, the first aim of the current study was to purify and characterize NC-derived EVs from porcine NCCM. The second aim was to determine the biologic effect of the NCCM-derived EVs on canine and human CLCs from degenerated IVDs in vitro.
RESULTS
NCCM-derived extracellular vesicle purification and characterization
NCCM-derived EVs were separated from soluble proteins by size-exclusion chromatography (SEC) and separately pelleted by UC at 100,000g. The UC pellets (containing either EVs or proteins) were labelled with PKH67. Thereafter, the EVs were floated in sucrose gradients to their buoyant density. Lastly, the EVs were quantitatively analyzed by high-resolution flow cytometry.
To confirm the presence of true EV within samples upon flow cytometric analysis, detergents can be added to disrupt the EV lipid bilayer [29]. Upon addition of 0.1% SDS or 0.1% Triton X-100 to the samples containing the highest number of EVs (density 1.12 g/mL), the event rate reduced considerably (Supplementary Figure 1a). This was accompanied by a loss of light scattering events (Supplementary Figure 1a). Taken together, this confirms that the majority of detected events during flow cytometric analysis were indeed EVs. To demonstrate that during flow cytometric analysis single EVs were measured and not EV swarms (presence of multiple EVs within the measuring spot [30]), the 1.12 g/mL sucrose gradient fractions were serially diluted and analyzed. Upon dilution, the scatter profiles of EVs did not change (Supplementary Figure 1c). A linear correlation (R2: 0.9996) was found between the number of measured events and the dilution (Supplementary Figure 1b), indicating that single EVs and not swarms of EVs were detected.
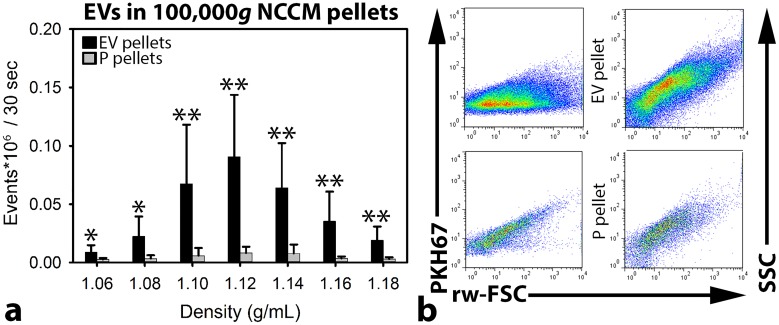
High-resolution flow cytometry of porcine NCCM-derived EV and protein (P) 100,000g UC pellets revealed that the number of EVs differed considerably between donors (Supplementary Figure 2). The majority of EVs were, however, detected at the same densities. Additionally, the EV scatter profiles were comparable between all donors (Figure 2). In all donors, the highest number of events was measured in the 1.10, 1.12, and 1.14 g/mL sucrose gradient fractions of the EV 100,000g UC pellets. In the P 100,000g UC pellets, most events were also detected in the 1.10-1.14 g/mL sucrose gradient fractions, but the number of events was significantly lower compared to the EV 100,000g UC pellets (p<0.05).
Figure 2. Quantitative extracellular vesicle (EV) analysis by high-resolution flow cytometry of notochordal cell-conditioned medium (NCCM).
(a) Number of measured events per sucrose gradient fraction (with different densities) (+ SD) from floated porcine NCCM EV 100,000g ultracentrifugation (UC) pellets (black bars) and floated protein (P) 100,000g UC pellets (grey bars). *,**: significantly more measured events per 30 sec in the sucrose gradient fractions of the EV 100,000g UC pellets than in those of the P 100,000g UC pellets (p<0.05, p<0.01, respectively). (b) Dot plots of the sucrose gradient fraction with the highest number of measured events (density 1.12 g/mL) from one representative donor (donor 2). Dot plots represent levels of PKH67 intensity (left) or side scatter (SCC; right) (y-axis) versus reduced wide-angle forward scatter (rw-FSC) (x-axis). Upper dot plots represent the 1.12 g/mL sucrose fraction of the EV 100,000g UC pellet, and lower dot plots represent the 1.12 g/mL sucrose fraction of the P 100,000g UC pellet. n = 7 porcine NCCM donors.
The effect of porcine NCCM-derived purified extracellular vesicles and proteins on canine and human CLCs
NCCM-derived EVs and proteins induce GAG deposition in canine and human CLC micro-aggregates
Based on the expected EV sizes and protein measurements, the three NCCM SEC fractions with most EVs and proteins (P) were separately collected (Supplementary Figure 3). Part was directly used in culture (EVqEV and PqEV), and part was subjected to 100,000g UC and thereafter used in culture (EVUC and PUC). After 7 days of culture, no treatment significantly influenced the canine micro-aggregates’ DNA content compared with controls (Figure 3a). The GAG and GAG/DNA content of the canine micro-aggregates were, however, significantly increased by 7-day unfractionated NCCM, EVqEV, EVUC, PqEV, and PUC treatment (p<0.05), with no differences between these treatments (Figure 3b and 3c).
Figure 3. Notochordal cell-conditioned medium (NCCM)-derived extracellular vesicles (EVs) and proteins (P) induce increased glycosaminoglycan (GAG) deposition in chondrocyte-like cell (CLC) micro-aggregates derived from degenerated discs.
(a, d) DNA content (b, e) GAG content, (c, f) GAG/DNA content (mean ± SD) of canine and human CLC micro-aggregates, respectively, cultured in control culture medium, unfractionated porcine NCCM, or porcine NCCM-derived EVs or proteins for 7 (canine) or 21 (human) days. EVqEV: NCCM-derived EVs obtained after size exclusion chromatography (SEC), EVUC: NCCM-derived EVs obtained after SEC and subsequent 100,000g ultracentrifugation (UC), PqEV: NCCM-derived proteins obtained after SEC, PUC: NCCM-derived proteins obtained after SEC and subsequent 100,000g UC. Bars indicate significant differences between conditions (p<0.05); *, **,***: significantly different from controls (p<0.05, p<0.01, p<0.001, respectively); (g) Safranin O/Fast Green staining and collagen type I and II immunohistochemistry of human CLC micro-aggregates after 21 days of culture. Arrowheads indicate collagen type II deposition. n = 7 porcine NCCM donors tested on a pool of 4 canine (in triplo) and 4 human CLC donors (in duplo). n.s.: not significantly different.
The DNA and GAG content of the human micro-aggregates were significantly induced by 21-day unfractionated NCCM, EVqEV, EVUC, PqEV, and PUC treatment (p<0.01, Figure 3d, 3e, and 3g). Unfractionated NCCM was most potent in this respect, followed by EVqEV and PqEV, and lastly EVUC and PUC. EVqEV induced a similar DNA and GAG content in human micro-aggregates as PqEV treatment. Also EVUC was equally potent in increasing the DNA and GAG content of the human micro-aggregates as PUC. Both were, however, less potent than EVqEV and PqEV in this respect. Only 21-day EVqEV and unfractionated NCCM treatment significantly increased the GAG/DNA content of the human micro-aggregates compared with control, PqEV and PUC treatment (p<0.05, Figure 3f). All treatments increased collagen type I deposition compared with controls (Figure 3g). Only in micro-aggregates treated with EVUC and PUC, limited collagen type II was deposited (Figure 3g).
The total number of EVs in the sucrose fractions (density 1.06-1.18 g/mL) of the porcine NCCM EV 100,000g UC pellets did not correlate with the GAG content of the canine and human micro-aggregates treated with these EVs (Supplementary Figure 4a-4h). Notably, the number of measured EVs and the canine, but not the human, micro-aggregates’ GAG/DNA content after 7-day EVqEV treatment displayed a moderate correlation (r:0.600, p<0.05; Supplementary Figure 4c).
Collagen and GAG concentration of the different culture media
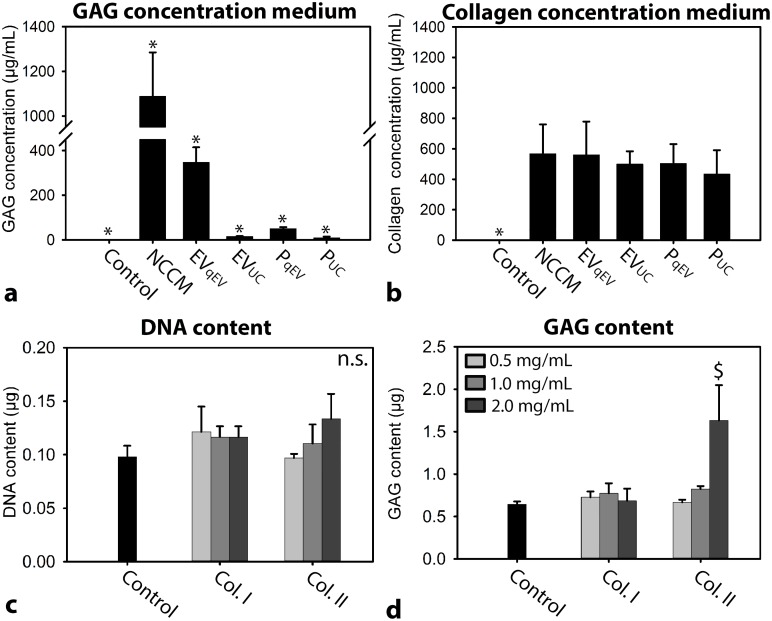
The GAG concentration of unfractionated NCCM, EVqEV, EVUC, PqEV, and PUC media was significantly higher than that of control media (in which GAGs were undetectable; p<0.05; Figure 4a). The GAG concentration of unfractionated NCCM was the highest, followed by EVqEV, PqEV, EVUC, and lastly PUC (p<0.05). This indicates that with 100,000g UC, GAGs were lost. The collagen concentration of unfractionated NCCM, EVqEV, EVUC, PqEV, and PUC media was significantly higher than that of control media (in which collagen was undetectable; p<0.05; Figure 4b). No differences in collagen concentration were detected between NCCM, EV, and P media.
Figure 4.
(a) GAG and (b) collagen concentration of the different culture media. n = 7 porcine NCCM donors. (c) DNA content (d) GAG content (mean + SD) of canine CLC micro-aggregates cultured in basal culture medium (control), supplemented with/without 0.5, 1.0 or 2.0 mg/mL collagen type I or II for 7 days. n = 6, tested on a pool of 4 canine CLC donors. *: significantly different from all other conditions (p<0.05); $: significantly different from control (p<0.05); GAG: glycosaminoglycan. n.s.: not significantly different.
Since in all NCCM-derived culture media collagen was present, it was determined whether collagen alone could exert effects on CLCs when it was applied at a concentration as present in NCCM(-derived EV/P media) (0.5 mg/mL). Interestingly, 0.5 and 1 mg/mL collagen type I or II supplementation did not exert regenerative effects on canine CLCs (Figure 4c and 4d). Only 2.0 mg/mL collagen type II increased the canine micro-aggregates GAG content compared with controls (p<0.05).
Serial dilution of NCCM(-derived EVs and proteins)
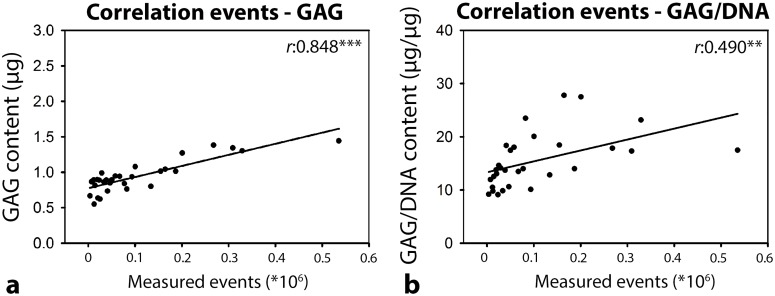
In follow-up experiments, canine CLC micro-aggregates were cultured for 7 days in serial (1-16 times) dilutions of unfractionated NCCM, EVUC, and PUC to determine the dose-dependency. A considerable amount of GAGs was present in PqEV and especially EVqEV, whereas this was almost absent in EVUC and PUC media. Therefore, PUC and EVUC were used in this experiment to exclude GAG interference. No treatment significantly influenced the micro-aggregates’ DNA content (Figure 5a). The micro-aggregates’ GAG content was significantly induced by one until sixteen times diluted unfractionated NCCM (p<0.05, Figure 5b). NCCM-derived EVs and proteins also induced the micro-aggregates’ GAG content, but only until a concentration as present in two (EVUC) or four (PUC) times diluted NCCM (p<0.05). The micro-aggregates’ GAG content significantly decreased with serial dilution of unfractionated NCCM, EVUC, and PUC. The micro-aggregates’ GAG/DNA content was only significantly induced by one times concentrated unfractionated NCCM, NCCM-derived EVs and proteins (p<0.05, Figure 5c). The micro-aggregates’ GAG/DNA content significantly decreased with serial dilution of NCCM-derived EVs, and unfractionated NCCM, but not with serial dilution of NCCM-derived proteins. There was a significant correlation between the total number of EVs in the porcine NCCM 100,000g EV UC pellets and the GAG (r:0.848, p<0.001) and GAG/DNA (r:0.490, p<0.01) content of the canine micro-aggregates treated with these EVs (Figure 6).
Figure 5. Serial dilution of notochordal cell-conditioned medium (NCCM), and NCCM-derived extracellular vesicles (EVs) and proteins (P).
(a) DNA content, (b) GAG content, (c) GAG/DNA content (mean ± SD) of canine CLC micro-aggregates cultured for 7 days in control culture medium, unfractionated porcine NCCM, or porcine NCCM-derived EVs or proteins. EVUC: NCCM-derived EVs obtained after size exclusion chromatography (SEC) and subsequent 100,000g ultracentrifugation (UC), PUC: NCCM-derived proteins obtained after SEC and subsequent 100,000g UC. Grey line represents controls (set at 100%). Black bars indicate significant differences between conditions (p<0.05); *, **,***: significantly different from controls (p<0.05, p<0.01, p<0.001, respectively). n = 6 porcine NCCM, EV, and P donors tested on a pool of 4 canine CLC donors. GAG: glycosaminoglycan. n.s.: not significantly different.
Figure 6.
Pearson correlation between the total number of EVs (as determined by high-resolution flow cytometry) in the sucrose fractions of the porcine notochordal cell-conditioned medium (NCCM) extracellular vesicle (EV) pellets after size exclusion chromatography (SEC) and 100,000g ultracentrifugation (UC) and the GAG (a) and GAG/DNA (b) content of canine CLC micro-aggregates treated with a serial dilution of EVUC (NCCM-derived EVs obtained after SEC and subsequent 100,000g UC). n = 6 porcine NCCM donors tested on a pool of 4 canine CLC donors. **,***: significant correlation (p<0.01, p<0.001, respectively). GAG: glycosaminoglycan.
DISCUSSION
Porcine NCCM contained a considerable amount of EVs which exerted regenerative effects on CLCs
Using SEC, differential UC, density gradient floatation, and quantitative high-resolution flow cytometric analysis, this study is the first to demonstrate that porcine NCs secrete a considerable amount of EVs. We furthermore demonstrate that these EVs have biologic effects across species that suffer from clinical intervertebral disc disease. Notably, NCCM-derived EVs induced GAG deposition in canine CLCs to a comparable level as NCCM-derived soluble proteins and unfractionated NCCM. In contrast, NCCM-derived EVs increased the DNA and GAG content of human CLC micro-aggregates to a similar level as NCCM-derived proteins, but to a lesser extent than unfractionated NCCM. These species-dependent differences could be explained by differences in culture period. Canine micro-aggregate disintegration (in NCCM-derived media, but not in basal culture medium) prohibited a 21-day culture. As such, the 7-day canine CLC culture may be too short to demonstrate the full regenerative potential of NCCM. With serial dilution, NCCM-derived EVs and proteins lost their regenerative effect on canine CLCs earlier than unfractionated NCCM. Taken together, this may indicate that also in canine CLCs, unfractionated NCCM may have a stronger regenerative potential than NCCM-derived EVs or proteins employed at the same concentration. Based on their pronounced loss of biological activity with serial dilution compared with unfractionated NCCM, the EVs may interact with proteins present in NCCM. Notably, there were still some EVs present in the PUC pellets, which are probably smaller in size given the SEC procedure. We cannot exclude that these EVs are another, possibly very potent EV subset. They may, however, also be part of the size continuum of the functional EVs (e.g. not another type, but only smaller EVs).
Correlation between the number of EVs and their regenerative effect
In human CLCs, no linear correlation was found between the number of EVs (when applied at a concentration as present in undiluted NCCM) and their regenerative effect. Previous work could also not find an NCCM dose-dependent effect for neuronal inhibition [20]. In canine CLCs, however, we found a moderate correlation between the number of EVs and the micro-aggregates’ GAG/DNA content after 7-day EVqEV treatment. These results can possibly be explained by the difference in culture period between the two species. After 7 days, canine CLCs were presumably in a dynamic phase of ECM production. After the 21-day human culture, however, secreted GAGs have already been deposited and only show a cumulative effect over time. Additionally, with serial dilution we found a significant positive correlation between the number of EVs and the GAG content of the canine micro-aggregates treated with these EVs. In conclusion, the results of this study may indicate that EVs induce a dose-dependent regenerative effect. However, a maximal response may be reached at an EV concentration as present in undiluted NCCM and/or after 21-day treatment. It remains to be determined if the EV-mediated anabolic effect can be further maximized with higher EV numbers.
Ultracentrifugation mildly affected the biological activity of EVs on human, but not on canine CLCs
In the current study, the effect of 100,000g UC was compared with SEC-based EV purification, since previous work indicated that UC may not fully preserve the EV biological properties [27, 31]. In canine CLCs, 100,000g UC did not affect the biological activity of NC-derived EVs. In contrast, in human CLCs, UC slightly reduced the biological effect of EVs, since the DNA and GAG content of EVqEV-treated micro-aggregates was slightly higher than those of EVUC-treated micro-aggregates. Altogether, we have indications that 100,000g UC only mildly affected the biological activity of NCCM-derived EVs on CLCs from degenerated IVDs. Interestingly, collagen type I deposition was similarly induced by all treatments and collagen type II was only mildly deposited in EVUC- and PUC-treated micro-aggregates. To explain the slight differences in deposited ECM, a comprehensive analysis of the different EV preparations needs to be performed.
NC-derived EVs exert regenerative effects in the absence of GAGs
When EVs were purified with SEC alone, interfering ECM residues (GAGs and collagens) were present. Affirmatively, previous work indicated that preparations obtained by limited processing resulted in ECM-embedded EVs, whereas sequential UC removed this contamination [28]. EVs bind to ECM compartments such as GAGs [32], collagens [33, 34], and hyaluronic acid [35] using integrins and other cell surface receptors such as CD44. With UC, interfering GAGs were lost from the preparations. Importantly, EVUC and PUC devoid of GAGs induced clear regenerative effects, indicating that the regenerative effects of NCCM-derived media were not mediated by GAGs. This finding seems to be in contrast with the notion that the NC-secreted GAG chondroitin sulphate is responsible for NCCM-induced neurite [20] and angiogenesis [36] inhibition. However, it cannot be excluded that neuro- and angiogenesis are differently regulated processes than ECM production.
ECM compartments may provide a favorable micro-environment for CLCs [37]. Unlike GAGs, collagens were not lost with UC. For this reason, we could not determine whether NC-secreted EVs/proteins or collagens were responsible for the regenerative effect induced by EVUC and PUC. Collagen type I or II are the most abundant collagen types in the AF and NP, respectively [12, 38]. When collagen type I and II were supplemented at 0.5 mg/mL (a concentration at which collagen is present in NCCM), they did not induce regenerative effects in CLCs. This may suggest that the regenerative effects of EVUC and PUC were not caused by collagens, but by NC-secreted EVs and other proteins, respectively. Supplementation of 2.0 mg/mL collagen type II, but not collagen type I, induced GAG deposition in canine CLCs. This is in accordance with previous work on human MSCs, in which collagen type II activated MAPK/ERK signalling and was involved in Smad signalling [37, 39].
Conclusions and future perspectives
In conclusion, porcine NCCM contained a considerable amount of EVs. These NC-derived EVs exerted comparable anabolic effects as NCCM-derived proteins, while unfractionated NCCM was more potent in human CLCs. The results of this study further imply that NCCM-derived GAGs and collagens were not responsible for the observed EV-mediated effects. Thus, NC-derived EVs have true regenerative potential. Based on their pronounced loss of biological activity with serial dilution compared with unfractionated NCCM, the EVs may interact with proteins present in NCCM. With a clinical directive in mind, the optimal combination of NC-secreted factors and the role of the EV as carrier need to be determined to fully exploit the regenerative potential of NC-derived treatment strategies. The next step would be to identify the bioactive substances which are transported with the EVs and the subsets of EVs that contain them. Thereafter, the (bioactive substances of the) EV subsets of interest could be used in functional in vivo studies.
MATERIALS AND METHODS
Sources of porcine NC-rich NP tissue and generation of NCCM
Healthy NP tissue was collected from 7 complete porcine spines (1.5 months of age, Thompson grade I) from the slaughterhouse in accordance with national regulations. To compare NCCM-derived EV and protein fractions with unfractionated NCCM in terms of EV and protein concentration, NCCM generation was slightly modified [12]. Briefly, NP tissue (1 gram/20 mL) was cultured for 4 days in hgDMEM+Glutamax (31966, Invitrogen) with 1% P/S (P11-010, GE Healthcare Life Sciences) at 37°C, 5% CO2, 5% O2. After 4 days, tissue was removed by 70 μm cell strainer filtration. The filtrate was centrifuged twice at 200g and 500g (ten minutes each, 4°C) to remove cells and to prevent the release of vesicles due to cell damage [40]. Subsequently, the supernatant was centrifuged at 4000g (45 minutes, 4°C) using 3 kDa Amicon Ultra-15 filter tubes (Merck Millipore) to remove small metabolites and waste products due to altered pH [20]. Substances with a molecular weight >3 kDa were suspended in fresh hgDMEM+Glutamax.
Extracellular vesicle purification from porcine NCCM
EVs present within porcine NCCM were purified by differential UC [40] (Beckman Coulter Optima L-90K ultracentrifuge) and SEC. First, 37 mL of NCCM (previously centrifuged at 200, 500, and 4000g) was centrifuged at 10,000g (SW28 rotor; 4°C; 30 minutes; 8,700 rpm; RCF average 10,016g; RCF max 13,648g; κ-factor 2543.1) to remove cellular debris and apoptotic bodies. The supernatant was aliquoted and stored at -70°C until use. Per donor, 3 mL of 10,000g NCCM supernatant was subjected to qEV SEC-Columns (iZON Science; 1 mL/column) according to the manufacturer’s instructions (Figure 1). The qEV columns were calibrated and eluted with either sterile hgDMEM+Glutamax (one column per donor; control, EVqEV and PqEV conditions, see later) or PBS/0.1% BSA (two columns per donor; EVUC and PUC conditions, see later) before use. PBS/0.1% BSA was depleted from aggregates by overnight (O/N) UC at 100,000g. Twenty-five fractions of 0.5 mL were collected per qEV column and the respective protein concentration was determined (Nanodrop 2000, A280). Based on expected EV sizes and measured protein content, the three fractions with most EVs (between fraction 6 and 11) and proteins (between fraction 18 and 24) were separately collected and pooled per donor (Supplementary Figure 3), yielding 4.5 mL enriched with EVs and 4.5 mL enriched with proteins per donor. One and a half mL hgDMEM+Glutamax with NCCM-derived EVs or proteins were directly employed in culture experiments (EVqEV and PqEV). The remaining 3 mL PBS/0.1% BSA with EVs or proteins was topped up with aggregate-depleted PBS/0.1% BSA in SW40 tubes and ultracentrifuged at 100,000g (SW40 rotor; 4°C; 65 minutes; 23000 rpm; RCF average 10,016g; RCF max 13,648g; κ-factor 2543.1). The 100,000g UC pellets of the pooled EV and protein fractions were separately resuspended in 40 μL aggregate-depleted PBS/0.1% BSA. Thirty-five μL of this 40 μL were employed in culture experiments (EVUC and PUC). To the remaining 5 μL of the 100,000g UC pellets, 15 μL aggregate-depleted PBS/0.1% BSA was added, yielding 20 μL that where further employed for EV characterization.
Figure 1. Schematic representation of the experimental setup.
Notochordal cell-conditioned medium (NCCM) was generated by culturing porcine NC-rich nucleus pulposus tissue for 4 days and (ultra) centrifuged to remove cells and cell debris. For extracellular vesicle (EV) purification, 10,000g NCCM supernatant was subjected to size-exclusion chromatography (SEC). Based on expected EV size and protein measurements, the three fractions with most EVs and proteins (P) were separately collected and pooled (4.5 mL). Part (1.5 mL) was directly used in micro-aggregate culture experiments (EVqEV and PqEV), and part (3 mL) was subjected to ultracentrifugation (UC) at 100,000g for EV enrichment. The 100,000g UC pellet (40 μL) was then partly (35 μL) used in culture experiments (EVUC and PUC), whereas the remainder (5 μL) was used for quantitative EV analysis using high-resolution flow cytometric analysis. n = 7 porcine NCCM donors, tested on a pool of 4 canine (in triplo) and 4 human chondrocyte-like cell donors (in duplo) in culture.
NCCM extracellular vesicle characterization
The EVs present in both 100,000g UC pellets (containing either EVs or proteins) were labelled with PKH67 (MIDI67, Sigma-Aldrich), floated to their buoyant density by O/N sucrose density gradient floatation, and quantitatively analyzed by high-resolution flow cytometry (BD Influx) as described previously [40]. In brief, 100,000g UC pellets were mixed with 180 μL Diluent C (MIDI67, Sigma-Aldrich) and 1.5 μL PKH67 and incubated for 3 minutes in SW40 tubes. One hundred μL IMDM (BE12-726F, Lonza) containing 10% EV-depleted Fetal Bovine Serum (generated by O/N UC at 100,000g) was added to stop the labelling process. These suspensions were mixed with 1.5 mL 2.5 M sucrose and overlaid with fifteen 700 μL sucrose fractions with decreasing molarity (2.0 until 0.4 M) in SW40 tubes. Sucrose gradients were then subjected to density gradient floatation for 16 hours at 200,000g (SW40 rotor; 4°C; 39,000 rpm; RCF average 192,072g; RCF max 270,519g; κ-factor 144.5). Twelve sucrose gradient fractions of 1 mL were collected from which the density was determined by refractometry. Lastly, density fractions were 20 times diluted in PBS (50 μL sample + 950 μL PBS) and analyzed for EV content using high-resolution flow cytometry, according to an earlier described method [40]. Detergents (0.1% Triton X-100 and 0.1% SDS) were added to the fraction with the highest number of events to determine whether measured events were truly EVs [29]. Additionally, serial dilution of the fraction with the highest number of measured events was performed to exclude swarm detection [30]. Data were analyzed using FlowJo software.
Cell culture
Canine and human CLCs were collected from early degenerated IVDs (Thompson score II-III) as described previously [12]. Briefly, NP tissue was digested with 0.15% pronase (45 minutes) and 0.15% collagenase type II (O/N) at 37°C. Complete canine spines were collected from dogs euthanized in unrelated research studies, approved by the Utrecht University Animal Ethics Committee. Human IVDs (L2-L5, ≤48 hours after death) were obtained in the course of standard post mortem diagnostics, as approved by the scientific committee of the Pathology department of the University Medical Centre Utrecht (UMCU). Anonymous use of redundant tissue for research purposes is part of the standard treatment agreement with UMCU patients (Local Medical Ethical Committee number 12-364). The material was used in line with the code ‘Proper Secondary Use of Human Tissue’, installed by the Federation of Biomedical Scientific Societies.
CLCs from four canine (2-10 years of age, Beagles) and four human (50-63 years of age) donors were expanded as described previously [12]. At passage 2, the CLCs were pooled per species to assess the effect of donor-specific (EVs/proteins from) porcine NCCM on a representative human and canine CLC population. For micro-aggregate formation, 35,000 CLCs were plated per well in low-adherence cell-repellent surface 96-well plates (650970, CELLSTAR® Greiner Bio-one) in 50 μL basal culture medium (hgDMEM+Glutamax with 1% P/S, 1% ITS+ premix (354352, Corning Life Sciences), 0.04 mg/mL L-proline (P5607, Sigma-Aldrich), 0.1 mM Ascorbic acid 2-phosphate (A8960, Sigma-Aldrich), 1.25 mg/mL Bovine Serum Albumin (A9418, Sigma-Aldrich)) supplemented with 10 ng/mL TGF-β1 (240-B-010, R&D Systems). The 96-well plates were centrifuged at 50g for 5 minutes to induce micro-aggregate formation. The next day, the culture medium was replaced with (a) hgDMEM+Glutamax that underwent SEC using qEV columns (similarly as 10,000g NCCM supernatant; control), (b) 10,000g NCCM supernatant (NCCM), (c) NCCM-derived EVs obtained by SEC (EVqEV), (d) NCCM-derived proteins obtained by SEC (PqEV), (e) NCCM-derived EVs obtained by SEC followed by 100,000g UC (EVUC), or (f) NCCM-derived proteins obtained by SEC followed by 100,000g UC (PUC). All different culture media were supplemented with the factors as present in basal culture medium. EVs and proteins were applied to the CLCs at a similar concentration as present in 10,000g NCCM supernatant. EVUC and PUC conditions were included to determine whether 100,000g UC affected the biological activity of the NCCM-derived EVs/proteins. The micro-aggregates were cultured for 7 (canine) or 21 (human) days at 37°C, 5% CO2, 5% O2. Canine CLC micro-aggregates disintegrated after 7 days of culture in NCCM(-derived factors), which prevented a 21-day culture. Culture medium was changed twice weekly.
The micro-aggregates’ DNA (dsDNA High Sensitivity Assay Kit, Q32851, Invitrogen) and GAG content (DMMB assay [41]) were determined at day 7 (canine) or 21 (human) as described previously [12] (n=7, in duplo (human) or triplo (canine)). Since canine CLC micro-aggregates disintegrated after about 7 days of culture in NCCM(-derived factors), only human CLC micro-aggregates were histologically analyzed. Safranin O/Fast Green staining and collagen type I and II immunohistochemistry were performed as described previously [12]. The collagen and GAG concentration of the different culture media was analyzed using a hydroxyproline [42] and DMMB [41] assay, respectively.
In follow up culture experiments, canine CLCs were cultured for 7 days in basal culture medium, supplemented with or without 0.5, 1 and 2 mg/mL collagen type I (C9791, Sigma-Aldrich) or II (C9301, Sigma-Aldrich) to determine whether collagen (applied at a concentration as present in NCCM, EVqEV, EVUC, PqEV and PUC) exerted regenerative effects on CLCs. Lastly, canine CLC micro-aggregates were cultured for 7 days in serial dilutions of 10,000g NCCM supernatant (NCCM), EVUC, and PUC (1, 1:2, 1:4, 1:8 and 1:16 times concentrated) to determine when NCCM(-derived factors) lost their regenerative potential. The different media were diluted in basal culture medium. The same canine CLC and porcine NCCM donors were used as described earlier. The micro-aggregates’ DNA and GAG content were determined as described previously.
Statistical analysis
Statistical analysis was performed using IBM SPSS (version 24). All data were examined for normal distribution (Shapiro Wilks test). Kruskal Wallis and Mann-Whitney U tests were performed on non-normally distributed data, whereas general linear regression models based on ANOVAs were used for normally distributed data. Benjamini & Hochberg False Discovery Rate post-hoc corrections for multiple comparisons were performed. To find correlations between the number of measured events and the micro-aggregates’ GAG(/DNA) content, Pearsons correlations were determined. A p-value <0.05 was considered significant.
SUPPLEMENTARY MATERIALS FIGURES
Acknowledgments
The authors would like to thank Willem de Jong and Stefan Wouters for help with the execution of experiments and Anita Krouwels and Imke Jansen for supplying human CLCs.
Abbreviations
- AF
annulus fibrosus
- CLC
chondrocyte-like cell
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- EV
extracellular vesicles
- GAG
glycosaminoglycan
- IVD
intervertebral disc
- MSC
mesenchymal stromal cell
- NC
notochordal cell
- NCCM
notochordal cell-conditioned medium
- NP
nucleus pulposus
- P
protein
- SEC
size-exclusion chromatography
- TGF-β1
transforming growth factor-β1
- UC
ultracentrifugation
Author contributions
The manuscript has been read and approved by ALL named authors. There are no other persons who satisfied the criteria for authorship. Each author has contributed to a minimum of two of the four major parts of the submitted work and there are no “Gift Authorships”. Given the extent of the study, 7 authors have been listed. They contributed as follows: FCB collected material, performed most of the experiments, mined and analyzed the data, participated in the design of the study, and drafted the manuscript. SFWML assisted in performance of experiments, mined the data and participated in the study design. LBC provided human CLCs. MHMW, LBC, KI, BPM, MAT contributed to the study design and data analysis. MAT conceived the study, and coordinated the process.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This work was funded by an AOSpine International (SRN2011_11) and the Dutch Arthritis Foundation (LLP22 and LLP12).
REFERENCES
- 1.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg. 2006;88:21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 2.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 3.Zheng CJ, Chen J. Disc degeneration implies low back pain. Theor Biol Med Model. 2015;12:24. doi: 10.1186/s12976-015-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergknut N, Rutges JP, Kranenburg HJ, Smolders LA, Hagman R, Smidt HJ, Lagerstedt AS, Penning LC, Voorhout G, Hazewinkel HA, Grinwis GC, Creemers LB, Meij BP, et al. The dog as an animal model for intervertebral disc degeneration? Spine. 2012;37:351–358. doi: 10.1097/BRS.0b013e31821e5665. [DOI] [PubMed] [Google Scholar]
- 5.Tellegen AR, Willems N, Beukers M, Grinwis GC, Plomp SG, Bos C, van Dijk M, de Leeuw M, Creemers LB, Tryfonidou MA, Meij BP. Intradiscal application of a PCLA-PEG-PCLA hydrogel loaded with celecoxib for the treatment of back pain in canines: what’s in it for humans? J Tissue Eng Regen Med. 2017 doi: 10.1002/term.2483. [DOI] [PubMed] [Google Scholar]
- 6.Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239:2141–2148. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergknut N, Smolders LA, Grinwis GC, Hagman R, Lagerstedt AS, Hazewinkel HA, Tryfonidou MA, Meij BP. Intervertebral disc degeneration in the dog. Part 1: anatomy and physiology of the intervertebral disc and characteristics of intervertebral disc degeneration. Vet J. 2013;195:282–291. doi: 10.1016/j.tvjl.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Benneker LM, Andersson G, Iatridis JC, Sakai D, Hartl R, Ito K, Grad S. Cell therapy for intervertebral disc repair: advancing cell therapy from bench to clinics. Eur Cell Mater. 2014;27:5–11. doi: 10.22203/ecm.v027sa02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach FC, Willems N, Penning LC, Ito K, Meij BP, Tryfonidou MA. Potential regenerative treatment strategies for intervertebral disc degeneration in dogs. BMC Vet Res. 2014;10:3. doi: 10.1186/1746-6148-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159–171. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Purmessur D, Cornejo MC, Cho SK, Hecht AC, Iatridis JC. Notochordal cell-derived therapeutic strategies for discogenic back pain. Global Spine J. 2013;3:201–218. doi: 10.1055/s-0033-1350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach FC, de Vries SA, Krouwels A, Creemers LB, Ito K, Meij BP, Tryfonidou MA. The species-specific regenerative effects of notochordal cell-conditioned medium on chondrocyte-like cells derived from degenerated human intervertebral discs. Eur Cell Mater. 2015;30:132–146. doi: 10.22203/ecm.v030a10. [DOI] [PubMed] [Google Scholar]
- 13.Abbott RD, Purmessur D, Monsey RD, Iatridis JC. Regenerative potential of TGFbeta3 + Dex and notochordal cell conditioned media on degenerated human intervertebral disc cells. J Orthopaed Res. 2012;30:482–488. doi: 10.1002/jor.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potier E, de Vries S, van Doeselaar M, Ito K. Potential application of notochordal cells for intervertebral disc regeneration: an in vitro assessment. Eur Cell Mater. 2014;28:68–80. doi: 10.22203/ecm.v028a06. [DOI] [PubMed] [Google Scholar]
- 15.Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purmessur D, Schek RM, Abbott RD, Ballif BA, Godburn KE, Iatridis JC. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res Ther. 2011;13:R81. doi: 10.1186/ar3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries SA, Potier E, van Doeselaar M, Meij BP, Tryfonidou MA, Ito K. Conditioned medium derived from notochordal cell-rich nucleus pulposus tissue stimulates matrix production by canine nucleus pulposus cells and bone marrow-derived stromal cells. Tissue Eng. 2015;21:1077–1084. doi: 10.1089/ten.tea.2014.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries SA, van Doeselaar M, Meij BP, Tryfonidou MA, Ito K. The stimulatory effect of notochordal cell-conditioned medium in a nucleus pulposus explant culture. Tissue Eng. 2016;22:103–110. doi: 10.1089/ten.TEA.2015.0121. [DOI] [PubMed] [Google Scholar]
- 19.Matta A, Karim MZ, Isenman DE, Erwin WM. Molecular therapy for degenerative disc disease: clues from secretome analysis of the notochordal cell-rich nucleus pulposus. Sci Rep. 2017;7:45623. doi: 10.1038/srep45623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purmessur D, Cornejo MC, Cho SK, Roughley PJ, Linhardt RJ, Hecht AC, Iatridis JC. Intact glycosaminoglycans from intervertebral disc-derived notochordal cell-conditioned media inhibit neurite growth while maintaining neuronal cell viability. Spine J. 2015;15:1060–1069. doi: 10.1016/j.spinee.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erwin WM, Ashman K, O’Donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- 22.Gantenbein B, Calandriello E, Wuertz-Kozak K, Benneker LM, Keel MJ, Chan SC. Activation of intervertebral disc cells by co-culture with notochordal cells, conditioned medium and hypoxia. BMC Musculoskeletal Disord. 2014;15:422. doi: 10.1186/1471-2474-15-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malda J, Boere J, van de Lest CH, van Weeren PR, Wauben MH. Extracellular vesicles - new tool for joint repair and regeneration. Nat Rev Rheumatol. 2016;12:243–249. doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva AM, Teixeira JH, Almeida MI, Goncalves RM, Barbosa MA, Santos SG. Extracellular vesicles: immunomodulatory messengers in the context of tissue repair/regeneration. Eur J Pharm Sci. 2017;98:86–95. doi: 10.1016/j.ejps.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Bach FC, de Vries SA, Riemers FM, Boere J, van Heel FW, van Doeselaar M, Goerdaya SS, Nikkels PG, Benz K, Creemers LB, Maarten Altelaar AF, Meij BP, Ito K, et al. Soluble and pelletable factors in porcine, canine and human notochordal cell-conditioned medium: implications for IVD regeneration. Eur Cell Mater. 2016;32:163–180. doi: 10.22203/ecm.v032a11. [DOI] [PubMed] [Google Scholar]
- 27.Nordin JZ, Lee Y, Vader P, Mager I, Johansson HJ, Heusermann W, Wiklander OP, Hallbrink M, Seow Y, Bultema JJ, Gilthorpe J, Davies T, Fairchild PJ, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11:879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Paolini L, Zendrini A, Di Noto G, Busatto S, Lottini E, Radeghieri A, Dossi A, Caneschi A, Ricotta D, Bergese P. Residual matrix from different separation techniques impacts exosome biological activity. Sci Rep. 2016;6:23550. doi: 10.1038/srep23550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osteikoetxea X, Sodar B, Nemeth A, Szabo-Taylor K, Paloczi K, Vukman KV, Tamasi V, Balogh A, Kittel A, Pallinger E, Buzas EI. Differential detergent sensitivity of extracellular vesicle subpopulations. Org Biomol Chem. 2015;13:9775–9782. doi: 10.1039/c5ob01451d. [DOI] [PubMed] [Google Scholar]
- 30.Groot Kormelink T, Arkesteijn GJ, Nauwelaers FA, van den Engh G, Nolte-’t Hoen EN, Wauben MH. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytometryt A. 2016;89:135–147. doi: 10.1002/cyto.a.22644. [DOI] [PubMed] [Google Scholar]
- 31.Deregibus MC, Figliolini F, D’Antico S, Manzini PM, Pasquino C, De Lena M, Tetta C, Brizzi MF, Camussi G. Charge-based precipitation of extracellular vesicles. Int J Mol medicine. 2016;38:1359–1366. doi: 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu W, Rana S, Zoller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15:875–887. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayanan R, Huang CC, Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016;2016:3808674. doi: 10.1155/2016/3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boere J, van de Lest CH, Libregts SF, Arkesteijn GJ, Geerts WJ, Nolte-’t Hoen EN, Malda J, van Weeren PR, Wauben MH. Synovial fluid pretreatment with hyaluronidase facilitates isolation of CD44+ extracellular vesicles. J Extracell Vesicles. 2016;5:31751. doi: 10.3402/jev.v5.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornejo MC, Cho SK, Giannarelli C, Iatridis JC, Purmessur D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthritis Cartilage. 2015;23:487–496. doi: 10.1016/j.joca.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu ZF, Doulabi BZ, Wuisman PI, Bank RA, Helder MN. Influence of collagen type II and nucleus pulposus cells on aggregation and differentiation of adipose tissue-derived stem cells. J Cell Mol Med. 2008;12:2812–2822. doi: 10.1111/j.1582-4934.2008.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao Y, Zhou X, Liu D, Li H, Liang C, Li F, Chen Q. Proportion of collagen type II in the extracellular matrix promotes the differentiation of human adipose-derived mesenchymal stem cells into nucleus pulposus cells. Biofactors. 2016;42:212–223. doi: 10.1002/biof.1266. [DOI] [PubMed] [Google Scholar]
- 40.van der Vlist EJ, Nolte-’t Hoen EN, Stoorvogel W, Arkesteijn GJ, Wauben MH. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc. 2012;7:1311–1326. doi: 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- 41.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 42.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184:299–306. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.