Abstract
Computational analysis of promoters is hindered by the complexity of their architecture. In less studied genomes with complex organization, false positive promoter predictions are common. Accurate identification of transcription start sites and core promoter regions remains an unsolved problem. In this paper, we present a comprehensive analysis of genomic features associated with promoters and show that probabilistic integrative algorithms-driven models allow accurate classification of DNA sequence into “promoters” and “non-promoters” even in absence of the full-length cDNA sequences. These models may be built upon the maps of the distributions of sequence polymorphisms, RNA sequencing reads on genomic DNA, methylated nucleotides, transcription factor binding sites, as well as relative frequencies of nucleotides and their combinations. Positional clustering of binding sites shows that the cells of Oryza sativa utilize three distinct classes of transcription factors: those that bind preferentially to the [-500,0] region (188 “promoter-specific” transcription factors), those that bind preferentially to the [0,500] region (282 “5′ UTR-specific” TFs), and 207 of the “promiscuous” transcription factors with little or no location preference with respect to TSS. For the most informative motifs, their positional preferences are conserved between dicots and monocots.
Introduction
Core promoters are the 5' regions adjacent to the transcriptional start site (TSS) and containing binding sites for transcription factors (TFBS). Computational analysis of the eukaryotic promoters is hindered by their complex architecture [1–3]. Each gene contains one or more TSS, and, respectively, one or more promoters, which initiate transcription of a gene. Depending on species, from 30% to 60% of eukaryotic genes contain the TATA motif approximately 30 nucleotides upstream of TSS. Most commonly, TATA-containing core promoters are associated with stress-related, tissue-specific and/or highly expressed genes [4]. Broadly expressed genes frequently have TATA-less promoters with a relatively broad transcription start region (TSR) replacing pronounced TSS [3, 5]. To predict the position of the TSR, characteristic promoter initiation regions (Inr) or the downstream promoter elements (DPE) may be used [1, 2].
A majority of TSS prediction software tools use sophisticated algorithms, such as oligonucleotide content-based neural network and linear discriminant approaches, while focusing on specific sequence features of the promoter region (e.g. TATA-box or CA-motif) [6]. Genome complexity affects quality of promoter predictions: for example, presence of several tissue-specific, alternative TSSs negatively affects the prediction accuracy. For the model plant Arabidopsis thaliana, modern algorithms identify TATA-containing promoters with sensitivities up to 95% and specificities up to 97% [2, 4, 5, 7–10]. For Homo sapiens and Oryza sativa prediction accuracies are substantially lower [10]. In case of even less studied genomes with complex organization, false positive and false negative error rates can be large, with a spurious promoter prediction occurring once per every 700–1000 nucleotides of the genome [11].
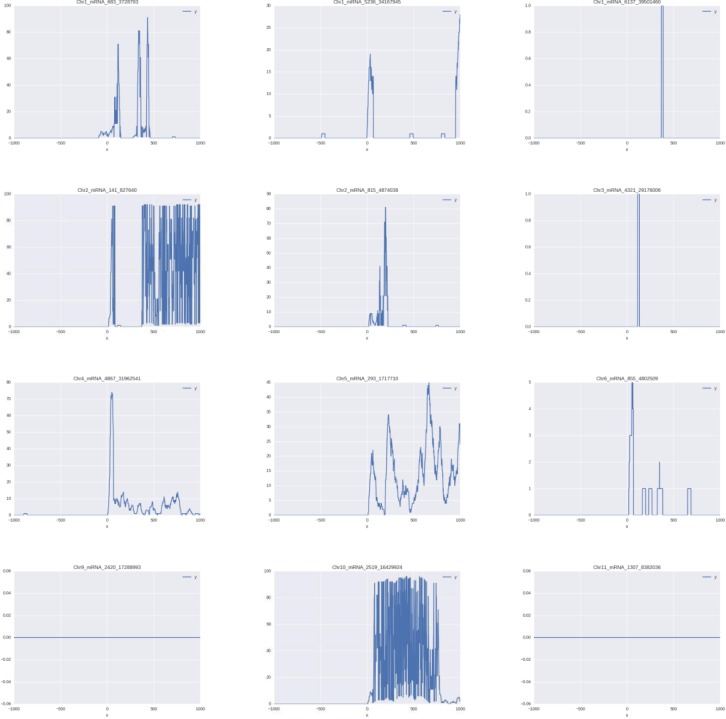
Even the best modern methods of promoter mapping, including genomic sequencing coupled with full-length cDNA capture and ascertainment [4, 12, 13], CAGE [14, 15], 3PEAT[16], or RAMPAGE [17] are incapable to predict TSS positions with 100% accuracy [5]. For example, the mapping of CAGE tags onto existing human cDNA/mRNA sequences revealed that less than 10% of these tags fall within 10 nucleotides from TSS [18]. To illustrate this, we mapped RNA-Seq reads onto the regions TSS+/-1000 nt corresponding to 12 well-studied, experimentally validated O. sativa promoters from Plant Prom DB [3, 19], the resultant plots showed that the peaks of RNA-Seq coverage did not match the positions of known TSS, supporting the idea that correct mapping of eukaryotic promoters possibly requires multiple sources of data (Fig 1).
Fig 1. RNA-Seq coverage near 12 randomly selected promoters with experimentally validated transcription start sites.
As me mentioned above, promoters contain transcription factor binding sites (TFBS) regulating transcription. Two most commonly used techniques to predict eukaryotic promoter by distribution of TFBS were proposed in 1995 by Kondrakhin and Kel [20] and by Prestridge [21]. The method of Kondrakhin and Kel [20] pairs up the detection of TATA boxes with the distribution of computed weight matrices of TFBS, improving the prediction accuracy compared to using the TATA box alone. Prestridge [21] combined density ratios of all individual TFBSs into a scoring profile, which was further augmented by the weighted TATA matrix. This approach reported a relatively low false positive rate. Real-world applicability of both tools, however, remains limited due to lack of species-specific TFBS models for training and failure to pinpoint locations of individual TSSs.
In the last decade, several improvements in the promoter prediction process were made. Troukhan [22] combined positional frequency of 5′ EST matches onto genomic DNA with the gene models. This approach, known as TSSer, is, in a nutshell, a deterministic method that predicts one transcription start site per locus. For Arabidopsis thaliana promoters, it achieves remarkable accuracy. However, even the most reliable prediction of a single promoter per gene cannot adequately reflect biological complexity underlying its regulation due to common occurrence of alternative promoters, which are often tissue-specific or responsive to the changes in architecture of chromatin [23]. In 2013, the TSSer approach was improved by incorporation of a non-parametric maximum likelihood approach to be reborn as NPEST algorithm [5], that allows prediction positions of alternative TSSs in the A. thaliana genome with better accuracy than the sequences identified in the several “gold standard” databases, such as TAIR [24, 25], Plant Prom DB [19] and Plant Promoter Database [26]. For example, for the set of 15,875 Arabidopsis promoters derived by both TAIR and NPEST, 11,304 (71%) were predicted within 50 nucleotides of each other, and 7,192 (45%) within 10 nucleotides of each other. Thirty percent of TAIR-predicted and 44% of NPEST-predicted promoters identified the “TATA” sequence within the interval [–40, –20] nucleotides upstream from the respective TSS. At the TSS, nucleotide consensus scores (46% of T and 49% of C followed by 65% of A) were stronger for NPEST then for TAIR (43% of T and 35% of C followed by 53% of A). When NPEST predictions were compared to experimentally confirmed promoters from other databases, similar patterns of nucleotide consensus were observed.
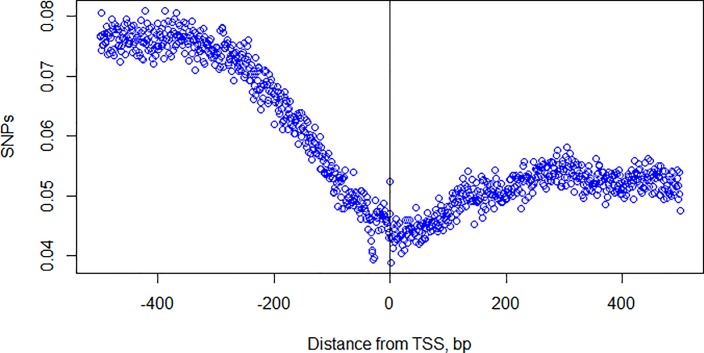
Recently, many more types of experimental and computational observations highlighting the TSS positions became available. For example, forty million single nucleotide polymorphisms (SNPs) from the 3,000 Rice Genomes Project (http://snp-seek.irri.org), the largest and the most dense SNP collection for higher plants [27], were shared to facilitate an analysis of genetic variants across the Oryza sativa cultivars [28]. Observed clusters of reduced nucleotide variability were shown to highlight functionally important genomic regions. Interestingly, a sharp decline in SNP density was noted about 250 nucleotides upstream of TSS elements; this decline reaches its minimum exactly at the TSS.
In plant genes with multiple promoters, precise mapping of TSSs requires incorporation of diverse data types including tissue/stress specificity of each transcript. Unfortunately, most of currently available techniques cannot incorporate a variety of available data, and also must ignore alternative promoters. Therefore, accurate identification of TSS and core promoter regions remains an open problem. Since evidences for location of TSS are imprecise, the best approach for promoter production should embed probabilistic integrative algorithms. In this paper, we present a comprehensive analysis of genomic features associated with the promoters and show that probabilistic integrative algorithms-driven models allow accurate classification of DNA sequence into “promoters” and “non-promoters” even in absence of full-length cDNA sequences. These models may be built upon the maps of the distributions of SNPs, RNA sequencing reads on genomic DNA, methylated nucleotides, TFBS as well as relative frequencies of nucleotides and their combinations.
Results
Selection of the “gold standard” gene prediction models
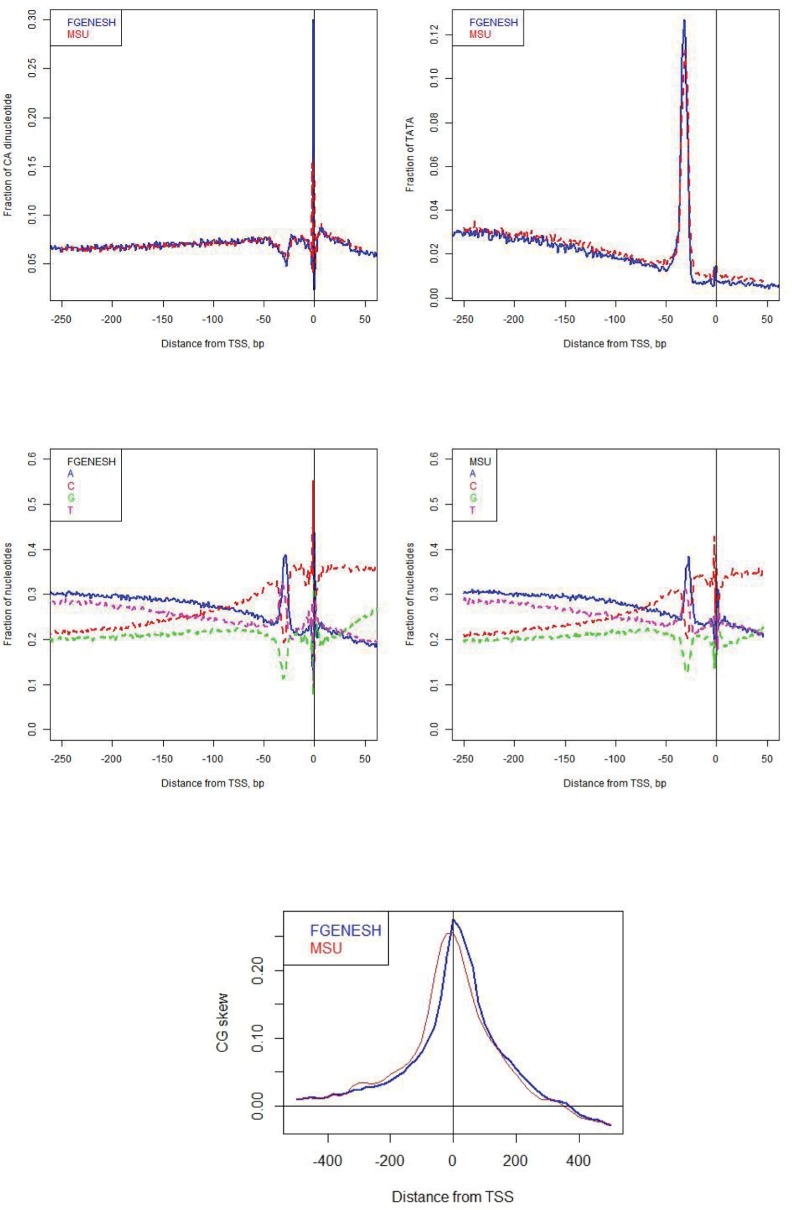
To aid a selection of the best available rice genome annotation, Fgenesh and MSU mRNA-based gene prediction models were compared. Fgenesh gene prediction set contains 18,389 high quality (5′ full, with mRNA support) gene models, while the MSU gene prediction set contains 20,367 high quality gene models [28, 29]. For every gene in both models, we extracted a 1,000 nt long sequence centered at the TSS, and calculated distributions of genomic features previously associated with the start of transcription: (1) frequency of dinucleotide CA [1, 30, 31]; (2) frequency of TATA [1, 4, 32]; (3) nucleotide consensus around TSS [12, 13, 33]; (4) CG skew ( where #C and #G refers to the counts of nucleotides C and G in a certain genomic window) [34]. Fig 2C and 2D shows that Fgenesh-annotated promoters have a more pronounced nucleotide consensus as compared to the promoters annotated by MSU. Fgenesh promoters also have higher frequency of the exact TATA motif at -30 (B), and more CA dinucleotides at the position of TSS (A). Fig 2(F) shows peak of the CG skew at TSS, calculated in the window of 40 nt both annotations; Fgenesh-annotated CG skew peak is higher than the MSU one. Based on the assumption that these features reliable reflect the quality of promoter annotation, for further analysis the Fgenesh model was selected.
Fig 2. Features of the nucleotide consensus around TSS.
A top left) Frequency of CA, B top right) Frequency of TATA motif, D middle feft) Frequencies of nucleotides A, C, G, T around TSS for Fgenesh, E middle right) Frequencies of A, C, G, T around TSS for MSU, F bottom) CG skew (), calculated in the window of 40 nt.
Distribution of transcription factor binding sites
The distributions of the transcription factor binding sites (TFBS) in promoters and the UTRs of high-confidence rice genes in the regions of -1000 +1000 around TSS were investigated with MATCH algorithm [35] incorporated in geneXplain platform (www.genexplain.com). MATCH uses the TRANSFAC database [36] comprising 764 plant position weight matrices (PWM) with a strict similarity score threshold of 0.95. MATCH scans the targets promoter sequences with a sliding window equal to the length of the PWM and calculates a score for each of the windows. The maximum value of the score (1.0) corresponds to the sequence that fully fits the consensus of the given PWM. Score threshold of 0.95 allows very little mismatches to the consensus, with few permitted mismatches limited to less conserved positions. In addition, the MATCH score considers the nucleotide position-specific entropy measures. In a recent study, MATCH performed with accuracy superior to other motif-finding algorithms [37].
In the Fgenesh-predicted rice promoters, MATCH search against the TRASNFAC database resulted in mapping of 3.2 million potential TFBS corresponding to 667 plant PWMs, while 97 PWMs remained matchless, possibly due to their exclusive role in the dicots or to the binding to distal promoters not analyzed in the present study (see S2 Table). Interestingly, 487 out of 667 TFBS (73%) were found in proximal promoters of Oryza sativa more than 1000 times; the most frequent sites were that for the transcription factors ASR1, DOF56 and PBF. When the frequencies of TFBS found in the proximal promoters were compared with the frequencies for the same PWMs found in randomly shuffled sequences, the most significant promoter-specific TFBS enrichments (twice or more) were observed for SPL12, SPL5, GBF1, ABI5, BZIP68, LEC2, and GT1 transcription factors.
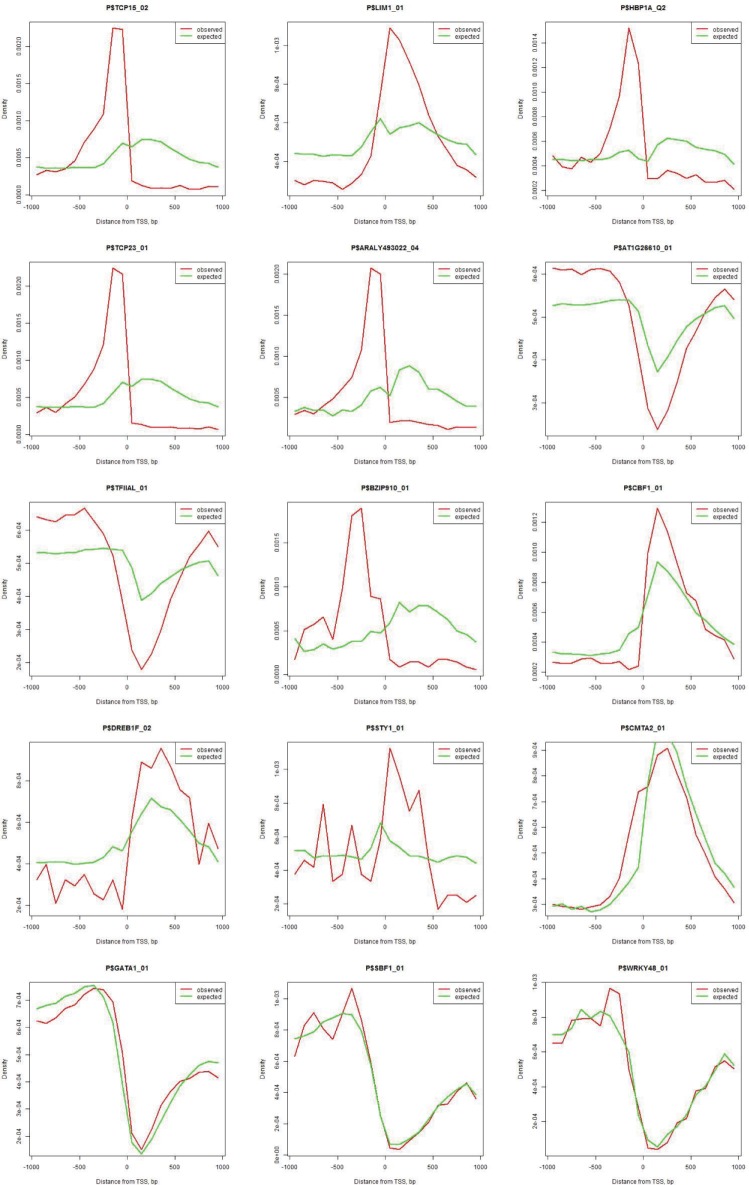
To account for dinucleotide statistics matching that of the Fgenesh rice promoter regions, another set of randomly shuffled sequences was generated as described by Stepanova, Tiazhelova [38]. Briefly, the 2000 nt regions [TSS-1000, TSS+1000] were divided onto non-overlapping 100 nt windows, then the dinucleotide statistics were calculated for each window. For each promoter, a 2000 nt long sequence with matching dinucleotide composition was generated and subjected to MATCH prediction of TFBS [35]. After selecting the motifs that occur at least in 100 different rice promoters, Kolmogorov-Smirnov test was applied to find significantly over-represented sequence motifs (S3 Table). Fig 3 shows examples of TFBS that occur at frequencies that differ and do not differ significantly between real and simulated sequences. The most pronounced differences (p-value < 0.002) were detected for the distributions of binding sites for TCP15, LIM1, HBP1A, and TCP23. On the other hand, occurrences of binding sites for CMTA2, GATA1, SBF1, and WRKY48 in real and simulated sequences were not different (p-value> 0.99999).
Fig 3. Examples of observed and expected occurrences of TFBS in rice promoters.
Different: TCP15, LIM1, HBP1A, TCP23, ARALY493022, AT1G26610, TFIIAL, BZIP910, CBF1, DREB1F, STY1. Observations agree with expectations: CMTA2, GATA1, SBF1, WRKY48.
Positional specificity of TFBS distribution
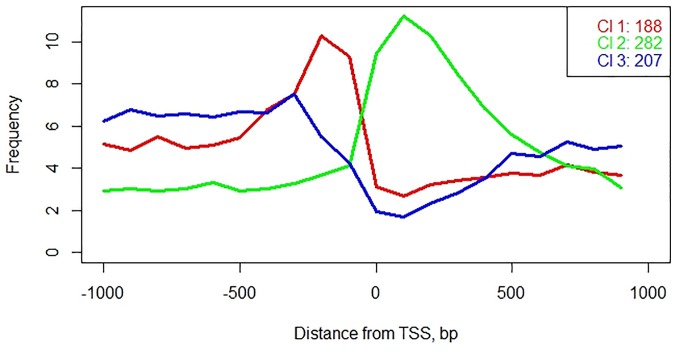
A phenomenon of the positional preference in TF binding was previously described by Weirauch, Yang [39], who showed that positions of TFBS are not randomly distributed in respect to the start of transcription (TSS); this observation holds across evolutionary kingdoms. To illustrate this phenomenon in rice, we divided the [TSS-1000, TSS+1000] regions into 100 nt long bins and calculated frequency histograms of TFBS occurrence in each bin; then we used K-means algorithm to cluster these histograms, with value at each bin treated as a separate dimension.
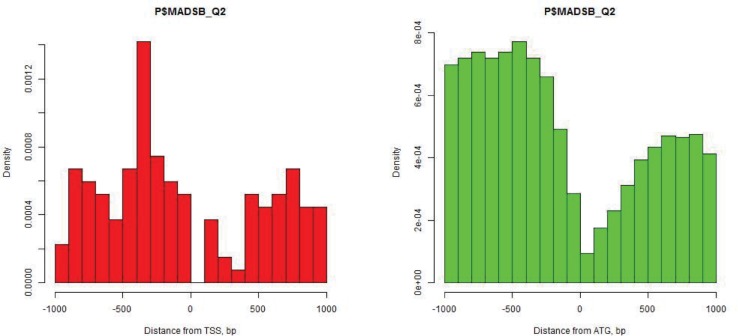
Positional clustering of TFBS demonstrates that the cells of Oryza sativa utilize three distinct classes of transcription factors: Class 1, which binds preferentially to the [-500,0] region (“promoter-specific”, N = 188); Class 2, which binds preferentially to the [0,500] region (“5′ UTR-specific”, N = 282); and Class 3, which includes predominantly “promiscuous” transcription factors with weak or no location preference for respective TSS (N = 207), see S4 Table and Fig 4. Note that some Class 3 TFs cannot be classified as promiscuous (Fig 5) as they are characterized by regular patterns of positional distribution. around the translation start rather than around the transcription start. Examples of the position frequency preference are shown in the Fig 6.
Fig 4. Positional specificity of TFBS distribution.
Fig 5. The distribution pattern for MADSB binding sites highlight the start codon (ATG) rather than the respective TSS.
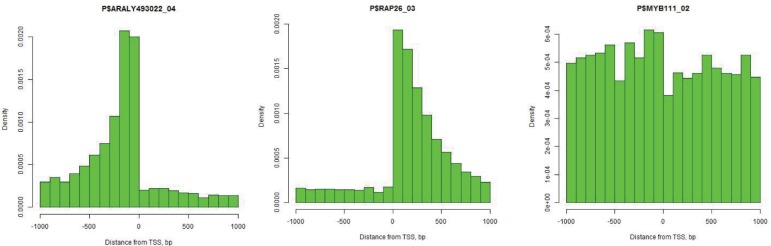
Fig 6. Frequency distributions of TFBS may have different patterns around the start of transcription (position 0 on the horizontal axis).
X-axis shows the distance from TSS, Y-axis reflects the frequency of motif in each window. Frequencies of ARALY493022_04 TFBS (Class 1) are plotted on the left panel, of RAP26_03 TFBS (Class 2) on the middle panel, and of MYB111_02 (Class 3) on the right panel.
To conduct the comparative gene ontology analysis of Class 1, 2 and 3 transcription factors (Table 1), the chi-square “Goodness of Fit” tests were used: , where Oi and Ei correspond to observed and expected numbers of genes in ith category.
Table 1. GO categories that are significantly different between three TF classes.
| GO | Class 1 | Class 2 | Class 3 | P-value |
|---|---|---|---|---|
| sequence-specific DNA binding | 43 | 22 | 61 | 7.78E-06 |
| protein dimerization activity | 21 | 4 | 18 | 0.000438 |
| systemic acquired resistance, salicylic acid mediated signaling pathway | 15 | 2 | 6 | 0.000504 |
| regulation of transcription from RNA polymerase II promoter | 5 | 2 | 16 | 0.000558 |
| response to bacterium | 14 | 3 | 4 | 0.000955 |
| jasmonic acid mediated signaling pathway | 15 | 4 | 5 | 0.001897 |
| carpel development | 6 | 1 | 13 | 0.002606 |
| protein binding | 51 | 31 | 35 | 0.002904 |
| negative regulation of defense response | 11 | 1 | 5 | 0.003011 |
| DNA binding | 90 | 146 | 85 | 0.007339 |
| protein targeting to membrane | 14 | 4 | 7 | 0.010643 |
| regulation of plant-type hypersensitive response | 14 | 4 | 7 | 0.010643 |
| ethylene-activated signaling pathway | 10 | 20 | 4 | 0.012518 |
| response to water deprivation | 48 | 76 | 38 | 0.016138 |
| plant ovule development | 12 | 4 | 15 | 0.017373 |
| Nucleus | 76 | 126 | 75 | 0.017489 |
| response to ozone | 9 | 4 | 14 | 0.033868 |
| cellular response to nitrogen levels | 13 | 11 | 23 | 0.044478 |
Total number of genes with GO categories for Class 1, 2 and 3 were 130, 164, and 144, respectively. P-values were calculated using the chi-square “Goodness of Fit” procedure.
Class 1 TFs of the rice are enriched in the following GO terms: “sequence-specific DNA binding”, “protein dimerization activity”, “systemic acquired resistance, salicylic acid mediated signaling pathway”, “regulation of transcription from RNA polymerase II promoter”, “response to bacterium”, “jasmonic acid mediated signaling pathway”, “carpel development”, “protein binding”, “negative regulation of defense response”, “protein targeting to membrane”, “regulation of plant-type hypersensitive response”, “plant ovule development”, “response to ozone”. Class 2 TFs are enriched in GO terms “DNA binding”, “ethylene-activated signaling pathway”, “response to water deprivation”. Class 3 TFs are enriched in “cellular response to nitrogen levels”.
To compare expression specificity of Class 1 and Class 2 transcription factors, we used the difference of proportions test (Table 2): , where . Genes encoding the Class 1 TFs are predominantly expressed in the petals, the sepals and the embryos of plants, while mRNAs encoding Class 2 TFs are overrepresented in the roots. This may explain previous observations of significant association of TATA motifs with expression in plant roots [4, 40]: possibly, most root-specific transcription factors bind to the 5′ UTR region rather than the region upstream of TSS.
Table 2. Expression specificity of TF from Class 1 and 2.
| Expression pattern | Class 1 (N = 99) | Class 2 (N = 134) | Z-score |
|---|---|---|---|
| Root | 66 | 114 | -3.31344 |
| Pollen | 57 | 60 | 1.93163 |
| Carpel | 72 | 77 | 2.39883 |
| Seed | 70 | 74 | 2.40454 |
| Leaf lamina base | 61 | 61 | 2.43144 |
| Cauline leaf | 64 | 65 | 2.4497 |
| Collective leaf structure | 80 | 87 | 2.65976 |
| Petal | 72 | 74 | 2.73045 |
| Plant embryo | 78 | 81 | 2.97259 |
| Sepal | 76 | 76 | 3.17706 |
Z-score is calculated using the difference of proportions test.
Fig 6 shows frequency profiles for TFBSs of ARALY493022_04 (Class 1, left panel), RAP26_03 (Class 2, middle panel), and MYB111_02 (Class 3, right panel). ARALY493022 is basic helix-loop-helix factor, with GGGCCC consensus sequence. Presence of GGGCCC in the region upstream of TSS is associated with the elevated level of gene expression [4, 39, 41, 42]. RAP2.6 is a defense-related, ethylene response transcription factor which recognizes the GCC-box and characterized by high affinity to DNA sequence GCGCCGCCG [43]. Ali, Abbas [44] experimentally showed that RAP2.6 works both in tissue-specific and stress-specific manner. Under normal conditions, expression of RAP26 is elevated in roots and stems, while being significantly reduced when plant is infected with pathogenic nematodes, such as H. schachtii. To suppress resistance responses, nematodes downregulate expression of RAP2.6 in host cells. MYB111 is involved in the regulation of several genes of the flavonoid biosynthesis pathway in cotyledons and leaves [45, 46]; it confers tolerance to UV-B [47]. Its binding site MYB111_02 has consensus G[G/T]TAGGT[A/G] [43]. MYB111 is an example of TFs with relatively weak position specificity related to TSS. The TFBS motifs occur no very often; they usually provide condition-specific regulation of genes. Fig 6 demonstrates utility of Class 1 and Class 2 TFBS for TSS prediction, while the mapping of the Class 3 TFBS does not convey additional positional information about the TSS.
According to the Kolmogorov-Smirnov test, three classes of TFs differ in the significance of over-representation of their TFBS in promoters and in the randomly shuffled sequences: thirty-seven percent of the Class 1 TFs with motifs located predominantly upstream of TSS were significantly overrepresented (p-values <0.05), In the Class 2 TFs with TFBS located in 5′ UTRs, overrepresentation was confirmed for 20% of the PWMs. The TFBS for Class 3 TFs were distributed evenly. For the latter group, significant over-representation was detected for 15% of class members (S2 Table).
In summary, three classes of TFBS differ in their position specificity, percentages of PWMS significantly over-represented in real promoters, functional classification of their downstream genes, and the patterns of their gene expression.
Evolutionary conservation of TFBS position information content
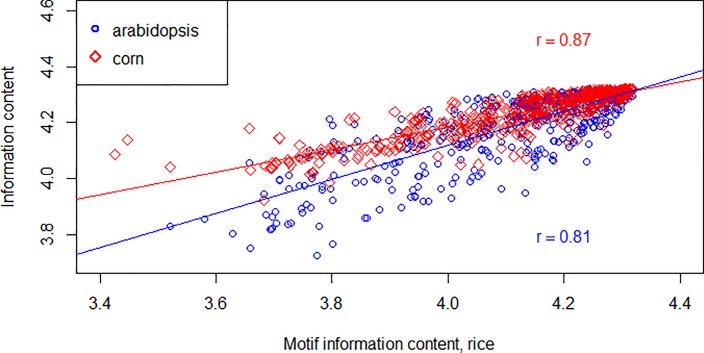
We have analyzed evolutionary conservation of the TFBS position information content (a measure of unevenness of the motif distribution along promoter regions, see Method section) in monocots Oryza sativa and Zea mays, and in the dicot Arabidopsis thaliana (Fig 7). Following correlations between these measures were identified:
Multiple R2 = 0.7504, Adjusted R2 = 0.75, F-statistic: 1819 on 1 and 605 DF, p-value: < 2.2E-16
Multiple R2 = 0.6512, Adjusted R2 = 0.6506, F-statistic: 1083 on 1 and 590 DF, p-value: < 2.2E-16.
Fig 7. Relationship between information content of TFBS positions in rice, corn and Arabidopsis.
Each point corresponds to one transcription factor; X axis shows information content in rice, Y axis–information content in corn and Arabidopsis.
Correlation of the TFBS position information content in two monocots (rice and corn) were higher than that for the rice and a dicot plant Arabidopsis. By extracting TFBS with more than 10,000 matches in each of three plant genomes, a list of 46 “common” informative TFBS was compiled. Each of these TFBS was classified into either “promoter-specific” or “5′ UTR-specific” category in each species. Between rice and corn, 42 of 46 “common” TFBS are consistent in their position preference (see S7 Table). Between rice and arabidopsis, the agreement is seemingly higher, with 45 of 46 TFBS of the common set having the same positional preference (see Supplemental Data). We hypothesize that this discrepancy is due to lower reliability of the TSS map in corn genome as compared to arabidopsis and rice genomes (Fig 8). Importantly, this phenomenon may lead to a systematic “shifting” of the TFBS peaks from promoters to 5′ UTRs and vice versa. Fortunately, incorrect prediction of TSS in corn does not affect the information content of a TFBS, and correlation coefficient of motif information content between two grasses (rice and corn) is 0.87, which is above the correlation between rice and arabidopsis (Fig 7). In summary, positional preference of the most informative motifs remains conserved between dicots and monocots.
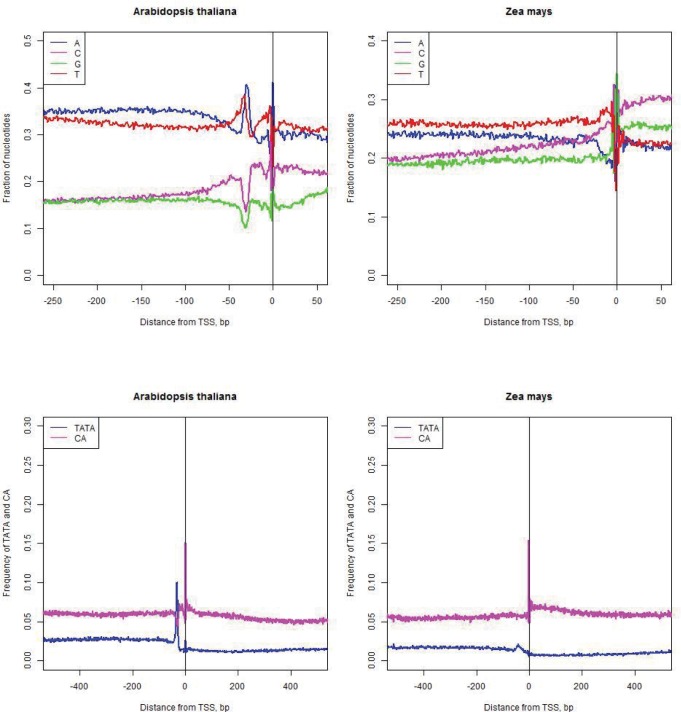
Fig 8. Assessment of promoter prediction quality in Arabidopsis (left) and corn (right).
Arabidopsis genome shows more pronounced consensus at TSS, with higher frequency of TATA motif at -30 and CA at TSS.
Identification of similar TFBS
Since TRANSFAC database tends to accumulate all published motifs, some of collected motifs appear to be redundant. For example, several PWMs may be independently built and reported for the same transcription factor (Fig 9). Also, transcription factors of the same protein family may recognize highly similar motifs, which will be reflected by similarities of respective PWMs. Fig 9 shows TFBS logo plots for a group of transcription factors with highly similar motifs. Although regulatory functions of these may vary, for a practical use in promoter prediction, these motifs should be clustered into a non-redundant set based on similarity of their PWMs. By clustering 764 plant PWMs, a non-redundant set of 376 sequence motifs was obtained, among which, forty-six were found informative with the scores above 0.0138 (see S1 Table).
Fig 9. An example of five distinct TFBS entries in the TRANSFAC database with very similar position weight matrices (PWMs).
Nucleotide variants resulting in the TFBS loss and gain
Core promoters and 5′ UTR regions located within 200 bp around the TSS are both protected against accumulation of nucleotide variants (Fig 10). This protective effect is due to selection constraints, which prevents disruption on regulatory elements located near TSS by neutral or near-neutral genomic variants. Cross-analysis of comprehensive collection of plant TFBS [37] and an extensive dataset of the genomic variants detected in various rice cultivars [27] allowed us to classify regulatory elements of these plants according to their tolerance to the mutations (see S5 Table).
Fig 10. Frequency of SNPs located near the TSS in rice.
To achieve that, we considered distribution of SNPs and their effects on loss and gain of TFBS. For each nucleotide change, we have calculated Δ = |q − q*| for the TFBS scores before (q) and after (q*) nucleotide change, and compared its values to empirically determined thresholds. Calculations of the scores q and q* were done according to the MATCH scoring formula (see Materials and Methods). If Δ ≥ Δ0, the site was considered as “lost” or “gained” depending which score value was larger, q or q*.
Frequencies of site losses and site gains for the promoters and for the random subset of 18,389 intergenic sequences, each 2,000 nt in length, were compared. We hypothesized that functionally important promoter motifs will have less variation causing the loss of sites. For each TF, we calculated the ratio of the site losses in intergenic sequences to the site losses in promoters. All entries were than ranked according to these ratios, which reflected relative “suppression” of the site losses by SNPs (Table 3). Relative suppressions of the site gains were calculated in a similar fashion (see Table 4). The binding sites for ABF (CACGTGGC) and CBF4 transcription factors were the most protected from the site loss. In abscisic acid signaling, ABF factors govern osmotic stress response through modulation of the gene expression downstream of SnRK2 kinases, while CBF4 regulates adaptation to drought. For several important transcription factors, such as MADS8 (involved in the control of flowering time), GT-1 and GATA-1 (response to light), we observed that variation was avoided in positions where nucleotide change can lead to the site gain. Additional data and the results of the analysis of SNPs in TFBS could be seen in the Table 3, Table 4, and S5 Table.
Table 3. Suppression of site loss caused by nucleotide variants in promoters.
| ID | Frequency intergenic/ Frequency promoters | #Promoter Sites | #Intergenic Sites | P-values |
|---|---|---|---|---|
| P$AT2G20350_01 | 1.856 | 1909 | 3660 | 6.15E-112 |
| P$ARF1_01 | 1.814 | 856 | 1604 | 3.30E-47 |
| P$DREBIII4_01 | 1.677 | 870 | 1507 | 2.78E-35 |
| P$AT2G41690_01 | 1.648 | 1072 | 1825 | 5.37E-40 |
| P$CBF1_03 | 1.540 | 2803 | 4459 | 8.89E-74 |
| P$DREB1F_01 | 1.534 | 4873 | 7720 | 4.52E-124 |
| P$ORA47_01 | 1.529 | 4129 | 6521 | 8.35E-104 |
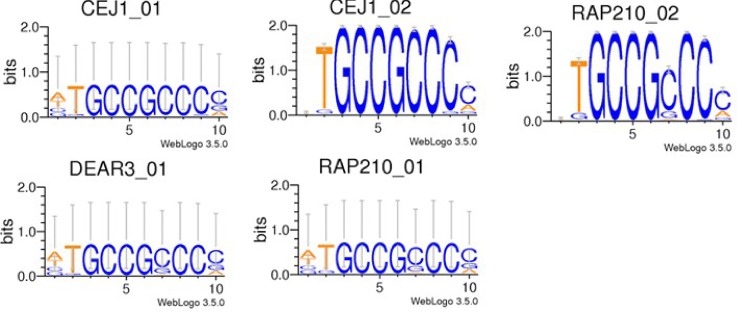
| P$RAP210_01 | 1.527 | 4519 | 7128 | 1.11E-112 |
| P$DEAR3_01 | 1.526 | 4525 | 7134 | 1.51E-112 |
| P$RAP210_02 | 1.526 | 4525 | 7134 | 1.51E-112 |
| P$ERF019_01 | 1.526 | 4199 | 6620 | 1.36E-104 |
| P$RAP21_01 | 1.526 | 4236 | 6675 | 3.17E-105 |
| P$AT1G71520_01 | 1.525 | 4242 | 6682 | 3.57E-105 |
| P$DREB1B_01 | 1.449 | 2544 | 3808 | 1.16E-48 |
| P$HSF3_01 | 1.423 | 1262 | 1855 | 1.08E-22 |
| P$AT4G16610_01 | 1.374 | 8852 | 12563 | 7.08E-118 |
| P$AT4G16750_01 | 1.347 | 1175 | 1635 | 2.62E-15 |
| P$AT2G44940_01 | 1.338 | 1231 | 1701 | 2.99E-15 |
| P$MADS17_01 | 1.307 | 7134 | 9628 | 1.37E-66 |
“Frequency intergenic”/“Frequency promoters” is the ratio between frequencies of site loss due to SNPs located in intergenic regions and the site loss due to SNPs located in promoters.
Table 4. Suppression of site gain caused by nucleotide variation in promoters.
| ID | Frequency intergenic/ Frequency promoters | #Promoter Sites | #Intergenic Sites | P-values |
|---|---|---|---|---|
| P$WRKY23_01 | 1.376 | 1691 | 2403 | 2.59E-24 |
| P$FUS3_Q2 | 1.331 | 2249 | 3091 | 2.07E-25 |
| P$BHLH112_01 | 1.319 | 1813 | 2471 | 1.14E-19 |
| P$MYB46_02 | 1.290 | 1015 | 1352 | 4.37E-10 |
| P$WRKY_Q2 | 1.286 | 10925 | 14507 | 1.56E-88 |
| P$TGA2_Q2 | 1.275 | 6140 | 8084 | 3.08E-47 |
| P$CDC5_01 | 1.269 | 3653 | 4787 | 8.44E-28 |
| P$MADS4_01 | 1.266 | 1948 | 2548 | 1.89E-15 |
Column “Frequency intergenic”/“Frequency promoters” contains the ratio of the frequency of site gain due to SNPs located in intergenic regions to the frequency of the site gain due to SNPs located in promoters.
The binding sites for AT2G20350 and ARF1 transcription factors were the most “protected” from the site loss. AT2G20350 factors regulate activity of ethylene-activated signaling pathway. The plant hormone ethylene is involved in many aspects of the plant life cycle, including seed germination, root hair development, root nodulation, flower senescence, abscission, and fruit ripening (Johnson and Ecker, 1998). ARF1 is a member of the auxin response factor family, involved in hyperosmotic salinity response. For several important transcription factors, such as WRKY23 (involved in hyperosmotic salinity response and response to auxin), FUS3 (plays a role in embryonic development ending in seed dormancy and response to auxin stimulus), we observed that variation was avoided in positions where nucleotide change can lead to the site gain.
Distribution of RNA-Seq reads
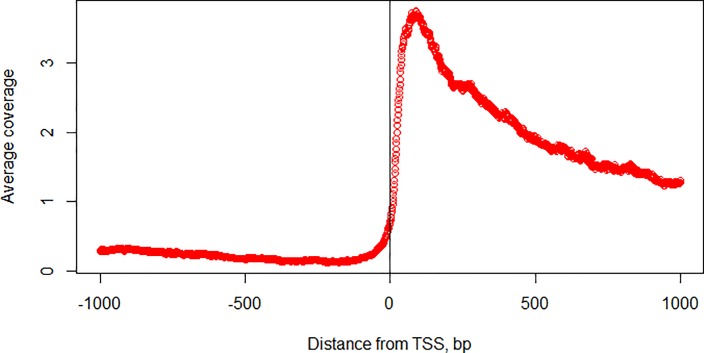
Predictably, an analysis of mapped RNA-Seq reads near TSS [-1000; +1000] showed that, on average, coverage peaks are observed immediately downstream of TSS (Fig 11). However, some genes lack a peak of RNS-Seq reads at their TSS. Notably, only 26% of rice genes display a maximum of the coverage in the range [-50, +250], and only 60% of genes display this maximum in the range [-50, +550].
Fig 11. RNA-Seq coverage near the transcription start site.
R-loop forming sequences (RLFS)
Three-stranded nucleic acid R-loop structure is formed between nascent RNA transcript and DNA template [48]. Length of the R-loop sequence varies between 150 to 650 nt. R-loops aid in the prevention of methylation within promoters [49–51] and are associated with initiation of transcription and other important gene-level features [48]. In particular, R-loops accumulate at the G-rich 5′-UTR regions immediately downstream of the CpG-non-methylated human promoters [50]. To map the R-loop forming structures in the area [TSS-1000, TSS+1000], we used the QmRRFS tool [48, 52, 53]. QmRRFS partitions R-loops into three segments, the RIZ (DNA region of initiation of R-loops containing at least three contiguous guanines), the linker (a spacer up to 50 nt between RIZ and REZ), and the REZ (G-rich region supporting extension of R-loop, up to 2000 nt long). In agreement with Ginno, Lott [50], QmRRFS-driven analysis showed that 22% of rice genes are associated with at least one R-loop in the area [TSS-1000, TSS+1000], with the predominant localization in 5′-UTR. The observed distribution of RLFS was unimodal, with the peak of the distribution located at the position around 200 nt downstream from the TSS; over a half of RLFS (52%) were found in the 5′-UTR [TSS, TSS+400] (S6 Table). Notable, this peak coincides with the region where polymerase typically pauses after the initiation of transcription [48, 52, 53].
DNA methylation
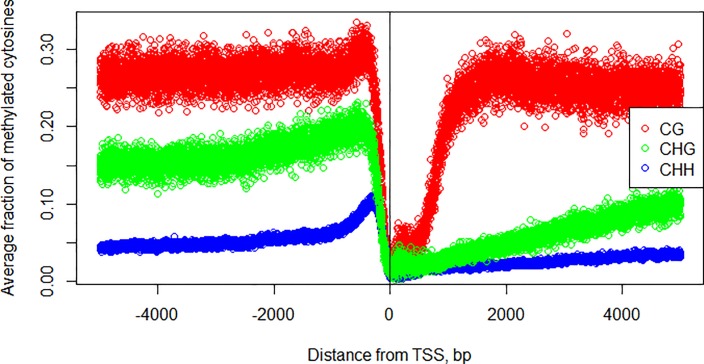
In the intergenic regions and within functional classes of genes and their promoters, the patterns of DNA methylation predictably differ [54, 55]. The most pronounced effect was observed for the methylated CpGs (see Fig 12). Intergenic level of CpG methylation was at 0.27, with sharp decline starting around 600 bp upstream of TSS to about 50% of that in intergenic region level at the position of -170, then proceeds to its minimum (0.01) at 8 bp upstream from the TSS.
Fig 12. Methylation around transcription start site in rice in different sequence contexts.
Red–CG, green—CHG, blue–CHH, where H denotes A, C or T nucleotide.
Combining the characteristic features of TSS into promoter classifier
We used 18,389 “promoter” (positives) and 18,389 “non-promoter” (negatives) sequences. To train the model, we used 14,711 positives and negatives; and the remaining 3,678 positives and negatives were used for testing. The binary classifier interrogates the candidate sequence and reports whether the sequence is “promoter” or “non-promoter”. The best combination of features was: composition of DNA sequence, GC-skew value and presence/absence of the CA-motif in every position. It achieved the best accuracy (0.9995) and has the Matthews correlation coefficient of 0.9989 (see Table 5). Other features also improve the classification accuracy in comparison with the DNA sequence alone, however, not performing as well as the combination of DNA sequence, GC-skew and CA-motif distribution.
Table 5. Promoter classification accuracy.
| Features | TP | TN | FP | FN | Accuracy | Sensitivity | Specificity | CC |
|---|---|---|---|---|---|---|---|---|
| DNA sequence | 3424 | 3030 | 648 | 254 | 0. 8774 | 0. 9309 | 0. 8238 | 0.7591 |
| DNA sequence + CG skew | 3635 | 3653 | 25 | 43 | 0.9907 | 0.9883 | 0.9932 | 0.9832 |
| DNA sequence + CG skew + frequency of CA motif | 3674 | 3678 | 0 | 4 | 0.9994 | 0.9989 | 1.0 | 0.9989 |
| DNA sequence + CG skew + RNA-Seq coverage | 3658 | 3666 | 12 | 20 | 0.9956 | 0.9945 | 0.9967 | 0.9913 |
| DNA sequence + CG skew + frequency of TATA motif | 3653 | 3608 | 70 | 25 | 0.9870 | 0.9932 | 0.9810 | 0.9742 |
| DNA sequence + CG skew + DNA methylation | 3657 | 3563 | 115 | 21 | 0.9815 | 0.9942 | 0.9687 | 0.9633 |
| DNA sequence + all TFBS | 3241 | 3386 | 292 | 437 | 0.9009 | 0.8812 | 0.9206 | 0.8024 |
| DNA sequence + all TFBS +CG skew | 3619 | 3668 | 10 | 59 | 0.9906 | 0.9839 |
0.9973 | 0.9813 |
| DNA sequence + selected TFBS+CG skew | 3628 | 3674 | 4 | 50 | 0.9927 | 0.9864 | 0.9989 | 0.9854 |
| DNA sequence + SNP | 3430 | 3138 | 540 | 248 | 0.8929 | 0.9326 | 0.8532 | 0.7882 |
| DNA sequence + SNP+CG skew | 3348 | 3296 | 382 | 330 | 0.9032 | 0.9103 | 0.8962 | 0.8065 |
| DNA sequence + CG skew+ RNA-Seq coverage +selected TFBS |
3653 | 3663 | 15 | 25 | 0.9946 | 0.9932 | 0.9959 | 0.9891 |
| DNA sequence + CG skew + frequency of CA motif + RNA-Seq | 3665 | 3638 | 40 | 13 | 0.9928 | 0.9965 | 0.9891 | 0.9856 |
Discussion
In this work, we have investigated several features of promoter area, identified characteristic patterns of their distribution and assessed utility of these features for identification of TSS location. Accuracy of TSS identification affects the overall quality of regulatory region analysis. To date, large amounts of the “mapped” TSS are, in fact, defined only approximately. A significant fraction of promoters has multiple alternative TSS, many of which are not yet annotated. These features make prediction of exact positions of TSS a very complex problem. Further work toward exact mapping of all TSS positions using various promoter characteristics in multiple species is warranted. It is essential to find and annotate tissue- and condition-specific transcription start sites and associate them with alternative splice form, gene regulatory network, and protein function.
Intelligent integration of multiple types of genomic information (DNA composition, regulatory elements, DNA methylation, RNA-Seq coverage data, SNP distribution etc.) may improve annotation of tissue- and developmental stage-specific genes that are often misidentified due to their atypical sequence composition in grasses [54–56]. We showed that the region containing promoter-UTR boundaries could be defined using the following pronounced trends: (1) drop in SNP density, (2) evolutionary conserved peaks and valleys of the positions of regulatory elements, (3) peak of RNA-Seq coverage immediately downstream from the TSS, (4) peak of CG skew, (5) drop in DNA methylation density in CpG, CHH and CHG contexts, where H denotes A, C or T nucleotide. Integration of multiple noisy features of promoter regions can result in 99% classification accuracy. Features identified as important by deep learning based classification can now be used to build a scoring function for promoter prediction.
In our work, we focused on the 2000 nucleotide long region around rice TSS, supported by experimental evidence. In rice, the median length of 5′ UTR is 120 nt; with less than 1.2% of 5′ UTRs being larger than 1000 nt [28]. Therefore, for the vast majority of loci, the considered regions covered both transcription and translation start sites, being sufficient for description and classification of rice promoters.
Analysis of SNPs in the context of TFBS in promoter and non-promoter region indicated that TFBS differ by their tolerance to nucleotide variation. It is of note that the binding sites for AT2G20350 and ARF1 transcription factors were the most “protected” from the site loss. Both of these factors are involved in plant hormone signaling [57]. We conclude that sites for these transcription factors are “protected” in evolution from being lost due to their importance for regulation of plant lifecycle. It was interesting to observe that for several transcription factors nucleotide variations were avoided in positions where nucleotide change can lead to the site gain. Among such factors were WRKY23 and FUS3, involved in gene regulation in response to the plant hormone auxin. We propose that spurious generation of novel sites for these transcription factors may significantly alter cellular timing. We conclude that TRANSFAC analysis may results in functional observations as it provided clear evidence of interplay between SNPs and TF binding sites in rice genome.
Materials and methods
Fgenesh++ rice gene prediction
Fgenesh++ (Find genes using Hidden Markov Models) [58–60] is a HMM-based ab initio gene prediction program [61]. We used the rice chromosomes (version MSU 7, [29]) to make the initial gene prediction set, applying the Fgenesh gene finder with generic parameters for monocot plants. From this set, we selected a subset of predicted genes that encode highly homologous proteins (using BLAST with E-value cut-off 1.0E-10) to known plant proteins from the NCBI non-redundant (NR) database. Based on this subset, we computed gene-finding parameters, optimized for the rice genome, and executed the Fgenesh++ pipeline to annotate the genes in the genomic scaffolds. The Fgenesh++ pipeline used all available supporting data, such as known transcripts and homologous protein sequences. NR plant and, specifically, rice transcripts were mapped to the rice genomic sequences, therefore identifying a set of potential splice sites. Plant proteins were mapped to the rice genomic contigs, and the high scoring matches were selected to generate protein-supported gene predictions, so that only the highly homologous proteins were used in gene identification.
Amino acid sequences from predicted rice genes were then compared to the protein sequences from plant NR database using the 'bl2seq' routine, and the similarity was significant if it had a BLAST percent identity ≥ 50, BLAST score ≥ 100, coverage of predicted protein ≥ 80% and coverage of homologous protein ≥ 80%. BLAST analysis of the predicted sequences was also carried out against the O. sativa mRNA dataset, using an identify cutoff of >90%. Predictions that have both NR plant RefSeq and O. sativa mRNA support, as well as the 5′ UTR longer than 20 nucleotides and shorter than 1000 were selected for the analysis.
GFF file with Fgenesh++ gene prediction is available as a Supplemental Data file.
MSU rice gene models
The current MSUv7 annotation (http://rice.plantbiology.msu.edu) of rice genome contains 55,986 predicted genes and 66,338 gene models [29]. Upon exclusion of pseudogenes, transposable elements, and genes with atypical lengths of 5′ UTR (below 20 nt or above 1000 nt long), a high-confidence set contains 20,367 expressed protein-coding rice genes.
Arabidopsis gene and promoter models
Genome annotation files for TAIR 10 version and sequences for 3000 nucleotides upstream from ATG were obtained from The Arabidopsis Information Resource (TAIR) [24, 62]. The upstream sequences were truncated based on the position of the nearest upstream locus. 290,085 EST sequences were obtained from NCBI and TAIR and mapped onto the 27,199 upstream sequences using nucleotide BLAST + (minimum identity percent: 95%; maximum query start of alignment: 5; only plus strand alignments were used). Using the text search, we removed ESTs annotated as 3′ or partial. NPEST [5] algorithm was used and resulted in prediction of 17,452 transcription start sites for 16,520 protein-coding loci.
Corn gene and promoter models
Genome annotation of maize (B73, 6a) contains 40,602 predicted protein-coding genes [63]. We excluded genes with atypical lengths of 5′ UTR (below 20 nt or above 1000 nt long), genes without full-length mRNA support, without valid start and stop codon, or no PFAM annotation. This filtering resulted in 16,180 putative corn TSS.
Positional information content of transcription factor binding sites
We selected TFBS that occur at least 10,000 times in promoters of a given species. In rice, it amounted to 487, in Arabidopsis -559, and in corn—171 TFBS. To calculate the information content of each TFBS, we divided the region around the start of transcription (TSS-1000, TSS+1000) into 100 nt long bins, and calculated the observed frequency of TFBS matches in every window as a ratio of matches within the window to the total number of matches . The expected frequency is calculated as . The information content (a.k.a. Shannon’s entropy) is defined as . The binding sites were ranked from highest to lowest information content.
RNA-Seq data
We used following publicly available rice RNA-Seq datasets: SRR034580, SRR034581, SRR034582, SRR034583, SRR034584, SRR034585, SRR034586, SRR034587, SRR034588, SRR034589, SRR034590, SRR034591, SRR034592, SRR034593, SRR034594, SRR034595, SRR034596, SRR034597, SRR034598, SRR034599, SRR042529, SRR074125, SRR074126, SRR074127, SRR074128, SRR074129, SRR074130, SRR074131, SRR074132, SRR074133, SRR074134, SRR074135, SRR074136, SRR074137, SRR074139, SRR074140, SRR074142, SRR074143, SRR074144, SRR074145, SRR074146, SRR074147, SRR074149, SRR074150.
The datasets were processed using the following protocol:
Duplicates were removed using tool clumpify (http://jgi.doe.gov/data-and-tools/bbtools/bb-tools-user-guide/clumpify-guide/) allowing for up to two errors per read.
Quality trimmed using trimmomatic [64] with minimum read length = 16, minimum quality 28 (sliding window of length 10)
Aligned to the MSU 7 rice genome using Hisat2 [65] aligner.
Summary statistics is shown in the Table 6.
Table 6. RNA-Seq dataset quality.
| Experiments | Reads | Read length | Quality | Aligned |
|---|---|---|---|---|
| SRR034580-SRR034599 | ~5.5 M | 35 | Poor | 67–72% |
| SRR042529 | 8.5M | 36 | Good | 84% |
| SRR074125-SRR074150 | 2–5M | 26 | Good | ~1.5% (!) |
Identification of transcription factor binding sites
The prediction of TF binding sites is done using the MATCH tool, which is based on the usage of information vector-based PWM model. This model calculates the matrix similarity score (q) defined in [35]. This model is a common additive model, which uses a transformed matrix instead of an initial matrix, where each column of the transformed matrix is determined with the help of weighting the corresponding initial column by information content. The matrix similarity score q is calculated according to the following formula:
here, L is the length of the weight matrix; bi is the nucleotide that is observed in the position i of the sequence of TF binding site; f(bi,i) is the frequency of nucleotide bi in the position i of the weight matrix; fmin(i) is the frequency of the nucleotide which is the rarest in the weight matrix in the given matrix position i; fmax(i) is the highest frequency the given matrix position i. The information content I(i) in the position i is defined as followed:
It describes conservation of the position i of the weight matrix. Multiplication of the nucleotide frequency by the information content imposes penalty on consensus mismatches in highly conserved regions of the matrix. We have recently demonstrated that this strategy is superior to the common alternative approaches of computing the TFBS scores [37].
Site loss and gain
We analyzed distribution of SNPs and their effect on TF binding site loss and gain. The effect of a SNP on TF binding sites was computed as the follows. For each SNP and for each PWM model we computed two matrix similarity scores (see above): q and q* corresponding to two nucleotides in the SNP–the reference and alternative nucleotides.
Next, we calculated Δ = |q − q*|, and compared its value to the empirically determined threshold Δ0. If Δ ≥ Δ0, the site was considered as “lost” or “gained” depending on sign of the difference q − q*.
We then calculated frequencies of site loss and site gain for all considered SNPs to identify which transcription factor binding sites (TFBS) are significantly enriched by the effect of nucleotide changes in SNPs analyzed. As a background, we considered random nucleotide changes in random genomic positions. We denote study and background sets briefly as “Yes” and “No” sets (the “Yes” set is the set of TFBS sequences overlapping SNPs with either the reference nucleotide or alternative nucleotide; the “No” set is the set created by random nucleotide substitutions in random genomic positions). The algorithm for TFBS enrichment analysis, called F-Match, has been described in Kel, Konovalova [66] and Koschmann, Bhar [67]. Briefly, the procedure finds a critical value (a threshold) for the differences between scores q and q* (the threshold Δ0) of each PWM in the library that maximizes the “Yes/No” ratio RYN as defined in Eq (1) under the constraint of statistical significance:
| (1) |
In Eq (1), #Sites and #Seq are the sites and sequences counted in “Yes” and “No” sets. A high “Yes/No” ratio indicates strong enrichment of binding sites for a given PWM in the “Yes” sequences. The statistical significance is computed as follows:
| (2) |
The Yes/No ratio and P-value is computed separately for the site gain and for the site loss. If “Yes/No” ratio >1 and a P-value < 0.01 for a given PWM we consider this as an indication of an enrichment of SNPs by the sites for the given PWM. We can say that sites of this PWM are frequently effected by the SNPs and, therefore, the gene regulation by the respective TFs is significantly altered by the considered SNPs.
Matrix clustering
Many matrices in the TRANSFAC database are highly similar, up to the point being undistinguishable. To lower the complexity of the training data, we performed hierarchical clustering and used only one matrix from each cluster for promoter classification. The distance between two motifs is calculated as sum of squared differences between all matrix elements. If matrices were not the same size, we slide the shorter matrix over the longer one and take minimal distance. The cut-off for merging clusters was determined empirically by considering the sequence logos of matrices to be merged at each step and deciding which matrices we consider duplicates.
Classification of promoter regions
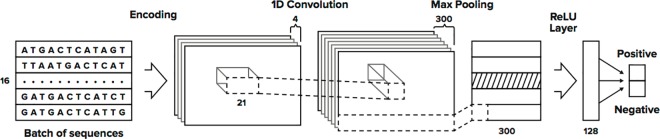
There are many network architectures and the task is to choose a suitable one for a given research problem. We used Convolutional Neural Networks (CNN) architecture for building promoter recognition models developed by Umarov and Solovyev [10]. The software consists of several modules. In the learnCNN.py modules the CNN model was implemented using Keras—a minimalist, highly modular neural networks library, written in Python. It uses the Theano library as a backend and utilizes GPU for fast neural network training. Adam optimizer was used for training with categorical cross-entropy as a loss function. Our CNN architecture (Fig 13) consists of one convolutional layer with 200 filters of length 21. After the convolutional layer, there is a standard Max-Pooling layer. The output from the Max-Pooling layer is fed into a standard fully connected ReLU layer with 128 neurons. Pooling size was equal to 2. The ReLU layer is connected to the output layer with sigmoid activation, where neurons correspond to promoter and non-promoter classes. The batch size used for training was 16.
Fig 13. Basic CNN architecture that was used in building promoter models implemented in the learnCNN.py program [3, 10].
Input of the network consisted of nucleotide sequences where each nucleotide is encoded by a four-dimensional vector A (1,0,0,0), T (0,1,0,0), G (0,0,1,0) and C (0,0,0,1) and other dimensions filled by other promoter features such as: GC-skew, DNA methylation, SNP, presence of CA motif, presence of TATA motifs, TFBS. The output is a two-dimensional vector: “promoter” (1, 0) and “non-promoter” (0, 1) prediction. learnCNN.py learns parameters of the CNN model and outputs the accuracy of promoter prediction for the test set of sequences. It also writes the computed CNN Model into a file, which can be used later in programs for promoter identification in each sequence. We used 70% of these examples for learning, 10% for validation (to find an optimal number of learning epochs) and 20% for testing.
We have extracted 18,389 sequences around transcription start site determined by full-length mRNA. Sequence [TSS-199, TSS+50], containing 200 nucleotides from promoter and 50 nucleotides from 5’ UTR, was designated as the “promoter” region, and sequence [TSS+751, TSS+1000], from the coding part of the gene, as “non-promoter”.
Quality of prediction was assessed using the following measures: True Positives (TP), True Negatives (TN), False Positive (FP), False Negative (FN), Accuracy, Sensitivity, Specificity, Matthews correlation coefficient (CC):
Supporting information
(ZIP)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
Financial disclosure
"AK was supported by a grant of the Federal Targeted Program “Research and development on priority directions of science and technology in Russia, 2014–2010”, Contract № 14.604.21.0101, unique identifier of the applied scientific project: RFMEFI60414X0101. AK's work was also supported by the following grants of the EU FP7 program: “SYSCOL”, “SysMedIBD”, “RESOLVE” and “MIMOMICS”. TT and MT were supported by the NSF Division of Environmental Biology (1456634). TT, MT and AB were supported by NSF STTR award 1622840. Additional funding was provided by GeneXplain GmbH in the form of salaries for AK, and by Softberry, Inc in the form salaries for VS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
Competing interests statement
AK is the Founder and Chief Scientific Officer of GeneXplain GmbH. VS is the Chief Scientific Officer of Softberry, Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Authors contribution
Conceived and designed the study: TT and AK. Developed algorithms: VS, AK, TT. Analyzed the data: MT, VS, TT, AK. Contributed analysis tools: VS, AK. Wrote the paper: TT, AB, MT, AK. Participated in the editing of the manuscript: MT, VS, AB, AK, TT. All authors read and approved the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
AK was supported by a grant of the Federal Targeted Program “Research and development on priority directions of science and technology in Russia, 2014–2010”, Contract № 14.604.21.0101, unique identifier of the applied scientific project: RFMEFI60414X0101. AK's work was also supported by the following grants of the EU FP7 program: “SYSCOL”, “SysMedIBD”, “RESOLVE” and “MIMOMICS”. TT and MT were supported by the NSF Division of Environmental Biology (1456634). TT, MT and AB were supported by NSF STTR award 1622840. Additional funding was provided by GeneXplain GmbH in the form of salaries for AK, and by Softberry, Inc in the form of salary for VS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Sandelin A, Carninci P, Lenhard B, Ponjavic J, Hayashizaki Y, Hume DA. Mammalian RNA polymerase II core promoters: insights from genome-wide studies. Nat Rev Genet. 2007;8(6):424–36. doi: 10.1038/nrg2026 . [DOI] [PubMed] [Google Scholar]
- 2.Solovyev VV, Shahmuradov IA, Salamov AA. Identification of promoter regions and regulatory sites. Methods Mol Biol. 2010;674:57–83. doi: 10.1007/978-1-60761-854-6_5 . [DOI] [PubMed] [Google Scholar]
- 3.Shahmuradov IA, Umarov RK, Solovyev VV. TSSPlant: a new tool for prediction of plant Pol II promoters. Nucleic Acids Res. 2017. doi: 10.1093/nar/gkw1353 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troukhan M, Tatarinova T, Bouck J, Flavell RB, Alexandrov NN. Genome-wide discovery of cis-elements in promoter sequences using gene expression. OMICS. 2009;13(2):139–51. doi: 10.1089/omi.2008.0034 . [DOI] [PubMed] [Google Scholar]
- 5.Tatarinova T, Kryshchenko A, Triska M, Hassan M, Murphy D, Neely M, et al. NPEST: a nonparametric method and a database for transcription start site prediction. Quant Biol. 2014;1(4):261–71. doi: 10.1007/s40484-013-0022-2 ; PubMed Central PMCID: PMCPMC4156414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fickett JW, Hatzigeorgiou AG. Eukaryotic promoter recognition. Genome Res. 1997;7(9):861–78. . [DOI] [PubMed] [Google Scholar]
- 7.Anwar F, Baker SM, Jabid T, Mehedi Hasan M, Shoyaib M, Khan H, et al. Pol II promoter prediction using characteristic 4-mer motifs: a machine learning approach. BMC Bioinformatics. 2008;9:414 doi: 10.1186/1471-2105-9-414 ; PubMed Central PMCID: PMCPMC2575220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azad AK, Shahid S, Noman N, Lee H. Prediction of plant promoters based on hexamers and random triplet pair analysis. Algorithms Mol Biol. 2011;6:19 doi: 10.1186/1748-7188-6-19 ; PubMed Central PMCID: PMCPMC3160368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahmuradov IA, Solovyev VV, Gammerman AJ. Plant promoter prediction with confidence estimation. Nucleic Acids Res. 2005;33(3):1069–76. doi: 10.1093/nar/gki247 ; PubMed Central PMCID: PMCPMC549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umarov RK, Solovyev VV. Recognition of prokaryotic and eukaryotic promoters using convolutional deep learning neural networks. PLoS One. 2017;12(2):e0171410 doi: 10.1371/journal.pone.0171410 ; PubMed Central PMCID: PMCPMC5291440 VS's employment by Softberry Inc. does not alter the authors' adherence to PLOS ONE policies on sharing data and materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solovyev V, Shahmuradov I. PromH: promoters identification using orthologous genomic sequences. Nucleic Acids Research. 2003;31(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexandrov NN, Brover VV, Freidin S, Troukhan ME, Tatarinova TV, Zhang H, et al. Insights into corn genes derived from large-scale cDNA sequencing. Plant Mol Biol. 2009;69(1–2):179–94. doi: 10.1007/s11103-008-9415-4 ; PubMed Central PMCID: PMCPMC2709227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandrov NN, Troukhan ME, Brover VV, Tatarinova T, Flavell RB, Feldmann KA. Features of Arabidopsis genes and genome discovered using full-length cDNAs. Plant Mol Biol. 2006;60(1):69–85. doi: 10.1007/s11103-005-2564-9 . [DOI] [PubMed] [Google Scholar]
- 14.Kawaji H, Kasukawa T, Fukuda S, Katayama S, Kai C, Kawai J, et al. CAGE Basic/Analysis Databases: the CAGE resource for comprehensive promoter analysis. Nucleic Acids Res. 2006;34(Database issue):D632–6. doi: 10.1093/nar/gkj034 ; PubMed Central PMCID: PMCPMC1347397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaji H, Lizio M, Itoh M, Kanamori-Katayama M, Kaiho A, Nishiyori-Sueki H, et al. Comparison of CAGE and RNA-seq transcriptome profiling using clonally amplified and single-molecule next-generation sequencing. Genome Res. 2014;24(4):708–17. doi: 10.1101/gr.156232.113 ; PubMed Central PMCID: PMCPMC3975069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton T, Petricka J, Corcoran DL, Li S, Winter CM, Carda A, et al. Paired-end analysis of transcription start sites in Arabidopsis reveals plant-specific promoter signatures. Plant Cell. 2014;26(7):2746–60. doi: 10.1105/tpc.114.125617 ; PubMed Central PMCID: PMCPMC4145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batut P, Gingeras TR. RAMPAGE: promoter activity profiling by paired-end sequencing of 5'-complete cDNAs. Curr Protoc Mol Biol. 2013;104:Unit 25B 11. doi: 10.1002/0471142727.mb25b11s104 ; PubMed Central PMCID: PMCPMC4372803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieterich C, Wang H., Rateitschak K., Luz H. and Vingron M. CORG: a database for COmparative Regulatory Genomics. Nucleic Acids Res. 2003;31:55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahmuradov IA, Abdulazimova A, Khan FZ, Solovyev V, Mustafaev N, Akbarova Y, et al. The PlantProm DB: Recent Updates. In: IEEE, editor. 2012 International Conference on Biomedical Engineering and Biotechnology (iCBEB); Macau, Macao2012.
- 20.Kondrakhin YV, Kel AE, Kolchanov NA, Romashchenko AG, Milanesi L. Eukaryotic promoter recognition by binding sites for transcription factors. Comput Appl Biosci. 1995;11(5):477–88. . [DOI] [PubMed] [Google Scholar]
- 21.Prestridge DS. Predicting Pol II promoter sequences using transcription factor binding sites. J Mol Biol. 1995;249(5):923–32. doi: 10.1006/jmbi.1995.0349 . [DOI] [PubMed] [Google Scholar]
- 22.Troukhan M, Tatarinova T. Bouck J., Flawell R., Alexandrov N. Genome-wide discovery of cis-elements in promoter sequences using gene expression data. Omics. 2009;13(1). [DOI] [PubMed] [Google Scholar]
- 23.Rye M, Sandve GK, Daub CO, Kawaji H, Carninci P, Forrest AR, et al. Chromatin states reveal functional associations for globally defined transcription start sites in four human cell lines. BMC genomics. 2014;15:120 doi: 10.1186/1471-2164-15-120 ; PubMed Central PMCID: PMC3986914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berardini TZ, Reiser L, Li D, Mezheritsky Y, Muller R, Strait E, et al. The Arabidopsis information resource: Making and mining the "gold standard" annotated reference plant genome. Genesis. 2015;53(8):474–85. doi: 10.1002/dvg.22877 ; PubMed Central PMCID: PMCPMC4545719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huala E, Dickerman AW, Garcia-Hernandez M, Weems D, Reiser L, LaFond F, et al. The Arabidopsis Information Resource (TAIR): a comprehensive database and web-based information retrieval, analysis, and visualization system for a model plant. Nucleic Acids Res. 2001;29(1):102–5. ; PubMed Central PMCID: PMCPMC29827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hieno A, Naznin HA, Hyakumachi M, Sakurai T, Tokizawa M, Koyama H, et al. ppdb: plant promoter database version 3.0. Nucleic Acids Res. 2014;42(Database issue):D1188–92. doi: 10.1093/nar/gkt1027 ; PubMed Central PMCID: PMCPMC3965062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexandrov N, Tai S, Wang W, Mansueto L, Palis K, Fuentes RR, et al. SNP-Seek database of SNPs derived from 3000 rice genomes. Nucleic Acids Res. 2015;43(Database issue):D1023–7. doi: 10.1093/nar/gku1039 ; PubMed Central PMCID: PMCPMC4383887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tatarinova TV, Chekalin E, Nikolsky Y, Bruskin S, Chebotarov D, McNally KL, et al. Nucleotide diversity analysis highlights functionally important genomic regions. Sci Rep. 2016;6:35730 doi: 10.1038/srep35730 ; PubMed Central PMCID: PMCPMC5075931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice (N Y). 2013;6(1):4 doi: 10.1186/1939-8433-6-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pullen SS, Friesen PD. The CAGT motif functions as an initiator element during early transcription of the baculovirus transregulator ie-1. J Virol. 1995;69(6):3575–83. ; PubMed Central PMCID: PMCPMC189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinya E, Shimada T. Identification of two initiator elements in the bidirectional promoter of the human dihydrofolate reductase and mismatch repair protein 1 genes. Nucleic Acids Res. 1994;22(11):2143–9. ; PubMed Central PMCID: PMCPMC308133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiran K, Ansari SA, Srivastava R, Lodhi N, Chaturvedi CP, Sawant SV, et al. The TATA-box sequence in the basal promoter contributes to determining light-dependent gene expression in plants. Plant Physiol. 2006;142(1):364–76. doi: 10.1104/pp.106.084319 ; PubMed Central PMCID: PMCPMC1557599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Heeringen SJ, Akhtar W, Jacobi UG, Akkers RC, Suzuki Y, Veenstra GJ. Nucleotide composition-linked divergence of vertebrate core promoter architecture. Genome Res. 2011;21(3):410–21. doi: 10.1101/gr.111724.110 ; PubMed Central PMCID: PMCPMC3044855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatarinova T, Brover V, Troukhan M, Alexandrov N. Skew in CG content near the transcription start site in Arabidopsis thaliana. Bioinformatics. 2003;19 Suppl 1:i313–4. . [DOI] [PubMed] [Google Scholar]
- 35.Kel AE, Gossling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, Wingender E. MATCH: A tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res. 2003;31(13):3576–9. ; PubMed Central PMCID: PMCPMC169193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006;34(Database issue):D108–10. doi: 10.1093/nar/gkj143 ; PubMed Central PMCID: PMCPMC1347505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondrakhin Y, Valeev T, Sharipov R, Yevshin I, Kolpakov F, Kel A. Prediction of protein-DNA interactions of transcription factors linking proteomics and transcriptomics data. EuPA Open Proteomics. 2016;13:14–23. Epub December 2016. j.euprot.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stepanova M, Tiazhelova T, Skoblov M, Baranova A. A comparative analysis of relative occurrence of transcription factor binding sites in vertebrate genomes and gene promoter areas. Bioinformatics. 2005;21(9):1789–96. doi: 10.1093/bioinformatics/bti307 [DOI] [PubMed] [Google Scholar]
- 39.Weirauch MT, Yang A, Albu M, Cote AG, Montenegro-Montero A, Drewe P, et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell. 2014;158(6):1431–43. doi: 10.1016/j.cell.2014.08.009 ; PubMed Central PMCID: PMCPMC4163041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Triska M, Grocutt D, Southern J, Murphy DJ, Tatarinova T. cisExpress: motif detection in DNA sequences. Bioinformatics. 2013;29(17):2203–5. doi: 10.1093/bioinformatics/btt366 ; PubMed Central PMCID: PMCPMC3740630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viola IL, Uberti Manassero NG, Ripoll R, Gonzalez DH. The Arabidopsis class I TCP transcription factor AtTCP11 is a developmental regulator with distinct DNA-binding properties due to the presence of a threonine residue at position 15 of the TCP domain. Biochem J. 2011;435(1):143–55. doi: 10.1042/BJ20101019 . [DOI] [PubMed] [Google Scholar]
- 42.Mathelier A, Fornes O, Arenillas DJ, Chen CY, Denay G, Lee J, et al. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2016;44(D1):D110–5. doi: 10.1093/nar/gkv1176 ; PubMed Central PMCID: PMCPMC4702842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco-Zorrilla JM, Lopez-Vidriero I, Carrasco JL, Godoy M, Vera P, Solano R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci U S A. 2014;111(6):2367–72. doi: 10.1073/pnas.1316278111 ; PubMed Central PMCID: PMCPMC3926073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali MA, Abbas A, Kreil DP, Bohlmann H. Overexpression of the transcription factor RAP2.6 leads to enhanced callose deposition in syncytia and enhanced resistance against the beet cyst nematode Heterodera schachtii in Arabidopsis roots. BMC Plant Biol. 2013;13:47 doi: 10.1186/1471-2229-13-47 ; PubMed Central PMCID: PMCPMC3623832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stracke R, Jahns O, Keck M, Tohge T, Niehaus K, Fernie AR, et al. Analysis of PRODUCTION OF FLAVONOL GLYCOSIDES-dependent flavonol glycoside accumulation in Arabidopsis thaliana plants reveals MYB11-, MYB12- and MYB111-independent flavonol glycoside accumulation. New Phytol. 2010;188(4):985–1000. doi: 10.1111/j.1469-8137.2010.03421.x . [DOI] [PubMed] [Google Scholar]
- 46.Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, et al. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50(4):660–77. doi: 10.1111/j.1365-313X.2007.03078.x ; PubMed Central PMCID: PMCPMC1976380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stracke R, Favory JJ, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, et al. The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 2010;33(1):88–103. doi: 10.1111/j.1365-3040.2009.02061.x . [DOI] [PubMed] [Google Scholar]
- 48.Wongsurawat T, Jenjaroenpun P, Kwoh CK, Kuznetsov V. Quantitative model of R-loop forming structures reveals a novel level of RNA-DNA interactome complexity. Nucleic Acids Res. 2012;40(2):e16 doi: 10.1093/nar/gkr1075 ; PubMed Central PMCID: PMCPMC3258121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ginno PA, Lim YW, Lott PL, Korf I, Chedin F. GC skew at the 5' and 3' ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res. 2013;23(10):1590–600. doi: 10.1101/gr.158436.113 ; PubMed Central PMCID: PMCPMC3787257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginno PA, Lott PL, Christensen HC, Korf I, Chedin F. R-loop formation is a distinctive characteristic of unmethylated human CpG island promoters. Mol Cell. 2012;45(6):814–25. doi: 10.1016/j.molcel.2012.01.017 ; PubMed Central PMCID: PMCPMC3319272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanz LA, Hartono SR, Lim YW, Steyaert S, Rajpurkar A, Ginno PA, et al. Prevalent, Dynamic, and Conserved R-Loop Structures Associate with Specific Epigenomic Signatures in Mammals. Mol Cell. 2016;63(1):167–78. doi: 10.1016/j.molcel.2016.05.032 ; PubMed Central PMCID: PMCPMC4955522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenjaroenpun P, Chew CS, Yong TP, Choowongkomon K, Thammasorn W, Kuznetsov VA. The TTSMI database: a catalog of triplex target DNA sites associated with genes and regulatory elements in the human genome. Nucleic Acids Res. 2015;43(Database issue):D110–6. doi: 10.1093/nar/gku970 ; PubMed Central PMCID: PMCPMC4384029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenjaroenpun P, Wongsurawat T, Yenamandra SP, Kuznetsov VA. QmRLFS-finder: a model, web server and stand-alone tool for prediction and analysis of R-loop forming sequences. Nucleic Acids Res. 2015;43(20):10081 doi: 10.1093/nar/gkv974 ; PubMed Central PMCID: PMCPMC4787779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elhaik E, Pellegrini M, Tatarinova TV. Gene expression and nucleotide composition are associated with genic methylation level in Oryza sativa. BMC Bioinformatics. 2014;15:23 doi: 10.1186/1471-2105-15-23 ; PubMed Central PMCID: PMCPMC3903047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tatarinova T, Elhaik E, Pellegrini M. Cross-species analysis of genic GC3 content and DNA methylation patterns. Genome Biol Evol. 2013;5(8):1443–56. doi: 10.1093/gbe/evt103 ; PubMed Central PMCID: PMCPMC3762193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatarinova T, Alexandrov N., Bouck J., Feldmann K. GC3 Biology in Corn, Rice, Sorghum and other grasses. BMC genomics. 2010;11(308). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–54. doi: 10.1146/annurev.genet.32.1.227 . [DOI] [PubMed] [Google Scholar]
- 58.Yao H, Guo L, Fu Y, Borsuk LA, Wen TJ, Skibbe DS, et al. Evaluation of five ab initio gene prediction programs for the discovery of maize genes. Plant Mol Biol. 2005;57(3):445–60. doi: 10.1007/s11103-005-0271-1 . [DOI] [PubMed] [Google Scholar]
- 59.Bajic VB, Brent MR, Brown RH, Frankish A, Harrow J, Ohler U, et al. Performance assessment of promoter predictions on ENCODE regions in the EGASP experiment. Genome Biol. 2006;7 Suppl 1:S3 1–13. doi: 10.1186/gb-2006-7-s1-s3 ; PubMed Central PMCID: PMCPMC1810552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solovyev V, Kosarev P, Seledsov I, Vorobyev D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006;7 Suppl 1:S10 1–2. doi: 10.1186/gb-2006-7-s1-s10 ; PubMed Central PMCID: PMCPMC1810547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salamov AA, Solovyev VV. Ab initio gene finding in Drosophila genomic DNA. Genome Res. 2000;10(4):516–22. ; PubMed Central PMCID: PMCPMC310882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2012;40(Database issue):D1202–10. doi: 10.1093/nar/gkr1090 ; PubMed Central PMCID: PMCPMC3245047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Law M, Childs KL, Campbell MS, Stein JC, Olson AJ, Holt C, et al. Automated update, revision, and quality control of the maize genome annotations using MAKER-P improves the B73 RefGen_v3 gene models and identifies new genes. Plant Physiol. 2015;167(1):25–39. doi: 10.1104/pp.114.245027 ; PubMed Central PMCID: PMCPMC4280997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. doi: 10.1093/bioinformatics/btu170 ; PubMed Central PMCID: PMCPMC4103590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11(9):1650–67. doi: 10.1038/nprot.2016.095 ; PubMed Central PMCID: PMCPMC5032908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kel A, Konovalova T, Waleev T, Cheremushkin E, Kel-Margoulis O, Wingender E. Composite Module Analyst: a fitness-based tool for identification of transcription factor binding site combinations. Bioinformatics. 2006;22(10):1190–7. doi: 10.1093/bioinformatics/btl041 . [DOI] [PubMed] [Google Scholar]
- 67.Koschmann J, Bhar A, Stegmaier P, Kel AE, Wingender E. "Upstream Analysis": An Integrated Promoter-Pathway Analysis Approach to Causal Interpretation of Microarray Data. Microarrays (Basel). 2015;4(2):270–86. doi: 10.3390/microarrays4020270 ; PubMed Central PMCID: PMCPMC4996392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.