Abstract
Background
Most recently, an emerging theme in the field of tumor immunology predominates: chimeric antigen receptor (CAR) therapy in treating solid tumors. The number of related preclinical trials was surging. However, an evaluation of the effects of preclinical studies remained absent. Hence, a meta-analysis was conducted on the efficacy of CAR in animal models for solid tumors.
Methods
The authors searched PubMed/Medline, Embase, and Google scholar up to April 2017. HR for survival was extracted based on the survival curve. The authors used fixed effect models to combine the results of all the trials. Heterogeneity was assessed by I-square statistic. Quality assessment was conducted following the Stroke Therapy Academic Industry Roundtable standard. Publication bias was assessed using Egger's test.
Results
Eleven trials were included, including 54 experiments with a total of 362 animals involved. CAR immunotherapy significantly improved the survival of animals (HR: 0.25, 95% CI: 0.13–0.37, P < 0.001). The quality assessment revealed that no study reported whether allocation concealment and blinded outcome assessment were conducted, and only five studies implemented randomization.
Conclusions
This meta-analysis indicated that CAR therapy may be a potential clinical strategy in treating solid tumors.
Introduction
Cancer is one of the leading causes of death around the world[1]. However, the leaps and bounds of chimeric antigen receptor (CAR) immunotherapy is changing such a situation. CARs can target specific antigen of tumor cells, therefore activating T cells and inducing robust antitumor effects. In the field of hematologic malignancies, the surging of CAR immunotherapy has demonstrated remarkable success[2].
Compared with hematologic malignancies, solid tumors remained a significant challenge to CAR-T immunotherapy. So far, an increasing number of preclinical trials have focused on solid tumors, targeting at carcinoembryonic antigen (CEA), interleukin 13 receptor (IL-13R), human epidermal growth factor receptor 2 (HER2), fibroblast activation protein (FAP) and so on. At present, more and more scientists are devoted to searching for potential targets.
Most recently, publications on preclinical trials of solid tumors have abounded, and relevant phase I or phase I/II clinical trials have just been initiated[3]. However, an evaluation of the effects of preclinical studies remains absent. Which target of CARs will induce better or worse outcomes? What is the role of CARs in treating different types of cancer? Are the outcomes reliable in preclinical studies? These questions still remain unknown.
Here, we conducted a meta-analysis of animal models in order to evaluate the potential value of CAR-T therapy for solid tumors based on the preclinical trials. Also, we attempted to explore the experimental design features of current studies in order to point out the possible shortcomings of the preclinical experimental designs and the future clinical trials.
Materials and methods
Literature search
We search trials among PubMed/Medline, Embase, and Google scholar up to April 2017. Key words included "chimeric antigen receptor", "CAR", "solid tumor", "GBM", "lung cancer", "colorectal cancer", "pancreatic cancer", "prostate cancer", "ovarian cancer", "breast cancer", "preclinical". All additional studies of potential interest were retrieved for further analysis. All publications were written in English.
All the related publications were screened independently by two reviewers (YW and RX) to identify studies that met the inclusion criteria (below).
Inclusion and exclusion criteria
Eligible studies should meet these standards. (1) Participant: the trials be conducted in animal models. (2) Intervention: CAR immunotherapy. (3) Control: the researchers should make at least one comparison between CAR T cell group and NT T cell group or untreated group. (4) Outcome: the survival curve should be reported. For trials that compared CAR and control group in more than one tumor model, the survival curve of each tumor model was included. If there was a disagreement between the two reviews, another reviewer (HS) reviewed it and a final consensus was reached.
Data extraction
Three reviewers (YW, RX, and KJ) independently extracted data with a extraction form, and we checked all the data very carefully. We identified all the studies with the first author and the year of publication. We extracted the following information from the reports: first-author; year of publication; animal species; age; experimental group; control group; animal number; type of model; target; the generation of CAR; the type of cancer; the Kaplan-Meier survival curve. When the data was reported merely in image format, we attempted to contact the correspondence author of the publication to ask for the original data. If there was no reply or no useful information, Engauge Digitizer software V9.7 for macOS 10.12.3 was used to measure graphically the data as presented. When different CAR T cells were evaluated in multiple groups in one publication, the data in each group were extracted as an individual experiment for analysis. If the efficacy of different doses of CAR T cells were evaluated, all the valid hazard ratios for survival would be extracted.
Quality assessment
A latest 2009 version of the initial Stroke Therapy Academic Industry Roundtable (STAIR) standard was applied to assess the quality of the studies[4]. It includes: (1) sample-size calculation; (2) inclusion and exclusion criteria; (3) randomization; (4) allocation concealment; (5) reporting of animals excluded from analysis; (6) blinded assessment of outcome; (7) reporting potential conflicts of interest and study funding. Three reviews (YW, RX, and KJ) assessed the qualities in all included studies and presented as a "yes" or "no". The "unclear" means the quality was not clear. The image was made with Numbers V4.1 software.
Data analysis
Statistical analysis, forest plots and detection of publication bias were carried out with Stata SE 14.1 for macOS 10.12.3 (StataCorp, College Station, TX, USA). The data of survival was extracted by Engauge Digitizer software V9.7 for macOS 10.12.3. The ln(HR) value and se(ln(HR)) value were calculated based on an Excel spreadsheet developed by Matthew Sydes and Jayne Tierney of the MRC Clinical Trials Unit, London, the United Kingdom[5, 6]. P≤0.05 was used to indicate a statistical significance. Heterogeneity was considered low, moderate or high for I- squared values <25%, 25–50% and >50%[7]. A fixed effect model would be used if the heterogeneity was low or moderate. If the heterogeneity was high, the analysis would be performed with a random effects model. Publication bias was assessed by Egger's test. If the p value is more than 0.1 in the Egger's test, it was considered insignificant for publication bias[8].
Results
Literature selection and study characteristics
The preliminary literature search included 3,199 relevant publications (S1 Fig). Of these, 3,157 studies contained commentaries, editorials, study protocols, and irrelevant themes. And they were excluded afterwards based on their titles or abstracts. The remaining 32 studies were reviewed in full text. After removing duplicated literatures, literatures without usable data and some ineligible literatures, we identified articles eligible for further review by screening texts. We identified fifteen trials including 54 experiments with a total of 362 animals involved[9–23]. The whole research process can be seen in the S1 Fig. All the studies reported the survival curve. The characteristics among these studies varied considerably. Main characteristics of those trials are available in the Table 1.
Table 1. Characteristics of the included animal studies.
| First author | Year | Cancer | Target | n | CAR generation | Animals | immunocompetent / immunocompromised | Exp | Ctrl | model |
|---|---|---|---|---|---|---|---|---|---|---|
| Choi | 2013 | GBM | EGFRvIII | 5/5/5/5 | 3 | 5-6-week-old NSG female mice | immunocompromised | 5×10^5 EGFRvIII CAR T cells; 5×10^4 EGFRvIII CAR T cells; 5×10^3 EGFRvIII CAR T cells; | 5×10^5 untreated CAR T cells | Intracranial glioma xenograft |
| Ohno | 2013 | GBM | EGFRvIII | 10/5 | 3 | 5-6-week-old NOG female mice | immunocompromised | 5×10^4 U87-EGFRvIII-Luc cells | 5×10^4 mock-transduced T-cells | mice bearing human GBM xenografts |
| Chow | 2013 | GBM | EphA2 | 12/8/9 | 2 | 8-12-week-old ICR-SCID male mice | immunocompromised | 1×10^6 EphA2 CAR T cells | 1×10^6 NT T cells; untreated | Orthotopic xenograft SCID mouse model |
| Kong | 2012 | GBM | IL13R | 13/12/4 | 2 | 6-8-week-old female nude rats | immunocompetent | 5×10^6 IL13Rα2 CAR T cells | 5×10^6 NT T cells; tumor only | a human glioma xenograft model |
| Krebs | 2014 | GBM | IL13R | 11/11/11/11/9/10 | 2 | ICR-SCID mice | immunocompromised | 2×10^6 IL13KR CAR T cells; 2×10^6 IL13K CAR T cells; 2×10^6 IL13YR CAR T cells; 2×10^6 IL13Y CAR T cells; | 2×10^6 NT T cells; untreated | an orthotopic xenograft SCID mouse model of GBM |
| Zhou | 2013 | lung cancer | EGFR | 5/5 | 2 | 5-6-week-old NSG CB-17 mice | immunocompromised | 2×10^6 EGFR CAR T cells | 2×10^6 mock T cell | a xenogeneic model of advanced lung metastatic A549 cancer; an A431 tumorigenicity model; an A2780 s.c. tumor model |
| Kakarla | 2013 | lung cancer | FAP | 8/9/9 | 2 | 8-12-week-old ICR-SCID male mice | immunocompromised | 10×10^6 FAP CAR T cells | 10×10^6 NT T cells; untreated | a human A549 lung cancer xenograft model; a loco-regional tumor model |
| Ahmed | 2009 | lung cancer | HER2 | 10/9/5/5 | 1 | 9-12-week-old ICR-SCID male mice | immunocompromised | 10×10^6 HER2 CAR T cells(treated day 2); 10×10^6 HER2 CAR T cells(treated day 8); | 10×10^6 NT T cells; Tumor | LM7 xenogeneic lung metastases model; a xenogeneic SCID mouse model |
| MALIAR | 2012 | pancreatic cancer | HER2 | 7/7 | 1 | ICR-SCID male mice | immunocompromised | 1×10^7 HER2 CAR T cells | 1×10^7 CD24 CAR T cells | PAC Wapac-4 and Wapac-5 xenograft models |
| Blat | 2014 | colorectal cancer | CEA | 7/7/7/7/7 | 2 | CEABAC-2 and CEABAC-10 mice | immunocompetent | 0.75×10^6 CEA CAR T cells; 1.5×10^6 CEA CAR T cells | 0.75×10^6 irrelevant CAR T cells; 1.5×10^6 irrelevant CAR T cells; untreated | T-cell-transfer colitis and azoxymethane–dextran sodium sulfate model for colitis-associated colorectal cancer |
| Zhu | 2015 | GBM | CD133 | 7/7 | 3 | 6–8-week-old male NMRI nude mice | immunocompromised | 2×10^6 CD133 CAR T cells | 2×10^6 NT T cells | an orthotopic mouse model of GBM |
| Slaney | 2016 | breast cancer | HER2 | 7/7 | 2 | 8–12-week-old C57BL/6 gender-matched mice | immunocompetent | 1×10^7 HER2 CAR T cells | untreated | an immunocompetent, self-antigen preclinical mouse model of orthotopic breast cancer |
| Wu | 2015 | ovarian cancer | B7H6 | 23/23 | 2 | 7–12-week-old C57BL/6 mice | immunocompetent | 5×10^6 B7H6 CAR T cells | 5×10^6 mock HER2 CAR T cells | a systemic T cell lymphoma model; an ovarian cancer model |
| Shiina | 2016 | GBM | PDPN | 12/14/14 | 3 | 5–6-week-old NOG female mice | immunocompromised | 2×10^6 NZ-1 CAR T cells | 2×10^6 mock-transduced PBMCs | an intracranial glioma xenograft model |
| Hong | 2016 | ovarian cancer | L1-CAM | 6/6/6/6 | 2 | 8-week-old NSG female mice | immunocompromised | 5×10^6 L1-CAM CAR T cells | 5×10^6 CD19 CAR T cells;5×10^6 mock-transduced T cells; PBS | a xenograft mouse model of ovarian cancer |
Abbreviations: NOD, nonobese diabetic; SCID, severe combined immunodeficient; NT, nontransduced; SCID, severe combined immunodeficiency; s.c., subcutaneous; GBM, Glioblastoma; PBL, peripheral blood lymphocytes; PBMC, peripheral blood mononuclear cells; ICR, inverted cytokine receptor; 1G, first generation; 2G, second generation; PBS, phosphate buffer saline.
Meta-analyses
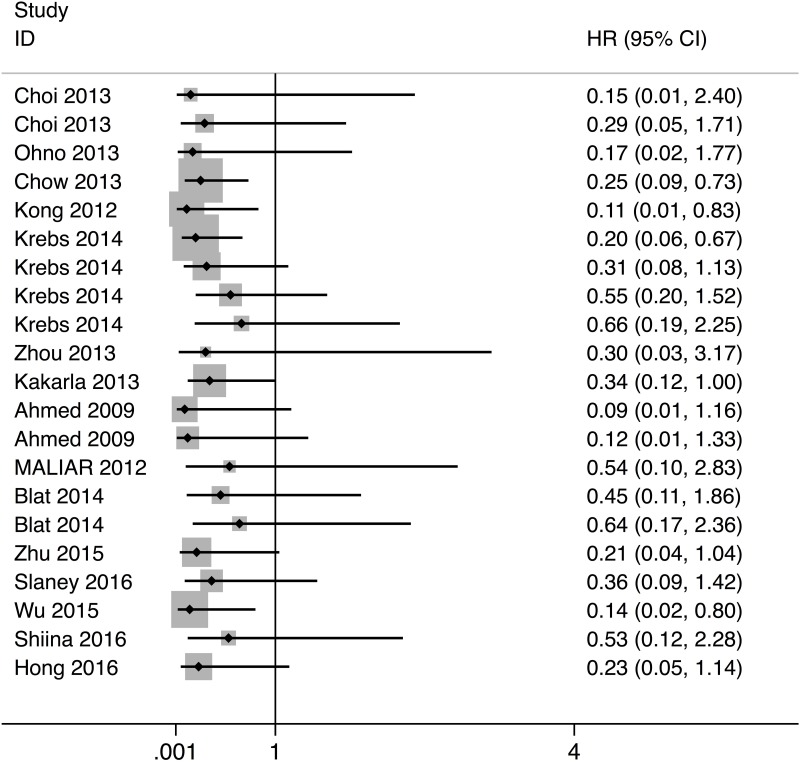
The meta-analysis on survival time indicated that CAR immunotherapy was associated with a significantly prolonged survival (HR: 0.25, 95% CI: 0.13–0.37, P < 0.001) (Fig 1). And the heterogeneity was low (I-squared = 0.0%). We then conducted a subgroup analyses of year of publication, generation of CAR, type of cancer, type of animal model, and target (Table 2). The subgroup analysis manifested that, among all types of cancers, CAR immunotherapy was most efficient in ovarian cancer animals (HR: 0.170, 95% CI: -0.147–0.488). The subgroup analysis by target showed that HER2-CAR-T therapy is most efficient (HR: 0.203, 95% CI: -0.148–0.554). Also, a comparison between immunocompromised and immunocompetent animal models was also performed. Notably, no significant difference was observed between immunocompromised and immunocompetent animals (P = 0.712). This finding could be due to the lack of statistical power.
Fig 1. Forest plot of the meta-analysis for the hazard ratio.
Table 2. Subgroup analysis by cancer type, target, generation, animal model, and publication year.
| Cancer type | HR | 95% CI |
|---|---|---|
| GBM | 0.247 | 0.092–0.402 |
| lung cancer | 0.223 | -0.080–0.526 |
| colorectal cancer | 0.524 | -0.160–1.208 |
| ovarian cancer | 0.17 | -0.147–0.488 |
| Target | ||
| EGFRvIII | 0.216 | -0.321–0.754 |
| IL13 | 0.247 | 0.041–0.453 |
| HER2 | 0.203 | -0.148–0.554 |
| CEA | 0.524 | -0.160–1.208 |
| Generation | ||
| 1 | 0.143 | -0.270–0.556 |
| 2 | 0.258 | 0.121–0.396 |
| 3 | 0.246 | -0.101–0.592 |
| Animal model | ||
| immunocompromised | 0.26 | 0.119–0.401 |
| immunocompetent | 0.207 | -0.036–0.450 |
| Publication year | ||
| 2009 | 0.103 | -0.331–0.536 |
| 2012 | 0.146 | -0.247–0.538 |
| 2013 | 0.270 | 0.039–0.500 |
| 2014 | 0.318 | 0.093–0.543 |
| 2015 | 0.166 | -0.141–0.474 |
| 2016 | 0.315 | -0.078–0.708 |
Subgroup analyses of less than two experiments were not performed due to the small sample size.
Quality assessments and risk of bias
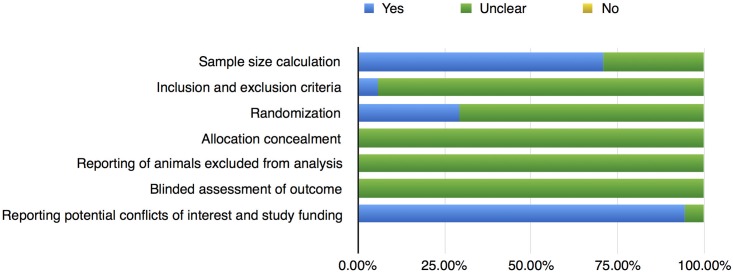
The quality of the seventeen studies was assessed by the STAIR tool (Fig 2 and Table 3). According to the Egger's test, the P value was 0.013, which manifests that the publication bias did not exist.
Fig 2. STAIR's risk of bias: Yes = low risk of bias, No = high risk bias, Unclear = unclear risk of bias.
Table 3. Quality assessment of the included trials.
| Study | Sample size calculation | Inclusion and exclusion criteria | Randomization | Allocation concealment | reporting of animals excluded from analysis | blinded assessment of outcome | reporting potential conflicts of interest and study funding |
|---|---|---|---|---|---|---|---|
| Choi 2013 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| Ohno 2013 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| Chow 2013 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| Kong 2012 | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Yes |
| Krebs 2014 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| Zhou 2013 | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | Yes |
| Kakarla 2013 | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes |
| Ahmed 2009 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| MALIAR 2012 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| Blat 2014 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| Zhu 2015 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Slaney 2016 | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Yes |
| Wu 2015 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| Shiina 2016 | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Yes |
| Hong 2016 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
This meta-analysis revealed that many common practices including randomization were not implemented in most of the trials. None of the published studies reported whether blinded assessment of outcome was carried out. Whether there existed any expectations or personal preferences was unclear. This made it difficult to find out that some outcome of experiments was in fact invalid.
Discussion
To the best of our knowledge, this is the first meta-analysis which assessed efficacy of chimeric antigen receptor (CAR) immunotherapy in animal models for solid tumors. Publications on preclinical trials of solid tumors have abounded recently. Also, phase I and phase I/II clinical trials of CAR on solid tumors have just been initiated. The aim of this study is to assess the potential value of CAR-T therapy for solid tumors based on the preclinical trials.
Main findings
Based on our analyses, CAR-T immunotherapy proved to generate a robust antitumor efficacy in animal models. The quality assessment manifested that there were some defects in the field of CAR preclinical research. No trials reported whether blinded outcome assessment or allocation concealment was performed. Only five studies implemented randomization, which may have induced uncertainties.
Our subgroup analysis illustrated that CAR immunotherapy was most efficient in ovarian cancer animals, and HER2-CAR-T cell therapy was demonstrated to be more effective. Inserestingly, between immunocompromised and immunocompetent animals models, no significant difference of efficacy was observed. This finding could be due to a lack of statistical power.
Agreement/disagreement with previous study
To date, there is no meta-analysis evaluating CAR immunotherapy in animal models. A meta-analysis tended to evaluate the efficiency of CD19 CAR T cells for treatment of B cell malignancies [24]. Base on results of that meta-analysis, the number of CD19-CAR T cells have positive correlations with the clinical efficiency. Also, a systematic review had a discussion about the the increasing number of CAR trials[25].
Limitations
This study does have some limitations. Firstly, all the preclinical trials evaluating CARs have comparatively small group sizes, leading to some uncertainties of outcomes. Secondly, doses of CAR-T cells varies in different experiments, ranging from 5×10^3 to 1×10^7. Therefore, some of the comparisons between CARs and control groups may be invalid, although we have excluded the invalid comparisons according to our criteria. Thirdly, we used Engauge Digitizer software in order to extract data from the survival curve. Minor distortion of effect sizes were likely to occurred. Fourth, the meta-analysis didn't directly address some elements, including duration of trial or selection of model.
Conclusions
CAR immunotherapy appeared to inhibit the growth of solid tumors in animal models. CAR therapy may be a potential clinical strategy in treating solid tumors.
Supporting information
(PDF)
Acknowledgments
We thanks the data provided by the authors of included trials. We acknowledged Bei Qian, Keren Jia, Defu He, and Xiangyu Cao who helped us in methodology.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332 . [DOI] [PubMed] [Google Scholar]
- 2.Johnson LA, June CH. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2017;27(1):38–58. doi: 10.1038/cr.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Jiang M. The revolution of lung cancer treatment: from vaccines, to immune checkpoint inhibitors, to chimeric antigen receptor T therapy. Biotarget. 2017;1:7. [Google Scholar]
- 4.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–50. doi: 10.1161/STROKEAHA.108.541128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parmar MKB, Torri V, Stewart LA. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–34. [DOI] [PubMed] [Google Scholar]
- 6.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Statistics in Medicine. 2002;21(22):3337–51. doi: 10.1002/sim.1303 [DOI] [PubMed] [Google Scholar]
- 7.Dersimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 8.Vandenbroucke JP. Bias in meta-analysis detected by a simple, graphical test. Experts' views are still needed. BMJ (Clinical research ed). 1997;315(7109):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N, Salsman VS, Yvon E, Louis CU, Perlaky L, Wels WS, et al. Immunotherapy for Osteosarcoma: Genetic Modification of T cells Overcomes Low Levels of Tumor Antigen Expression. Molecular Therapy. 2009;17(10):1779 doi: 10.1038/mt.2009.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi BD, Suryadevara CM, Gedeon PC, Nd HJ, Sanchezperez L, Bigner DD, et al. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. Journal of Clinical Neuroscience. 2014;21(1):189–90. doi: 10.1016/j.jocn.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Molecular Therapy. 2013;21(3):629–37. doi: 10.1038/mt.2012.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakarla S, Chow KK, Mata M, Shaffer DR, Song XT, Wu MF, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Molecular Therapy. 2013;21(8):1611 doi: 10.1038/mt.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong S, Sengupta S, Tyler B, Bais AJ, Ma Q, Doucette S, et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2012;18(21):5949 doi: 10.1158/1078-0432.CCR-12-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs S, Chow KK, Yi Z, Rodriguez-Cruz T, Hegde M, Gerken C, et al. T cells redirected to interleukin-13Ralpha2 with interleukin-13 mutein—chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Ralpha1. Cytotherapy. 2014;16(8):1121–31. doi: 10.1016/j.jcyt.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maliar A, Servais C, Waks T, Chmielewski M, Lavy R, Altevogt P, et al. Redirected T Cells That Target Pancreatic Adenocarcinoma Antigens Eliminate Tumors and Metastases in Mice. Gastroenterology. 2012;143(5):1375–84. doi: 10.1053/j.gastro.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 16.Ohno M, Ohkuri T, Kosaka A, Tanahashi K, June CH, Natsume A, et al. Expression of miR-17-92 enhances anti-tumor activity of T-cells transduced with the anti-EGFRvIII chimeric antigen receptor in mice bearing human GBM xenografts. Journal for ImmunoTherapy of Cancer. 2013;1(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong H, Brown CE, Ostberg JR, Priceman SJ, Chang WC, Weng L, et al. L1 Cell Adhesion Molecule-Specific Chimeric Antigen Receptor-Redirected Human T Cells Exhibit Specific and Efficient Antitumor Activity against Human Ovarian Cancer in Mice. Plos One. 2016;11(1):e0146885 doi: 10.1371/journal.pone.0146885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Li J, Wang Z, Chen Z, Qiu J, Zhang Y, et al. Cellular Immunotherapy for Carcinoma Using Genetically Modified EGFR-Specific T Lymphocytes. Neoplasia. 2013;15(5):544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed S, Sukumaran S, Bajgain P, Watanabe N, Heslop HE, Rooney CM, et al. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Molecular Therapy the Journal of the American Society of Gene Therapy. 2017;25(1):249 doi: 10.1016/j.ymthe.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiina S, Ohno M, Ohka F, Kuramitsu S, Yamamichi A, Kato A, et al. CAR T cells targeting podoplanin reduce orthotopic glioblastomas in mouse brains. Cancer Immunol Res. 2016. [DOI] [PubMed] [Google Scholar]
- 21.Slaney CY, Von SB, Davenport AJ, Beavis PA, Westwood JA, Mardiana S, et al. Dual-specific Chimeric Antigen Receptor T Cells and an Indirect Vaccine Eradicate a Variety of Large Solid Tumors in an Immunocompetent, Self-antigen Setting. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu MR, Zhang T, Demars LR, Sentman CL. B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity. Gene Therapy. 2015;22(8):675–84. doi: 10.1038/gt.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Prasad S, Gaedicke S, Hettich M, Firat E, Niedermann G. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget. 2015;6(1):171–84. doi: 10.18632/oncotarget.2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Cao L, Xie J, Shi N, Zhang Z, Luo Z, et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget. 2015;6(32):33961–71. doi: 10.18632/oncotarget.5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzinger A, Barden M, Abken H. The growing world of CAR T cell trials: a systematic review. Cancer Immunol Immunother. 2016;65(12):1433–50. doi: 10.1007/s00262-016-1895-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.