Abstract
Anhedonia is defined as a diminished ability to obtain pleasure from otherwise positive stimuli. Anxiety and mood disorders have been previously associated with dysregulation of the reward system, with anhedonia as a core element of major depressive disorder (MDD). The aim of the present study was to investigate whether stress-induced anhedonia could be prevented by treatments with escitalopram or novel herbal treatment (NHT) in an animal model of depression. Unpredictable chronic mild stress (UCMS) was administered for 4 weeks on ICR outbred mice. Following stress exposure, animals were randomly assigned to pharmacological treatment groups (i.e., saline, escitalopram or NHT). Treatments were delivered for 3 weeks. Hedonic tone was examined via ethanol and sucrose preferences. Biological indices pertinent to MDD and anhedonia were assessed: namely, hippocampal brain-derived neurotrophic factor (BDNF) and striatal dopamine receptor D2 (Drd2) mRNA expression levels. The results indicate that the UCMS-induced reductions in ethanol or sucrose preferences were normalized by escitalopram or NHT. This implies a resemblance between sucrose and ethanol in their hedonic-eliciting property. On a neurobiological aspect, UCMS-induced reduction in hippocampal BDNF levels was normalized by escitalopram or NHT, while UCMS-induced reduction in striatal Drd2 mRNA levels was normalized solely by NHT. The results accentuate the association of stress and anhedonia, and pinpoint a distinct effect for NHT on striatal Drd2 expression.
Introduction
Since the time of Epicurus [1], an ancient Greek philosopher, pleasure has been stipulated as a vital ingredient of human well-being. DSM-V [2] defines anhedonia as the diminished ability to obtain pleasure from otherwise positive stimuli and as a keystone symptom of various neuropsychiatric disorders, such as major depressive disorder (MDD). Pizzagali [3] proceeds further and solicits anhedonia as one of the most promising endophenotypes of MDD. The current treatise aims to further examine the relationship between hedonic faculty and bio-behavioral state.
As remission rate following selective serotonin reuptake inhibitors (SSRIs) treatment for MDD is roughly 45% [4,5] and SSRIs treatment is associated with frequent adverse effects, such as orgasm dysfunction in as up to 37% of the patients [4], it is of utmost importance to develop new efficacious pharmacotherapies that also mitigate the reward-related adverse effects of SSRIs. In previous studies from our lab we demonstrated a therapeutic effect of a novel herbal treatment (NHT) in reducing depressive- and anxiety-like behaviors in the unpredictable chronic mild stress (UCMS) animal model of depression. Specifically, we showed how chronic NHT administration prevented the UCMS-induced increments in time of immobility in the forced swim test (FST), passive coping in the tail suspension test (TST) and anxiety-like behavior in the elevated plus maze (EPM) [6,7]. Moreover, we demonstrated how NHT had no negative effect on sexual function in mice, in contrast to the SSRI escitalopram [6]. The effect of NHT on UCMS-induced anhedonia was not examined yet.
Alcohol is perceived by consumers as a pleasurable substance of choice [8]. The consumption of alcohol promotes activity of the brain reward system (BRS), as depicted by dopamine secretion in the nucleus accumbens (NAc) in both rodents [9] and humans [10]. The BRS has an important functional role by regulating hedonic state, motivation, decision-making and learning processes [11]. The inability to attain hedonic state in an adequate manner is characteristic of destabilized BRS [12] and is correlated with poorer well-being as expressed in self-report surveys [13] and underlain by dopaminergic alterations [14]. Most individuals consume alcohol for recreational purposes, as merely 15.4% of all alcohol users were reported with alcohol dependence [15]. The transition from controlled to maladaptive alcohol or other drug use is an intricate process affected by varying genetic and environmental factors. The neurobiological and molecular implications of chronic, addiction-related versus acute or sub-chronic, moderate drug use differ significantly, thus stating that ample portion of alcohol use has no negative implications on the BRS, and might even imply normative hedonic-prompting behavior [16].
Ethanol has been widely employed in animal models of addiction [17], but is not frequently applied in models screening hedonic tone. The present design aims to place under scrutiny the possible utilization of ethanol preference test as a pre-clinical instrument for testing the reward system. Our conjecture was that moderate ethanol preference is an indicator of regulated hedonic quality. We conducted an experiment in which ethanol preference was obtained following stress exposure and pharmacological treatments. The applied drugs were the SSRI escitalopram and NHT. Consequently, a second experiment was designed to study the effects of stress and escitalopram / NHT treatments on the hedonic tone prompted by sucrose, a primary reinforcer vastly used in animal models of anhedonia [18–20]. This design makes it feasible to discriminate between the rewarding potencies of the two substances (ethanol and sucrose) under naïve and stress conditions, with or without the aforementioned treatments.
Mice were exposed to UCMS for 4 weeks, treated with escitalopram, NHT or vehicle for 3 weeks, and then screened for hedonic tone and pertinent neurobiological markers. We examined whether NHT will attenuate the UCMS-induced anhedonia and whether NHT has a specific effect on an important factor of the BRS, i.e., dopamine receptor D2 (Drd2) gene expression in the striatum. We additionally assessed hippocampal brain-derived-neurotrophic-factor (BDNF) to confirm our prior finding that UCMS-induced down-regulation in BDNF levels can be averted by NHT [7]. The behavioral and neuromolecular effects of NHT were compared with those of escitalopram.
Materials and methods
Animals
One-month-old ICR outbred male mice (Envigo, Israel) were kept in the vivarium of the ‘Academic College of Tel-Aviv-Yaffo’. Mice were housed in standard group cages (5 mice per cage, each cage containing mice from all experimental groups) and kept on a reversed 12 h light/dark cycle (light on 19:00–7:00). Mice had ad-libitum access to food and water except during stressor application (with the exclusion of the light/dark cycle reversal). All experiments were carried out in strict accordance with the recommendations in The Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the 'Academic College of Tel-Aviv-Yaffo' (Permit Number: mta-2015-09-5). Animal sacrifice was executed via cervical dislocation by an experienced experimenter. All efforts were made to minimize animal suffering.
The ICR outbred mouse is a species that entails high genetic variability between animals, and therefore has a relatively better ecological validity compared to other transgenic mice and was utilized for this study [21].
UCMS
The procedure is grounded on the paradigm originally designed for rats by Katz [22] and subsequently Willner [23]. It was previously adapted to mice, whilst applying an unpredictable stressor regime [24]. The following stressors were applied: cages with 1 cm of water at the bottom (water stress), inversed light/dark cycle, cages with wet sawdust, tilted cages at 45 degrees, mice restrain, empty cages and cages with the sawdust of different mice. A single stressor was applied for 4 h daily, during a period of 4 weeks. Contrastingly, the light/dark cycle disruption was applied from mid-day Friday until Sunday morning. To prevent habituation and to provide an unpredictable feature, stressors' schedules were altered daily.
Drugs
NHT is composed of Crataegus Pinnatifida, Triticum Aestivu, Lilium Brownie and Fructus Zizyphi Jujubae. Herbs were purchased as freeze-dried granules from KPC Products Inc. (Irvine, CA, USA). NHT was prepared by dissolving the 4 herbs (together) in saline, containing 1% DMSO to give a final concentration of 0.47 mg/ml (each). NHT was administered daily (30 mg/kg; i.p.). The dose was opted based on our previous study [6].
Escitalopram was kindly donated by TEVA Pharmaceutical Industries Ltd. and was administered daily (15 mg/kg; i.p.). The dose was opted based on previous studies [25,26].
Saline was administered at a weighed dose of 1% of the mice current weight (i.p.).
Behavioral assessment: Two bottle choice (sucrose/ethanol)
After the treatment phase, mice were single housed for a period of 6 days. Two drinking nozzles were set at the cage through which the animal could intake distilled water and either a 10% ethanol solution (experiment 1) or a 2% sucrose solution (experiment 2). The nozzles' positions were switched after 3 days to counterbalance the effect of position preference, in acquiescence with previous reports[19]. Fresh fluids were supplied after bottles' weight assessments. Sucrose and ethanol solutions were introduced for the first time during the assessment period, and there were neither prior acclimation nor habituation phases. Six-day preference was calculated per mouse as ratio of sucrose or ethanol mean intake from total fluid mean intake (i.e., ethanol or sucrose / ethanol or sucrose + water).
Assessment of BDNF levels
Mice' brains were removed and rinsed of blood after sacrifice and the hippocampus and striatum were dissected out entirely. Tissues were homogenized in a cold extraction buffer (Tris-buffered saline, pH 8.0, with 1% NP-40, 10% glycerol, 5 mM NaMetavanadate, 10 mM PMSF, 100 μg/ml aprotinin and 10 μg/ml leupeptin). Homogenates were acidified with 0.1 M HCl (pH 3.0), incubated at room temperature (22–24°C) for 15 min, and neutralized with 0.1 M NaOH (pH 7.6). Homogenates were then microfuged at 7,000 g for 10 min. BDNF levels were evaluated using sandwich enzyme-linked immunosorbent assay (ELISA) as previously described [27]. BDNF concentrations are presented after normalization to total protein levels.
Assessment of Drd2 mRNA expression levels
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was conducted as previously described [28]. Briefly, RNA was executed with TRIzol reagent and precipitated with 100% ethanol and 0.3 M NaAcetate. mRNA was reverse transcribed with RevertAid cDNA synthesis kit. Expression was quantified via quantitative real time PCR (StepOnePlus: Applied Biosystems, Foster City, CA, USA) using the ∆∆Ct method. We used the following primers to amplify specific cDNA regions: Drd2, forward 5'-GACACCACTCAAGGGCAACT-3'; reverse 5'-TCCATTCTCCGCCTGCCTGTTCAC-3'; Gapdh, forward 5'-GCAAGAGAGAGGCCCTCAG-3'; reverse 5'-TGTGAGGGAGATGCTCAGTG-3'.
Study design
Experiment 1—ethanol
UCMS procedure was administered on ICR outbred mice. Escitalopram or NHT, were injected for 3 weeks following stress protocol, balanced with saline-injected mice and home cage naïve controls. Thereafter, mice were subjected to two-bottle-choice procedure in which they were tested for their ethanol preference (see Fig 1A for study design).
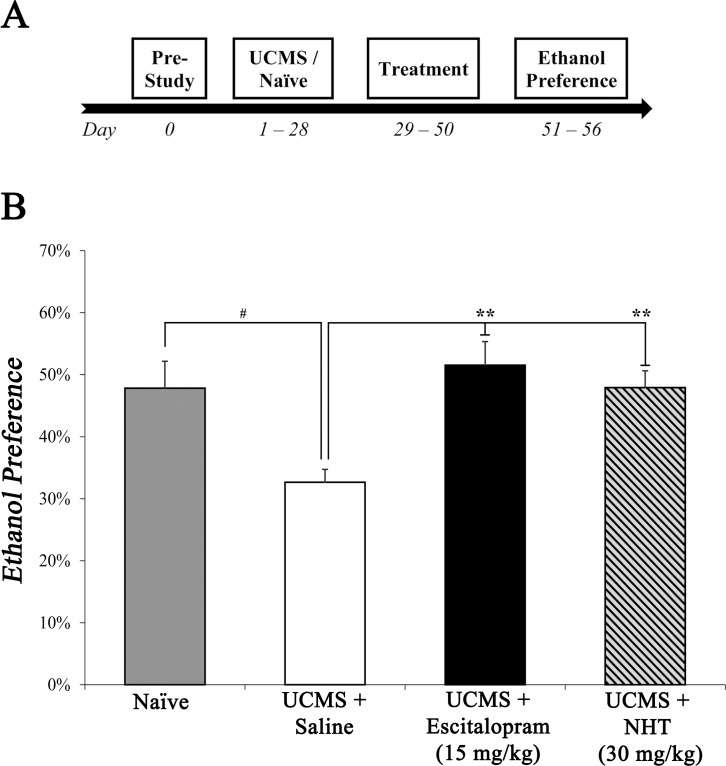
Fig 1. The effect of escitalopram (15 mg/kg) and NHT (30 mg/kg) treatments on stress-induced alterations in ethanol preference.
(A) A diagram depicting study design of experiment 1. After acclimation, mice were submitted to UCMS or naïve conditions (4 weeks), subsequently treated with saline, escitalopram or NHT (3 weeks) and screened for ethanol preference (6 days). (B) Stress diminished ethanol preference, while both NHT and escitalopram reversed this stress-induced diminution. n = 15–17 mice per group. #P<0.05 vs. naïve group. **P<0.01 vs. UCMS + saline group.
Experiment 2 –sucrose
Mice were subjected to UCMS or remained non-stressed (naïve). Stressed and naïve mice were then treated with escitalopram, NHT or saline for a period of 3 weeks and subsequently underwent the sucrose preference test (see Fig 2A for study design). Shortly after, mice were sacrificed, and their brains were removed. Biological indices pertinent to MDD and anhedonia were assessed: namely, hippocampal BDNF levels and striatal Drd2 mRNA levels.
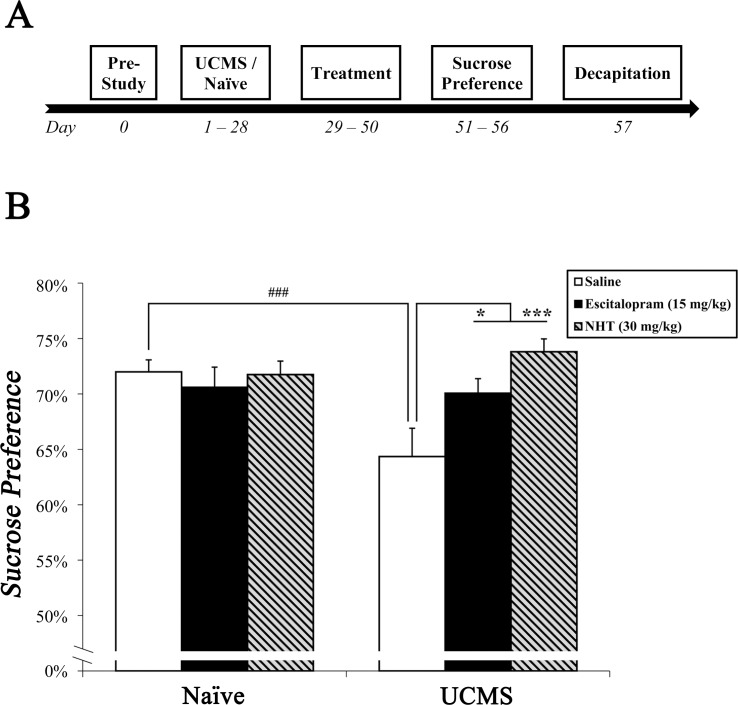
Fig 2. The effect of escitalopram (15 mg/kg) and NHT (30 mg/kg) treatments on stress-induced alterations in sucrose preference.
(A) A diagram depicting study design of experiment 2. After acclimation, mice were submitted to UCMS or naïve conditions (4 weeks), subsequently treated with saline, escitalopram or NHT (3 weeks), screened for sucrose preference (6 days) and prepared for neurobiological assessments. (B) Stress diminished sucrose preference, while both NHT and escitalopram reversed this stress-induced diminution. n = 15–17 mice per group. ###P<0.001 vs. naïve + saline group. *P<0.05, ***P<0.001 vs. UCMS + saline group.
Data analysis and interpretation of results
Results are expressed as mean +/- SEM. In the ethanol experiment, data was analyzed using one-way ANOVA. Other data was analyzed using two-way ANOVA with pharmacological treatment and stress manipulation as between subject variables. ANOVA was followed by Sidak post-hoc analysis. Significance was assumed as P<0.05.
Results
Ethanol preference
NHT and escitalopram normalized the stress-induced reduction in ethanol preference
One-way ANOVA revealed a significant main effect for treatment (F(3,61) = 6.785, P<0.001; Fig 1B). Sidak post-hoc analysis revealed that saline-treated stressed mice exhibited lower ethanol preference compared to naïve mice (non-stressed, non-treated) (P<0.05). In Addition, escitalopram- and NHT-treated stressed mice showed significantly higher ethanol preference compared to saline-treated stressed mice (P<0.01 in both contrasts). No significant differences were found between the escitalopram or NHT groups to the naïve group (N.S.).
Sucrose preference
NHT and escitalopram normalized the stress-induced reduction in sucrose preference
Two-way ANOVA revealed a significant manipulation × treatment interaction (F(2,92) = 4.917, P<0.01; Fig 2B). Saline-treated stressed mice exhibited lower sucrose preference compared to saline-treated naïve mice (post-hoc: P<0.001). In addition, escitalopram- and NHT-treated stressed mice exhibited higher sucrose preference compared to saline-treated stressed mice (post-hoc: P<0.05 and P<0.001, respectively), which was analogous to the sucrose preference demonstrated by naïve mice (N.S.).
Hippocampal BDNF levels
NHT and escitalopram normalized the stress-induced reduction in BDNF levels
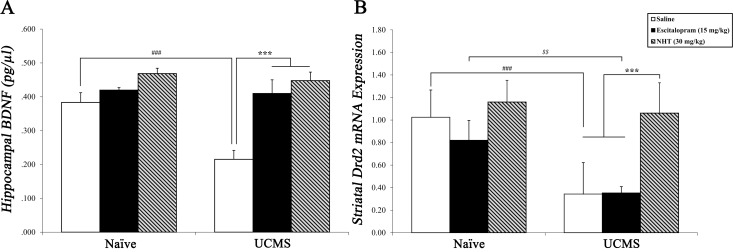
Two-way ANOVA revealed a significant manipulation × treatment interaction (F(2,22) = 5.188, P<0.05; Fig 3A). Saline-treated stressed mice exhibited lower BDNF levels compared to saline-treated naïve mice (post-hoc: P<0.001). In addition, escitalopram and NHT-treated mice showed higher BDNF levels compared to saline-treated stressed mice (post-hoc: P<0.001 in both contrasts). No significant differences in BDNF levels were found between the escitalopram-, NHT- and naïve-saline groups (N.S.).
Fig 3. The effects of escitalopram (15 mg/kg) and NHT (30 mg/kg) treatments on stress-induced alterations in hippocampal BDNF and striatal Drd2 levels.
(A) Stress reduced hippocampal BDNF concentration, while both NHT and escitalopram normalized this stress-induced reduction. n = 4–5 mice per group. ###P<0.001 vs. naïve + saline group. ***P<0.001 vs. UCMS + saline group. (B) Stress reduced striatal Drd2 mRNA expression under both saline and escitalopram treatments, but not under NHT treatment. n = 4–6 mice per group. ###P<0.001 vs. naïve + saline group. $ $P<0.01 vs. naïve + escitalopram group. ***P<0.001 vs. UCMS + NHT group.
Striatal Drd2 mRNA levels
NHT averted the stress-induced down-regulation in Drd2 mRNA levels, as opposed to escitalopram
Two-way ANOVA revealed a significant manipulation × treatment interaction (F(2,23) = 4.522, P<0.05; Fig 3B). Saline-treated stressed mice exhibited lower striatal Drd2 levels compared to saline-treated naïve mice (post-hoc: P<0.001); similar stress-induced down-regulation was found among escitalopram-treated mice (post-hoc: P<0.01). Unlike escitalopram-treated stressed mice, NHT-treated stressed mice showed significantly higher levels of striatal Drd2 compared to saline-treated stressed mice (post-hoc: P<0.001).
Discussion
The present study explored the hedonic tone in an animal model of depression and the effects of escitalopram and NHT. It yielded several important findings: [1] UCMS reduced ethanol/sucrose preferences and escitalopram or NHT restored baseline preference. In our study ethanol played a parallel role to sucrose, suggesting that in ICR mice ethanol consumption could function as an immediate reinforcer, instigating the reward system; [2] the behavioral outcome was supplemented by neurobiological alteration, viz. restoration of UCMS-induced diminution in hippocampal BDNF levels found in both escitalopram- and NHT-treated mice; and [3] striatal Drd2 mRNA levels were reduced by stress manipulation. NHT had an enhancing and balancing effect on Drd2 expression, whilst escitalopram did not.
All the presented biochemical data was obtained in the sucrose experiment. We did not apply those tests in the ethanol group, since previous studies reported on upregulation in Bdnf expression after acute ethanol consumption [29], which might have confounded the results. The same logic was applied to the Drd2 assessments following the datum that ethanol exposure yields significant alterations in DRD2 expression [30]. Data was obtained through the sucrose group, where a precise delineation was more attainable. The dopaminergic reaction in the NAc to sucrose consumption wanes rapidly, and has no effect in the following tests [31]. This is in contrast to the robust dopaminergic alterations exhibited after exposure to various drugs of abuse, including ethanol [32,33].
The validity of UCMS as a construct reflecting the pathogenesis, bio-symptomatology and phenomenology of depression in humans has been frequently debated [34], being chiefly accepted as a valid model for pharmacological screenings and neurobiological examinations reminiscing mood and anxiety disorders [35]. One of the most bolstering features of UCMS pertaining to this debate is the elicitation of anhedonia [36], a phenomena intrinsically twined with MDD. Loas [37] suggested a model centered on anhedonia in the etiology of depression. His model emphasized that minor anhedonic tone during childhood, also due to exposure to environmental stressors [38,39], is a strong predictor of anterior formation of MDD. Within the realm of MDD, anhedonia was found to be a core domain, stressed by evidence of poorer treatment outcome and more severe depressive symptoms for MDD patients with prominent anhedonia [40,41]. The current results revealed a strong yoke between environmental stress and anhedonia in mice. Nonetheless, the current design does not fully discriminate between anhedonia and other domains of MDD. Such discrimination could be proven fruitful in future research.
In congruence with our previous findings [6,42], a significant diminution of hippocampal BDNF concentration was observed following stress manipulation. Vast literature affirms and typifies the eminent role of BDNF in the etiology of human depression [43,44]. Diminished hippocampal BDNF expression was found in untreated depressive human patients [45] and in stress induction animal models [46]. The 'neurotrophin hypothesis' suggests that BDNF is a gene of utmost importance in the etiology of MDD [47]. Contradictory to the initial 'neurotrophin hypothesis', studies have shown that BDNF plays a diverse role in different brain systems. In the hippocampus and hypothalamus-pituitary-adrenal (HPA) stress-related pathways, lowered BDNF levels are indicators of dysregulation, as exhibited in MDD. In contrast, in the ventral-tegmental area and NAc, reward-related pathways, ascended BDNF expression is signaling imbalance in affect [48,49]. Chronic stress led to dendritic atrophy in the hippocampus and pre-frontal cortex (PFC), and to impaired long-term-potentiation induction in the hippocampal-PFC circuitry [50,51]. In the NAc, on the other hand, chronic stress led to dendritic hypertrophy [52]. The neurobiology of MDD involves deficiencies in both the stress and reward systems; the presented BDNF results convey reinforcement to the established HPA-dysregulation hypothesis of MDD in its relation to BDNF expression.
Although the links between depression, stress, antidepressants and BDNF are well recognized, the mechanisms underlying their interactions are still not soundly established. It is recognized that serotonin and BDNF exert bidirectional (rather than unidirectional) influence, promoting signaling and gene expression of each other [53]. One conjecture regarding the mechanism of this influence suggests that chronic SSRIs treatment (as opposed to stress) facilitates the expression and synthesis of BDNF in hippocampal astrocytic cells; thereby, eliciting neuro-protective and anti-depressive effects [54,55]. Other postulations have suggested that SSRIs can alter phosphorylation of CREB (a transcription factor which is a catalyst of BDNF synthesis) in pertinent signaling pathways; thus, promoting BDNF expression [53,56]. Insight is lacking vis-à-vis the mechanism by which NHT affects BDNF expression. In a previous study [6] we found that NHT upregulated serotonin transporter (SERT) expression in the hypothalamus. Both the hypothalamus and the hippocampus are involved in the inhibitory feedback of the HPA-axis, and are implicated in affective disorders [57,58]. Hence, further studies should be aimed to elucidate whether the effect of NHT on hippocampal BDNF expression is obtained through serotonergic alterations in stress regulatory structures or through other neural mechanisms.
In our study NHT had a balancing effect on Drd2 expression in the striatum following stress, while escitalopram did not. Previous works reported that pharmacologically induced DRD2 blockaded rats showed a reduced tendency to work for sucrose [59] and chronic mild stress caused a decrease in striatal Drd2 expression [60,61]. In addition, social isolation of Flinders Sensitive Line rats, genetic model of depression [62], reduced Drd2 expression in several areas in the striatum [63]. Contrastingly, Zhang et al. [64] reported that chronic unpredictable stress in rats up-regulated Drd2 mRNA expression in the striatum, with no effect for escitalopram administration compared to saline. Nonetheless, the stress paradigm they applied comprises more severe stressors (e.g., electric footshocks), therefore, might elicit other reactions than the mild stress paradigm we applied. The rational for utilizing mild stressors is that they are more resembling of the pathogenic environmental factor in MDD formation [65], as opposed to severe stress resemblance to trauma-related pathologies.
Neuroimaging studies have illustrated an abnormal activity in several BRS areas of MDD patients, among them the striatum [66,67]. Additionally, the ventral striatum was clinically supported as an effective region for deep brain stimulation (DBS) treatment of refractory depression [68,69]. Studies have indicated a significant involvement of DRD2 in the BRS dysregulation affecting MDD patients, though there are incongruous findings regarding the nature of this involvement [14]. One of the suggested models postulates that recurring activation of the HPA-axis, accompanied by increased secretion of glucocorticoids from the adrenal gland, results in sensitization of the dopaminergic-mesolimbic system. Such hypercortisolemia alters dopamine binding and DRD2 availability in the striatum, which might underlie the changes in hedonic reactivity [70,71]. DRD2 plays an important role in the motivational facet of the reward system [72]; clinical studies found that striatal DRD2 upregulation is a sign of MDD treatment responsiveness to SSRIs [73,74]. Moreover, the use of the DRD2/3 antagonist, sulpiride, annulled the antidepressant effect of SSRI treatment in MDD patients [75]. Deducing from the stated findings it has been hypothesized that sensitization of DRD2 in mesolimbic terminal regions is one of the central mechanisms by which SSRIs exert their therapeutic action [75,76]. Divergent to the aforementioned studies that utilized paroxetine or fluoxetine, escitalopram did not sustain striatal Drd2 expression following stress in our study. A putative explanation to this finding is that escitalopram is an SSRI with relatively lower affinity to dopamine transporter (DAT) [77]. The mechanism by which NHT altered striatal Drd2 expression is still unclear and remains to be further examined in future neurobiological and molecular studies. Such attempts are currently being conducted in our lab, in which we aim to identify specific active ingredients of NHT and their pharmacodynamics and biomolecular interactions.
One of the main adverse effects of SSRIs is sexual dysfunction [78]. Dopamine has a focal function in the regulation of sexual behavior [79]. Drugs that enhance dopamine transmission cultivate sexual activity. On the pharmacological aspect, the antidepressant that impairs sexual function the least is bupropion which is a dopaminergic agonist (apart from its effects on norepinephrine and serotonin) [80]. In a previous study we demonstrated that treatment with escitalopram reduced the sexual behavior of mice in comparison with NHT treatment, which had no such negative effect [6]. Our current results may suggest that this difference is underlain by a discrete effect of NHT on the dopaminergic system. The sustainment of striatal Drd2 levels held by NHT treatment might interact with reactivity to dopamine in the BRS by the receptors, a mechanism that might putatively explain the differences in sexual activity. This distinction could prove fertile in the development of antidepressant medicine free of the frequent adverse effect of sexual dysfunction. However, such presupposition could only be viewed as an initial hypothesis that remains to be examined and corroborated with further pre-clinical and clinical data. Moreover, the Drd2 stated effect did not reflect per se the behavioral hedonic tone observed in the solution preference tests, where both drugs yielded hedonic effects. This implies that the some aspects of hedonic behavior depend on striatal Drd2 expression, while others might not.
A resemblance between the patterns of sucrose and ethanol preferences in ICR mice was observed. Some addiction researchers maintain the presupposition that rodents have a natural tendency to avoid alcohol or consume it in an unsatisfactory manner [81]. It is perhaps so when the task at hand is manipulating substance-dependency, with substantial voluntary drug self-administration as an important component in the model's validity [82]. This obstacle seems to ebb in the case of hedonic-related consumption. Numerous animal species display a significant ethanol intake in naïve voluntary conditions including differing inbred mice species [83], rats [84] and primates [85]. In rats, this voluntary consumption phenomena is insufficient to elicit abuse-like phenotype without induction of intermittent withdrawals [86]. Other studies exploring the properties of ethanol in animals found a diminution in ethanol preference in deficient DRD2 mice [87] and a reduction in ethanol preference of rats subdued to chronic mild stress [88,89]. The inferred resemblance we found between ethanol and sucrose preferences highlights the possibility of operationalizing ethanol preference test not solely for addiction research but also for experiments concerning hedonic tone. The use of ICR outbred mice strengthens the suggested notion, considering the 'genetically-based-noise' they entail in their between-animal DNA variability.
Conclusions
The current research has emphasized the relation between stress and anhedonia, and pinpointed the possible involvement of striatal Drd2 and hippocampal BDNF levels in their association. Ethanol was shown as a substance eliciting reward behavior, implying the possibility of utilization of ethanol in reward-related experiments and not merely in animal addiction models. Stress had a lessening effect on mice sucrose and ethanol preferences. These effects were reversed via two pharmacotherapies (escitalopram and NHT). Both therapies prevented the down-regulation in hippocampal BDNF levels but bared a distinct impact on striatal Drd2 expression.
Acknowledgments
The authors would like to thank Nadav Kately for inventing and developing the NHT herbal formula. We thank Dr. Ohad Shaham for technical support.
Data Availability
All data files are available from the Dryad database (https://doi.org/10.5061/dryad.c0v5r).
Funding Statement
Funding for this study was provided by the Israel Science Foundation (ISF, http://www.isf.org.il, Grant 738/11), by the National Institute for Psychobiology in Israel (NIPI, http://www.psychobiology.org.il, Grant 7-2011-12) and by the Open University of Israel research fund (http://www.openu.ac.il) (all funds received by R.D.); the ISF, NIPI and the Open University of Israel had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
References
- 1.Epicurus. Letter to Menoeceus. Monadnock Valley Press; 2011. Available from: http://www.monadnock.net/epicurus/letter.html#n6 [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5). Washington: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10: 393–423. doi: 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, et al. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: A meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry. 2005;66(8): 974–81. [DOI] [PubMed] [Google Scholar]
- 5.Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001;178 March: 234–41. [DOI] [PubMed] [Google Scholar]
- 6.Doron R, Lotan D, Einat N, Yaffe R, Winer A, Marom I, et al. A novel herbal treatment reduces depressive-like behaviors and increases BDNF levels in the brain of stressed mice. Life Sci. 2014;94(2): 151–7. doi: 10.1016/j.lfs.2013.10.025 [DOI] [PubMed] [Google Scholar]
- 7.Doron R, Lotan D, Versano Z, Benatav L, Franko M, Armoza S, et al. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS One. 2014; 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keane H. Intoxication, harm and pleasure: an analysis of the Australian National Alcohol Strategy. Crit Public Health. 2009;19(2): 135–42. [Google Scholar]
- 9.Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9(1): 17–22. [DOI] [PubMed] [Google Scholar]
- 10.Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4): 226–31. doi: 10.1002/syn.10226 [DOI] [PubMed] [Google Scholar]
- 11.Berridge KC, Kringelbach ML. Neuroscience of affect: Brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013;23(3): 294–303. doi: 10.1016/j.conb.2013.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012; 35(1): 68–77. doi: 10.1016/j.tins.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology. 2008;199(3): 457–80. doi: 10.1007/s00213-008-1099-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlop BW, Nemeroff CB. The Role of Dopamine in the Pathophysiology of Depression. Arch Gen Psychiatry. 2007;64(3): 327–37. doi: 10.1001/archpsyc.64.3.327 [DOI] [PubMed] [Google Scholar]
- 15.Anthony JC, Warner L a., Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2(3): 244–68. [Google Scholar]
- 16.Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162(4): 712–25. doi: 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 17.Spanagel R. Alcohol addiction research: from animal models to clinics. Best Pract Res Clin Gastroenterol. 2003;17(3): 507–18. [DOI] [PubMed] [Google Scholar]
- 18.Katz RJ. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16(6): 965–8. [DOI] [PubMed] [Google Scholar]
- 19.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29(11): 2007–17. doi: 10.1038/sj.npp.1300532 [DOI] [PubMed] [Google Scholar]
- 20.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl). 1987;93(3): 358–64. [DOI] [PubMed] [Google Scholar]
- 21.Lutz CM, Linder CC, Davisson MT. Strains, Stocks and Mutant Mice In: Hedrich HJ, editor. The Laboratory Mouse, 2nd ed. Elsevier; 2012. pp. 37–56. [Google Scholar]
- 22.Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci Biobehav Rev. 1981;5(2): 247–51. [DOI] [PubMed] [Google Scholar]
- 23.Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 24.Surget A, Belzung C. Unpredictable chronic mild stress in mice In: Kalueff A V., LaPorte JL, editors. Experimental Animal Models in Neurobehavioral Research. New-York: Nova Science Publishers; 2009. pp. 79–112. [Google Scholar]
- 25.Sánchez C, Bergqvist PBF, Brennum LT, Gupta S, Hogg S, Larsen a, et al. Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology (Berl). 2003;167(4): 353–62. [DOI] [PubMed] [Google Scholar]
- 26.Pandey DK, Yadav SK, Mahesh R, Rajkumar R. Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: A model of comorbid depression and anxiety? Behav Brain Res. 2009;205(2): 436–42. doi: 10.1016/j.bbr.2009.07.027 [DOI] [PubMed] [Google Scholar]
- 27.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7(1): 48–55. doi: 10.1038/nn1166 [DOI] [PubMed] [Google Scholar]
- 28.Zipori D, Sadot-Sogrin Y, Goltseker K, Even-Chen O, Rahamim F, Shaham O, et al. Re-exposure to nicotine-associated context from adolescence enhances alcohol intake in adulthood. Sci Rep. 2017;7: 2479 doi: 10.1038/s41598-017-02177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGough NNH, He D- Y, Logrip ML, Jeanblanc J, Phamluong K, Luong K, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24(46): 10542–52. doi: 10.1523/JNEUROSCI.3714-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hruska RE. Effect of Ethanol Administration on Striatal D 1 and D 2 Dopamine Receptors. J Neurochem. 1988. June;50(6): 1929–33. [DOI] [PubMed] [Google Scholar]
- 31.Timofeeva E, Mitra A. The effects of sucrose on neuronal activity In: Magazù S, editor. Sucrose: Properties, Biosynthesis and Health Implications. New-York: Nova Science Publishers; 2013. pp. 75–114. [Google Scholar]
- 32.Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl). 1995;120(1): 10–20. [DOI] [PubMed] [Google Scholar]
- 33.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(July): 5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1(1):9 doi: 10.1186/2045-5380-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nollet M, Le Guisquet A- M, Belzung C. Models of depression: unpredictable chronic mild stress in mice. Curr Protoc Pharmacol. 2013;61(5.65): 5.65.1–5.65.17. [DOI] [PubMed] [Google Scholar]
- 36.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4(9): 775–90. doi: 10.1038/nrd1825 [DOI] [PubMed] [Google Scholar]
- 37.Loas G. Vulnerability to depression: A model centered on anhedonia. J Affect Disord. 1996;41(1): 39–53. [DOI] [PubMed] [Google Scholar]
- 38.Jansen K, Cardoso TA, Fries GR, Branco JC, Silva RA, Kauer-Sant’Anna M, et al. Childhood trauma, family history, and their association with mood disorders in early adulthood. Acta Psychiatr Scand. 2016;134(4): 281–86 doi: 10.1111/acps.12551 [DOI] [PubMed] [Google Scholar]
- 39.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48(1): 191–214. [DOI] [PubMed] [Google Scholar]
- 40.Kasch KL, Rottenberg J, Arnow B a, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111(4): 589–97. [DOI] [PubMed] [Google Scholar]
- 41.Vrieze E, Demyttenaere K, Bruffaerts R, Hermans D, Pizzagalli DA, Sienaert P, et al. Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord. 2014;155(1): 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doron R, Lotan D, Rak-Rabl A, Raskin-Ramot A, Lavi K, Rehavi M. Anxiolytic effects of a novel herbal treatment in mice models of anxiety. Life Sci. 2012;90(25–26): 995–1000. doi: 10.1016/j.lfs.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 43.Molendijk ML, Spinhoven P, Polak M, Bus BAA, Penninx BWJH, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol Psychiatry. 2014;19(7): 791–800. doi: 10.1038/mp.2013.105 [DOI] [PubMed] [Google Scholar]
- 44.Licinio J, Dong C, Wong M- L. Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. Arch Gen Psychiatry. 2009;66(5): 488–97. doi: 10.1001/archgenpsychiatry.2009.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50(4): 260–5. [DOI] [PubMed] [Google Scholar]
- 46.Duman RS, Monteggia LM. A Neurotrophic Model for Stress-Related Mood Disorders. Biol Psychiatry. 2006;59(12): 1116–27. doi: 10.1016/j.biopsych.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 47.Chourbaji S, Brandwein C, Gass P. Altering BDNF expression by genetics and/or environment: Impact for emotional and depression-like behaviour in laboratory mice. Neurosci Biobehav Rev. 2011;35(3): 599–611. doi: 10.1016/j.neubiorev.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 48.Martinowich K, Manji HK, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9): 1089–93. doi: 10.1038/nn1971 [DOI] [PubMed] [Google Scholar]
- 49.Eisch AJ, Bolaños CA, De Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol Psychiatry. 2003;54(10): 994–1005. [DOI] [PubMed] [Google Scholar]
- 50.Cerqueira JJ, Mailliet F, Almeida OOFX, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27(11): 2781–7. doi: 10.1523/JNEUROSCI.4372-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JJ, Diamond DM, Haven N, Blvd BBD. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6): 453–62. doi: 10.1038/nrn849 [DOI] [PubMed] [Google Scholar]
- 52.Bessa JM, Morais M, Marques F, Pinto L, Palha JA, Almeida OFX, et al. Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl Psychiatry. 2013;3(6): e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinowich K, Lu B. Interaction between BDNF and Serotonin: Role in Mood Disorders. Neuropsychopharmacology. 2008;33(1): 73–83. doi: 10.1038/sj.npp.1301571 [DOI] [PubMed] [Google Scholar]
- 54.Quesseveur G, David DJ, Gaillard MC, Pla P, Wu M V, Nguyen HT, et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry. 2013;3(4): e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moraga-Amaro R, Jerez-Baraona JM, Simon F, Stehberg J. Role of astrocytes in memory and psychiatric disorders. J Physiol Paris. 2014;108(4–6): 240–51. doi: 10.1016/j.jphysparis.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 56.Chen ACH, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49(9): 753–62. [DOI] [PubMed] [Google Scholar]
- 57.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9): 464–8. doi: 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 58.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1): 13–25. [DOI] [PubMed] [Google Scholar]
- 59.Pardo M, López-Cruz L, Miguel NS, Salamone JD, Correa M. Selection of sucrose concentration depends on the effort required to obtain it: Studies using tetrabenazine, D1, D2, and D3 receptor antagonists. Psychopharmacology (Berl). 2015;232(13): 2377–91. [DOI] [PubMed] [Google Scholar]
- 60.Zhu X, Peng S, Zhang S, Zhang X. Stress-induced depressive behaviors are correlated with Par-4 and DRD2 expression in rat striatum. Behav Brain Res. 2011;223(2): 329–35. doi: 10.1016/j.bbr.2011.04.052 [DOI] [PubMed] [Google Scholar]
- 61.Dziedzicka-Wasylewska M, Willner P, Papp M. Changes in dopamine receptor mRNA expression following chronic mild stress and chronic antidepressant treatment. Behav Pharmacol. 1997;8(6–7): 607–18. [DOI] [PubMed] [Google Scholar]
- 62.Overstreet DH, Friedman E, Mathé AA, Yadid G. The Flinders Sensitive Line rat: A selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29(4–5): 739–59. doi: 10.1016/j.neubiorev.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 63.Bjørnebekk A, Mathé A a, Brené S. Isolated Flinders Sensitive Line rats have decreased dopamine D2 receptor mRNA. Neuroreport. 2007;18(10): 1039–43. doi: 10.1097/WNR.0b013e3281668bf7 [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Wang Y, Wang L, Bai M, Zhang X, Zhu X. Dopamine receptor D2 and associated microRNAs are involved in stress susceptibility and resistance to escitalopram treatment. Int J Neuropsychopharmacol. 2015;18(8): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 1992;16(4): 525–34. [DOI] [PubMed] [Google Scholar]
- 66.Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor SJ, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62(11): 1228–36. doi: 10.1001/archpsyc.62.11.1228 [DOI] [PubMed] [Google Scholar]
- 67.Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2): 240–9. [DOI] [PubMed] [Google Scholar]
- 68.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2): 368–77. doi: 10.1038/sj.npp.1301408 [DOI] [PubMed] [Google Scholar]
- 69.Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Treatment-Resistant Depression. Biol Psychiatry. 2009;65(4): 267–75. doi: 10.1016/j.biopsych.2008.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30(4): 821–32. doi: 10.1038/sj.npp.1300667 [DOI] [PubMed] [Google Scholar]
- 71.Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression—striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacol. 1996;126(1): 91–4. [DOI] [PubMed] [Google Scholar]
- 72.Blum K, Oscar-Berman M, Gardner EL, Simpatico T, Braverman ER, Gold MS. Neurogenetics and neurobiology of dopamine in anhedonia In: Ritsner MS, editor. Anhedonia: A Comprehensive Handbook Vol 1. Dordrecht: Springer; 2014. pp. 179–208. [Google Scholar]
- 73.Larisch R, Klimke a, Vosberg H, Löffler S, Gaebel W, Müller-Gärtner HW. In vivo evidence for the involvement of dopamine-D2 receptors in striatum and anterior cingulate gyrus in major depression. Neuroimage. 1997;5(5): 251–60. [DOI] [PubMed] [Google Scholar]
- 74.Klimke A, Larisch R, Janz A, Vosberg H, Müller-Gärtner HW, Gaebel W. Dopamine D2 receptor binding before and after treatment of major depression measured by [123I]IBZM SPECT. Psychiatry Res [Internet]. 1999;90(2): 91–101. [DOI] [PubMed] [Google Scholar]
- 75.Willner P, Hale AS, Argyropoulos S. Dopaminergic mechanism of antidepressant action in depressed patients. J Affect Disord. 2005;86(1): 37–45. doi: 10.1016/j.jad.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 76.Nestler EJ, Carlezon WA. The Mesolimbic Dopamine Reward Circuit in Depression. Biol Psychiatry. 2006;59(12): 1151–9. doi: 10.1016/j.biopsych.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 77.Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: Human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50(5): 345–50. [DOI] [PubMed] [Google Scholar]
- 78.Clayton AH, Pradko JF, Croft HA, Brendan Montano C, Leadbetter RA, Bolden-Watson C, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63(4): 357–66. [DOI] [PubMed] [Google Scholar]
- 79.Paredes RG, Ågmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73(3): 179–225. doi: 10.1016/j.pneurobio.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 80.Keltner NL, McAfee KM, Taylor CL. Mechanisms and treatments of SSRI-induced sexual dysfunction. Perspect Psychiatr Care. 2002;38(3): 111–6. [PubMed] [Google Scholar]
- 81.Becker HC. Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci. 2013;13:355–77. doi: 10.1007/7854_2012_203 [DOI] [PubMed] [Google Scholar]
- 82.Cicero TJ. A critique of animal analogues of alcoholism In: Majchrowicz E, Noble EP, editors. Biochemistry and pharmacology of ethanol, vol 2. New-York: Plenum Press; 1979. pp. 533–60. [Google Scholar]
- 83.Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3): 149–60. doi: 10.1016/j.alcohol.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palm S, Roman E, Nylander I. Differences in voluntary ethanol consumption in Wistar rats from five different suppliers. Alcohol. 2011;45(6): 607–14. doi: 10.1016/j.alcohol.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 85.Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100(3): 235–55. [DOI] [PubMed] [Google Scholar]
- 86.Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48(3): 243–52. doi: 10.1016/j.alcohol.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly M a, Rubinstein M, et al. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1(7): 610–5. doi: 10.1038/2843 [DOI] [PubMed] [Google Scholar]
- 88.Smith JW, Maurel Remy S, Schreiber R, DeVry J. Chronic mild stress causes a decrease in the preference for low ethanol concentrations in male Wistar rats. Eur Neuropsychopharmacol. 1996;6: S4–131. [Google Scholar]
- 89.Smith JW, Willner P, Little HJ. Chronic mild stress induces a decrease in voluntary intake of 10% ethanol in a four bottle choice paradigm. Br J Pharmacol. 1996;118: 64P. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data files are available from the Dryad database (https://doi.org/10.5061/dryad.c0v5r).