Abstract
Background
Carbohydrate antigen 19–9 (CA 19–9) is one of the most frequently used tumor markers for gastrointestinal cancer, particularly for diagnostic purposes. However, its value in predicting prognosis remains controversial. In this study, we sought to clarify this by conducting a meta-analysis of relevant studies.
Methods
We systematically searched several databases, including PubMed, EMBASE and Web of Science for articles pertaining to the relationship between pretreatment serum CA 19–9 levels and prognosis in patients with colorectal cancer (CRC). The reported hazard ratios (HR) of overall survival (OS), disease-free survival (DFS), pooled progression-free survival (PFS) and recurrence-free survival (RFS) in the analyzed studies were compared by fixed effects/random effects models.
Results
Seventeen studies involving 6434 patients with CRC were included in our meta-analysis. A comprehensive analysis of the collected data revealed that high serum CA 19–9 levels before treatment were significantly associated with poor OS (HR: 1.58, 95% CI: 1.36–1.83, P<0.001), DFS (HR: 1.71, 95% CI: 1.38–2.13, P<0.001), PFS (HR: 1.30,95%CI:0.93–1.82, P = 0.121) and RFS (HR: 1.43, 95% CI: 1.11–1.83, P = 0.006). This association between high pretreatment serum CA 19–9 levels and poor survival held true across different geographical regions, analysis types, methods used for HR determination, sample size, and treatment methods.
Conclusions
The results of this study indicate that pretreatment serum CA 19–9 level can be used as a prognostic indicator for patients with CRC.
1. Introduction
Colorectal cancer (CRC) ranks third and second among the most common cancers detected in men and women, respectively, with an incidence of over 1.2 million and a mortality of 608,700 in 2008[1]. Over the years, considerable advances have been made in the treatment of CRC. Surgical resection still remains the mainstay in the treatment of patients with non-metastatic disease, but unfortunately, curative resection may not be possible at the time of diagnosis in most cases [2]. Therefore, the five-year survival rate for metastatic CRC remains poor[3]. Furthermore, there is still a lack of clarity regarding the optimal treatment for advanced CRC, the prognostic value of treatment, and effective prognostic markers.
Several screening modalities are currently available for colorectal cancer, including stool examination, colonoscopy, and computed tomography (CT). Many of these methods are invasive or have limited sensitivity and do not offer much value in prognostic evaluation. Nevertheless, the detection of cancer markers is a non-invasive method in the diagnosis of cancers. It is easily accepted by patients and is a simple procedure [4]. In clinical practice, tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19–9 are often used for the detection of adenocarcinomas [3,5,6]. Generally, serum CEA levels are elevated in the case of cancer recurrence, and therefore, this parameter is widely considered a marker for postoperative surveillance in CRC [7–9]. Although the specificity of CA 19–9 for detecting colorectal cancer is 96% [10], its sensitivity is only 23%, and its utility in predicting prognosis remains controversial [11]. In other words, elevated CA 19–9 levels have been reported to be strongly associated with poor prognosis in nodal-positive CRC after completion of adjuvant chemotherapy[12,13]. However, this method is not useful in predicting the prognosis in cases of nodal-negative CRC.
To date, there has been no systematic meta-analysis on the relationship between pretreatment serum CA 19–9 levels and the prognosis in patients with CRC. We aimed to overcome this gap in knowledge through this meta-analysis.
2. Methods
2.1. Search strategies
We conducted a systematic search of various databases, such as PubMed, EMBASE and Web of Science using the following search terms: “CRC,” “colorectal cancer,” “colorectal tumor,” “colorectal neoplasms,” “colon cancer,” or “rectal cancer;” and “survival,” “prognostic,” “prognosis,” or “outcome;” and “CA 19–9 antigen,” “gastrointestinal cancer antigen,” “CA 19–9,” or “carbohydrate antigen 19–9.” All entries meeting these criteria were manually retrieved.
2.2. Inclusion and exclusion criteria
Two investigators independently selected articles according to inclusion criteria. Disagreements were discussed with a third reviewer. Articles meeting the following criteria were included in the meta-analysis: (1) the diagnosis of CRC was confirmed by pathological examination; (2) data on OS, DFS, PFS, and/or RFS were provided to allow for the assessment of the relationship between serum CA19-9 levels before treatment and prognosis; (3) hazard ratio (HR) and 95% confidence interval (CI) values were directly provided or could be calculated. We excluded animal studies, editorials, reviews, comments, abstracts, meetings, or case reports.
2.3. Data extraction
The following data were extracted from the included studies: (1) study characteristics including the first author, country of origin, year of publication, number of patients, duration of follow-up, and method of survival analysis; (2) patient characteristics including geographical area, tumor site, age, gender, metastasis, treatment and cut-off value; (3) survival measures including HRs of OS, DFS, RFS, PFS and their 95% CIs. The HRs were extracted from multivariate or univariate analyses or estimated from Kaplan–Meier survival curves [14]. E-mails were also sent to the corresponding author to requesting the requested data.
2.4. Quality assessment
The quality of each study was assessed using the Newcastle–Ottawa Scale (NOS) [15]. If the score was more than 6, the study was considered to be of high quality.
2.5. Statistical analysis
From the data provided in each study, the HRs and their 95% CIs were calculated to assess the relationship between prognosis and pretreatment serum CA19-9 levels. We applied the random-effects model (DerSimonian–Laird method) in case of significant heterogeneity (I2≥50% and P<0.1) and the fixed-effects model (Mantel–Haenszel method) in the absence of heterogeneity. Data analysis was conducted using the Stata 12 Edition (Stata, College Station, TX, USA). Subgroup analysis was used to evaluate the data pertaining to geographical area, distant metastasis, the type of analysis, source of HRs, sample size, and the treatment method.
3. Results
3.1. Search result
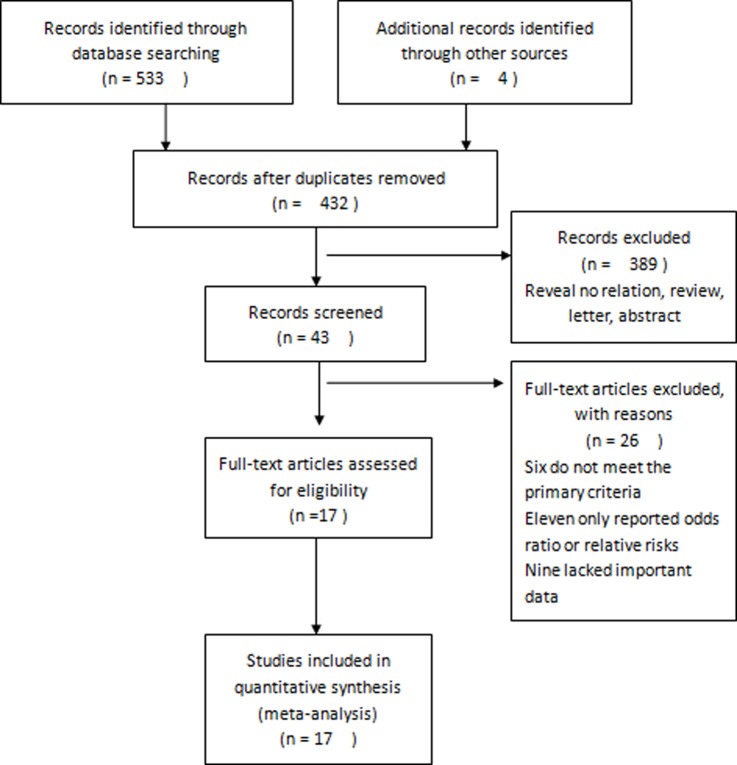
The systematic database search retrieved 537 articles. Each of these extracted articles was read, and 520 articles that did not meet the inclusion criteria were excluded from further analysis (Fig 1). Thus, 17 studies [11,16–31] comprising 6434 CRC patients were included in this meta-analysis, in order to assess the pretreatment levels of serum CA 19–9 as a prognostic biomarker in CRC.
Fig 1. Flow diagram of the study selection process.
The main characteristics of all 17 studies are summarized in Table 1. Fifteen of these studies provided data on the relationship between OS and pretreatment serum CA 19–9 levels. Five and two of these studies analyzed the relationship of pretreatment serum CA 19–9 level with DFS and with RFS and PFS, respectively. Among all 17 eligible articles, six studies were from China, six cohorts were from Japan, four studies were from the Korea, one study each was from Turkey and Czech Republic. HRs were recorded from the univariate analysis in four studies, determined from the multivariate analysis in 13 studies, and extracted from survival curves in two studies.
Table 1. Main characteristics of all studies included in the meta-analysis.
| Author | Country/Year | Area | Tumor site | Case number | Age (years) | Gender (M/F) | Metastasis | Treatment | Follow-up (months) | Survival analysis | Cut-off value | Analysis | HR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sookyung Lee | Korea /2016 |
Eastern | Colon/rectum 88/32 |
120 | 82/38 (<65/≥ 65) |
59/61 | No/yes 45/75 |
Mixed | median 7.6 |
OS | 27 | UV | report |
| Anna Song | Korea /2015 |
Eastern | Colon/rectum 125/52 |
177 | 123/54 (<65/≥ 65) |
83/94 | No/yes 69/108 |
Mixed | median 8.3 |
OS | 27 | UV | report |
| Mitsuru Ishizuka | Japan/2016 | Eastern | Colon/rectum 418/209 |
627 | 169/458 (≤60/>60) | 400/227 | No/yes 491/136 |
surgery | median 29.9 | OS | 9.5 | MV | report |
| Yuchen Wu | China/2016 | Eastern | Colon/rectum 25/30 |
55 | 28/27 (<60/≥60) |
35/20 | No/yes 0/55 |
Mixed | NR | OS/PFS | 37 | NR | report |
| Yukiya Narita | Japan/2014 | Eastern | Colon/rectum 148/104 |
252 | Median 61 |
155/97 | No/yes 0/252 |
chemotherapy | median 36.7 | OS | 37 | MV | report |
| Jingtao Wang | China/2015 | Eastern | Colon/rectum 176/134 |
310 | 138/172 (<65/≥ 65) |
152/158 | No/yes 310/0 |
surgery | median 71 | OS/DFS | 35 | MV | report |
| Masatsune Shibutani | Japan/2015 | Eastern | Colon/rectum 131/123 |
254 | median 66 | 139/115 | No/yes 254/0 |
surgery | median 1 | OS | 37 | UV | report |
| Ondrej Fiala | Czech/2015 | Western | Colon/rectum 86/66 |
152 | median 61.1 | 104/48 | No/yes 0/152 |
Mixed | median 18.9 | OS/PFS | 28 | MV | report |
| Tsuyoshi Ozawa | Japan/2016 | Eastern | Colon/rectum 96/77 |
173 | mean 61 | 98/75 | No/yes 0/173 |
surgery | median 36.9 | OS/RFS | 37 | MV | report |
| Xian-Hua Gao | China/2013 | Eastern | Colon/rectum 217/206 |
742 | mean 60 | 423/319 | No/yes 687/55 |
surgery | median 56 | OS/DFS | 37 | MV | report |
| Z.-M Li | China/2016 | Eastern | Colon/rectum 110/0 |
110 | mean 62.9 | 58/52 | No/yes 0/110 |
surgery | median 10.4 | OS | 37 | MV | report |
| Ruixue Yuan | China/2013 | Eastern | Colon/rectum/unspecified 184/182/5 |
371 | mean 58.4 | 207/164 | No/yes 341/30 |
surgery | mean 45.3 |
OS/DFS | 37.5 | MV | report |
| Fatih Selcukbiricik | Turkey/2013 | Western | Colon/rectum 127/88 |
215 | 125/90 (≤60/>60) | 133/82 | No/yes 94/121 |
chemotherapy | median 30.8 | OS | 37 | MV | report |
| HARUNOBU SATO | Japan/2011 | Eastern | Colon/rectum 1476/0 |
1476 | 179/1296 (≤50/>50) | 881/595 | No/yes 1476/0 |
surgery | median Re/NRe 52.5/101.5 |
OS | 37 | MV | report |
| Shinya Abe | Japan/2016 | Eastern | Colon/rectum 67/62 |
129 | 60/69 (<60/≥60) |
80/49 | No/yes 0/129 |
surgery | median 33.6 |
OS/RFS | 50 | MV | SC |
| IN JA PARK | Korea /2009 |
Eastern | Colon/rectum 534/575 |
1109 | NR | 614/501 | No/yes 1109/0 |
surgery | median 48 |
DFS | 37 | MV | SC |
| Injae Hong | Korea /2015 |
Eastern | Colon/rectum 88/74 |
162 | 88/74 (≤62/>62) | 90/72 | No/yes 146/16 |
surgery | Mean 83 |
DFS | 37 | MV | report |
Abbreviation: OS overall survival, DFS disease-free survival, PFS progression-free survival, RFS relapse-free survival, HR hazard ratio, NR not report, MV Multivariate analysis, UV univariate analysis, SC survival curve.
3.2. Meta-analysis
3.2.1. Overall survival
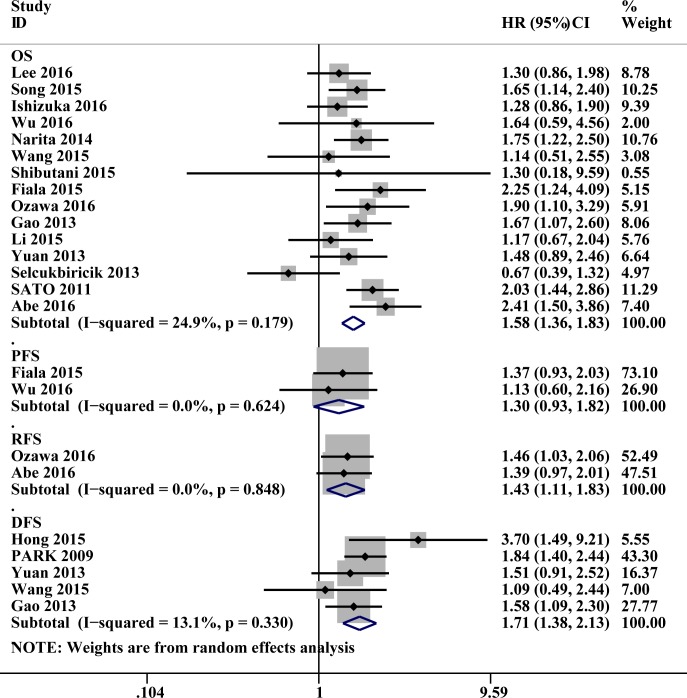
Data collected from 15 studies involving 5163 patients with CRC were investigated to determine the association between pretreatment serum CA 19–9 level and OS. High pretreatment serum CA 19–9 levels were found to be associated with poor prognosis and low OS (HR: 1.58, 95% CI: 1.36–1.83, P<0.001), and there was no significant heterogeneity between these studies (P = 0.179, I2 = 24.9%; Fig 2).
Fig 2. Forest plots of studies evaluating hazard ratios of pretreatment serum carbohydrate antigen 19–9 level (CA199) in patients with colorectal cancer (CRC).
(1) Pretreatment serum CA199 level was associated with shorter overall survival (OS) in CRC; (2) Pretreatment serum CA199 level was associated with shorter disease-free survival, progression-free survival, recurrence-free survival in CRC.
3.2.2. Disease-free survival
Data on DFS were provided by five studies involving 2694 patients. The data indicated an association between high pretreatment serum CA 19–9 levels and poor DFS (HR: 1.71, 95% CI: 1.38–2.13, P<0.001), and there was no heterogeneity between studies (P = 0.330, I2 = 13.1%; Fig 2).
3.2.3. Progression-free survival and recurrence-free survival
Overall, data on PFS were available for 207 patients and showed that elevated pretreatment levels of serum CA 19–9 were associated with poor prognosis (HR: 1.30,95%CI:0.93–1.82, P = 0.121), we have got the same conclusion in RFS with 302 CRC patients (HR: 1.43, 95% CI: 1.11–1.83, P = 0.006). Subgroup analysis was not performed in view of the small number of RFS and PFS.
3.2.4. Subgroup analysis
Subgroup analysis was used to evaluate the data pertaining to geographical area, distant metastasis, the type of analysis, source of HRs, sample size, and the treatment method. Heterogeneity occurred in the area subgroup and the data do not account for the small sample size. There was no significant difference between the rest subgroups (Table 2).
Table 2. Pooled hazard ratios (HRs) for OS according to subgroup analyses.
| Variables | Outcome | Studies | Patients | HR (95% CI) | P value | Model | Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||
| All | OS | 15 | 5163 | 1.58(1.36, 1.83) | <0.001 | fixed | 24.9% | 0.179 |
| DFS | 5 | 2694 | 1.71(1.38, 2.13) | <0.001 | fixed | 13.1% | 0.330 | |
| Area | ||||||||
| Eastern | OS | 13 | 4796 | 1.63 (1.42, 1.87) | <0.001 | fixed | 0.0% | 0.579 |
| DFS | 5 | 2694 | 1.71(1.38, 2.13) | <0.001 | fixed | 13.1% | 0.330 | |
| Western | OS | 2 | 367 | 1.23(0.38, 4.03) | 0.773 | random | 87.1% | 0.005 |
| DFS | 0 | 0 | - | - | - | - | - | |
| Analysis type* | ||||||||
| Univariate | OS | 4 | 606 | 1.50(1.15, 1.95) | <0.001 | fixed | 0.0% | 0.865 |
| Multivariate | OS | 11 | 4557 | 1.58(1.30, 1.93) | <0.001 | fixed | 43.3% | 0.062 |
| DFS | 5 | 2694 | 1.71(1.38, 2.13) | <0.001 | fixed | 13.1% | 0.330 | |
| Metastasis* | ||||||||
| Mixed | OS | 11 | 2871 | 1.51(1.25, 1.83) | <0.001 | fixed | 34.9% | 0.119 |
| DFS | 3 | 1275 | 1.70(1.27,2.26) | 0.004 | fixed | 36.2% | 0.209 | |
| No | OS | 4 | 2292 | 1.80(1.42, 2.28) | <0.001 | fixed | 0.0% | 0.608 |
| DFS | 2 | 1419 | 1.63 (1.06, 2.51) | 0.026 | fixed | 31.2% | 0.228 | |
| HR obtain method | ||||||||
| Reported in text | OS | 14 | 5034 | 1.53(1.32, 1.77) | <0.001 | fixed | 16.0% | 0.279 |
| DFS | 4 | 1585 | 1.63(1.25, 2.12) | 0.001 | fixed | 6.5% | 0.370 | |
| Data-extrapolated | OS | 1 | 129 | 2.41(1.50,3.86) | - | - | - | - |
| DFS | 1 | 1109 | 1.84(1.40,2.44) | - | - | - | - | |
| Sample size* | ||||||||
| >200 | OS | 8 | 4247 | 1.46(1.16,1.84) | <0.001 | fixed | 39.9% | 0.113 |
| DFS | 4 | 2532 | 1.66(1.36,2.02) | <0.001 | fixed | 0.0% | 0.622 | |
| <200 | OS | 7 | 916 | 1.69(1.39,2.04) | <0.001 | fixed | 5.9% | 0.382 |
| DFS | 1 | 162 | 3.699(1.486,9.209) | - | - | - | - | |
| Treatment method* | ||||||||
| operate | OS | 9 | 3300 | 1.66(1.40,1.96) | <0.001 | fixed | 2.3% | 0.413 |
| DFS | 5 | 2694 | 1.71(1.38, 2.13) | <0.001 | fixed | 13.1% | 0.330 | |
| mixed | OS | 6 | 1863 | 1.57(1.28,1.94) | <0.001 | random | 52.8% | 0.048 |
Abbreviation: OS overall survival, DFS disease-free survival, PFS progression-free survival, RFS relapse-free survival, HR hazard ratio, CI confidence intervals.
*indicates that the difference was statistically significant
3.3. Sensitivity analyses
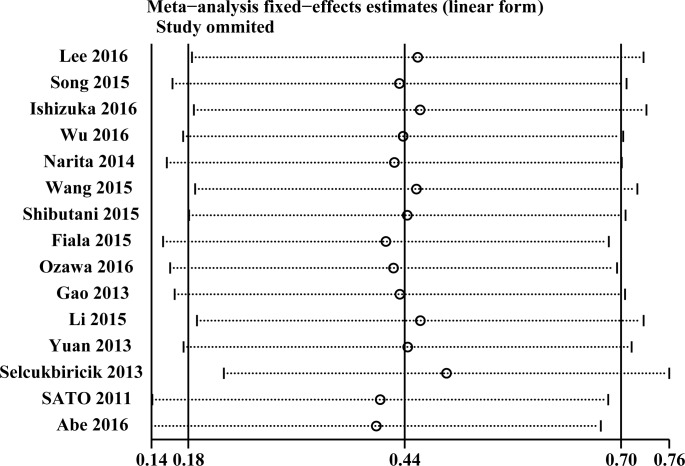
We did sensitivity analysis only for OS. We removed each of the articles to assess the impact of the individual data of each of these studies on the results of this study and found no significant change in the combinations of HRs and 95% CIs. This finding reflected the stability of the outcome (Fig 3).
Fig 3. Sensitivity analyses for confirming robustness of OS by removing 1 study each time.
3.4. Publication bias
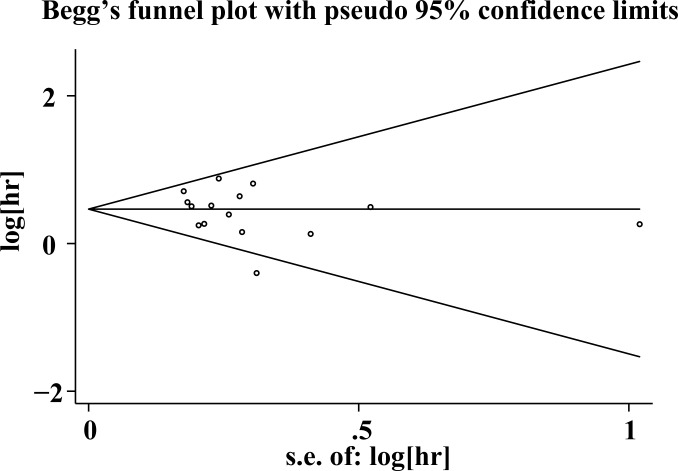
The publication bias in each of the included studies was calculated only for OS by funnel plots as well as Egger’s and Begg’s tests. The funnel plots were almost symmetrical (Fig 4). Furthermore, Egger’s and Begg’s test results revealed no significant publication bias in our study (OS: P = 0.347, 0.373).
Fig 4. Funnel plots for the evaluation of potential publication bias of OS for CRC.
4. Discussion
In this meta-analysis, we investigated the relationship between the pretreatment serum CA 19–9 levels and prognosis in 17 studies comprising 6434 patients with CRC. Our final results indicate a significant correlation between elevated pretreatment serum CA 19–9 levels and poor prognosis in CRC patients, with a combined HR of 1.58 (95% CI: 1.36–1.83, P<0.001), for OS, 1.71 (95% CI: 1.38–2.13, P<0.001for DFS, 1.30 (95% CI: 0.93–1.82) for RFS and 1.43 (95% CI: 1.11–1.83) for PFS. Furthermore, subgroup analyses were conducted according to the tumor site, occurrence of distant metastasis, type of analysis, method of calculating HR, sample size, and treatment. The results of analyses with all these subgroups revealed that a high pretreatment serum CA 19–9 level indicates poor prognosis, and further indicated that there was no significant difference among these different subgroups.
Although several studies have attested to the association between pretreatment serum CA 19–9 levels and the prognosis of CRC, the underlying reasons continue to remain elusive. CA 19–9 has been used in the diagnosis and prognosis of pancreatic cancer, CRC, gastric cancer, and other gastrointestinal tumors [32–34]. In CRC patients, the role of CA 19–9 remains controversial since it has a lower sensitivity as compared to CEA[35]. In the body, CA19-9 has been found to occur in salivary mucin and is distributed across the normal pancreas, gallbladder, liver, intestine, bile duct epithelium, etc. It is a cell surface glycoprotein and is involved in cellular adhesion, cancer cells expressing this protein may have greater metastatic and invasive potential [27]. Additionally, it has been reported to mediate the adhesion of tumor cells to the endothelial cells of blood vessels, thereby contributing to tumor metastasis [36]. Moreover, the presence of CA 19–9 correlate with the occurrence of tumor cell-induced platelet aggregation, which is an important process involved in the distant metastasis of CRC [37]. Furthermore, the possible involvement of CA 19–9 in tumor recurrence and survival may be attributed to differences in chemotherapy resistance or biological features of stage IV CRC, compared to other stages [17]. Such as it is reported that the baseline CA 19–9 level was an independent prognostic factor in metastatic CRC and also a predictive factor of bevacizumab efficacy, and the baseline CA 19–9 level correlated with the KRAS/BRAF mutation status [27]. This is also reported in another research [29]. CA 19–9 has also been reported to play a role in the occurrence of cancer invasion by enhancing cell adhesion and indirectly promoting angiogenesis [38]. Put together, these explanations, at least partly, explain the negative correlation of pretreatment serum CA 19–9 levels and prognosis.
This study has some limitations. First, our sample size was relatively small. Second, owing to the diversity of the cut-off values defined in each of these studies, we could not perform further subgroup analysis. Third, these included studies were heterogeneous due to differences in design, which also did not allow for the assessment of other clinical features.
5. Conclusion
Thus, our meta-analysis revealed an association between high pre-treatment serum CA 19–9 levels and poor survival in patients with CRC. Hence, serum CA 19–9 levels may be used as a marker to facilitate the treatment planning and prognostic evaluation of CRC patients. As a common tumor marker today, it has the advantage that it is cheaper, more convenient and more acceptable to patients. Further investigations are necessary to determine whether any association exists between serum CA 19–9 levels and survival.
Supporting information
(DOCX)
(DOC)
Abbreviations
- CA 19–9
Carbohydrate antigen 19–9
- CRC
colorectal cancer
- OS
overall survival
- DFS
disease-free survival
- PFS
progression-free survival
- RFS
recurrence-free survival
- HR
hazard ratio
- CI
confidence interval.
Data Availability
All files are available from the Pubmed database (accession number yz20161234).
Funding Statement
This work was supported by Changzhou Science and Technology Bureau, CE20125020, YGW.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64: 104–117. doi: 10.3322/caac.21220 [DOI] [PubMed] [Google Scholar]
- 3.Oliphant R, Nicholson GA, Horgan PG, Molloy RG, McMillan DC, Morrison DS, et al. (2013) Deprivation and colorectal cancer surgery: longer-term survival inequalities are due to differential postoperative mortality between socioeconomic groups. Ann Surg Oncol 20: 2132–2139. doi: 10.1245/s10434-013-2959-9 [DOI] [PubMed] [Google Scholar]
- 4.Zhong W, Yu Z, Zhan J, Yu T, Lin Y, Xia ZS, et al. (2015) Association of serum levels of CEA, CA199, CA125, CYFRA21-1 and CA72-4 and disease characteristics in colorectal cancer. Pathol Oncol Res 21: 83–95. doi: 10.1007/s12253-014-9791-9 [DOI] [PubMed] [Google Scholar]
- 5.Staab HJ, Brummendorf T, Hornung A, Anderer FA, Kieninger G (1985) The clinical validity of circulating tumor-associated antigens CEA and CA 19–9 in primary diagnosis and follow-up of patients with gastrointestinal malignancies. Klin Wochenschr 63: 106–115. [DOI] [PubMed] [Google Scholar]
- 6.Mennini G, Silecchia G, Zanna C, Cucchiara G, Spadaro G, Greco E, et al. (1985) Clinical value of CA 19–9 (carbohydrate antigen) in gastrointestinal adenocarcinoma. Ital J Surg Sci 15: 37–43. [PubMed] [Google Scholar]
- 7.Yakabe T, Nakafusa Y, Sumi K, Miyoshi A, Kitajima Y, Sato S, et al. (2010) Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol 17: 2349–2356. doi: 10.1245/s10434-010-1004-5 [DOI] [PubMed] [Google Scholar]
- 8.Park IJ, Choi GS, Lim KH, Kang BM, Jun SH (2009) Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann Surg Oncol 16: 3087–3093. doi: 10.1245/s10434-009-0625-z [DOI] [PubMed] [Google Scholar]
- 9.Zeng Z, Cohen AM, Urmacher C (1993) Usefulness of carcinoembryonic antigen monitoring despite normal preoperative values in node-positive colon cancer patients. Dis Colon Rectum 36: 1063–1068. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg EM, Simunovic LM, Drake SL, Mueller WF Jr., Verrill HL (1989) Comparison of serum CA 19–9 and CEA levels in a population at high risk for colorectal cancer. Hybridoma 8: 569–575. doi: 10.1089/hyb.1989.8.569 [DOI] [PubMed] [Google Scholar]
- 11.Park IJ, Choi GS, Jun SH (2009) Prognostic value of serum tumor antigen CA19-9 after curative resection of colorectal cancer. Anticancer Res 29: 4303–4308. [PubMed] [Google Scholar]
- 12.Reiter W, Stieber P, Reuter C, Nagel D, Lau-Werner U, Lamerz R (2000) Multivariate analysis of the prognostic value of CEA and CA 19–9 serum levels in colorectal cancer. Anticancer Res 20: 5195–5198. [PubMed] [Google Scholar]
- 13.Behbehani AI, Al-Sayer H, Farghaly M, Kanawati N, Mathew A, al-Bader A, et al. (2000) Prognostic significance of CEA and CA 19–9 in colorectal cancer in Kuwait. Int J Biol Markers 15: 51–55. [DOI] [PubMed] [Google Scholar]
- 14.Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 15.Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25: 603–605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Li C, Zhao J, Yang L, Liu F, Zheng H, et al. (2016) Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol 14: 289 doi: 10.1186/s12957-016-1044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozawa T, Ishihara S, Kawai K, Nozawa H, Yamaguchi H, Kitayama J, et al. (2016) Prognostic Significance of Preoperative Serum Carbohydrate Antigen 19–9 in Patients With Stage IV Colorectal Cancer. Clin Colorectal Cancer 15: e157–e163. doi: 10.1016/j.clcc.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 18.Li ZM, Peng YF, Du CZ, Gu J (2016) Colon cancer with unresectable synchronous metastases: the AAAP scoring system for predicting the outcome after primary tumour resection. Colorectal Dis 18: 255–263. doi: 10.1111/codi.13123 [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Song A, Eo W (2016) Serum Ferritin as a Prognostic Biomarker for Survival in Relapsed or Refractory Metastatic Colorectal Cancer. J Cancer 7: 957–964. doi: 10.7150/jca.14797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K (2016) Clinical Significance of the C-Reactive Protein to Albumin Ratio for Survival After Surgery for Colorectal Cancer. Ann Surg Oncol 23: 900–907. doi: 10.1245/s10434-015-4948-7 [DOI] [PubMed] [Google Scholar]
- 21.Abe S, Kawai K, Ishihara S, Nozawa H, Hata K, Kiyomatsu T, et al. (2016) Prognostic impact of carcinoembryonic antigen and carbohydrate antigen 19–9 in stage IV colorectal cancer patients after R0 resection. J Surg Res 205: 384–392. doi: 10.1016/j.jss.2016.06.078 [DOI] [PubMed] [Google Scholar]
- 22.Song A, Eo W, Lee S (2015) Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol 21: 12410–12420. doi: 10.3748/wjg.v21.i43.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibutani M, Maeda K, Nagahara H, Ohtani H, Iseki Y, Ikeya T, et al. (2015) The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World J Surg Oncol 13: 194 doi: 10.1186/s12957-015-0609-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Jingtao1 W X, Yu Fudong1, Chen Jian1, Zhao Senlin1, Zhang Dongyuan1, Yu Yang1, Liu Xisheng1, Tang Huamei2 P Z (2015) Combined detection of preoperative serum CEA, CA19-9 and CA242 improve prognostic prediction of surgically treated colorectal cancer patients. Int J Clin Exp Pathol 8: 14853–14863. [PMC free article] [PubMed] [Google Scholar]
- 25.Hong I, Hong SW, Chang YG, Lee WY, Lee B, Kang YK, et al. (2015) Expression of the Cancer Stem Cell Markers CD44 and CD133 in Colorectal Cancer: An Immunohistochemical Staining Analysis. Ann Coloproctol 31: 84–91. doi: 10.3393/ac.2015.31.3.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiala O, Finek J, Buchler T, Matejka VM, Holubec L, Kulhankova J, et al. (2015) The Association of Serum Carcinoembryonic Antigen, Carbohydrate Antigen 19–9, Thymidine Kinase, and Tissue Polypeptide Specific Antigen with Outcomes of Patients with Metastatic Colorectal Cancer Treated with Bevacizumab: a Retrospective Study. Target Oncol 10: 549–555. doi: 10.1007/s11523-015-0365-x [DOI] [PubMed] [Google Scholar]
- 27.Narita Y, Taniguchi H, Komori A, Nitta S, Yamaguchi K, Kondo C, et al. (2014) CA19-9 level as a prognostic and predictive factor of bevacizumab efficacy in metastatic colorectal cancer patients undergoing oxaliplatin-based chemotherapy. Cancer Chemother Pharmacol 73: 409–416. doi: 10.1007/s00280-013-2367-7 [DOI] [PubMed] [Google Scholar]
- 28.Yuan R, Chen Y, He X, Wu X, Ke J, Zou Y, et al. (2013) CCL18 as an independent favorable prognostic biomarker in patients with colorectal cancer. J Surg Res 183: 163–169. doi: 10.1016/j.jss.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 29.Selcukbiricik F, Bilici A, Tural D, Erdamar S, Soyluk O, Buyukunal E, et al. (2013) Are high initial CEA and CA 19–9 levels associated with the presence of K-ras mutation in patients with metastatic colorectal cancer? Tumour Biol 34: 2233–2239. doi: 10.1007/s13277-013-0763-6 [DOI] [PubMed] [Google Scholar]
- 30.Gao XH, Liu QZ, Chang W, Xu XD, Du Y, Han Y, et al. (2013) Expression of ZNF148 in different developing stages of colorectal cancer and its prognostic value: a large Chinese study based on tissue microarray. Cancer 119: 2212–2222. doi: 10.1002/cncr.28052 [DOI] [PubMed] [Google Scholar]
- 31.Sato H, Maeda K, Sugihara K, Mochizuki H, Kotake K, Teramoto T, et al. (2011) High-risk stage II colon cancer after curative resection. J Surg Oncol 104: 45–52. doi: 10.1002/jso.21914 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Gao SG, Chen JM, Wang GP, Wang ZF, Zhou B, et al. (2015) Serum CA242, CA199, CA125, CEA, and TSGF are Biomarkers for the Efficacy and Prognosis of Cryoablation in Pancreatic Cancer Patients. Cell Biochem Biophys 71: 1287–1291. doi: 10.1007/s12013-014-0345-2 [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Yang Y, Zhang YP, Zou Z, Qian X, Liu B, et al. (2014) Prognostic value of carbohydrate tumor markers and inflammation-based markers in metastatic or recurrent gastric cancer. Med Oncol 31: 289 doi: 10.1007/s12032-014-0289-9 [DOI] [PubMed] [Google Scholar]
- 34.Polat E, Duman U, Duman M, Atici AE, Reyhan E, Dalgic T, et al. (2014) Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19–9 in colorectal cancer. Curr Oncol 21: e1–7. doi: 10.3747/co.21.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galli C, Basso D, Plebani M (2013) CA 19–9: handle with care. Clin Chem Lab Med 51: 1369–1383. doi: 10.1515/cclm-2012-0744 [DOI] [PubMed] [Google Scholar]
- 36.Dabelsteen E (1996) Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol 179: 358–369. doi: 10.1002/(SICI)1096-9896(199608)179:4<358::AID-PATH564>3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- 37.Martini F, Guadagni F, Lenti L, D'Alessandro R, Aloe S, Roselli M, et al. (2000) CA 19–9 monosialoganglioside content of human colorectal tumor cells correlates with tumor cell-induced platelet aggregation. Anticancer Res 20: 1609–1614. [PubMed] [Google Scholar]
- 38.Ballehaninna UK, Chamberlain RS (2012) The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 3: 105–119. doi: 10.3978/j.issn.2078-6891.2011.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All files are available from the Pubmed database (accession number yz20161234).