Abstract
Scrub typhus is a serious public health problem in the Asia-Pacific area. It threatens one billion people globally, and causes illness in one million people each year. Caused by Orientia tsutsugamushi, scrub typhus can result in severe multiorgan failure with a case fatality rate up to 70% without appropriate treatment. The antigenic heterogeneity of O. tsutsugamushi precludes generic immunity and allows reinfection. As a neglected disease, there is still a large gap in our knowledge of the disease, as evidenced by the sporadic epidemiologic data and other related public health information regarding scrub typhus in its endemic areas. Our objective is to provide a systematic analysis of current epidemiology, prevention and control of scrub typhus in its long-standing endemic areas and recently recognized foci of infection.
Author summary
Scrub typhus is a serious public health problem in the Asia-Pacific area. There is an estimated one million new scrub typhus infections each year, and over one billion people around the world are at risk. Without appropriate treatment, the case fatality rate of scrub typhus can reach 30% or even higher. Scrub typhus has long been a neglected infectious disease so many aspects of the disease, including its diagnosis to prevention, are unknown. We here provide a comprehensive review of the epidemiology, prevention and control of scrub typhus.
Introduction
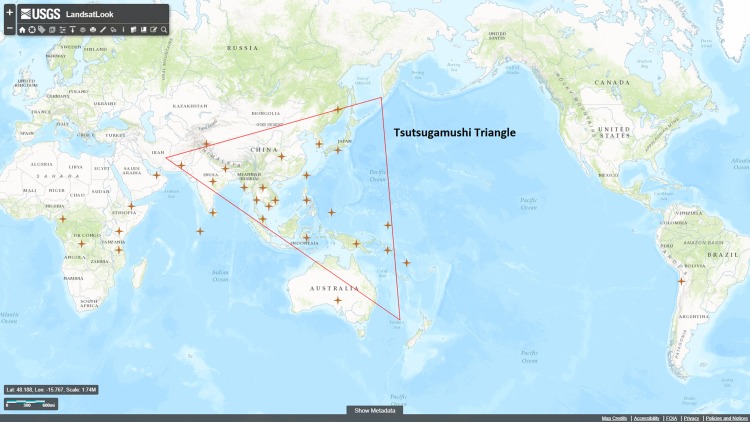
Scrub typhus is a serious public health problem in the Asia-Pacific area including, but not limited to, Korea, Japan, China, Taiwan, India, Indonesia, Thailand, Sri Lanka, and the Philippines (Fig 1). It threatens one billion people globally, and causes illness in one million people each year [1]. Scrub typhus, also known as tsutsugamushi disease, is caused by the arthropod-borne gram-negative obligately intracellular bacillus Orientia tsutsugamushi [2,3,4]. Approximately 5 to 14 days after being bitten by an infected vector, a Leptotrombidium mite, patients begin to exhibit manifestations of infection such as non-specific flu-like symptoms, fever, rash, eschar at the bite site, headache, myalgia, cough, generalized lymphadenopathy, nausea, vomiting, and abdominal pain [5,6,7]. Fever and headache are the most common features among scrub typhus patients. Between 95% and 100% of confirmed cases were noted to have fever in several studies [8,9].
Fig 1. Worldwide map of countries with reported scrub typhus cases.
The majority of scrub typhus cases occur in the “tsutsugamushi triangle” in the Asia-Pacific area. Countries with human cases are labeled with a star. [Modified from https://landsatlook.usgs.gov/viewer.html].
An eschar at the site of chigger feeding is a classic clinical feature of scrub typhus. It begins as a papule at the site of chigger feeding and then ulcerates and forms a black crust like a skin burn from a cigarette. When present, it occurs prior to the onset of fever and other symptoms [6,10]. The presentation of eschar varies from 1%–97% of scrub typhus patients depending on the geographic areas and studies [8,11,12]. It is more easily found on Caucasian and East Asian patients than on dark skinned South Asian patients [13]. Most eschars develop on the front of the body (~80%). In male patients, eschars are primarily within 30 cm below the umbilicus. The other common locations are lower extremities and anterior chest. There is a different pattern in female patients, whose anterior chest, head and neck are the most prevalent areas [13,14]. Eschars are also commonly present in the axillae of children in addition to the sites mentioned above [15,16].
Severe complications such as multiorgan failure occur in some cases. The severe multiorgan manifestations include jaundice, acute renal failure, pneumonitis, acute respiratory distress syndrome (ARDS), myocarditis, septic shock, meningoencephalitis, pericarditis, and disseminated intravascular coagulation (DIC) [6,7,9,17,18]. The lung is one of the main target organs for Orientia, leading to pulmonary complications of variable severity. Interstitial pneumonia may occur in severe cases [7,19]. Meningitis and/or encephalitis can develop in severe illness, causing patients to become agitated, delirious or even have seizures. Focal neurological signs are rare but have been known to occur. Laboratory tests may demonstrate changes in cerebrospinal fluid similar to those found in viral or tuberculous meningitis [20,21,22,23]. Patients may also develop the complications of hearing loss or hearing impairment during scrub typhus infection [24,25]. The case fatality rate of scrub typhus varies among different countries, regions, and areas as well as different studies [26]. The case fatality can be up to 30–70% if no appropriate treatment is received while the median case fatality rate for untreated patients is 6% and for treated patient is 1.4% [26,27,28]. Therefore, development of effective measures to treat, control and prevent the disease is a critical public health issue.
Other signs can be observed in scrub typhus patients. Marked hyperemia and even hemorrhage can be found on the conjunctiva during the acute phase of the disease. There are reports of hemorrhages and coagulation disorders, mostly gastrointestinal complications, among scrub typhus patients. Severely ill patients can suffer gastrointestinal mucosal hemorrhage, multiple erosions and ulcers. [6,23,29].
Orientia tsutsugamushi is transmitted to mammalian hosts including humans by the larval stage of Leptotrombidium mites, also called chiggers [30]. Mites act as the primary reservoirs for O. tsutsugamushi. They remain infected through their life cycle (larva, nymph, adult and egg) [7]. It is thought that larvae of mites only feed once on a mammal host. Chiggers usually feed on thin, tender or wrinkled skin. The feeding lasts 2 to 4 days [31]. It has been shown that chiggers do not pierce the host skin but rather take advantage of hair follicles or pores [6]. The saliva that the mites secrete can dissolve host tissue around the feeding site, and the mites ingest the liquefied tissue. Orientia tsutsugamushi has been found in the salivary glands of mites [6]. Transovarial and transstadial transmission are the main mechanisms for maintaining Orientia in the mite, and there are a limited number of reports that the bacteria can also be transmitted to mites during co-feeding and/or from wild rodents [32,33,34,35]. Transstadial transmission is maintenance of the pathogen in the vector from one life stage to the next, i.e., passage of O. tsutsugamushi from mite larva to nymph, and nymph to adult. Transovarial transmission is the process of passing O. tsutsugamushi from the female to offspring through eggs [12,35]. Both methods of transmission are involved in vertical transmission. There have been rare documented occurrences of horizontal transmission of Orientia among mites [36]. During horizontal transmission, a chigger acquires Orientia from an infected host, and its offspring infects a new host. Not enough evidence exists to demonstrate that such horizontal transmission is an important means of maintenance of O. tsutsugamushi in nature [34,36,37,38]. There are no reports of person-to-person transmission of scrub typhus [7]. Further studies on mites and their engorgement on the host can facilitate the control and prevention of scrub typhus.
There are still many unknowns regarding the mechanisms of pathogenesis and the cell biology of the interaction of this bacterium with its host cell, due to the extra research obstacles of studying an obligately intracellular bacterium [39]. The antigenic heterogeneity of, reemergence of, and short-lived immunity to Orientia result in a substantial number of primary infections and reinfections. However, both basic research and epidemiological studies of O. tsutsugamushi have been largely neglected during recent decades [40,41,42]. Preliminary studies demonstrated that the disease has existed in the endemic areas for some time [43,44,45,46,47]. There are only sporadic epidemiological data available regarding scrub typhus in the endemic areas, as well as other parts of the world, resulting in a current gap in knowledge.
We provide here a systematic analysis of current epidemiology, prevention and control of scrub typhus in the endemic areas as well as among travelers from the rest of the world.
Epidemiology of scrub typhus
The traditional endemic area of scrub typhus is known as the “tsutsugamushi triangle”. It is a region covering more than 8 million km2, from the Russian Far East in the north, to Pakistan in the west, Australia in the south, and the Japan in the east [23,48,49]. There are one billion people at risk of infection; the endemic area is highly populated [1]. The progress of globalization and associated travel contributes to the exportation of the infected persons to non-endemic areas [50]. The antigenic and genetic diversity of O. tsutsugamushi strains, and their unclear correlation with virulence for humans, confound the epidemiological study of scrub typhus [51]. Better understanding of the epidemiology of scrub typhus will help efforts to prevent and control the disease. This part of the article describes studies of the geographic distribution and risk factors of scrub typhus in both the endemic areas and in travelers from the rest of the world.
Methods
A systematic literature review of epidemiological studies and case reports of scrub typhus was carried out using the methods of Hemingway et al. and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (S1 Table) [52,53].
We first defined the research question as the epidemiology of scrub typhus. Literature was searched in the PubMed and Google Scholar databases. The following search terms were used: (scrub typhus) AND (epidemiology OR distribution OR case report). There was a total of 898 records identified, and 793 records were screened after removal of duplicates. Eighty-two records were further filtered out in PubMed due to being nonhuman studies. Titles and abstracts were used to assess the eligibility of each study. Only English language peer-reviewed articles were included in the study. A number of publications in Chinese, Japanese, Korean, Russian, and other languages were not included in the analysis. Two hundred and forty-two records were excluded due to non-English language and non-human studies. Among the 711 records that remained, 647 records were either simple case reports for which the abstracts provided complete case information, or only abstracts were available during analysis. Sixty-four full-text articles were obtained and assessed. Eight articles which reported imported cases in Belgium, France, Germany, Italy, the Netherlands, New Zealand, Scandinavia, and the U.S. were excluded [54,55,56,57,58,59,60,61,62]. Fifty-six articles were included in this qualitative synthesis and analysis.
We summarized lists of countries or regions with human case reports or other epidemiological studies of scrub typhus in tables. For those countries or areas having long history of endemic transmission, or who which multiple studies were reported, we further did a literature review using Pubmed and Google Scholar to retrieve detailed epidemiological characteristics of the specific country or region, such as Japan, China, South Korea, Thailand, and India. To make the results easier to understand, we have prepared a list of seroprevalence of scrub typhus by county, and the outbreaks of scrub typhus, in separate tables.
Transmission of scrub typhus
Even with its recent re-emergence in the traditionally endemic areas and worldwide, scrub typhus is still a neglected infectious disease [6,63,64]. The geographic distribution of scrub typhus is determined by the distribution of its vector and reservoir—mites, primarily of the genus Leptotrombidium. Humans are accidental hosts [1,51].
Outdoor workers, especially field workers in rural areas, have a higher risk of acquiring the disease [65]. It is reported that rice fields are an under-appreciated location where the biting of mites and transmission of O. tsutsugamushi occurs in the endemic areas [63]. Tropical weather provides stable and ideal conditions for transmission of the disease. High temperature and high humidity are optimal for mite activity. In more temperate climates, the transmission of scrub typhus is more seasonal due to the temporal activity of chiggers [45,63,66].
Scrub typhus has been a nationally notifiable disease in Bhutan, China, Japan, South Korea, Thailand, and Taiwan [67,68,69,70,71,72]. The reported seroprevalence of O. tsutsugamushi for each country is shown in Table 1 [73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106]. The reports of outbreaks of scrub typhus are summarized in Table 2[43,94,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137]. The list of other countries with published cases of scrub typhus is found in S2 Table [80,136,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154]. All countries with reported cases are designated in Fig 1.
Table 1. Global seroprevalence of scrub typhus by Region/Country.
| Region and Country / Reported by | Collection Year | Tested Number | Tested Population | Sero-prevalence |
|---|---|---|---|---|
| Australia | ||||
| (Graves, Wang et al. 1999) | 1996 | 920 | General population | 2.6% |
| Bangladesh | ||||
| (Maude, Maude et al. 2014) | 2010 | 1,209 | General population | 23.7% |
| China | ||||
| (Horton, Jiang et al. 2016) | 2009 | 100 | General population | 10% |
| India | ||||
| (Shivalli 2016) | Unknown | 721 | General population | 31.8% |
| (Jakharia, Borkakoty et al. 2016) | Unknown | 300 | General population | 40.0% |
| (Kalal, Puranik et al. 2016) | 2010–2012 | 103 | Hospitalized children with clinical features of rickettsial illness | 60.2% |
| (Sengupta, Anandan et al. 2015) | 2013 | 100 | General population | 15.0% |
| (Ramyasree, Kalawat et al. 2015) | 2013 | 100 | Clinically suspected cases | 39.0% |
| (Sharma, Mahajan et al. 2005) | 2003–2004 | 150 | Patients with fever of unknown origin, diagnosed with the Weil-Felix test. PCR results were negative | 34.7% |
| Indonesia | ||||
| (Richards, Ratiwayanto et al. 2003) | 1997 | 53 | Local villagers and mining employees enrolled in a study of in vivo resistance of malaria and lymphatic filariasis | 9.4% |
| (Richards, Soeatmadji et al. 1997) | 1994 | 464 | General population | 1.3% |
| Japan | ||||
| (Ogawa and Ono 2008) | 1984–2005 | 561 | General population | 68.4% |
| Kenya | ||||
| (Thiga, Mutai et al. 2015) | Unknown | 1,401 | Patients with fever (>38°C) | 4.8% |
| Lao | ||||
| (Vallee, Thaojaikong et al. 2010) | 2006 | 2,001 | Randomly selected adults (≥35 years) in urban and peri-urban Vientiane City | 20.3% |
| Malaysia | ||||
| (Tay, Mohamed Zan et al. 2013) | 2007–2010 | 280 | General population | 17.9% |
| (Tay, Kamalanathan et al. 2003) | 1998–1999 | 240 | Blood donors | 5.4% |
| 1998–1999 | 292 | Febrile patients | 43.5% | |
| (Sagin, Ismail et al. 2000) | N/A | 261 | General population | 1.5% |
| (Tay, Ho et al. 2000) | 1995–1997 | 1596 | Febrile patients | 24.9% |
| (Tee, Kamalanathan et al. 1999) | 1996–1997 | 378 | 70 out of the 300 rubber estate workers in Dec (humid), and 31of 184 examined workers in Mar (dry, 106 were bled previously) were seropositive | 16.8–23.3% |
| (Cadigan, Andre et al. 1972) | 690 | Blood from 245 adult (>20 yr) and 445 under 20 were collected from Orange Asli in central West Malaysia | 0–73% | |
| Nepal | ||||
| (Blacksell, Sharma et al. 2007) | 2002–2004 | 103 | Patients with fever (defined as axillary temperature >38°C). | 22% |
| Palau | ||||
| (Demma, McQuiston et al. 2006) | 2003 | 212 | General population | 47.6% |
| Papua New Guinea | ||||
| (Faa, Graves et al. 2006) | 2002–2003 | 113 | Pregnant patients during routine antenatal blood test | 0.0% |
| (Kende and Graves 2003) | 1997 | 191 | Non-randomly residents living in Port Moresby (n = 93) and in the highland villages of Samberigi (n = 98) | 1.6% |
| South Korea | ||||
| (Jang, Kim et al. 2004) | 1992–1993 | 3,401 | General population | 35.2% |
| Sri Lanka | ||||
| (Premaratna, Ariyaratna et al. 2014) | 2008 | 106 | General population | 38.7% |
| (Liyanapathirana and Thevanesam 2011) | 2009–2010 | 615 | General population | 27.3% |
| Thailand | ||||
| (Bhengsri, Baggett et al. 2016) | 2002–2005 | 2,225 | General population | 4.2% |
| (Blacksell, Tanganuchitcharnchai et al. 2015) | 2006–2007 | 152 | Hospitalized patients in a subset of a febrile illness study | 28.3% |
| (Singhsilarak, Phongtananant et al. 2006) | N/A | 194 | Adult patients with falciparum malaria, who were enrolled in antimalarial drug trials | 14.9% |
| (Chanyasanha, Kaeburong et al. 1998) | 1994 | 200 | Malaria patients at 6 malaria clinics | 59.5%15 |
| (Frances, Eamsila et al. 1997) | 1991–1992 | 403 | Royal Thai Army deployed to Thai-Cambodian border between September, 1991 and October, 1992 | 2.7%16 |
| (Eamsila, Singsawat et al. 1996) | 1989–1991 | 1,218–1,888 | Soldiers from royal Thai Army, Border Patrol Police, and Thai Rangers | 7.8–10.3% |
| (Strickman, Tanskul et al. 1994) | N/A | 215 | General population | 21.0% |
| Vietnam | ||||
| (Trung, Hoi et al. 2017) | 2011–2012 | 908 | General population | 1.1% |
| (Nadjm, Thuy et al. 2014) | 2001–2003 | 7226 | Febrile adults & children patients admitted to hospital | 3.5% |
Table 2. Global outbreaks of scrub typhus.
| Region and Country | Reported Year | Case # | Notes |
|---|---|---|---|
| Australia | |||
| (Harris, Oltvolgyi et al. 2016) | 2011 | 45 | |
| (Faa, McBride et al. 2003) | 2000–2001 | 10 | |
| (McBride, Taylor et al. 1999) | 1996–1997 | 28 | 17 cases in the 1st outbreak in north Queensland in 1996, 11 cases in another outbreak in 1997 |
| China | |||
| (Cao, Che et al. 2016) | 2011 | 45 | |
| (Hu, Tan et al. 2015) | 2013 | 271 | |
| (Wei, Luo et al. 2014) | 2012 | 29 | |
| (Zhang, Jin et al. 2007) | 2005 | 32 | |
| (Xiangrui, Jinju et al. 1991) | 1986 | 138 | |
| India | |||
| (Borkakoty, Jakharia et al. 2016) | 2013 | 30 | |
| (Stephen, Sangeetha et al. 2015) | 2012–2013 | 28 | |
| (Sharma, Krishna et al. 2014) | 2012–2013 | 125 | 2 outbreaks occurred in Rajasthan in 2012 and 2013 |
| (Subbalaxmi, Madisetty et al. 2014) | 2011–2012 | 176 | |
| (Krishna, Vasuki et al. 2015) | 2010–2011 | 52 | |
| (Sinha, Gupta et al. 2014) | 2012 | 42 | |
| (Sethi, Prasad et al. 2014) | 2009–2012 | 45 | |
| (Gurung, Pradhan et al. 2013) | 2011 | 63 | |
| (Tilak, Kunwar et al. 2011) | 2005–2007 | 61 | |
| (Dass, Deka et al. 2011) | 2009–2010 | 24 | |
| (Vivekanandan, Mani et al. 2010) | 2006–2008 | 50 | |
| (Singh, Devi et al. 2010) | 2007 | 38 | |
| (Vaz and Gupta 2006) | 2002 | 12 | |
| (Kumar, Saxena et al. 2004) | 2003 | 113 | |
| (Sharma, Mahajan et al. 2005) | 2003 | 45 | Diagnosed by Weil-Felix test, 12 of the 45 positive cases were tested negative by PCR |
| (Mathai, Rolain et al. 2003) | 2001–2002 | 28 | |
| Japan | |||
| (Jiang, Marienau et al. 2003) | 2000, 2001 | 17 | 2 outbreaks occurred among U.S. Marines training at Camp Fuji, Japan, 9 cases in ~800 Marines in 2000, 8 cases in ~900 Marines in 2011 |
| Maldives | |||
| (Lewis, Yousuf et al. 2003) | 2002–2003 | 14+ | 168 suspected and confirmed cases. AFRIM tested 28 of them, and 14 were positive |
| Palau | |||
| (Durand, Kuartei et al. 2004) | 2001–2003 | 15 | |
| Papua New Guinea | |||
| (Spicer, Taufa et al. 2007) | 2001 | 39 | |
| Solomon Island | |||
| (Marks, Joshua et al. 2016) | 2014 | 9 | |
| Thailand | |||
| (Rodkvamtook, Gaywee et al. 2013) | 2006–2007 | 26+ | 142 febrile children with clinically suspected ST, 30 Hmong hill tribe children were tested serologically and genetically. |
| (Rodkvamtook, Ruang-Areerate et al. 2011) | 2002 | 17 | |
| Taiwan | |||
| (Bourgeois, Olson et al. 1977) | 1975 | 69 | |
| (Gale, Irving et al. 1974) | 1970 | 19 |
Epidemiology in the Asia Pacific region
The literature review confirmed that the majority of scrub typhus cases were reported in the “tsutsugamushi triangle” in the Asia-Pacific region (Table 3) [23,45,105,155,156,157,158]. There were a few cases reported in Central Asia and the Middle East, which are outside the traditional definition of the Asia Pacific region, but neighboring it [49,145,159].
Table 3. Characteristics of scrub typhus in main endemic areas.
| Country/Region | Age Distribution | Gender Ratio (F:M) | High Risk Season |
|---|---|---|---|
| China | <10 yr (11.84%) | 1:1 | June & July |
| 10–19 yr (5.10%) | |||
| 20–29 yr (6.39%) | |||
| (Zhang, Wang et al. 2013) | 30–39 yr (10.45%) | ||
| 40–49 yr (18.06%) | |||
| 50–59 yr (21.36%) | |||
| 60–69 yr (16.00%) | |||
| ≥70 yr (10.81%) | |||
| Japan | 0–25 yr (3%) | 1:1 | November |
| (Ogawa, Hagiwara et al. 2002) | 25–50 yr (21%) | ||
| 51–75 yr (62%) | |||
| ≥76 yr (14%) | |||
| South Korea | <10 yr (1.04%) | 13:7 | October & November |
| 10–19 yr (1.21%) | |||
| 20–29 yr (2.24%) | |||
| 30–39 yr (4.84%) | |||
| 40–49 yr (12.05%) | |||
| (Lee, Cho et al. 2015) | |||
| 50–59 yr (21.98%) | |||
| 60–69 yr (27.48%) | |||
| 70–79 yr (22.76%) | |||
| 80–89 yr (6.04%) | |||
| ≥90 yr (0.37%) | |||
| India | N/A | N/A | August—October |
| (Kalra 1952) | |||
| Thailand | 11–29 yr (15.5%) | 1:2 | N/A |
| 30–39 yr (21.2%) | |||
| 40–49 yr (20.1%) | |||
| (Suputtamongkol, Suttinont et al. 2009) | |||
| 50–59 yr (22.3%) | |||
| 60–69 yr (14.0%) | |||
| ≥70 yr (6.8%) | |||
| Vietnam | N/A | N/A | Summer |
| (Nadjm, Thuy et al. 2014) |
N/A means the data is not available from literature search.
China
The cases are primarily found in southwest China, and the southeast coastal and eastern regions of China. May is usually the start of the scrub typhus season, and June and July are the peak months. The pattern correlates with the weather and life cycle of mites [160]. Recent studies showed that the geographic distribution of the disease has expanded to northern China. It has existed in southern China for thousands of years [158,161]. Scrub typhus cases can be divided into 6 clusters in China. Cluster 1, the significant primary cluster, is located in southern and southeast China, which includes provinces of Guangdong, southern Fujian, Jiangxi, and Guangxi. The secondary cluster is mainly in southwest China, which includes Yunnan and Sichuan Provinces. Jiangsu, Anhui and Shandong provinces in East China are the third cluster for scrub typhus. Shaanxi province in the Northwest and Beijing Municipality were recognized as the fourth and fifth clusters in the analysis done by Zhang et al. The provinces of Zhejiang and northern Fujian were cluster 6 [158]. In other studies, there were reported cases in Hunan Province and Tibet [160].
Data collected between 2006 and 2012 show that the highest cumulative incidence was in the 60–69 year-old age group (0.66 per 100,000), and the lowest one was in the 10–19 year-old age group (0.11 per 100,000). The 50–60 year-old group accounted for the largest portion of all scrub typhus patients in China (21.36%). There was no difference in incidence between genders [158].
Japan
A remarkable resurgence and a prominent outbreak occurred between 1976 and 1984 due to an increase of mite populations carrying O. tsutsugamushi. There was no explanation available for the increased number of mites during that period [162]. The disease has now been found in almost all areas of Japan except in Okinawa and Hokkaido prefectures. A retrospective study in 1998 demonstrated that Kyushu (51% of total cases), Tohoku-Hokuriku (27%) and Kanto (19%) had the largest numbers of cases [45]. In contrast to China, November accounted for the largest proportion of reported cases driven by the large number of cases in Kanto and Kyushu. May had the second highest monthly number of cases due to the cases in Tohoku-Hokuriku [45]. The age distribution differs between Japan and China. In Japan, 62% of cases were 51–75 years old, while in China this age group accounted for less than half of the total patients (~48.2%) [45,158]. No significant gender differences were observed in Orientia infection in Japan. Not surprisingly, working in farming and forestry is an important risk factor for scrub typhus [45,162].
South Korea
Scrub typhus was first reported in South Korea during the Korean War, but it was still unfamiliar to Korean civilians until 1986 [163]. The disease has subsequently been recognized as the most common rickettsial disease in South Korea [156,163,164]. Nation-wide seroepidemiologic and microbiologic surveys demonstrated that 27.7% to 51% of acute febrile illness patients in South Korea were seropositive for O. tsutsugamushi between 1986 and 1993 [163]. The study confirmed that scrub typhus was widely spread in the country, and that it was frequently underdiagnosed [163]. Scrub typhus became a reportable disease in South Korea in 1994. Physicians must report all confirmed or suspected scrub typhus cases to both the local health bureau and the Korean Centers for Disease Control and Prevention (CDC). The gender inequality of scrub patients is unique in this country. Several studies confirmed that more female patients were reported than male patients (~65% vs 35%) [70,156]. One possible explanation is due to the conventional working behavior in farms in South Korea. Female workers typically work in a squatting position in dry fields, while male farmers tend to stand with tools in rice fields during work [70]. Other characteristics of the epidemiology of scrub typhus in South Korea are the increasing incidence in urban areas and expansion to northern regions [70,164,165]. The proportion of cases identified in urban areas increased from 20% (388 cases) in 2002 to 26.9% (1,345 cases) in 2009, while that in farmers decreased from 43.3% to 25%. However, further analysis revealed that outdoor activity in urban areas is the most common risk factor [70,164]. Similar to Japan, October and November are the peak months for scrub typhus cases. The age group 60–69 years old is the largest group for scrub typhus cases in South Korea (27.48%), and 72.2% patients were 50–79 years-old [70,156].
India
Scrub typhus was recognized as a typhus-like fever in India in 1917 [1,166]. It was a major cause of fever among military personnel along the Assam-India-Myanmar (formerly Burma) border during World War II, and the 1965 Indo-Pak war [1,43]. The disease resurged at the Pakistan border of India in 1990 [43]. The widespread use of insecticides and empiric treatment of febrile illness as well as changes in lifestyle all contributed to the subsequent decrease in incidence [43,167]. However, scrub typhus is still an under-diagnosed disease in India [43]. Field epidemiology studies indicate that the disease occurs all over India, from South India to Northeast India and Northwest India. There were cases reported from Maharashtra, Tamil Nadu, Karnataka, Kerala, Himachal Pradesh, Jammu and Kashmir, Uttaranchal, Rajasthan, West Bengal, Bihar, Meghalaya, and Nagaland [27,43,110,168,169]. The peak of the disease is between August and October. Leptotrombidium deliense is reported as the primary vector of O. tsutsugamushi [1,155]. Socioeconomic status and occupation are important risk factors for scrub typhus. Most scrub typhus patients in India are uneducated and live in rural areas [27,43].
Thailand
The tropical climate of Thailand provides an ideal environment for the vectors of O. tsutsugamushi, L. deliense and L. chiangraiensis [1]. Nationwide sero-epidemiological studies revealed a high prevalence of scrub typhus in Thailand [157,170]. The rates of O. tsutsugamushi antibodies varied from 13% to 31% of residents in suburban Bangkok, to 59% to 77% of residents in the northern and northeastern regions [157]. A human case was first reported from the central region of Thailand in 1952 [157,171]. The pathogen was first isolated from rodents in the same part of the country two years later [172]. There was a substantial increase in the number of confirmed cases in Thailand from the 1980s to the 2000s [157]. Growing awareness of the disease and development of new diagnostic tools may at least partially contribute to this trend [157,173,174]. Different from China and Japan, the male to female gender ratio of scrub typhus patients in Thailand is 2:1 [157]. The age distribution of the disease in Thailand is that the 50–59 year old group is the largest group (22.3%), but both the 30–39 year old and 40–49 year old groups are similar, ~20%. Outdoor activity, especially occupational exposure, is a critical risk factor [157].
Vietnam
The earliest reports of scrub typhus cases in Vietnam can be dated back to the 1960s during the Vietnam War [175,176,177,178]. Most of the patients in the 1960s and 1970s were military personnel, especially American servicemen in South Vietnam [46,178]. The disease had been neglected in Vietnam since then until the end of the last century and the beginning of 21st century, resulting in a gap in publications during that time [179]. Differing from past studies, current studies and case reports of scrub typhus in Vietnam have been focused on the northern part of the country. There was only one study in the central part of Vietnam, Quang Nam province, which identified that the main genotype in the area was the Karp group [180]. Recent studies of scrub typhus in North Vietnam demonstrated that the cumulative incidence of scrub typhus was about 1.1% among the general population, and ~3.5% among patients admitted to hospitals [103,105]. The peak season in North Vietnam is summer though cases occur throughout the year. The transmission pattern of scrub typhus in tropical South Vietnam may be different because a seasonal pattern is more obvious in a temperate climate [66,105]. There was no significant difference between urban and rural areas [103].
Other countries
In addition to the countries described above, there are quite a few other countries with reported scrub typhus cases in the tsutsugamushi triangle. Scrub typhus has been recognized on the islands of the southwest Pacific including Indonesia and the Philippines, and the continent of Australia for almost a century [149,181,182,183,184]. It was recognized as “coastal fever” in 1913 and scrub typhus after the 1920s in Australia. The endemic areas in Australia are the tropical coastal periphery of northeastern Queensland, the tropical region of the Northern Territory, and the adjacent Kimberly region of Western Australia [1,181,182]. A new strain, Litchfield, different from those from other Asia-Pacific area countries was isolated in Australia in 1998 [185]. The Philippines did not confirm the occurrence of scrub typhus until World War II. The first but failed US scrub typhus vaccine was prepared from the lungs and spleens of white rats infected with Volner strain of O. tsutsugamushi. The Volner strain was originally isolated from the blood of a soldier in the Philippines [149,186]. The history of scrub typhus in Malaysia could be traced back to 1915 [187,188,189]. World War II made the disease known in the Solomon Islands, Republic of Vanuatu, and Papua New Guinea [128,146,190,191,192]. Our literature search found isolated Orientia infection in human patients in Far East Russia and Pakistan, and further studies may be necessary to confirm the distribution and other epidemiological features of scrub typhus in these countries [48,153,154,193,194,195,196]. The distribution of scrub typhus covers a large and diverse area. In the Asia-Pacific region alone, different countries in the endemic area have different climates, environment, and culture, which all contribute to the different characteristics of epidemiology of the disease [1,64]. Our study has the limitation that we only analyzed literature in English within the databases of PubMed and Google Scholar. There were quite a few publications in Chinese, Japanese, Korean, Russian, and other languages not included in the analysis.
Epidemiology outside the “tsutsugamushi triangle”
Our literature review determined that there are a few sporadic scrub typhus cases from countries and regions outside the traditional “tsutsugamushi triangle” in the Asia-Pacific area (Fig 1).
United Arab Emirates (UAE)
UAE is outside the traditional endemic triangle. However, the case reported in 2010 demonstrated a scrub typhus case confirmed to be caused by a new Orientia species, O. chuto [49]. The Australian patient concerned had traveled to Dubai, UAE and the United Kingdom before the onset of her febrile illness. She noticed an eschar on her abdomen after the Dubai visit. An IFA, polymerase chain reaction (PCR), and sequencing were employed to determine the etiologic pathogen. The molecular variance of the 47-kDa gene, 56-kDa gene and other nucleotide sequences, and geographical difference led the researchers to propose the new Orientia species. There was only one known Orientia species, i.e., O. tsutsugamushi, before this case [23,49].
Chile
Before the two scrub typhus reports in Chile, there was no reported autochthonous scrub typhus case in the Western Hemisphere. A patient was bitten by terrestrial leeches on Chiloé Island in southern Chile in 2006 [139]. Both PCR, a molecular biological test, and, IFA, a serological test, showed diagnostic confirmation of O. tsutsugamushi infection. The molecular analysis further indicated that the pathogen is closely related but not identical to other O. tsutsugamushi and O. chuto species. It was significantly closer to Orientia sp. than to other rickettsiae. The results suggested that the pathogen from the Chilean sample was not a simple import from an endemic area. In addition, the case also reminds us that there might be other vectors, such as leeches, for Orientia [139]. The latest study reported three native cases on the same island in 2016 [151]. The researchers used both serological testing and molecular analysis to diagnose the patients. At least one patient received diagnosis by two serological tests from both the local hospital and the Mahidol-Oxford Tropical Medicine Research Unit in Thailand, and molecular analysis at the Lao-Oxford-Mahosot-Hospital-Wellcome Trust Research Unit. They used paired antibody titer comparison for diagnosis, which is more reliable than a single titer diagnosis [197]. The second patient was documented by molecular analysis of the agent but half of the IFA serologic tests were negative. The third patient had two high single-titer IFA results but negative PCR results. No leech bite was observed in these cases [151]. More follow-up studies could fill these gaps.
Africa
There are case reports from Cameroon, Kenya, Congo, Djibouti and Tanzania in Africa [102,143,144,147]. The only case report from Cameroon was an American who visited Cameroon before developing a febrile illness [143]. The patient’s IFA titer to O. tsutsugamushi increased from 1:256 to >1:1,024 two weeks after admission, but PCR analysis of formalin-fixed, paraffin-embedded skin samples was negative. Several clinical features were not typical [143]. The researchers in Kenya screened samples of reactive sera from patients with febrile illness in Kenya. Western blot was performed to confirm the specificity. About 5% of the serum samples contained antibodies reactive with O. tsutsugamushi [102]. The clinical features and serological test, IIP, confirmed the diagnosis of O. tsutsugamushi Kato strain in the only case in Congo. However, the patient resided and was diagnosed in Japan. The patient visited Congo for 23 days, and noticed symptoms 8 days after he left Congo. The researchers contacted local centers for disease control in Japan where the patient lived and worked. They did not find similar reports in Japan so they concluded that the patient contracted the disease in Congo. No other case in Congo has been reported [147]. Therefore, it is reasonable to suspect the possibility of domestic infection in Japan instead of acquisition in Congo. More epidemiologic studies of scrub typhus are necessary to confirm the existence of scrub typhus in Congo. A new study of 49 abattoir workers in Djibouti demonstrated that three workers were seropositive against Orientia, and one worker seroconverted during the study [80]. The titers observed in the study were 100–400 for ELISAs, and 1:128 for IFA. The cut-off titer and methods used in this study may be controversial, and require further validation [197]. In addition, the study did not provide the participants’ travel history even though the authors played down this confounder due to the subjects’ socioeconomic status [80]. A Dutch traveler to Tanzania contracted O. tsutsugamushi there. Researchers in the Netherlands confirmed the case with clinical features (fever, eschar, etc.) and serological tests, i.e., IFA [144]. No other case has been reported in Tanzania.
Discussion
Prevention and control of scrub typhus
Diagnosis
Orientia causes a flu-like febrile illness similar to many other diseases, which makes the clinical diagnosis of scrub typhus quite difficult. Generally, the diagnosis is based on the patient’s clinical presentation and history. Presence of an eschar and history of travel to or residence in an endemic area favors the diagnosis. An eschar is not observed in every confirmed patient, and cutaneous lesions from other diseases, e.g., spider bites, leishmaniasis, spotted fever rickettsiosis and anthrax, may make the presence of an eschar less than a definitive diagnostic sign. Scrub typhus can be misdiagnosed as many acute febrile illnesses, including malaria, dengue, leptospirosis, other rickettsioses, meningococcal disease, typhoid fever, infectious mononucleosis and HIV [6,198,199].
Laboratory methods for diagnosing rickettsial diseases including scrub typhus are mainly based on serological tests and molecular assays. Cross-reactivity of Orientia with other rickettsiae is rare [200,201,202]. The gold standard test for diagnosis of scrub typhus is the indirect immunofluorescence assay (IFA) [199,203]. However, IFA is expensive and complicated, and requires extensive training and a biocontainment facility for production of the reagents. Even though the assay has been available for many years, the application of IFA in the endemic area is still limited for the above mentioned reasons. The test fails to provide diagnostic results at early stages of infection because those antibodies represent adaptive immunity and are not generated during the early acute infection stage. It is more reliable to interpret the serological tests when the antibody titer has a 4-fold rise [197]. There are other limitations of IFA, from the controversial cut-off antibody titer, and subjective determination of results, to imperfect specificity of the test [203,204].
Other serological tests include the indirect immunoperoxidase assay (IIP), Weil-Felix test (W-F), enzyme-linked immunoassays (ELISAs), and various commercially available immunochromatographic tests (ICT) [203,205]. The W-F agglutination test has been commercially available for many years. This test using the Proteus mirabilis OXK strain lacks both specificity and sensitivity, especially the latter, for routine diagnosis [95,206]. Studies have shown that the sensitivity is only 50% during the second week of illness [6,199].
IIP is a modification of IFA, which provides a comparable sensitivity and specificity without the requirement for an ultraviolet microscope in diagnosis of scrub typhus [202,207]. Both IFA and IIP have been used as the reference standard. No significant difference was found in the accuracy of the two tests, except for one study which claimed that IIP was more sensitive than the IFA with acute sera (79.6% vs. 68.5% at titer ≥1:400) [208,209].
ELISAs and their variants, such as commercially available dipstick tests, use either pooled cell lysates of different strains of O. tsutsugamushi as antigen, or recombinant p56 or other outer membrane proteins as the antigen. ELISAs provide sensitive and specific test results. They eventually may replace the IFA and IIP assays. The sensitivity and specificity of dipstick assays are inferior to ELISAs, but these commercially available assays are easy to use, and could be performed in underserved areas [6,208,209] Blacksell et al. reported experience with InBios Scrub Typhus Detect IgM ELISA, which was proven to be easy-to-use, affordable, and to have adequate accuracy for screening and diagnosis [106]. ELISA-based tests should be considered a good alternative to the gold standard IFA.
ICT is another commercially available kit for early rapid diagnosis. The test also uses the recombinant Orientia outer membrane proteins to detect IgG, IgM and IgA antibodies to O. tsutsugamushi. Evaluation of the test indicated that ICT has moderate to high sensitivity (~70%) among scrub typhus patients. The sensitivity increases with the fever duration. Several studies claimed that, similar to the passive hemagglutination assay (PHA), ICT also has a substantial number of false negative results [204]. PHA was replaced by ICT due to the former’s lower sensitivity. However, Lim et al. demonstrated that the specificity of IFA IgM is low, which caused inaccurate comparisons between IFA and other diagnostic assays. By contrast, the IgM ICT has comparable sensitivity and significantly better specificity than IFA as assessed using Bayesian latent class models [203,204]. Kingston et al. demonstrated InBios Scrub Typhus Detect IgM Rapid test to have high specificity and promising accuracy [210].
The molecular method to diagnose scrub typhus is detecting the bacteria with PCR assays. PCRs usually target the genes of the outer membrane proteins of 56 kDa, 47 kDa, and groEL genes [203]. It was reported that nested PCR may be more sensitive than the serological tests including the gold standard, IFA [211,212]. This molecular biologic method can detect Orientia DNA in blood even during the persistent phase of the infection, when no obvious clinical symptoms are observed. However, the sensitivity of PCRs decreases with treatment [6,199,213,214]
Treatment
As a gram-negative bacterium, Orientia infection can be effectively treated with the appropriate antibiotics. Early treatment results in better outcomes, i.e., shortening the disease course and reducing fatalities [63]. Oral treatment is effective for mild cases, but the parenteral route is often necessary for severely ill patients. Similar to treatment for other rickettsial diseases, doxycycline is one of most effective antibiotics for treating scrub typhus. Antibiotics are usually able to abate patients’ fever rapidly, and this outcome is even used as a diagnostic indicator. Some randomized clinical trials determined that there is no significant difference in outcomes among tetracycline, doxycycline, telithromycin, and azithromycin, the latter of which is a recommended treatment considered to be as effective as doxycycline [215,216,217]. Rifampicin was shown to be more effective than tetracycline in patients responding poorly to doxycycline [6,217,218]. World Health Organization (WHO) recommends that pregnant women or children can use azithromycin or chloramphenicol. Multiple studies proved that azithromycin and other macrolides are as effective as doxycycline or chloramphenicol but without potential side effects as aplastic anemia in treatment of children with chloramphenicol [216,219,220].Antibiotic resistance has been reported in a few papers [221,222]. There is much unknown regarding antibiotic resistance. It will be useful for the development of new diagnostic tools and treatments to elucidate the mechanisms of poor response in certain patients, as well as the antibiotic resistance of the pathogen [6,63,223].
Current prevention methods
There is no vaccine available for any rickettsial infections including scrub typhus. The enormous antigenic variation in different O. tsutsugamushi strains, and weak and short lived cross protection among different strains hamper the development of an effective vaccine. Vaccine efforts are also hindered by the different antigenically divergent strains of O. tsutsugamushi in different endemic countries/regions, or even among different strains in the same location [50,224,225].
WHO recommends prophylactic treatment under special circumstances in the endemic areas [226]. A single oral dose of doxycycline, chloramphenicol or tetracycline every 5 days for a total of 35 days provided protection against Orientia infection [50]. A prospective randomized double blind study among Taiwanese military personnel confirmed that prophylactic treatment with doxycycline could decrease the incidence of scrub typhus to 1/5 of that in the placebo group [227]. The US army has used a weekly dose of doxycycline to prevent scrub typhus [228]. However, CDC in the U.S. does not recommend using antibiotics as prophylaxis for rickettsial diseases including scrub typhus because the preventive treatment may simply delay onset of disease and make diagnosis more difficult [224,229]. To treat rickettsial diseases more effectively, CDC suggests starting treatment based on clinical suspicion alone [229,230].
General protective measures can be followed to avoid the infection. These precautions are critical for people living in or visiting endemic areas [50,224,226,231]:
Avoid outbreaks. Persons should avoid known focal outbreak areas to the extent that this is possible. Travelers can check the regional disease transmission and outbreak information at http://www.cdc.gov/travel.
Avoid exposure conditions. Chiggers reside in grass, woodlands, and other vegetated areas. Persons are encouraged to avoid the outdoors or take preventive actions. Do not sit or lie on bare ground or grass; use a sheet or a cover on the ground instead.
Wear appropriate clothing. Persons should wear long-sleeved shirts, long pants, boots and hats to reduce exposure. Persons should tuck in shirts and pants, and wear closed shoes.
Insect and spatial repellents. Persons should apply insect repellent containing dibutyl phthalate, benzyl benzoate, diethyl toluamide or other chemicals to their skin and permethrin to their clothing, to prevent chigger bites.
Insecticides and habitat modification. Farmers and field workers can improve sanitation, clear vegetation, control rodents, use insecticides and chemically treat the soil. These steps can impede the propagation of chiggers and the transmission cycle.
Thorough cleaning after visiting high risk areas. Due to its small size of 0.2–0.4 mm, detection of mite larva on clothing or skin is extremely difficult. Prompt removal of clothing and thorough cleaning of skin and clothes with detergent after work or travel and at the end of the day can reduce the risk of infection.
Strategies for prevention and control
The reemergence and geographic expansion of scrub typhus in the Asia-Pacific area as well as the increase in antibiotic resistance reminds us of the urgency of developing and adopting effective control and preventive measures [50,225,227,232,233].
Though no vaccine is available, a number of other preventive measures can be taken. To make these measures work, public education on case recognition and personal protection is the priority. WHO recommends that advocacy, awareness and educational activities should be targeted at schoolchildren, teachers and women in endemic areas [226]. However, field workers and outdoor travelers may have higher risks of becoming infected, so the educational program should not be limited to the three groups mentioned above. Farms and other places with bush, wood piles, rodents, and domestic animals increase the risk of infection. One strategy is to avoid high risk areas. For field workers and those people who cannot avoid the risk factors, taking the precautions as described in the last section will help prevent the disease [50,224,225,226,227,231].
Kwak et al. simulated incidence of scrub typhus with selected meteorological predictors, such as temperature, precipitation, relative humidity, wind speed, duration of sunshine, and cloud cover[47]. The study indicated that the seasonality of meteorological factors affects model prediction. Korean CDC and researchers demonstrated the expansion of scrub typhus high incidence area to northern regions of South Korea, which they suggested might be associated with global warming [165]. We should consider these factors when predicting the disease cycle, and employ appropriate strategies of prevention and control [47,224,225,226]
Rodent control and habitat modification are helpful for disease control and prevention. Different rodent control strategies can be employed, from trapping, to poisoning, and use of natural predators [226,234]. Rodent control is important but difficult, especially maintaining the control long-term. Public education is a priority for the control of rodents and mites [234,235]. Poisoning is widely used in the agricultural sector in the endemic areas, but secondary poisoning poses hazards to other animals and humans. For this reason using the natural predators of rodents and other natural forms of control are preferred. Habitat modification, such as good sanitation in and around buildings, clearing vegetation around fields, and secure storage of grain, can make areas less suited for rodents, and prevent them from flourishing in high numbers [50,226,232,233].
The great clinical and public health challenge of scrub typhus is its difficulty in diagnosis, especially early diagnosis. Early diagnosis and early treatment can significantly reduce the complications and fatality rate caused by Orientia. WHO suggests improving the awareness of empiric therapy and also urges researchers to develop affordable and easy-to-use diagnostic assays with high sensitivity and specificity [6,50,199,203,225,226]. Broader deployment of diagnostic testing is likely to identify new areas where scrub typhus has emerged.
Even though scrub typhus can be a life-threatening disease, collaborative actions in the countries and regions in the endemic areas using the strategies mentioned above can effectively control and prevent the outbreak of this neglected disease.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Nicole L. Mendell, Patricia A. Valdes, and Dr. Cara L. Pennel for assistance with preparation and submission of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by UTMB McLaughlin Fellowship (to GX), the Carmage and Martha Walls Distinguished University Chair in Tropical Diseases (to DHW), and the Department of Preventive Medicine and Community Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kelly DJ, Fuerst PA, Ching WM, Richards AL (2009) Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis 48 Suppl 3: S203–230. [DOI] [PubMed] [Google Scholar]
- 2.Valbuena G, Walker DH (2013) Approaches to vaccines against Orientia tsutsugamushi. Frontiers in Cellular and Infection Microbiology 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim I-S, Walker DH (2011) Scrub Typhus In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: principles, pathogens and practice. Third ed: Elsevier Health Sciences; pp. 332–336. [Google Scholar]
- 4.Paris DH, Shelite TR, Day NP, Walker DH (2013) Unresolved problems related to scrub typhus: A seriously neglected life-threatening disease. American Journal of Tropical Medicine and Hygiene 89: 301–307. doi: 10.4269/ajtmh.13-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warrell DA, Cox TM, Firth JD (2003) Oxford textbook of medicine: Oxford University Press. [Google Scholar]
- 6.Mahajan SK (2005) Scrub typhus. J Assoc Physicians India 53: 954–958. [PubMed] [Google Scholar]
- 7.Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, et al. (2007) Scrub typhus: clinical, pathologic, and imaging findings. Radiographics. 2007/January/20 ed. pp. 161–172. doi: 10.1148/rg.271065074 [DOI] [PubMed] [Google Scholar]
- 8.Tsay RW, Chang FY (1998) Serious complications in scrub typhus. J Microbiol Immunol Infect 31: 240–244. [PubMed] [Google Scholar]
- 9.Jamil M, Lyngrah KG, Lyngdoh M, Hussain M (2014) Clinical manifestations and complications of scrub typhus: a hospital based study from north eastern India. J Assoc Physicians India 62: 19–23. [PubMed] [Google Scholar]
- 10.Gupta V, Gautam V (2004) Scrub Typhus—A short review. J Commun Dis 36: 284–289. [PubMed] [Google Scholar]
- 11.Kawamura A (1995) Tsutsugamushi disease: University of Tokyo Press. [Google Scholar]
- 12.Tang Y-W, Sussman M, Liu D, Poxton I, Schwartzman J (2014) Orientia. Molecular medical microbiology 2 ed: Academic press; pp. 2057–2096. [Google Scholar]
- 13.Kim DM, Won KJ, Park CY, Yu KD, Kim HS, et al. (2007) Distribution of eschars on the body of scrub typhus patients: a prospective study. Am J Trop Med Hyg 76: 806–809. [PubMed] [Google Scholar]
- 14.Thipmontree W, Tantibhedhyangkul W, Silpasakorn S, Wongsawat E, Waywa D, et al. (2016) Scrub typhus in Northeastern Thailand: Eschar distribution, abnormal electrocardiographic findings, and predictors of fatal outcome. Am J Trop Med Hyg 95: 769–773. doi: 10.4269/ajtmh.16-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose W, Rajan RJ, Punnen A, Ghosh U (2016) Distribution of eschar in pediatric scrub typhus. J Trop Pediatr 62: 415–420. doi: 10.1093/tropej/fmw027 [DOI] [PubMed] [Google Scholar]
- 16.Arun Babu T, Vijayadevagaran V, Ananthakrishnan S (2017) Characteristics of pediatric scrub typhus eschar in South Indian children. Pediatr Dermatol 34: 124–127. doi: 10.1111/pde.13048 [DOI] [PubMed] [Google Scholar]
- 17.Chi WC, Huang JJ, Sung JM, Lan RR, Ko WC, et al. (1997) Scrub typhus associated with multiorgan failure: a case report. Scand J Infect Dis 29: 634–635. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande GA, Mittal R, Jesudasan MR, Perakath B (2015) Surgical manifestations of scrub typhus: A diagnostic dilemma. Natl Med J India 28: 12–13. [PubMed] [Google Scholar]
- 19.Song SW, Kim KT, Ku YM, Park SH, Kim YS, et al. (2004) Clinical role of interstitial pneumonia in patients with scrub typhus: a possible marker of disease severity. J Korean Med Sci 19: 668–673. doi: 10.3346/jkms.2004.19.5.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben RJ, Feng NH, Ku CS (1999) Meningoencephalitis, myocarditis and disseminated intravascular coagulation in a patient with scrub typhus. J Microbiol Immunol Infect 32: 57–62. [PubMed] [Google Scholar]
- 21.Kim DE, Lee SH, Park KI, Chang KH, Roh JK (2000) Scrub typhus encephalomyelitis with prominent focal neurologic signs. Arch Neurol 57: 1770–1772. [DOI] [PubMed] [Google Scholar]
- 22.Silpapojakul K, Ukkachoke C, Krisanapan S (1991) Rickettsial meningitis and encephalitis. Arch Intern Med 151: 1753–1757. [PubMed] [Google Scholar]
- 23.Seong SY, Choi MS, Kim IS (2001) Orientia tsutsugamushi infection: overview and immune responses. Microbes and Infection 3: 11–21. [DOI] [PubMed] [Google Scholar]
- 24.Noad KB, Haymaker W (1953) The neurological features of Tsutsugamushi fever, with special reference to deafness. Brain 76: 113–131. [DOI] [PubMed] [Google Scholar]
- 25.Premaratna R, Chandrasena TG, Dassayake AS, Loftis AD, Dasch GA, et al. (2006) Acute hearing loss due to scrub typhus: a forgotten complication of a reemerging disease. Clin Infect Dis 42: e6–8. doi: 10.1086/498747 [DOI] [PubMed] [Google Scholar]
- 26.Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH (2017) Estimating the burden of scrub typhus: A systematic review. PLoS Negl Trop Dis 11: e0005838 doi: 10.1371/journal.pntd.0005838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varghese G, Abraham O, Mathai D, Thomas K, Aaron R, et al. (2006) Scrub typhus among hospitalised patients with febrile illness in South India: magnitude and clinical predictors. Journal of Infection 52: 56–60. doi: 10.1016/j.jinf.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 28.Taylor AJ, Paris DH, Newton PN (2015) A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis 9: e0003971 doi: 10.1371/journal.pntd.0003971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SJ, Chung IK, Chung IS, Song DH, Park SH, et al. (2000) The clinical significance of upper gastrointestinal endoscopy in gastrointestinal vasculitis related to scrub typhus. Endoscopy 32: 950–955. doi: 10.1055/s-2000-9621 [DOI] [PubMed] [Google Scholar]
- 30.Phasomkusolsil S, Tanskul P, Ratanatham S, Watcharapichat P, Phulsuksombati D, et al. (2012) Influence of Orientia tsutsugamushi infection on the developmental biology of Leptotrombidium imphalum and Leptotrombidium chiangraiensis (Acari: Trombiculidae). J Med Entomol 49: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 31.Philip CB (1948) Tsutsugamushi disease in World War II. J Parasitol 34: 169–191. [PubMed] [Google Scholar]
- 32.Lerdthusnee K, Khlaimanee N, Monkanna T, Sangjun N, Mungviriya S, et al. (2002) Efficiency of Leptotrombidium chiggers (Acari: Trombiculidae) at transmitting Orientia tsutsugamushi to laboratory mice. J Med Entomol. 2002/June/14 ed. pp. 521–525. [DOI] [PubMed] [Google Scholar]
- 33.Lerdthusnee K, Khuntirat B, Leepitakrat W, Tanskul P, Monkanna T, et al. (2003) Scrub typhus: vector competence of Leptotrombidium chiangraiensis chiggers and transmission efficacy and isolation of Orientia tsutsugamushi. Ann N Y Acad Sci 990: 25–35. [DOI] [PubMed] [Google Scholar]
- 34.Walker JS, Chan CT, Manikumaran C, Elisberg BL (1975) Attempts to infect and demonstrate transovarial transmission of R. tsutsugamushi in three species of Leptotrombidium mites. Ann N Y Acad Sci 266: 80–90. [DOI] [PubMed] [Google Scholar]
- 35.Phasomkusolsil S, Tanskul P, Ratanatham S, Watcharapichat P, Phulsuksombati D, et al. (2009) Transstadial and transovarial transmission of Orientia tsutsugamushi in Leptotrombidium imphalum and Leptotrombidium chiangraiensis (Acari: Trombiculidae). J Med Entomol 46: 1442–1445. [DOI] [PubMed] [Google Scholar]
- 36.Traub R, Wisseman CL Jr., Jones MR, O'Keefe JJ (1975) The acquisition of Rickettsia tsutsugamushi by chiggers (trombiculid mites) during the feeding process. Ann N Y Acad Sci 266: 91–114. [DOI] [PubMed] [Google Scholar]
- 37.Lerdthusnee K, Khlaimanee N, Monkanna T, Sangjun N, Mungviriya S, et al. (2002) Efficiency of Leptotrombidium chiggers (Acari: Trombiculidae) at transmitting Orientia tsutsugamushi to laboratory mice. Journal of Medical Entomology 39: 521–525. [DOI] [PubMed] [Google Scholar]
- 38.Frances SP, Watcharapichat P, Phulsuksombati D, Tanskul P (2000) Transmission of Orientia tsutsugamushi, the aetiological agent for scrub typhus, to co-feeding mites. Parasitology 120 (Pt 6): 601–607. [DOI] [PubMed] [Google Scholar]
- 39.Giengkam S, Blakes A, Utsahajit P, Chaemchuen S, Atwal S, et al. (2015) Improved Quantification, Propagation, Purification and Storage of the Obligate Intracellular Human Pathogen Orientia tsutsugamushi. Plos Neglected Tropical Diseases 9: e0004009 doi: 10.1371/journal.pntd.0004009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho BA, Cho NH, Min CK, Kim SY, Yang JS, et al. (2010) Global gene expression profile of Orientia tsutsugamushi. Proteomics 10: 1699–1715. doi: 10.1002/pmic.200900633 [DOI] [PubMed] [Google Scholar]
- 41.Ge Y, Rikihisa Y (2011) Subversion of host cell signaling by Orientia tsutsugamushi. Microbes and Infection 13: 638–648. doi: 10.1016/j.micinf.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Cho NH, Kim SY, Bang SY, Chu H, et al. (2008) Fibronectin facilitates the invasion of Orientia tsutsugamushi into host cells through interaction with a 56-kDa type-specific antigen. Journal of Infectious Diseases 198: 250–257. doi: 10.1086/589284 [DOI] [PubMed] [Google Scholar]
- 43.Sinha P, Gupta S, Dawra R, Rijhawan P (2014) Recent outbreak of scrub typhus in North Western part of India. Indian J Med Microbiol 32: 247–250. doi: 10.4103/0255-0857.136552 [DOI] [PubMed] [Google Scholar]
- 44.Yang LP, Liang SY, Wang XJ, Li XJ, Wu YL, et al. (2015) Burden of disease measured by disability-adjusted life years and a disease forecasting time series model of scrub typhus in Laiwu, China. PLoS Negl Trop Dis 9: e3420 doi: 10.1371/journal.pntd.0003420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa M, Hagiwara T, Kishimoto T, Shiga S, Yoshida Y, et al. (2002) Scrub typhus in Japan: epidemiology and clinical features of cases reported in 1998. Am J Trop Med Hyg 67: 162–165. [DOI] [PubMed] [Google Scholar]
- 46.Berman SJ, Kundin WD (1973) Scrub typhus in South Vietnam. A study of 87 cases. Ann Intern Med 79: 26–30. [DOI] [PubMed] [Google Scholar]
- 47.Kwak J, Kim S, Kim G, Singh VP, Hong S, et al. (2015) Scrub typhus incidence modeling with meteorological factors in South Korea. Int J Environ Res Public Health 12: 7254–7273. doi: 10.3390/ijerph120707254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rehácek J, Tarasevich IV (1988) Rickettsia tsutsugamushi. Acari-borne Rickettsiae & rickettsioses in Eurasia: Veda. pp. 146–202.
- 49.Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, et al. (2010) Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol 48: 4404–4409. doi: 10.1128/JCM.01526-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuo CC, Huang JL, Shu PY, Lee PL, Kelt DA, et al. (2012) Cascading effect of economic globalization on human risks of scrub typhus and tick-borne rickettsial diseases. Ecol Appl 22: 1803–1816. [DOI] [PubMed] [Google Scholar]
- 51.Walker DH, Fishbein DB (1991) Epidemiology of rickettsial diseases. Eur J Epidemiol 7: 237–245. [DOI] [PubMed] [Google Scholar]
- 52.Hemingway P, Brereton N (2009) What is a systematic review. What is.
- 53.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 54.Nachega JB, Bottieau E, Zech F, Van Gompel A (2007) Travel-acquired scrub typhus: emphasis on the differential diagnosis, treatment, and prevention strategies. J Travel Med 14: 352–355. doi: 10.1111/j.1708-8305.2007.00151.x [DOI] [PubMed] [Google Scholar]
- 55.Dupon M, Rogues AM, Malou M, d'Ivernois C, Lacut JY (1992) Scrub typhus: an imported rickettsial disease. Infection 20: 153–154. [DOI] [PubMed] [Google Scholar]
- 56.Marschang A, Nothdurft HD, Kumlien S, von Sonnenburg F (1995) Imported rickettsioses in German travelers. Infection 23: 94–97. [DOI] [PubMed] [Google Scholar]
- 57.Ciceroni L, Pinto A, Ciarrocchi S, Ciervo A (2006) Current knowledge of rickettsial diseases in Italy. Ann N Y Acad Sci 1078: 143–149. doi: 10.1196/annals.1374.024 [DOI] [PubMed] [Google Scholar]
- 58.Vliegenthart-Jongbloed K, de Mendonca Melo M, Slobbe L, Beersma MF, van Genderen PJ (2013) Imported scrub typhus in The Netherlands. Travel Med Infect Dis 11: 197–199. doi: 10.1016/j.tmaid.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 59.Henderson R, Reynolds R, Dickie A, Lang S (1986) Imported scrub typhus. N Z Med J 99: 126–127. [PubMed] [Google Scholar]
- 60.Jensenius M, Montelius R, Berild D, Vene S (2006) Scrub typhus imported to Scandinavia. Scand J Infect Dis 38: 200–202. doi: 10.1080/00365540500277342 [DOI] [PubMed] [Google Scholar]
- 61.McDonald JC, MacLean JD, McDade JE (1988) Imported rickettsial disease: clinical and epidemiologic features. Am J Med 85: 799–805. [DOI] [PubMed] [Google Scholar]
- 62.Maher PH (1976) Imported scrub typhus. Conn Med 40: 377–378. [PubMed] [Google Scholar]
- 63.Watt G, Parola P (2003) Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis 16: 429–436. doi: 10.1097/01.qco.0000092814.64370.70 [DOI] [PubMed] [Google Scholar]
- 64.Kelly DJ, Foley DH, Richards AL (2015) A spatiotemporal database to track human scrub typhus using the VectorMap Application. PLoS Negl Trop Dis 9: e0004161 doi: 10.1371/journal.pntd.0004161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, et al. (2009) A community-based case-control study of behavioral factors associated with scrub typhus during the autumn epidemic season in South Korea. Am J Trop Med Hyg 80: 442–446. [PubMed] [Google Scholar]
- 66.Matsui T, Kramer MH, Mendlein JM, Osaka K, Ohyama T, et al. (2002) Evaluation of national tsutsugamushi disease surveillance—Japan, 2000. Jpn J Infect Dis 55: 197–203. [PubMed] [Google Scholar]
- 67.Tshokey T, Choden T, Sharma R (2016) Scrub typhus in Bhutan: a synthesis of data from 2009 to 2014. WHO South East Asia J Public Health 5: 117–122. doi: 10.4103/2224-3151.206248 [DOI] [PubMed] [Google Scholar]
- 68.Wu YC, Qian Q, Magalhaes RJ, Han ZH, Haque U, et al. (2016) Rapid increase in scrub typhus incidence in mainland China, 2006–2014. Am J Trop Med Hyg 94: 532–536. doi: 10.4269/ajtmh.15-0663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hashimoto S, Kawado M, Murakami Y, Izumida M, Ohta A, et al. (2007) Epidemics of vector-borne diseases observed in infectious disease surveillance in Japan, 2000–2005. J Epidemiol 17 Suppl: S48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, et al. (2009) Rapid increase of scrub typhus, South Korea, 2001–2006. Emerg Infect Dis 15: 1127–1129. doi: 10.3201/eid1507.080399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leelarasamee A, Chupaprawan C, Chenchittikul M, Udompanthurat S (2004) Etiologies of acute undifferentiated febrile illness in Thailand. J Med Assoc Thai 87: 464–472. [PubMed] [Google Scholar]
- 72.Lee YS, Wang PH, Tseng SJ, Ko CF, Teng HJ (2006) Epidemiology of scrub typhus in eastern Taiwan, 2000–2004. Jpn J Infect Dis 59: 235–238. [PubMed] [Google Scholar]
- 73.Blacksell SD, Sharma NP, Phumratanaprapin W, Jenjaroen K, Peacock SJ, et al. (2007) Serological and blood culture investigations of Nepalese fever patients. Trans R Soc Trop Med Hyg 101: 686–690. doi: 10.1016/j.trstmh.2007.02.015 [DOI] [PubMed] [Google Scholar]
- 74.Chanyasanha C, Kaeburong K, Chenchittikul M, Sujirarat D (1998) Seroprevalence of scrub typhus infection in patients with pyrexia at some malaria clinics in three western provinces of Thailand. Asian Pac J Allergy Immunol 16: 119–125. [PubMed] [Google Scholar]
- 75.Demma LJ, McQuiston JH, Nicholson WL, Murphy SM, Marumoto P, et al. (2006) Scrub typhus, Republic of Palau. Emerg Infect Dis 12: 290–295. doi: 10.3201/eid1202.050967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eamsila C, Singsawat P, Duangvaraporn A, Strickman D (1996) Antibodies to Orientia tsutsugamushi in Thai soldiers. Am J Trop Med Hyg 55: 556–559. [DOI] [PubMed] [Google Scholar]
- 77.Faa AG, Graves SR, Nguyen C, Stenos J (2006) A serological survey of rickettsial infections in the Gazelle Peninsula, East New Britain and a review of the literature. P N G Med J 49: 43–46. [PubMed] [Google Scholar]
- 78.Frances SP, Eamsila C, Strickman D (1997) Antibodies to Orientia tsutsugamushi in soldiers in northeastern Thailand. Southeast Asian J Trop Med Public Health 28: 666–668. [PubMed] [Google Scholar]
- 79.Graves S, Wang L, Nack Z, Jones S (1999) Rickettsia serosurvey in Kimberley, Western Australia. Am J Trop Med Hyg 60: 786–789. [DOI] [PubMed] [Google Scholar]
- 80.Horton KC, Jiang J, Maina A, Dueger E, Zayed A, et al. (2016) Evidence of Rickettsia and Orientia infections among abattoir workers in Djibouti. The American journal of tropical medicine and hygiene 95: 462–465. doi: 10.4269/ajtmh.15-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jakharia A, Borkakoty B, Biswas D, Yadav K, Mahanta J (2016) Seroprevalence of scrub typhus infection in Arunachal Pradesh, India. Vector Borne Zoonotic Dis 16: 659–663. doi: 10.1089/vbz.2016.1970 [DOI] [PubMed] [Google Scholar]
- 82.Jang WJ, Kim JH, Choi YJ, Jung KD, Kim YG, et al. (2004) First serologic evidence of human spotted fever group rickettsiosis in Korea. J Clin Microbiol 42: 2310–2313. doi: 10.1128/JCM.42.5.2310-2313.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalal BS, Puranik P, Nagaraj S, Rego S, Shet A (2016) Scrub typhus and spotted fever among hospitalised children in South India: Clinical profile and serological epidemiology. Indian J Med Microbiol 34: 293–298. doi: 10.4103/0255-0857.188315 [DOI] [PubMed] [Google Scholar]
- 84.Kende M, Graves S (2003) Survey of rickettsial antibodies at two local sites and review of rickettsiosis in Papua New Guinea. P N G Med J 46: 53–62. [PubMed] [Google Scholar]
- 85.Liyanapathirana VC, Thevanesam V (2011) Seroepidemiology of rickettsioses in Sri Lanka: a patient based study. BMC Infect Dis 11: 328 doi: 10.1186/1471-2334-11-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maude RR, Maude RJ, Ghose A, Amin MR, Islam MB, et al. (2014) Serosurveillance of Orientia tsutsugamushi and Rickettsia typhi in Bangladesh. Am J Trop Med Hyg 91: 580–583. doi: 10.4269/ajtmh.13-0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogawa M, Ono T (2008) Epidemiological characteristics of tsutsugamushi disease in Oita Prefecture, Japan: yearly and monthly occurrences of its infections and serotypes of its causative agent, Orientia tsutsugamushi, during 1984–2005. Microbiol Immunol 52: 135–143. doi: 10.1111/j.1348-0421.2008.00024.x [DOI] [PubMed] [Google Scholar]
- 88.Premaratna R, Ariyaratna N, Attanayake C, Bandara W, Chandrasena N, et al. (2014) Rickettsial infection among military personnel deployed in Northern Sri Lanka. BMC Infect Dis 14: 3864 doi: 10.1186/s12879-014-0688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramyasree A, Kalawat U, Rani ND, Chaudhury A (2015) Seroprevalence of Scrub typhus at a tertiary care hospital in Andhra Pradesh. Indian J Med Microbiol 33: 68–72. doi: 10.4103/0255-0857.148381 [DOI] [PubMed] [Google Scholar]
- 90.Richards AL, Ratiwayanto S, Rahardjo E, Kelly DJ, Dasch GA, et al. (2003) Serologic evidence of infection with ehrlichiae and spotted fever group rickettsiae among residents of Gag Island, Indonesia. Am J Trop Med Hyg 68: 480–484. [PubMed] [Google Scholar]
- 91.Richards AL, Soeatmadji DW, Widodo MA, Sardjono TW, Yanuwiadi B, et al. (1997) Seroepidemiologic evidence for murine and scrub typhus in Malang, Indonesia. Am J Trop Med Hyg 57: 91–95. [DOI] [PubMed] [Google Scholar]
- 92.Sagin DD, Ismail G, Nasian LM, Jok JJ, Pang EK (2000) Rickettsial infection in five remote Orang Ulu villages in upper Rejang River, Sarawak, Malaysia. Southeast Asian J Trop Med Public Health 31: 733–735. [PubMed] [Google Scholar]
- 93.Sengupta M, Anandan S, Daniel D, Prakash JA (2015) Scrub typhus seroprevalence in healthy Indian population. J Clin Diagn Res 9: DM01–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V (2005) Investigation of an outbreak of scrub typhus in the Himalayan region of India. Jpn J Infect Dis 58: 208–210. [PubMed] [Google Scholar]
- 95.Shivalli S (2016) Diagnostic evaluation of rapid tests for scrub typhus in the Indian population is needed. Infect Dis Poverty 5: 40 doi: 10.1186/s40249-016-0137-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singhsilarak T, Phongtananant S, Jenjittikul M, Watt G, Tangpakdee N, et al. (2006) Possible acute coinfections in Thai malaria patients. Southeast Asian J Trop Med Public Health 37: 1–4. [PubMed] [Google Scholar]
- 97.Strickman D, Tanskul P, Eamsila C, Kelly DJ (1994) Prevalence of antibodies to rickettsiae in the human population of suburban Bangkok. Am J Trop Med Hyg 51: 149–153. [DOI] [PubMed] [Google Scholar]
- 98.Tay ST, Ho TM, Rohani MY, Devi S (2000) Antibodies to Orientia tsutsugamushi, Rickettsia typhi and spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans R Soc Trop Med Hyg 94: 280–284. [DOI] [PubMed] [Google Scholar]
- 99.Tay ST, Kamalanathan M, Rohani MY (2003) Antibody prevalence of Orientia tsutsugamushi, Rickettsia typhi and TT118 spotted fever group rickettsiae among Malaysian blood donors and febrile patients in the urban areas. Southeast Asian J Trop Med Public Health 34: 165–170. [PubMed] [Google Scholar]
- 100.Tay ST, Mohamed Zan HA, Lim YA, Ngui R (2013) Antibody prevalence and factors associated with exposure to Orientia tsutsugamushi in different aboriginal subgroups in West Malaysia. PLoS Negl Trop Dis 7: e2341 doi: 10.1371/journal.pntd.0002341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tee TS, Kamalanathan M, Suan KA, Chun SS, Ming HT, et al. (1999) Seroepidemiologic survey of Orientia tsutsugamushi, Rickettsia typhi, and TT118 spotted fever group rickettsiae in rubber estate workers in Malaysia. Am J Trop Med Hyg 61: 73–77. [DOI] [PubMed] [Google Scholar]
- 102.Thiga JW, Mutai BK, Eyako WK, Ng'ang'a Z, Jiang J, et al. (2015) High seroprevalence of antibodies against spotted fever and scrub typhus bacteria in patients with febrile Illness, Kenya. Emerg Infect Dis 21: 688–691. doi: 10.3201/eid2104.141387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trung NV, Hoi LT, Thuong NTH, Toan TK, Huong TTK, et al. (2017) Seroprevalence of scrub typhus, typhus, and spotted fever among rural and urban populations of northern Vietnam. Am J Trop Med Hyg 96: 1084–1087. doi: 10.4269/ajtmh.16-0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vallee J, Thaojaikong T, Moore CE, Phetsouvanh R, Richards AL, et al. (2010) Contrasting spatial distribution and risk factors for past infection with scrub typhus and murine typhus in Vientiane City, Lao PDR. PLoS Negl Trop Dis 4: e909 doi: 10.1371/journal.pntd.0000909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nadjm B, Thuy PT, Trang VD, Ha le D, Kinh NV, et al. (2014) Scrub typhus in the northern provinces of Vietnam: an observational study of admissions to a national referral hospital. Trans R Soc Trop Med Hyg 108: 739–740. doi: 10.1093/trstmh/tru145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blacksell SD, Tanganuchitcharnchai A, Nawtaisong P, Kantipong P, Laongnualpanich A, et al. (2015) Diagnostic accuracy of the InBios scrub typhus detect enzyme-linked immunoassay for the detection of IgM antibodies in Northern Thailand. Clin Vaccine Immunol 23: 148–154. doi: 10.1128/CVI.00553-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borkakoty B, Jakharia A, Biswas D, Mahanta J (2016) Co-infection of scrub typhus and leptospirosis in patients with pyrexia of unknown origin in Longding district of Arunachal Pradesh in 2013. Indian J Med Microbiol 34: 88–91. doi: 10.4103/0255-0857.174116 [DOI] [PubMed] [Google Scholar]
- 108.Bourgeois AL, Olson JG, Ho CM, Fang RC, Van Peenen PF (1977) Epidemiological and serological study of scrub typhus among Chinese military in the Pescadores islands of Taiwan. Trans R Soc Trop Med Hyg 71: 338–342. [DOI] [PubMed] [Google Scholar]
- 109.Cao M, Che L, Zhang J, Hu J, Srinivas S, et al. (2016) Determination of scrub typhus suggests a new epidemic focus in the Anhui Province of China. Sci Rep 6: 20737 doi: 10.1038/srep20737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dass R, Deka NM, Duwarah SG, Barman H, Hoque R, et al. (2011) Characteristics of pediatric scrub typhus during an outbreak in the North Eastern region of India: peculiarities in clinical presentation, laboratory findings and complications. Indian J Pediatr 78: 1365–1370. doi: 10.1007/s12098-011-0470-5 [DOI] [PubMed] [Google Scholar]
- 111.Faa AG, McBride WJ, Garstone G, Thompson RE, Holt P (2003) Scrub typhus in the Torres Strait islands of north Queensland, Australia. Emerg Infect Dis 9: 480–482. doi: 10.3201/eid0904.020509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gale JL, Irving GS, Wang HC, Lien JC, Chen WF, et al. (1974) Scrub typhus in eastern Taiwan, 1970. Am J Trop Med Hyg 23: 679–684. [DOI] [PubMed] [Google Scholar]
- 113.Gurung S, Pradhan J, Bhutia PY (2013) Outbreak of scrub typhus in the North East Himalayan region-Sikkim: an emerging threat. Indian J Med Microbiol 31: 72–74. doi: 10.4103/0255-0857.108729 [DOI] [PubMed] [Google Scholar]
- 114.Harris PN, Oltvolgyi C, Islam A, Hussain-Yusuf H, Loewenthal MR, et al. (2016) An outbreak of scrub typhus in military personnel despite protocols for antibiotic prophylaxis: doxycycline resistance excluded by a quantitative PCR-based susceptibility assay. Microbes Infect 18: 406–411. doi: 10.1016/j.micinf.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 115.Hu J, Tan Z, Ren D, Zhang X, He Y, et al. (2015) Clinical characteristics and risk factors of an outbreak with scrub typhus in previously unrecognized areas, Jiangsu province, China 2013. PLoS One 10: e0125999 doi: 10.1371/journal.pone.0125999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang J, Marienau KJ, May LA, Beecham HJ 3rd, Wilkinson R, et al. (2003) Laboratory diagnosis of two scrub typhus outbreaks at Camp Fuji, Japan in 2000 and 2001 by enzyme-linked immunosorbent assay, rapid flow assay, and Western blot assay using outer membrane 56-kD recombinant proteins. Am J Trop Med Hyg 69: 60–66. [PubMed] [Google Scholar]
- 117.Krishna MR, Vasuki B, Nagaraju K (2015) Scrub typhus: audit of an outbreak. Indian J Pediatr 82: 537–540. doi: 10.1007/s12098-014-1664-4 [DOI] [PubMed] [Google Scholar]
- 118.Kumar K, Saxena VK, Thomas TG, Lal S (2004) Outbreak investigation of scrub typhus in Himachal Pradesh (India). J Commun Dis 36: 277–283. [PubMed] [Google Scholar]
- 119.Lewis MD, Yousuf AA, Lerdthusnee K, Razee A, Chandranoi K, et al. (2003) Scrub typhus reemergence in the Maldives. Emerg Infect Dis 9: 1638–1641. doi: 10.3201/eid0912.030212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marks M, Joshua C, Longbottom J, Longbottom K, Sio A, et al. (2016) An outbreak investigation of scrub typhus in Western Province, Solomon Islands, 2014. Western Pac Surveill Response J 7: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, et al. (2003) Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci 990: 359–364. [DOI] [PubMed] [Google Scholar]
- 122.McBride WJ, Taylor CT, Pryor JA, Simpson JD (1999) Scrub typhus in north Queensland. Med J Aust 170: 318–320. [DOI] [PubMed] [Google Scholar]
- 123.Rodkvamtook W, Gaywee J, Kanjanavanit S, Ruangareerate T, Richards AL, et al. (2013) Scrub typhus outbreak, northern Thailand, 2006–2007. Emerg Infect Dis 19: 774–777. doi: 10.3201/eid1905.121445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rodkvamtook W, Ruang-Areerate T, Gaywee J, Richards AL, Jeamwattanalert P, et al. (2011) Isolation and characterization of Orientia tsutsugamushi from rodents captured following a scrub typhus outbreak at a military training base, Bothong district, Chonburi province, central Thailand. Am J Trop Med Hyg 84: 599–607. doi: 10.4269/ajtmh.2011.09-0768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sethi S, Prasad A, Biswal M, Hallur VK, Mewara A, et al. (2014) Outbreak of scrub typhus in North India: a re-emerging epidemic. Trop Doct 44: 156–159. doi: 10.1177/0049475514523761 24557641 [Google Scholar]
- 126.Sharma R, Krishna VP, Manjunath, Singh H, Shrivastava S, et al. (2014) Analysis of two outbreaks of scrub typhus in Rajasthan: A clinico-epidemiological study. J Assoc Physicians India 62: 24–29. [PubMed] [Google Scholar]
- 127.Singh SI, Devi KP, Tilotama R, Ningombam S, Gopalkrishna Y, et al. (2010) An outbreak of scrub typhus in Bishnupur district of Manipur, India, 2007. Trop Doct 40: 169–170. doi: 10.1258/td.2010.090468 [DOI] [PubMed] [Google Scholar]
- 128.Spicer PE, Taufa T, Benjamin AL (2007) Scrub typhus (Orientia tsutsugamushi), spotted fever (Rickettsia australis) and dengue fever as possible causes of mysterious deaths in the Strickland Gorge area of Southern Highlands and West Sepik Provinces of Papua New Guinea. P N G Med J 50: 172–183. [PubMed] [Google Scholar]
- 129.Stephen S, Sangeetha B, Ambroise S, Sarangapani K, Gunasekaran D, et al. (2015) Outbreak of scrub typhus in Puducherry & Tamil Nadu during cooler months. Indian J Med Res 142: 591–597. doi: 10.4103/0971-5916.171289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Subbalaxmi MV, Madisetty MK, Prasad AK, Teja VD, Swaroopa K, et al. (2014) Outbreak of scrub typhus in Andhra Pradesh—experience at a tertiary care hospital. J Assoc Physicians India 62: 490–496. [PubMed] [Google Scholar]
- 131.Vaz LS, Gupta NK (2006) Outbreak of scrub typhus in Jammu—A report. Med J Armed Forces India 62: 342–343. doi: 10.1016/S0377-1237(06)80103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, et al. (2010) Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India 58: 24–28. [PubMed] [Google Scholar]
- 133.Wei Y, Luo L, Jing Q, Li X, Huang Y, et al. (2014) A city park as a potential epidemic site of scrub typhus: a case-control study of an outbreak in Guangzhou, China. Parasit Vectors 7: 513 doi: 10.1186/s13071-014-0513-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xiangrui C, Jinju W, Yongguo Z, Yanan W, Chunmu Y, et al. (1991) Recent studies on scrub typhus and Rickettsia tsutsugamushi in Shandong Province—China. Eur J Epidemiol 7: 304–306. [DOI] [PubMed] [Google Scholar]
- 135.Zhang L, Jin Z, Xia S, Zhang J, Li M, et al. (2007) Follow-up analysis on the epidemic strains of Orientia tsutsugamushi in the first outbreak of scrub typhus in Henan Province, China. Southeast Asian J Trop Med Public Health 38: 482–486. [PubMed] [Google Scholar]
- 136.Durand AM, Kuartei S, Togamae I, Sengebau M, Demma L, et al. (2004) Scrub typhus in the Republic of Palau, Micronesia. Emerg Infect Dis 10: 1838–1840. doi: 10.3201/eid1010.040288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tilak R, Kunwar R, Wankhade UB, Tilak VW (2011) Emergence of Schoengastiella ligula as the vector of scrub typhus outbreak in Darjeeling: has Leptotrombidium deliense been replaced? Indian J Public Health 55: 92–99. doi: 10.4103/0019-557X.85239 [DOI] [PubMed] [Google Scholar]
- 138.Audy JR (1947) Epidemiology of scrub typhus in Assam and Burma. Trans R Soc Trop Med Hyg 40: 359. [PubMed] [Google Scholar]
- 139.Balcells ME, Rabagliati R, Garcia P, Poggi H, Oddo D, et al. (2011) Endemic scrub typhus-like illness, Chile. Emerg Infect Dis 17: 1659–1663. doi: 10.3201/eid1709.100960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chheng K, Carter MJ, Emary K, Chanpheaktra N, Moore CE, et al. (2013) A prospective study of the causes of febrile illness requiring hospitalization in children in Cambodia. PLoS One 8: e60634 doi: 10.1371/journal.pone.0060634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corwin AL, Soeprapto W, Widodo PS, Rahardjo E, Kelly DJ, et al. (1997) Short report: surveillance of rickettsial infections in Indonesian military personnel during peace keeping operations in Cambodia. Am J Trop Med Hyg 57: 569–570. [DOI] [PubMed] [Google Scholar]
- 142.Errington AF, King AN, et al. (1946) Tsutsugamushi disease on Samar, Philippine Islands. U S Nav Med Bull 46: 1669–1673. [PubMed] [Google Scholar]
- 143.Ghorbani RP, Ghorbani AJ, Jain MK, Walker DH (1997) A case of scrub typhus probably acquired in Africa. Clin Infect Dis 25: 1473–1474. [DOI] [PubMed] [Google Scholar]
- 144.Groen J, Nur YA, Dolmans W, Ligthelm RJ, Osterhaus AD (1999) Scrub and murine typhus among Dutch travellers. Infection 27: 291–292. [PubMed] [Google Scholar]
- 145.Kulagin SM, Tarasevic IV, Kudryasova NI, Plotnikova LF (1968) The investigation of scrub typhus in the USSR. J Hyg Epidemiol Microbiol Immunol 12: 257–264. [PubMed] [Google Scholar]
- 146.Miles JA, Austin FJ, Jennings LC (1981) Scrub typhus in the Eastern Solomon Islands and Northern Vanuatu (New Hebrides). Am J Trop Med Hyg 30: 849–854. [DOI] [PubMed] [Google Scholar]
- 147.Osuga K, Kimura M, Goto H, Shimada K, Suto T (1991) A case of Tsutsugamushi disease probably contracted in Africa. Eur J Clin Microbiol Infect Dis 10: 95–96. [DOI] [PubMed] [Google Scholar]
- 148.Parola P, Miller RS, McDaniel P, Telford SR 3rd, Rolain JM, et al. (2003) Emerging rickettsioses of the Thai-Myanmar border. Emerg Infect Dis 9: 592–595. doi: 10.3201/eid0905.020511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Philip CB, Woodward TE, Sullivan RR (1946) Tsutsugamushi disease (scrub or mite-borne typhus) in the Philippine Islands during American re-occupation in 1944–45. Am J Trop Med Hyg 26: 229–242. [DOI] [PubMed] [Google Scholar]
- 150.Sayen JJ, Pond HS, et al. (1946) Scrub typhus in Assam and Burma; a clinical study of 616 cases. Medicine (Baltimore) 25: 155–214. [DOI] [PubMed] [Google Scholar]
- 151.Weitzel T, Dittrich S, Lopez J, Phuklia W, Martinez-Valdebenito C, et al. (2016) Endemic scrub typhus in South America. N Engl J Med 375: 954–961. doi: 10.1056/NEJMoa1603657 [DOI] [PubMed] [Google Scholar]
- 152.Woodward TE, Philip CB, Loranger GL (1946) Endemic typhus in Manila, Philippine Islands; report of cases and identification of the murine rickettsial agent in domestic rats by complement fixation. J Infect Dis 78: 167–172. [DOI] [PubMed] [Google Scholar]
- 153.Shirai A, Wisseman CL Jr. (1975) Serologic classification of scrub typhus isolates from Pakistan. Am J Trop Med Hyg 24: 145–153. [DOI] [PubMed] [Google Scholar]
- 154.Tarasevich IV, Kulagin SM, Plotnikova LF, Mirolyubova LV (1968) Studies on antigenic characteristics of Rickettsia tsutsugamushi isolated in the U.S.S.R. Acta Virol 12: 63–67. [PubMed] [Google Scholar]
- 155.Kalra S (1952) Natural history of typhus fevers in India. Indian Journal of Medical Sciences 6: 569–575. [Google Scholar]
- 156.Lee HW, Cho PY, Moon SU, Na BK, Kang YJ, et al. (2015) Current situation of scrub typhus in South Korea from 2001–2013. Parasit Vectors 8: 238 doi: 10.1186/s13071-015-0858-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suputtamongkol Y, Suttinont C, Niwatayakul K, Hoontrakul S, Limpaiboon R, et al. (2009) Epidemiology and clinical aspects of rickettsioses in Thailand. Ann N Y Acad Sci 1166: 172–179. doi: 10.1111/j.1749-6632.2009.04514.x [DOI] [PubMed] [Google Scholar]
- 158.Zhang WY, Wang LY, Ding F, Hu WB, Soares Magalhaes RJ, et al. (2013) Scrub typhus in mainland China, 2006–2012: the need for targeted public health interventions. PLoS Negl Trop Dis 7: e2493 doi: 10.1371/journal.pntd.0002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kudriashova NI (1972) [Trombiculid mites and foci of tsutsugamushi fever in Tadzhikistan]. Med Parazitol (Mosk) 41: 287–290. [PubMed] [Google Scholar]
- 160.Fan MY, Walker DH, Yu SR, Liu QH (1987) Epidemiology and ecology of rickettsial diseases in the People's Republic of China. Rev Infect Dis 9: 823–840. [PubMed] [Google Scholar]
- 161.Zhang S, Song H, Liu Y, Li Q, Wang Y, et al. (2010) Scrub typhus in previously unrecognized areas of endemicity in China. J Clin Microbiol 48: 1241–1244. doi: 10.1128/JCM.01784-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kawamura A Jr., Tanaka H (1988) Rickettsiosis in Japan. Jpn J Exp Med 58: 169–184. [PubMed] [Google Scholar]
- 163.Chang WH (1995) Current status of tsutsugamushi disease in Korea. J Korean Med Sci 10: 227–238. doi: 10.3346/jkms.1995.10.4.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim S, Kim JS, Lee H (2010) Epidemiological characteristics of scrub typhus in Korea, 2009. Osong Public Health Res Perspect 1: 55–60. doi: 10.1016/j.phrp.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park SW, Ha NY, Ryu B, Bang JH, Song H, et al. (2015) Urbanization of scrub typhus disease in South Korea. PLoS Negl Trop Dis 9: e0003814 doi: 10.1371/journal.pntd.0003814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tattersall R (1945) Tsutsugamushi fever on the India-Burma border. The Lancet 246: 392–394. [Google Scholar]
- 167.Isaac R, Varghese GM, Mathai E, J M, Joseph I (2004) Scrub typhus: prevalence and diagnostic issues in rural Southern India. Clin Infect Dis 39: 1395–1396. doi: 10.1086/424748 [DOI] [PubMed] [Google Scholar]
- 168.Chrispal A, Boorugu H, Gopinath KG, Prakash JA, Chandy S, et al. (2010) Scrub typhus: an unrecognized threat in South India—clinical profile and predictors of mortality. Trop Doct 40: 129–133. doi: 10.1258/td.2010.090452 [DOI] [PubMed] [Google Scholar]
- 169.Khan SA, Dutta P, Khan AM, Topno R, Borah J, et al. (2012) Re-emergence of scrub typhus in northeast India. Int J Infect Dis 16: e889–890. doi: 10.1016/j.ijid.2012.05.1030 [DOI] [PubMed] [Google Scholar]
- 170.Puranavej S, Winter P, Sangkasuwan V, Pongpradit P (1968) SEATO medical research study on rickettsial diseases in Thailand. Annual research progress report Bangkok: US Army-SEATO Medical Research Unit: 444–448.
- 171.Trishnananda M, Vasuvat C, Harinasuta C (1964) Investigation of scrub typhus in Thailand. J Trop Med Hyg 67: 215–219. [PubMed] [Google Scholar]
- 172.Traub R, Johnson PT, Miesse ML, Elbel RE (1954) Isolation of Rickettsia tsutsugamushi from rodents from Thailand. Am J Trop Med Hyg 3: 356–359. [DOI] [PubMed] [Google Scholar]
- 173.Wongprompitak P, Anukool W, Wongsawat E, Silpasakorn S, Duong V, et al. (2013) Broad-coverage molecular epidemiology of Orientia tsutsugamushi in Thailand. Infect Genet Evol 15: 53–58. doi: 10.1016/j.meegid.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 174.Manosroi J, Chutipongvivate S, Auwanit W, Manosroi A (2006) Determination and geographic distribution of Orientia tsutsugamushi serotypes in Thailand by nested polymerase chain reaction. Diagn Microbiol Infect Dis 55: 185–190. doi: 10.1016/j.diagmicrobio.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 175.Deller JJ Jr., Russell PK(1967) An analysis of fevers of unknown origin in American soldiers in Vietnam. Ann Intern Med 66: 1129–1143. [DOI] [PubMed] [Google Scholar]
- 176.Colwell EJ, Brown JD, Russell PK, Boone SC, Legters LJ (1969) Catino D: Investigations on acute febrile illness in American servicemen in the Mekong Delta of Vietnam. Mil Med 134: 1409–1414. [PubMed] [Google Scholar]
- 177.Hazlett DR (1970) Scrub typhus in Vietnam: experience at the 8th Field Hospital. Mil Med 135: 31–34. [PubMed] [Google Scholar]
- 178.Berman SJ, Irving GS, Kundin WD, Gunning JJ, Watten RH (1973) Epidemiology of the acute fevers of unknown origin in South Vietnam: effect of laboratory support upon clinical diagnosis. Am J Trop Med Hyg 22: 796–801. [DOI] [PubMed] [Google Scholar]
- 179.Thiebaut MM, Bricaire F, Raoult D (1997) Scrub typhus after a trip to Vietnam. N Engl J Med 336: 1613–1614. [DOI] [PubMed] [Google Scholar]
- 180.Viet NL, Laroche M, Pham HLT, Viet NL, Mediannikov O, et al. (2017) Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. Plos Neglected Tropical Diseases 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Currie B, O'Connor L, Dwyer B (1993) A new focus of scrub typhus in tropical Australia. Am J Trop Med Hyg 49: 425–429. [DOI] [PubMed] [Google Scholar]
- 182.Graves S, Unsworth N, Stenos J (2006) Rickettsioses in Australia. Ann N Y Acad Sci 1078: 74–79. doi: 10.1196/annals.1374.008 [DOI] [PubMed] [Google Scholar]
- 183.Noad KB (1946) Tsutsugamushi fever in natives. Med J Aust 2: 20 [DOI] [PubMed] [Google Scholar]
- 184.Corwin A, Soderquist R, Suwanabun N, Sattabongkot J, Martin L, et al. (1999) Scrub typhus and military operations in Indochina. Clin Infect Dis 29: 940–941. doi: 10.1086/520468 [DOI] [PubMed] [Google Scholar]
- 185.Odorico DM, Graves SR, Currie B, Catmull J, Nack Z, et al. (1998) New Orientia tsutsugamushi strain from scrub typhus in Australia. Emerg Infect Dis 4: 641–644. doi: 10.3201/eid0404.980416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Berge TO, Gauld RL, Kitaoka M (1949) A field trial of a vaccine prepared from the Volner strain of Rickettsia tsutsugamushi. Am J Hyg 50: 337–342. [DOI] [PubMed] [Google Scholar]
- 187.Tay ST, Nazma S, Rohani MY (1996) Diagnosis of scrub typhus in Malaysian aborigines using nested polymerase chain reaction. Southeast Asian J Trop Med Public Health 27: 580–583. [PubMed] [Google Scholar]
- 188.Cadigan FC Jr., Andre RG, Bolton M, Gan E, Walker JS (1972) The effect of habitat on the prevalence of human scrub typhus in Malaysia. Trans R Soc Trop Med Hyg 66: 582–587. [DOI] [PubMed] [Google Scholar]
- 189.Robinson DM, Gan E, Donaldson JR (1976) The prevalence of scrub typhus antibodies in residents of West Malaysia. Trop Geogr Med 28: 303–308. [PubMed] [Google Scholar]
- 190.Irons EM, Armstrong HE (1947) Scrub typhus in Dutch New Guinea. Ann Intern Med 26: 201–220. [DOI] [PubMed] [Google Scholar]
- 191.Bekker BV, Dinger JE, Wolff HL (1964) Scrub typhus, an epidemiological study. Ann Soc Belges Med Trop Parasitol Mycol 44: 207–216. [PubMed] [Google Scholar]
- 192.Bekker BV, Dinger JE, Wolff HL (1968) Scrub typhus, an epidemiological study. Acta Leiden 36: 1–8. [PubMed] [Google Scholar]
- 193.Traub R, Wisseman CL Jr., Ahmad N (1967) The occurrence of scrub typhus infection in unusual habitats in West Pakistan. Trans R Soc Trop Med Hyg 61: 23–57. [DOI] [PubMed] [Google Scholar]
- 194.Wisseman CL Jr., Traub R, Ahmad N (1967) Scrub typhus in West Pakistan. Acta Med Biol (Niigata) 15: 37–39. [PubMed] [Google Scholar]
- 195.Nelyubov MV (2002) Astrakhan scrub typhus: time course of infectious process according to electron microscopy findings. Bull Exp Biol Med 134: 374–375. [DOI] [PubMed] [Google Scholar]
- 196.Urakami H, Tamura A, Tarasevich IV, Kadosaka T, Shubin FN (1999) Decreased prevalence of Orientia tsutsugamushi in trombiculid mites and wild rodents in the Primorye region, Far East Russia. Microbiol Immunol 43: 975–978. [DOI] [PubMed] [Google Scholar]
- 197.Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, et al. (2007) Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis 44: 391–401. doi: 10.1086/510585 [DOI] [PubMed] [Google Scholar]
- 198.Li W, Dou X, Zhang L, Lyu Y, Du Z, et al. (2013) Laboratory diagnosis and genotype identification of scrub typhus from Pinggu District, Beijing, 2008 and 2010. Am J Trop Med Hyg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Koraluru M, Bairy I, Varma M, Vidyasagar S (2015) Diagnostic validation of selected serological tests for detecting scrub typhus. Microbiol Immunol 59: 371–374. doi: 10.1111/1348-0421.12268 [DOI] [PubMed] [Google Scholar]
- 200.Tay ST, Kamalanathan M, Rohani MY (2003) Antibody prevalence of Orientia tsutsugamushi, Rickettsia typhi and TT118 spotted fever group rickettsiae among Malaysian blood donors and febrile patients in the urban areas. The Southeast Asian journal of tropical medicine and public health 34: 165–170. [PubMed] [Google Scholar]
- 201.Wilkinson R, Rowland D, Ching WM (2003) Development of an improved rapid lateral flow assay for the detection of Orientia tsutsugamushi-specific IgG/IgM antibodies. Ann N Y Acad Sci 990: 386–390. [DOI] [PubMed] [Google Scholar]
- 202.Kelly DJ, Wong PW, Gan E, Lewis GE Jr. (1988) Comparative evaluation of the indirect immunoperoxidase test for the serodiagnosis of rickettsial disease. Am J Trop Med Hyg 38: 400–406. [DOI] [PubMed] [Google Scholar]
- 203.Lim C, Paris DH, Blacksell SD, Laongnualpanich A, Kantipong P, et al. (2015) How to determine the accuracy of an alternative diagnostic test when it is actually better than the reference tests: a re-evaluation of diagnostic tests for scrub typhus using Bayesian LCMs. PLoS One 10: e0114930 doi: 10.1371/journal.pone.0114930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee K-D, Moon C, Oh WS, Sohn KM, Kim B-N (2014) Diagnosis of scrub typhus: introduction of the immunochromatographic test in Korea. The Korean Journal of Internal Medicine 29: 253 doi: 10.3904/kjim.2014.29.2.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD (2010) Diagnosis of scrub typhus. Am J Trop Med Hyg 82: 368–370. doi: 10.4269/ajtmh.2010.09-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.La Scola B, Raoult D (1997) Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol 35: 2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yamamoto S, Minamishima Y (1982) Serodiagnosis of tsutsugamushi fever (scrub typhus) by the indirect immunoperoxidase technique. J Clin Microbiol 15: 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pradutkanchana J, Silpapojakul K, Paxton H, Pradutkanchana S, Kelly DJ, et al. (1997) Comparative evaluation of four serodiagnostic tests for scrub typhus in Thailand. Trans R Soc Trop Med Hyg 91: 425–428. [DOI] [PubMed] [Google Scholar]
- 209.Coleman RE, Sangkasuwan V, Suwanabun N, Eamsila C, Mungviriya S, et al. (2002) Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg 67: 497–503. [DOI] [PubMed] [Google Scholar]
- 210.Kingston HW, Blacksell SD, Tanganuchitcharnchai A, Laongnualpanich A, Basnyat B, et al. (2015) Comparative accuracy of the InBios Scrub Typhus Detect IgM Rapid Test for the detection of IgM antibodies by using conventional serology. Clin Vaccine Immunol 22: 1130–1132. doi: 10.1128/CVI.00390-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Paris DH, Blacksell SD, Newton PN, Day NP (2008) Simple, rapid and sensitive detection of Orientia tsutsugamushi by loop-isothermal DNA amplification. Trans R Soc Trop Med Hyg 102: 1239–1246. doi: 10.1016/j.trstmh.2008.04.040 [DOI] [PubMed] [Google Scholar]
- 212.Saisongkorh W, Chenchittikul M, Silpapojakul K (2004) Evaluation of nested PCR for the diagnosis of scrub typhus among patients with acute pyrexia of unknown origin. Transactions of the Royal Society of Tropical Medicine and Hygiene 98: 360–366. doi: 10.1016/j.trstmh.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 213.Chung MH, Lee JS, Baek JH, Kim M, Kang JS (2012) Persistence of Orientia tsutsugamushi in humans. J Korean Med Sci 27: 231–235. doi: 10.3346/jkms.2012.27.3.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Smadel JE, Ley HL Jr., Diercks RH, Cameron JA (1952) Persistence of Rickettsia tsutsugamushi in tissues of patients recovered from scrub typhus. Am J Hyg 56: 294–302. [DOI] [PubMed] [Google Scholar]
- 215.Lee SC, Cheng YJ, Lin CH, Lei WT, Chang HY, et al. (2017) Comparative effectiveness of azithromycin for treating scrub typhus: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 96: e7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chanta C, Phloenchaiwanit P (2015) Randomized controlled trial of azithromycin versus doxycycline or chloramphenicol for treatment of uncomplicated pediatric scrub typhus. J Med Assoc Thai 98: 756–760. [PubMed] [Google Scholar]
- 217.Panpanich R, Garner P (2002) Antibiotics for treating scrub typhus. Cochrane Database Syst Rev: CD002150 doi: 10.1002/14651858.CD002150 [DOI] [PubMed] [Google Scholar]
- 218.Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, et al. (2000) Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet 356: 1057–1061. doi: 10.1016/S0140-6736(00)02728-8 [DOI] [PubMed] [Google Scholar]
- 219.Lee KY, Lee HS, Hong JH, Hur JK, Whang KT (2003) Roxithromycin treatment of scrub typhus (tsutsugamushi disease) in children. Pediatr Infect Dis J 22: 130–133. doi: 10.1097/01.inf.0000047864.80791.20 [DOI] [PubMed] [Google Scholar]
- 220.Rajapakse S, Rodrigo C, Fernando SD (2011) Drug treatment of scrub typhus. Trop Doct 41: 1–4. doi: 10.1258/td.2010.100311 [DOI] [PubMed] [Google Scholar]
- 221.Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, et al. (1996) Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 348: 86–89. [DOI] [PubMed] [Google Scholar]
- 222.Strickman D, Sheer T, Salata K, Hershey J, Dasch G, et al. (1995) In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob Agents Chemother 39: 2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chogle A (2010) Diagnosis and treatment of scrub typhus–the Indian scenario. J Assoc Physicians India 58: 11 [PubMed] [Google Scholar]
- 224.CDC (2015) Rickettsial (spotted & typhus fevers) & related infections (anaplasmosis & ehrlichiosis) Yellow book: Chapter 3 Infectious Diseases Related to Travel: Centers for Disease Control and Prevention. [Google Scholar]
- 225.Sharma R (2010) Scrub typhus: prevention and control. JK Science 12. [Google Scholar]
- 226.WHO (2016) Frequently asked questions: Scrub typhus World Health Organization. [Google Scholar]
- 227.Olson JG, Bourgeois AL, Fang RC, Coolbaugh JC, Dennis DT (1980) Prevention of scrub typhus. Prophylactic administration of doxycycline in a randomized double blind trial. Am J Trop Med Hyg 29: 989–997. [PubMed] [Google Scholar]
- 228.Twartz JC, Shirai A, Selvaraju G, Saunders JP, Huxsoll DL, et al. (1982) Doxycycline propylaxis for human scrub typhus. J Infect Dis 146: 811–818. [DOI] [PubMed] [Google Scholar]
- 229.CDC (2010) RMSF: Symptoms, diagnosis, and treatment Rocky Mountain spotted fever (RMSF): CDC. [Google Scholar]
- 230.Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, et al. (2006) Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 55: 1–27. [PubMed] [Google Scholar]
- 231.CDC (2015) Protection against mosquitoes, ticks, & other arthropods Yellow Book: Chapter 2 The Pre-Travel Consultation: Centers for Disease Control and Prevention. [Google Scholar]
- 232.Brezina R (1985) Diagnosis and control of rickettsial diseases. Acta Virol 29: 338–349. [PubMed] [Google Scholar]
- 233.Kazar J, Brezina R (1991) Control of rickettsial diseases. Eur J Epidemiol 7: 282–286. [DOI] [PubMed] [Google Scholar]
- 234.WHO (1974) Ecology and control of rodents of public health importance. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser: 1–42. [PubMed]
- 235.Brown LM, Laco J (2015) Rodent Control and Public Health: A Description of Local Rodent Control Programs. J Environ Health 78: 28–29. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.