Abstract
The prevalence of carbapenemase-producing Enterobacteriaceae (CPE) is increasing worldwide. Here we present associated patient data and molecular, epidemiological and phenotypic characteristics of all CPE isolates in Norway from 2007 to 2014 confirmed at the Norwegian National Advisory Unit on Detection of Antimicrobial Resistance. All confirmed CPE isolates were characterized pheno- and genotypically, including by whole genome sequencing (WGS). Patient data were reviewed retrospectively. In total 59 CPE isolates were identified from 53 patients. Urine was the dominant clinical sample source (37%) and only 15% of the isolates were obtained from faecal screening. The majority of cases (62%) were directly associated with travel or hospitalization abroad, but both intra-hospital transmission and one inter-hospital outbreak were observed. The number of CPE cases/year was low (2–14 cases/year), but an increasing trend was observed. Klebsiella spp. (n = 38) and E. coli (n = 14) were the dominant species and blaKPC (n = 20), blaNDM (n = 19), blaOXA-48-like (n = 12) and blaVIM (n = 7) were the dominant carbapenemase gene families. The CPE isolates were genetically diverse except for K. pneumoniae where clonal group 258 associated with blaKPC dominated. All isolates were multidrug-resistant and a significant proportion (21%) were resistant to colistin. Interestingly, all blaOXA-48-like, and a large proportion of blaNDM-positive Klebsiella spp. (89%) and E. coli (83%) isolates were susceptible in vitro to mecillinam. Thus, mecillinam could have a role in the treatment of uncomplicated urinary tract infections caused by OXA-48- or NDM-producing E. coli or K. pneumoniae. In conclusion, the impact of CPE in Norway is still limited and mainly associated with travel abroad, reflected in the diversity of clones and carbapenemase genes.
Introduction
Carbapenemase-producing Enterobacteriaceae (CPE) have emerged as a global public health concern during the last two decades [1, 2]. CPE isolates are usually multidrug-resistant (MDR) or even extensively- or pandrug-resistant (XDR/PDR), resulting in limited antibiotic treatment options [1, 3, 4]. Due to the lack of effective therapy, CPE infections have been associated with high mortality rates [5, 6]. Currently, colistin and various combination regimens are generally used for treatment of CPE infections. However, the clinical evidence is mainly based on case reports and observational retrospective studies [1, 4]. Worryingly, high rates of colistin resistance among CPE have been observed in certain regions [7, 8]. Although colistin resistance is often mutation-based, plasmid-mediated colistin resistance has now also been described [9–14], and observed in CPE isolates [11, 15–17].
The main carbapenemases among Enterobacteriaceae include KPC (Ambler class A), the metallo-β-lactamases NDM, VIM and IMP (Ambler class B), and OXA-48-like enzymes (Ambler class D) [1]. Certain carbapenemases dominate in specific regions and countries, i.e. NDM in the Indian subcontinent, KPC in Italy, Portugal, Israel, Greece and the US, and OXA-48-like in many Mediterranean (e.g. Turkey and Malta) and North African countries as well as some other European countries (e.g. Belgium, France, Germany and Spain) [7, 18–20]. Specific clones or clonal groups (CG) are often associated with specific carbapenemases, while other carbapenemases show a more broad diversity with respect to host genetic backgrounds [2, 21]. The global spread of KPC has mainly been associated with Klebsiella pneumoniae sequence type (ST) 258 or CG 258 [2, 21, 22]. In contrast, NDM and OXA-48-like enzymes are broadly distributed in various genetic backgrounds of K. pneumoniae and Escherichia coli and for blaNDM there is no clear link to a specific plasmid backbone [2, 21]. For blaOXA-48-like there is molecular evidence supporting an association with a specific internationally epidemic IncL plasmid backbone [23–25].
The emergence of CPE in the Nordic countries has mainly been associated with single sporadic cases associated with import [26–36], and the prevalence is low compared with other European countries [7, 19]. However, there are indications of local dissemination unrelated to travel in Denmark [37, 38].
The aim of this study was to analyse the epidemiological, phenotypic and molecular characteristics of CPE isolated in Norway from 2007 to 2014 to understand the molecular epidemiology associated with the emergence of CPE in Norway.
Materials and methods
Bacterial strains and demographic data
The study collection consisted of 59 CPE isolates genetically-verified at the Norwegian National Advisory Unit on Detection of Antimicrobial Resistance from 2007–2014. The criteria for submitting isolates to the Unit included reduced susceptibility to carbapenems according to the Norwegian Working Group for Antibiotics (AFA, https://unn.no/fag-og-forskning/arbeidsgruppen-for-antibiotikasporsmal-og-metoder-for-resistensbestemmelse-afa)/Nordic Committee on Antimicrobial Susceptibility Testing (NordicAST) guidelines (www.nordicast.org). In 2012 mandatory reporting of confirmed CPE cases to the Norwegian Surveillance System for Communicable Diseases (MSIS) was established. After confirmation at the Advisory Unit, MSIS and the primary lab are notified. The primary laboratory subsequently notifies the responsible clinician, who also reports data to MSIS. Clinical data were collected from the laboratory requisition. Multiple isolates from the same patient were included in the analysis if they were (i) of different species, (ii) the same species, but harboured a different carbapenemase gene or (iii) if the isolates were of the same species and harboured the same carbapenemase gene, but were identified >1 year apart.
Phenotypic analysis
Species identification was performed using MALDI-TOF MS (Bruker Daltonik GmbH, Bremen, Germany). MIC profiling was performed using gradient strips (Liofilchem, Roseto degli Abruzzi, Italy/bioMérieux, Marcy-l’Étolie, France) and broth microdilution for colistin using in-house designed premade Sensititre microtiter plates (TREK Diagnostic Systems/Thermo Fisher Scientific, East Grinstead, UK). Interpretation was according to EUCAST clinical breakpoints version 6.0 (www.eucast.org). Non-susceptibility included both the intermediate and resistant categories. The AmpC Confirm kit (ROSCO Diagnostica, Taastrup, Denmark), ESBL combination discs (Becton-Dickinson, Franklin Lakes, NJ, USA), KPC, MBL and OXA-48 Confirm kit (ROSCO Diagnostica) and the in-house version of Carba NP test were used for phenotypic typing of β-lactamases [39, 40].
Molecular analysis
The presence of carbapenemase genes was initially determined by various PCRs for blaKPC, blaIMI, blaVIM, blaNDM, blaIMP, blaGIM, blaSPM, blaSIM and blaOXA-48-like [41–44]. WGS was performed on all isolates using the MiSeq platform (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, genomic DNA was purified using the GenElute bacterial genomic DNA kit (Sigma-Aldrich, St. Louis, MO, USA). DNA libraries were prepared using Nextera/Nextera XT kits (Illumina) followed by paired-end sequencing. Contigs were assembled using SPAdes [45] through the iMetAMOS extension [46] of the MetAMOS package [47]. The presence of resistance genes/mutations, carbapenemase genes and single nucleotide polymorphisms (SNP) variations were determined using a customised algorithm that uses Bowtie 2 to map reads against a locally curated reference database and assembled from publically accessible databases. The database comprised sequences for all reported carbapenemase variants. Samtools was used to generate an mpileup file [48] which was then parsed based on read depth (> 10 reads per base) and base-call agreement (> 90%) to determine the base type at each nucleotide position relative to the closest reference sequence. Presence of reported carbapenemase variants were defined based on 100% identity across the whole length of the corresponding reference gene.
STs of Klebsiella spp., E. coli and Enterobacter cloacae complex were determined from WGS data using the Klebsiella MLST database (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html), EnteroBase (http://enterobase.warwick.ac.uk/species/index/ecoli) for E. coli, and the E. cloacae MLST database (http://pubmlst.org/ecloacae). Core genome MLST (cgMLST) was performed on K. pneumoniae isolates using 694 loci as previously described [22]. A phylogenetic tree was constructed based on the concatenated sequence alignments using RAxML [49] and FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Genbank accession numbers
WGS data have been deposited at the National Center for Biotechnology Information (NCBI) under BioProject PRJNA295003.
Ethical considerations
The study was reviewed and approved by the Regional Committee for Medical and Health Research Ethics North (reference no. 2016/2122/REK Nord and 2017/146/REK Nord) and the Data Protection Officer at the University Hospital of North Norway (reference no. 2017/1562). The need for patient consent was waived by the Regional Committee for Medical and Health Research Ethics North (reference no. 2017/146/REK nord)
Results
Bacterial isolates
In total 59 CPE were identified from 53 patients of which 44 were hospitalized patients. Samples from eight patients were taken at general practitioners or in other health care institutions (e.g. elderly care homes). For one patient no information was obtained. Of the 53 patients, four had multiple CPE isolates belonging to different species or different STs. One patient had four blaNDM-1-positive strains of different species (Proteus mirabilis, Providencia stuartii, Citrobacter sp. and K. pneumoniae) isolated within a four-month period. Another had blaKPC-2-positive K. pneumoniae and Enterobacter cloacae complex isolates in the same faecal screening sample. A third had blaNDM-1-positive E. coli and E. cloacae complex isolates identified in two different specimens (wound secretion and urine, respectively) within a one-month period. The fourth patient yielded two blaNDM-1-positive K. pneumoniae strains with unrelated STs from specimens taken 21 months apart.
Increasing number of CPE identified during the study period from a high proportion of clinical isolates
CPE isolates were identified in 14 of 22 clinical microbiology laboratories representing all health regions in Norway. The number of CPE cases per year, diversity of carbapenemase variants and species increased during the study period (Table 1), but with a trend towards dominance of NDM and OXA-48-like carbapenemase variants and increasing number of carbapenemase-producing E. coli. Fifty-six percent of the patients were male. The patient age ranged from 3–96 years (mean 63 and median 66 years). The majority of CPE were isolated from urine (n = 22, 37%), blood culture (n = 9, 15%) and faecal screening (n = 9, 15%).
Table 1. Time-line and distribution of identified CPEs and carbapenemase variants.
No. of isolates in parenthesis.
| Year | No. of isolates | No. of casesa | Klebsiella sp. | E. coli | Other Enterobacteriaceae |
|---|---|---|---|---|---|
| 2007 | 3 | 3 | KPC-2 (1), VIM-1 (2) | ||
| 2008 | 6 | 6 | KPC-2 (6) | ||
| 2009 | 2 | 2 | KPC-2 (2) | ||
| 2010 | 8 | 7 | KPC-2 (2), KPC-3 (1), VIM-27 (2), NDM-1 (1) | NDM-1 (1) | KPC-2 (1) |
| 2011 | 4 | 4 | KPC-2 (2), NDM-1+OXA-181 (1), OXA-48 (1) | ||
| 2012 | 16 | 14 | KPC-2 (1), VIM-1 (1), NDM-1 (2), NDM-7 (1), OXA-245 (1) | VIM-29 (1), NDM-1 (1), NDM-5 (1), NDM-7 (1), OXA-48 (2) | NDM-1 (3), IMI-9 (1) |
| 2013 | 8 | 7 | KPC-3 (1), NDM-1 (2), OXA-48 (1), OXA-245 (1) | NDM-1 (1),OXA-48 (2) | |
| 2014 | 12 | 10 | KPC-2 (2), NDM-1 (2), OXA-48 (1), OXA-162 (1) | VIM-4 (1), NDM-1 (1), IMP-26 (1), OXA-181 (1) | KPC-2 (1), NDM-1 (1) |
| Total 2007–2014 | 59 | 53 | KPC-2 (16), KPC-3 (2), VIM-1 (3), VIM-27 (2), NDM-1 (7), NDM-7 (1), NDM-1+OXA-181 (1), OXA-48 (3), OXA-162 (1), OXA-245 (2) | VIM-4 (1), VIM-29 (1), NDM-1 (4), NDM-4 (1), NDM-7 (1), IMP-26 (1), OXA-48 (4), OXA-181 (1) | KPC-2 (2), IMI-9 (1), NDM-1 (4) |
a Patients identified with multiple CPE defined as a single case.
Association with travel or hospitalization abroad
Thirty-three patients (62%) had a known history of travel and/or hospitalization abroad (Table 2). Sixteen patients (30%) reported no travel or hospitalization abroad and for four patients (8%), no information was obtained. With respect to the non-direct import cases, eight cases were associated with secondary spread from imported cases. This included six cases associated with a previously described, small but long-term outbreak of blaKPC-2-positive K. pneumoniae/E. cloacae complex in 2007–2010 [50]. In addition, two other intra-hospital transmissions of blaKPC-2-positive K. pneumoniae [28] and blaVIM-27-positive K. pneumoniae were observed involving one additional patient in each case.
Table 2. Distribution of isolates according to association with importation.
| Country | No. of isolates | Species | Sequence type (ST) | Carbapenemase |
|---|---|---|---|---|
| Greece | 7 | K. pneumoniae | ST258 | KPC-2 |
| 1 | K. pneumoniae | ST147 | VIM-27 | |
| India | 1 | K. pneumoniae | ST11 | NDM-1 |
| 1 | K. pneumoniae | ST17 | NDM-1 | |
| 1a | K. pneumoniae | ST147 | NDM-1 | |
| 1 | E. coli | ST101 | NDM-7 | |
| 1 | E. coli | ST131 | NDM-1 | |
| 1 | E. coli | ST410 | NDM-1 | |
| Turkey | 1 | K. pneumoniae | ST273 | VIM-1 |
| 1 | K. variicola | ST981 | OXA-48 | |
| 1 | E. coli | ST38 | OXA-48 | |
| Serbiab | 1 | K. pneumoniae | ST17 | NDM-1 |
| 1 | P. stuartii | - | NDM-1 | |
| 1 | P. mirabilis | - | NDM-1 | |
| 1 | Citrobacter sp. | - | NDM-1 | |
| Spain | 1 | K. pneumoniae | ST11 | OXA-245 |
| 1 | K. quasipneumoniae | ST1466 | VIM-1 | |
| 1 | E. cloacae complex | ST635 | IMI-9 | |
| Morocco | 1 | K. pneumoniae | ST405 | OXA-48 |
| 1 | K. pneumoniae | ST11 | OXA-245 | |
| Thailand | 1 | E. coli | ST405 | OXA-48 |
| 1 | E. coli | ST6355 | VIM-29 | |
| Brazil | 1 | K. pneumoniae | ST855 | KPC-2 |
| United Arab Emirates | 1 | K. pneumoniae | ST336 | NDM-7 |
| Syria/Jordan | 1 | E. coli | ST410 | VIM-4 |
| Jamaica | 1 | E. cloacae complex | ST456 | KPC-2 |
| Pakistan | 1 | E. coli | ST617 | NDM-1 |
| Romania | 1 | K. pneumoniae | ST525 | NDM-1+OXA-181 |
| Sri Lanka | 1 | K. pneumoniae | ST101 | NDM-1 |
| USA | 1 | K. pneumoniae | ST258 | KPC-3 |
| Unknown | 1 | K. pneumoniae | ST187 | OXA-48 |
| 2 | E. coli | ST38 | OXA-48 | |
| 1 | E. coli | ST95 | IMP-26 | |
| Norway (no reported overseas travel) | 9c, d | K. pneumoniae | ST258 | KPC-2 |
| 1 | K. pneumoniae | ST14 | OXA-162 | |
| 1a | K. pneumoniae | ST37 | NDM-1 | |
| 1e | K. pneumoniae | ST147 | VIM-27 | |
| 1c | K. pneumoniae | ST461 | KPC-2 | |
| 1 | K. pneumoniae | ST2134 | VIM-1 | |
| 1 | E. coli | ST410 | OXA-181 | |
| 1 | E. coli | ST636 | NDM-5 | |
| 1f | E. coli | ST681 | NDM-1 | |
| 1f | E. cloacae complex | ST92 | NDM-1 | |
| 1c | E. cloacae complex | ST484 | KPC-2 |
a Two blaNDM-1-positive K. pneumoniae isolates, one ST147 and one ST37, were isolated from the same patient. The isolates were identified 21 months apart where the first detection was associated with importation, but not for the second detection.
b All four blaNDM-1-positive isolates were isolated from the same patient.
c Six K. pneumoniae ST258, one K. pneumoniae ST461 and one E. cloacae complex ST484, all blaKPC-2-positive, were associated with a long-term outbreak [50]. The first case (K. pneumoniae ST258 with blaKPC-2) of the outbreak were associated with import from Greece.
d One blaKPC-2-positive K. pneumoniae ST258 associated with intra-hospital transmission (first case associated with import from Greece)[28].
e The blaVIM-27-positive isolate were associated with a case of intra-hospital transmission (first case associated with import from Greece).
f Both isolates identified from the same patient.
Bacterial species and carbapenemase diversity
Overall Klebsiella spp. (K. pneumoniae, n = 36; Klebsiella variicola n = 1; Klebsiella quasipneumoniae n = 1) were dominant, followed by E. coli (n = 14), E. cloacae complex (n = 4) and single isolates of P. stuartii, P. mirabilis and Citrobacter sp. (Table 1 and S1 Table). The most dominant carbapenemase gene family was blaKPC, found in K. pneumoniae (n = 18) and E. cloacae complex (n = 2), followed by blaNDM identified in K. pneumoniae (n = 8), E. coli (n = 6), E. cloacae complex (n = 1), P. stuartii (n = 1), P. mirabilis (n = 1) and Citrobacter sp. (n = 1). blaVIM was identified in K. pneumoniae (n = 4), E. coli (n = 2) and K. quasipneumoniae (n = 1) while blaOXA-48-like was identified in K. pneumoniae (n = 5), E. coli (n = 5) and K. variicola (n = 1). In addition, we identified one K. pneumoniae isolate harbouring both blaNDM and blaOXA-48-like and single isolates with blaIMI (E. cloacae complex) and blaIMP (E. coli). With respect to KPC, KPC-2 (n = 18) was the most predominant allele with the closest KPC-3 (n = 2) variant detected in only two isolates. The remaining carbapenemase genes encoded three different variants of NDM (NDM-1, n = 16; NDM-7, n = 2; and NDM-5, n = 1), four OXA-48-like (OXA-48, n = 7; OXA-181, n = 2; OXA-245, n = 2 and OXA-162, n = 1) and four VIM (VIM-1, n = 3; VIM-27, n = 2; VIM-4, n = 1; and VIM-29, n = 1). The single isolates with blaIMI and blaIMP encoded IMI-9 and IMP-26, respectively.
Bacterial population structure and linkage to specific carbapenemase alleles
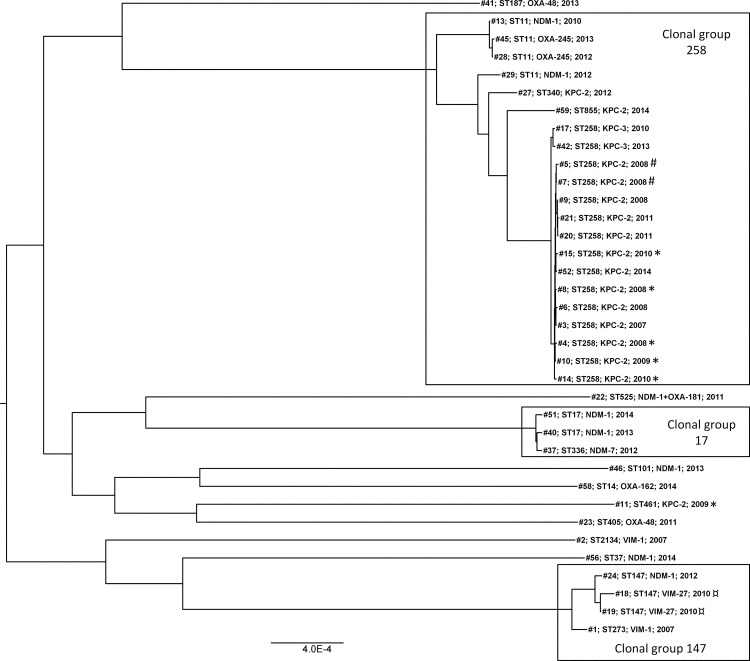
MLST and cgMLST (Fig 1) showed that K. pneumoniae was dominated by KPC-producing clonal group (CG) 258, more specifically ST258 (n = 15) and its single locus variants (SLV) ST855 (n = 1) and ST340 (n = 1). The CG258 cluster comprised 21 isolates and included nearly all KPC-producers (n = 17) in addition to four ST11 isolates carrying blaNDM-1 (n = 2) or blaOXA-245 (n = 2) genes. Outside CG258, blaKPC was only identified in one isolate belonging to ST461. Among the K. pneumoniae isolates cgMLST identified two other clusters represented by more than one isolate: one representing CG147 and including ST147 with blaVIM-27 (n = 2) or blaNDM-1 (n = 1) and ST273 with blaVIM-1 (n = 1), and one representing CG17 including ST17 with blaNDM-1 (n = 2) and ST336 with blaNDM-7 (n = 1). The remaining K. pneumoniae isolates represented genetically diverse single strains harbouring blaNDM-1 (ST37 and ST101), blaNDM-1 + blaOXA-181 (ST525), blaOXA-48 (ST187 and ST405), blaOXA-162 (ST14) and blaVIM-1 (ST2134). The K. quasipneumoniae isolate carrying blaVIM-1 belonged to ST1466 and the K. variicola with blaOXA-48 belonged to ST981.
Fig 1. Phylogenetic tree of K. pneumoniae isolates based on alignment of concatenated sequences of the 694 cgMLST scheme of K. pneumoniae [22].
The tree was constructed in RAxML [49] and visualized using FigTree (http://tree.bio.ed.ac.uk/software/figtree/). Clonal groups with >1 isolates are boxed. Sequence type (ST), carbapenemase gene and year of isolation is indicated for each isolate. Isolates associated with the long-term outbreak [50] and the two occurrences of intra-hospital transmission are labelled *, # and ¤, respectively.
Ten diverse genetic backgrounds were identified among the E. coli isolates (n = 14). None of the STs were SLVs or double locus variants (DLVs) of any other. Only ST38 (n = 3) and ST410 (n = 3) were represented by >1 isolate. All three ST38 isolates carried blaOXA-48, while the three ST410 strains harboured each a different carbapenemase gene (blaNDM-1, blaVIM-4 or blaOXA-181). The remaining strains were genetically diverse and carried various carbapenemase genes/variants: blaNDM-1 (ST131, ST617 and ST681), blaNDM-5 (ST636), blaNDM-7 (ST101), blaOXA-48 (ST405), blaVIM-29 (ST6355) and blaIMP-26 (ST95).
The four carbapenemase-producing E. cloacae complex isolates were all of different STs: ST456 and ST484 both with blaKPC-2, ST92 with blaNDM-1 and ST635 with blaIMI-9. All STs were defined as singletons (no SLVs) by BURST analysis of the E. cloacae MLST database (http://pubmlst.org/ecloacae/, last accessed 24.06.2016).
Antimicrobial susceptibility profile and performance of phenotypic methods for detection of CPE
All isolates were multidrug-resistant (MDR) according to the definitions by Magiorakos et al. [51]. (Table 3 and S1 Table). One isolate, a blaNDM-1-positive P. stuartii was non-susceptible to all relevant antimicrobial agents tested. Overall fosfomycin and colistin were the most active antimicrobial agents with 85% and 79% of the isolates being susceptible when excluding P. mirabilis and P. stuartii isolates which are intrinsically resistant to colistin [52] (Table 3). Seven of the twelve colistin-resistant isolates were K. pneumoniae ST258 with blaKPC-2 (n = 6) or blaKPC-3 (n = 1). The other colistin-resistant isolates included K. pneumoniae ST525 with blaNDM-1 + blaOXA-181, K. pneumoniae ST147 with blaNDM-1, K. pneumoniae ST336 with blaNDM-7, E. cloacae complex ST635 with blaIMI-9 and E. cloacae complex ST456 with blaKPC-2.
Table 3. Antimicrobial resistance profiles of CPE isolates according to species and carbapenemase variant.
| Percent non-susceptible (I+R)a | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Carbapenemase | TZP | MEC | CXM | CTX | CAZ | ATM | MEM | ETP | IPM | GEN | AMK | TOB | CIP | TGC | SXT | CST | FOS |
| Klebsiella spp. | KPC (n = 18) | 100 | 100 | 100 | 100 | 100 | 100 | 83 | 100 | 50 | 28 | 78 | 83 | 94 | 83 | 72 | 39 | 6 |
| VIM (n = 5) | 100 | 100 | 100 | 100 | 100 | 40 | 60 | 100 | 80 | 0 | 40 | 100 | 100 | 40 | 80 | 0 | 0 | |
| NDM (n = 9)b | 100 | 11 | 100 | 100 | 100 | 100 | 89 | 100 | 67 | 78 | 89 | 100 | 89 | 56 | 78 | 33 | 11 | |
| OXA-48-like (n = 6) | 100 | 0 | 67 | 50 | 67 | 50 | 17 | 100 | 33 | 17 | 0 | 50 | 83 | 83 | 33 | 0 | 33 | |
| E. coli | VIM/IMP (n = 3) | 67 | 67 | 100 | 100 | 100 | 100 | 33 | 67 | 67 | 100 | 100 | 100 | 33 | 33 | 100 | 0 | 33 |
| NDM (n = 6) | 100 | 17 | 100 | 100 | 100 | 83 | 33 | 100 | 83 | 83 | 83 | 83 | 67 | 0 | 33 | 0 | 0 | |
| OXA-48-like (n = 5) | 100 | 0 | 100 | 100 | 100 | 100 | 20 | 100 | 20 | 80 | 0 | 80 | 60 | 20 | 80 | 0 | 0 | |
| E. cloacae complex | KPC (n = 2) | 100 | -c | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 50 | 50 | 50 | 100 | 50 | 50 | 50 | 100 |
| NDM (n = 1) | 100 | - | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | |
| IMI (n = 1) | 0 | - | 0 | 0 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | 100 | 0 | |
| P. stuartii | NDM (n = 1) | 100 | - | - | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | - | 100 | - | 100 |
| P. mirabilis | NDM (n = 1) | 0 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 100 | 100 | 100 | 100 | 100 | - | 100 | - | 0 |
| Citrobacter spp. | NDM (n = 1) | 100 | - | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 0 |
| Totald | 95 | 53 | 95 | 93 | 95 | 88 | 59 | 97 | 61 | 51 | 63 | 83 | 83 | 58 | 68 | 21 | 15 | |
a according to EUCAST clinical breakpoint table v. 6.0. TZP: piperacillin-tazobactam; MEC: mecillinam; CXM: cefuroxime; CTX: cefotaxime; CAZ: ceftazidime; AZT: aztreonam; MEM: meropenem; ETP: ertapenem; IPM: imipenem; GEN: gentamicin; AMK: amikacin; TOB: tobramycin; CIP: ciprofloxacin; TGC: tigecycline; SXT: trimethoprim-sulfamethoxazole; CST: colistin; FOS: fosfomycin.
b includes one isolate co-harboring blaNDM-1 and blaOXA-181.
C “-”indicates lack of clinical breakpoint or intrinsic resistance according to EUCAST Expert Rules on Intrinsic Resistance and Exceptional Phenotypes v.3.1 (http://www.eucast.org/).
d calculations excludes species/antibiotic combinations with intrinsic resistance.
High levels of non-susceptibility were observed to aminoglycosides (gentamicin, 51%; amikacin, 63%; and tobramycin, 83%), tigecycline (58%) and ciprofloxacin (83%).
With respect to the carbapenems, 41% were susceptible to meropenem, 39% to imipenem and 3% to ertapenem. All isolates had meropenem and ertapenem MIC values above the EUCAST screening breakpoint for carbapenemase detection (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf) (S1 Table). For imipenem nine isolates had MIC values below the screening breakpoint. There was no clear correlation between carbapenemase variant and susceptibility to meropenem and imipenem with the exception that among the isolates harbouring blaOXA-48-like (excluding the strain with both blaNDM-1 and blaOXA-181) 9/11 and 8/11 were susceptible to meropenem and imipenem, respectively. As expected, a high level of resistance was observed against other β-lactams (Table 3 and S1 Table). Three isolates: one K. pneumoniae (blaOXA-48), one K. variicola (blaOXA-48) and the blaIMI-9-positive E. cloacae complex isolate were susceptible to extended-spectrum cephalosporins (cefotaxime, ceftazidime and cefuroxime) and aztreonam. Interestingly, all OXA-48-like-positive E. coli and Klebsiella spp. as well as 83% and 89% of NDM-positive E. coli and Klebsiella spp. isolates, respectively were susceptible to mecillinam. Nine (15%) of the isolates tested negative for carbapenemase-production with the in-house Carba NP test (S1 Table), including six blaNDM-1-positive isolates (E. coli n = 2, P. stuartii, P. mirabilis, Citrobacter sp. and K. pneumoniae), two blaOXA-48-like-positive isolates (E. coli and K. pneumoniae) and one E. cloacae complex isolate (blaIMI-9). The KPC, MBL and OXA-48 confirm kit correctly identified the presence of either an MBL or KPC in all relevant isolates except for one blaNDM-1-positive P. mirabilis strain (S1 Table). The single blaIMI-9-positive E. cloacae complex isolate also showed significant synergy with boronic acid only. With the exception of the isolate harbouring both blaNDM-1 and blaOXA-181, where synergy was observed between meropenem and dipicolinic acid, no synergy was observed with the β-lactamase inhibitors for all blaOXA-48-like-positive isolates. Moreover, with the exception of two isolates, all blaOXA-48–like-positive isolates showed no zones of inhibition around the temocillin tablet, which may indicate the presence of OXA-48-like carbapenemases according to the manufacturer’s guidelines. The meropenem-meropenem/EDTA gradient strip correctly identified all MBL-positive isolates, with the exception of the K. pneumoniae strain positive for both blaNDM-1 and blaOXA-181 where the test was inconclusive (S1 Table).
Association with other antibiotic resistance determinants
BlaCTX-M and specifically blaCTX-M-15 were the most common ESBL variants identified and were mainly associated with K. pneumoniae and E. coli isolates with blaNDM (10/15 isolates) or blaOXA-48-like (8/11 isolates) and E. coli isolates with blaVIM (2/2 isolates) (S1 Table). BlaCTX-M were not identified in blaKPC-positive K. pneumoniae isolates. One E. coli isolate with blaOXA-48 harboured both blaCTX-M-14 and blaCTX-M-15. blaCTX-M-15 was also identified in one blaKPC-2- and one blaNDM-1-positive E. cloacae complex. BlaCMY (n = 12) were the most common plasmid-mediated AmpC variants identified with blaCMY-6 particularly associated with blaNDM (n = 9). The two blaOXA-48-like-positive Klebsiella spp. isolates that were susceptible to extended-spectrum cephalosporins and aztreonam were negative for ESBL and plasmid-mediated AmpC genes.
In addition to various genes encoding aminoglycoside-modifying enzymes, the 16S rRNA methylase genes rmtC and armA, were identified in eight and five isolates, respectively (S1 Table). With the exception of the single isolate of E. coli with blaIMP-26, armA and rmtC were only associated with isolates harbouring blaNDM-1. In Klebsiella spp. insertional disruption of mgrB [53] associated with colistin resistance was identified in seven K. pneumoniae isolates (S1 Table). Insertional disruption of mgrB was also observed in two clinically colistin susceptible (MIC = 1 mg/L) K. pneumoniae isolates. One K. pneumoniae isolate with a disrupted mgrB also carried a nonsense mutation in pmrB leading to a truncated PmrB. Two colistin-resistant K. pneumoniae isolates had mutations in pmrA resulting in amino acid substitutions of G53C and D86E in one, and G53C in the other. In one colistin-resistant Klebsiella spp. isolate (MIC >8 mg/L) no previously described colistin resistance determinants were identified. The strain had mutations in pmrA (PmrA E57G) and pmrB (PmrB T246A) compared with the colistin-susceptible K. pneumoniae strain MGH 78578 [54], but neither mutation has been linked with colistin resistance and PmrB T246A is commonly found in K. pneumoniae [54]. No mutations were identified in phoP, phoQ or the mgrB promoter for this isolate. The plasmid-mediated colistin resistance genes mcr-1 [9], mcr-2 [10], mcr-3 [12], mcr-4 [13] and mcr-5 [14] were not detected.
All E. coli, K. pneumoniae and E. cloacae complex isolates with high-level ciprofloxacin resistance (MIC ≥32 mg/L) harboured mutations in both gyrA and parC (S1 Table). In addition, various plasmid-mediated quinolone resistance determinants were identified, including aac(6’)-Ib-cr (n = 24), qnrB1 (n = 8), qnrB4 (n = 1), qnrB19 (n = 2), qnrD (n = 1) and qnrS1 (n = 8).
Discussion
The main objective of this study was to gain a better understanding of the molecular epidemiology associated with the emergence of CPE in Norway. As observed in other Nordic countries [26, 27, 32–36] the emergence of CPE in Norway is also mainly associated with importation, highlighting the importance of targeted screening of patients hospitalized abroad and patients with a recent travel history to a country with a high prevalence of CPE. A relatively low number of cases (15%) were identified through faecal screening in contrast to Sweden (74,5%) and France (59.8%) [26, 55]. This difference is most likely due to dissimilarities in the use of targeted screening and that CPE screening in Norway was not fully implemented in the study period. This could also explain why a higher proportion of CPE cases in Sweden (81%) were associated with import [26]. Revised recommendations for infection prevention and control, including indications for screening for CPE, were introduced in Norway in August 2015 and in the first six months of 2016, 63% of CPE cases were identified through faecal screening. The occurrence of one long-term outbreak and two separate incidences of secondary transmission further highlights the importance of rapid implementation of infection prevention and control measures before confirmation of CPE if patients have risk factors (e.g. hospitalization abroad) or when an MDR isolate is identified.
The diversity of species and genetic backgrounds observed is probably due to the high degree of importation from a variety of countries (Table 2). Several studies have shown that the dissemination of resistance genes among clinical strains of Enterobacteriaceae is often associated with high-risk clones and the linkage between specific genetic backgrounds and resistance genes [2, 21, 56]. The cgMLST analysis of K. pneumoniae isolates showed that the observed epidemiology reflects the current global epidemiology (Fig 1), where blaKPC-2/-3 spread is primarily driven by strains associated with CG258 (and more specifically, ST258). In contrast, ST11 (a member of CG258, and a single locus variant of ST258) has been shown to be associated with a diversity of carbapenemase genes including blaKPC, blaNDM, blaVIM and blaOXA-48-like in different geographical regions [2, 57, 58]. Accordingly, the four ST11 strains in this study harboured either blaNDM-1 (n = 2) or blaOXA-245 (n = 2). Notably, cgMLST has shown that ST11 and ST340 represent a genetic sublineage within CG258 [22]. Isolates with blaNDM and blaVIM belonging to two other globally dispersed high-risk CGs like CG17 and CG147 [2] were also identified. The identification of blaVIM-1 and blaOXA-48 in K. quasipneumoniae and K. variicola, respectively shows that these Klebsiella species also contribute to the dissemination of carbapenemase genes and infections as both isolates were associated with infection. K. variicola have been shown to be frequently associated with bloodstream infections and associated with higher mortality than K. pneumoniae [59].
All three E. coli ST38 isolates harboured blaOXA-48, which is consistent with previous observations showing a prevalent linkage of ST38 to blaOXA-48 in a large collection of clinical isolates from European and North-African countries [23]. In contrast, the three E. coli isolates belonging to ST410 were associated with different carbapenemase genes (blaNDM-1, blaVIM-4 or blaOXA-181) indicating the ability of this genetic background to maintain different plasmids and resistance genes. ST410 E. coli isolates have also previously been identified harbouring blaKPC-2 [60]. The global dissemination of blaNDM has so far not been linked to specific high-risk clones or epidemic plasmids [21] and this is also reflected among the five blaNDM-positive E. coli isolates, which belonged to five different genetic backgrounds. However, one strain belonged to the international high-risk clone ST131 [21] and another to ST101, which has previously been found to be associated with blaNDM and other carbapenemases in several countries (e.g. Bangladesh [61], USA [62], Canada [63, 64] and Bulgaria [65]).
CPE frequently exhibit MDR or XDR phenotypes, limiting treatment options [1, 4]. This was also observed in our strain collection (Table 2 and S1 Table) due to the association with a wide variety of other acquired resistance genes, including 16S rRNA methylase genes conferring high-level broad-spectrum aminoglycoside resistance [66] and chromosomal mutations/insertions resulting in ciprofloxacin and colistin resistance (S1 Table). The mechanism(s) behind colistin resistance in one K. pneumoniae strain and the colistin-resistant E. cloacae isolates remains to be determined. Interestingly, a high prevalence of susceptibility to mecillinam among OXA-48- and NDM-producing E. coli and K. pneumoniae isolates was observed. Marrs et al. also showed high levels of in vitro susceptibility to mecillinam among NDM-producing E. coli and K. pneumoniae isolates from Pakistan [67], suggesting that mecillinam could have a role in the treatment of uncomplicated urinary tract infections caused by OXA-48- or NDM-producing E. coli or K. pneumoniae [68].
Rapid identification of CPE is essential for timely implementation of enhanced infection control measures to reduce transmission of CPE and prevent infections [3]. As observed in previous studies [69, 70] false-negative results (15%) for carbapenemase production were observed with the in-house version of the Carba NP test, particularly with NDM- and OXA-48-like-producing isolates. Identification of OXA-48-like-producers can be particularly challenging due to their relatively low level of activity against carbapenems and the lack of specific inhibitors [71]. The relatively high number of false-negative Carba NP results could also be due to the media used. In our study, colonies for the Carba NP test were harvested from MH agar and Literacka et al have recently reported that MH agar from different companies were associated with false-negative results for MBL-producers [72]. High-level resistance to temocillin is a sensitive and specific indicator for the presence of OXA-48-like enzymes [73]. All blaOXA-48-like–positive isolates in our collection showed high-level resistance (MIC>128 mg/L) to temocillin, but several isolates harboring blaVIM and blaNDM also had temocillin MIC >128mg/L showing that testing for synergy with metal chelators (e.g. EDTA or dipicolinic acid) is necessary to discriminate between isolates with OXA-48 and MBLs.
Conclusions
The low prevalence of clinical CPE in Norway is consistent with the general low level of antimicrobial resistance compared with other countries. The relatively low level of antibiotic consumption and the use of narrow spectrum antibiotics [74] have probably contributed to this situation. The low prevalence is also reflected in the epidemiology of Norwegian CPE; mainly associated with importation, exhibiting a broad diversity of genetic backgrounds and carbapenemase variants that mirror the global epidemiology. Only a few cases of secondary spread also support this notion. In order to limit the infection pressure brought by increasing travel and globalization, continued emphasis must be put on diagnostic capabilities, surveillance and infection control.
Supporting information
(XLSX)
Acknowledgments
Center for Bioinformatics at UiT The Arctic University of Norway is acknowledged for genome sequencing of part of the strain collection and initial bioinformatic analysis. We are grateful for assistance on the phylogenetic analysis of Klebsiella spp. by Jessin Janice and Ellen H. Josefsen for technical assistance. Tohru Miyoshi-Akiyama and colleagues are acknowledged for curating the Enterobacter cloacae MLST database and for providing novel STs.
This work was performed through a collaborative project with the diagnostic microbiology laboratories in Norway forming the Norwegian Study Group on CPE. We acknowledge the contributions of the representatives in the study group including: Trond E. Ranheim (Akershus University Hospital), Karianne Wiger Gammelsrud (Oslo University Hospital Rikshospitalet, Oslo University Hospital Aker and Oslo University Hospital Radiumhospitalet), Annette Onken (Vestre Viken Bærum Hospital), Kristina Papp (Vestre Viken Drammen Hospital), Liv Jorunn Sønsteby (Haugesund Hospital), Haima Mylvaganam (Haukeland University Hospital), Angela Kümmel (Levanger Hospital), Einar Nilsen (Molde Hospital), Hege Elisabeth Larsen (Nordland Hospital), Hans-Johnny Schjelderup Nilsen (St. Olavs University Hospital), Iren H. Löhr and Anita Brekken (Stavanger University Hospital), Anita Kanestrøm (Østfold Hospital), Guro Furset Jensen (Sørlandet Hospital), Nils Olav Hermansen (Oslo University Hospital Ullevål), Gunnar Skov Simonsen (University Hospital of North Norway), Dagfinn Skaare (Vestfold Hospital) and Reidar Hide (Ålesund Hospital).
Data Availability
Whole genome sequence data have been deposited at the National Center for Biotechnology Information (NCBI) under BioProject PRJNA295003. Original data regarding patient details have been aggregated to secure anonymity as approved by the Regional Ethical Committee and the Data Protection Officer at the University Hospital of North Norway. Complete data are available after consideration by the Regional Ethical Committee (rek-nord@asp.uit.no)/Data Protection Officer (personvernombudet@unn.no) for researchers who meet the criteria for access to confidential data. All other data contained within the paper and Supporting Information.
Funding Statement
The study was performed as part of our routine work and the authors received no specific funding for this study. There was no additional external funding received for this study.
References
- 1.Tangden T, Giske CG. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Int Med. 2015;277(5):501–12. [DOI] [PubMed] [Google Scholar]
- 2.Pitout JD, Nordmann P, Poirel L. Carbapenemase-producing Klebsiella pneumoniae: a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother. 2015;59(10):5873–84. doi: 10.1128/AAC.01019-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temkin E, Adler A, Lerner A, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci. 2014;1323:22–42. doi: 10.1111/nyas.12537 [DOI] [PubMed] [Google Scholar]
- 4.Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply "precision medicine" to antimicrobial chemotherapy? Expert Opin Pharmacother. 2016;17(6):761–81. doi: 10.1517/14656566.2016.1145658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133–43. doi: 10.1093/jac/dkv086 [DOI] [PubMed] [Google Scholar]
- 6.Doi Y, Paterson DL. Carbapenemase-producing Enterobacteriaceae. Semin Resp Crit Care Med. 2015;36(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasevic AT, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017;17(2):153–63. doi: 10.1016/S1473-3099(16)30257-2 [DOI] [PubMed] [Google Scholar]
- 8.Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, Pollini S, et al. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill 2014;19(42) pii: 20939. [DOI] [PubMed] [Google Scholar]
- 9.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–8. doi: 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 10.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. 2016;21(27) doi: 10.2807/1560-7917.ES.2016.21.27.30280 [DOI] [PubMed] [Google Scholar]
- 11.Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, Fortunato S, et al. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother. 2016;60(9):5612–5. doi: 10.1128/AAC.01075-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio. 2017;8(3) pii: e00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017;22(31) pii: 30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother. 2017;September 18 doi: 10.1093/jac/dkx327. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Falgenhauer L, Waezsada SE, Yao Y, Imirzalioglu C, Kasbohrer A, Roesler U, et al. Colistin resistance gene mcr-1 in extended-spectrum-β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis. 2016;16(3):282–3. doi: 10.1016/S1473-3099(16)00009-8 [DOI] [PubMed] [Google Scholar]
- 16.Mulvey MR, Mataseje LF, Robertson J, Nash JH, Boerlin P, Toye B, et al. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis. 2016;16(3):289–90. doi: 10.1016/S1473-3099(16)00067-0 [DOI] [PubMed] [Google Scholar]
- 17.Li A, Yang Y, Miao M, Chavda KD, Mediavilla JR, Xie X, et al. Complete sequences of mcr-1-harboring plasmids from extended-spectrum-β-lactamase- and carbapenemase-producing Enterobacteriaceae. Antimicrob Agents and Chemother. 2016;60(7):4351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014;20(9):821–30. doi: 10.1111/1469-0691.12719 [DOI] [PubMed] [Google Scholar]
- 19.Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae working group. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill. 2015;20(45) doi: 10.2807/1560-7917.ES.2015.20.45.30062 [DOI] [PubMed] [Google Scholar]
- 20.Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(suppl_1):S28–S36. doi: 10.1093/infdis/jiw282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathers AJ, Peirano G, Pitout JD. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev. 2015;28(3):565–91. doi: 10.1128/CMR.00116-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bialek-Davenet S, Criscuolo A, Ailloud F, Passet V, Jones L, Delannoy-Vieillard AS, et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis. 2014;20(11):1812–20. doi: 10.3201/eid2011.140206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potron A, Poirel L, Rondinaud E, Nordmann P. Intercontinental spread of OXA-48 β-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Euro Surveill. 2013;18(31) pii: 20549. [DOI] [PubMed] [Google Scholar]
- 24.Poirel L, Bonnin RA, Nordmann P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother. 2012;56(1):559–62. doi: 10.1128/AAC.05289-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carattoli A, Seiffert SN, Schwendener S, Perreten V, Endimiani A. Differentiation of IncL and IncM plasmids associated with the spread of clinically relevant antimicrobial resistance. PLoS ONE. 2015;10(5):e0123063 doi: 10.1371/journal.pone.0123063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lofmark S, Sjostrom K, Makitalo B, Edquist P, Tegmark Wisell K, Giske CG. Carbapenemase-producing Enterobacteriaceae in Sweden 2007–2013: Experiences from seven years of systematic surveillance and mandatory reporting. Drug Resist Updates. 2015;20:29–38. [DOI] [PubMed] [Google Scholar]
- 27.Osterblad M, Kirveskari J, Hakanen AJ, Tissari P, Vaara M, Jalava J. Carbapenemase-producing Enterobacteriaceae in Finland: the first years (2008–11). J Antimicrob Chemother. 2012;67(12):2860–4. doi: 10.1093/jac/dks299 [DOI] [PubMed] [Google Scholar]
- 28.Samuelsen Ø, Naseer U, Tofteland S, Skutlaberg DH, Onken A, Hjetland R, et al. Emergence of clonally related Klebsiella pneumoniae isolates of sequence type 258 producing plasmid-mediated KPC carbapenemase in Norway and Sweden. J Antimicrob Chemother. 2009;63(4):654–8. doi: 10.1093/jac/dkp018 [DOI] [PubMed] [Google Scholar]
- 29.Samuelsen Ø, Naseer U, Karah N, Lindemann PC, Kanestrom A, Leegaard TM, et al. Identification of Enterobacteriaceae isolates with OXA-48 and coproduction of OXA-181 and NDM-1 in Norway. J Antimicrob Chemother. 2013;68(7):1682–5. doi: 10.1093/jac/dkt058 [DOI] [PubMed] [Google Scholar]
- 30.Samuelsen Ø, Thilesen CM, Heggelund L, Vada AN, Kummel A, Sundsfjord A. Identification of NDM-1-producing Enterobacteriaceae in Norway. J Antimicrob Chemother. 2011;66(3):670–2. doi: 10.1093/jac/dkq483 [DOI] [PubMed] [Google Scholar]
- 31.Samuelsen Ø, Toleman MA, Hasseltvedt V, Fuursted K, Leegaard TM, Walsh TR, et al. Molecular characterization of VIM-producing Klebsiella pneumoniae from Scandinavia reveals genetic relatedness with international clonal complexes encoding transferable multidrug resistance. Clin Microbiol Infect. 2011;17(12):1811–6. doi: 10.1111/j.1469-0691.2011.03532.x [DOI] [PubMed] [Google Scholar]
- 32.Hammerum AM, Littauer P, Hansen F. Detection of Klebsiella pneumoniae co-producing NDM-7 and OXA-181, Escherichia coli producing NDM-5 and Acinetobacter baumannii producing OXA-23 in a single patient. Int J Antimicrob Agents. 2015;46(5):597–8. doi: 10.1016/j.ijantimicag.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 33.Jakobsen L, Hammerum AM, Hansen F, Fuglsang-Damgaard D. An ST405 NDM-4-producing Escherichia coli isolated from a Danish patient previously hospitalized in Vietnam. J Antimicrob Chemother. 2014;69(2):559–60. doi: 10.1093/jac/dkt356 [DOI] [PubMed] [Google Scholar]
- 34.Hammerum AM, Larsen AR, Hansen F, Justesen US, Friis-Moller A, Lemming LE, et al. Patients transferred from Libya to Denmark carried OXA-48-producing Klebsiella pneumoniae, NDM-1-producing Acinetobacter baumannii and meticillin-resistant Staphylococcus aureus. Int J Antimicrobial Agents. 2012;40(2):191–2. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen JB, Hansen F, Littauer P, Schonning K, Hammerum AM. An NDM-1-producing Escherichia coli obtained in Denmark has a genetic profile similar to an NDM-1-producing E. coli isolate from the UK. J Antimicrob Chemother. 2012;67(8):2049–51. doi: 10.1093/jac/dks149 [DOI] [PubMed] [Google Scholar]
- 36.Hammerum AM, Toleman MA, Hansen F, Kristensen B, Lester CH, Walsh TR, et al. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect Dis. 2010;10(12):829–30. doi: 10.1016/S1473-3099(10)70276-0 [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Ellermann-Eriksen S, Hansen DS, Kjerulf A, Fuglsang-Damgaard D, Holm A, et al. Epidemic increase in the incidence of carbapenemase-producing Enterobacteriaceae in Denmark. Ugeskr Laeger. 2016;178(45) pii: V06160422. [PubMed] [Google Scholar]
- 38.Hammerum AM, Hansen F, Nielsen HL, Jakobsen L, Stegger M, Andersen PS, et al. Use of WGS data for investigation of a long-term NDM-1-producing Citrobacter freundii outbreak and secondary in vivo spread of blaNDM-1 to Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca. J Antimicrob Chemother. 2016;71(11):3117–24. doi: 10.1093/jac/dkw289 [DOI] [PubMed] [Google Scholar]
- 39.Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2012;18(9):1503–7. doi: 10.3201/eid1809.120355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dortet L, Brechard L, Poirel L, Nordmann P. Impact of the isolation medium for detection of carbapenemase-producing Enterobacteriaceae using an updated version of the Carba NP test. J Med Microbiol. 2014;63(Pt 5):772–6. doi: 10.1099/jmm.0.071340-0 [DOI] [PubMed] [Google Scholar]
- 41.Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, Nordmann P. Genetic structures at the origin of acquisition of the β-lactamase blaKPC gene. Antimicrob Agents Chemother. 2008;52(4):1257–63. doi: 10.1128/AAC.01451-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendes RE, Kiyota KA, Monteiro J, Castanheira M, Andrade SS, Gales AC, et al. Rapid detection and identification of metallo-β-lactamase-encoding genes by multiplex real-time PCR assay and melt curve analysis. J Clin Microbiol. 2007;45(2):544–7. doi: 10.1128/JCM.01728-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swayne RL, Ludlam HA, Shet VG, Woodford N, Curran MD. Real-time TaqMan PCR for rapid detection of genes encoding five types of non-metallo- (class A and D) carbapenemases in Enterobacteriaceae. Int J Antimicrob Agents. 2011;38(1):35–8. doi: 10.1016/j.ijantimicag.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 44.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diag Microbiol Infect Dis. 2011;70(1):119–23. [DOI] [PubMed] [Google Scholar]
- 45.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–77. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koren S, Treangen TJ, Hill CM, Pop M, Phillippy AM. Automated ensemble assembly and validation of microbial genomes. BMC Bioinformatics. 2014;15:126 doi: 10.1186/1471-2105-15-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Treangen TJ, Ondov BD, Koren S, Phillippy AM. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014;15(11):524 doi: 10.1186/s13059-014-0524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doumith M, Day M, Ciesielczuk H, Hope R, Underwood A, Reynolds R, et al. Rapid identification of major Escherichia coli sequence types causing urinary tract and bloodstream infections. J Clin Microbiol. 2015;53(1):160–6. doi: 10.1128/JCM.02562-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tofteland S, Naseer U, Lislevand JH, Sundsfjord A, Samuelsen Ø. A long-term low-frequency hospital outbreak of KPC-producing Klebsiella pneumoniae involving Intergenus plasmid diffusion and a persisting environmental reservoir. PLoS ONE. 2013;8(3):e59015 doi: 10.1371/journal.pone.0059015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 52.Leclercq R, Canton R, Brown DF, Giske CG, Heisig P, MacGowan AP, et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect. 2013;19(2):141–60. doi: 10.1111/j.1469-0691.2011.03703.x [DOI] [PubMed] [Google Scholar]
- 53.Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol. 2014;5:643 doi: 10.3389/fmicb.2014.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olaitan AO, Diene SM, Kempf M, Berrazeg M, Bakour S, Gupta SK, et al. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int J Antimicrob Agents. 2014;44(6):500–7. doi: 10.1016/j.ijantimicag.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 55.Dortet L, Cuzon G, Nordmann P. Dissemination of carbapenemase-producing Enterobacteriaceae in France, 2012. J Antimicrob Chemother. 2014;69(3):623–7. doi: 10.1093/jac/dkt433 [DOI] [PubMed] [Google Scholar]
- 56.Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev. 2011;35(5):736–55. doi: 10.1111/j.1574-6976.2011.00268.x [DOI] [PubMed] [Google Scholar]
- 57.Kristof K, Toth A, Damjanova I, Janvari L, Konkoly-Thege M, Kocsis B, et al. Identification of a blaVIM-4 gene in the internationally successful Klebsiella pneumoniae ST11 clone and in a Klebsiella oxytoca strain in Hungary. J Antimicrob Chemother. 2010;65(6):1303–5. doi: 10.1093/jac/dkq133 [DOI] [PubMed] [Google Scholar]
- 58.Oteo J, Perez-Vazquez M, Bautista V, Ortega A, Zamarron P, Saez D, et al. The spread of KPC-producing Enterobacteriaceae in Spain: WGS analysis of the emerging high-risk clones of Klebsiella pneumoniae ST11/KPC-2, ST101/KPC-2 and ST512/KPC-3. J Antimicrob Chemother. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maatallah M, Vading M, Kabir MH, Bakhrouf A, Kalin M, Naucler P, et al. Klebsiella variicola is a frequent cause of bloodstream infection in the stockholm area, and associated with higher mortality compared to K. pneumoniae. PLoS ONE. 2014;9(11):e113539 doi: 10.1371/journal.pone.0113539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen YT, Lin JC, Fung CP, Lu PL, Chuang YC, Wu TL, et al. KPC-2-encoding plasmids from Escherichia coli and Klebsiella pneumoniae in Taiwan. J Antimicrob Chemother. 2014;69(3):628–31. doi: 10.1093/jac/dkt409 [DOI] [PubMed] [Google Scholar]
- 61.Toleman MA, Bugert JJ, Nizam SA. Extensively drug-resistant New Delhi metallo-β-lactamase-encoding bacteria in the environment, Dhaka, Bangladesh, 2012. Emerg Infect Dis. 2015;21(6):1027–30. doi: 10.3201/eid2106.141578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pannaraj PS, Bard JD, Cerini C, Weissman SJ. Pediatric carbapenem-resistant Enterobacteriaceae in Los Angeles, California, a high-prevalence region in the United States. Pediatr Infect Dis J. 2015;34(1):11–6. doi: 10.1097/INF.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peirano G, Ahmed-Bentley J, Fuller J, Rubin JE, Pitout JD. Travel-related carbapenemase-producing Gram-negative bacteria in Alberta, Canada: the first 3 years. J Clin Microbiol. 2014;52(5):1575–81. doi: 10.1128/JCM.00162-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mataseje LF, Abdesselam K, Vachon J, Mitchel R, Bryce E, Roscoe D, et al. Carbapenem-producing Enterobacteriaceae in Canada: results from the Canadian Nosocomial Infection Surveillance Program, 2010–2014. Antimicrob Agents Chemother. 2016;60(11):6787–94. doi: 10.1128/AAC.01359-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poirel L, Savov E, Nazli A, Trifonova A, Todorova I, Gergova I, et al. Outbreak caused by NDM-1- and RmtB-producing Escherichia coli in Bulgaria. Antimicrob Agents Chemother. 2014;58(4):2472–4. doi: 10.1128/AAC.02571-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wachino J, Arakawa Y. Exogenously acquired 16S rRNA methyltransferases found in aminoglycoside-resistant pathogenic Gram-negative bacteria: an update. Drug Resist Updates. 2012;15(3):133–48. [DOI] [PubMed] [Google Scholar]
- 67.Marrs EC, Day KM, Perry JD. In vitro activity of mecillinam against Enterobacteriaceae with NDM-1 carbapenemase. J Antimicrob Chemother. 2014;69(10):2873–5. doi: 10.1093/jac/dku204 [DOI] [PubMed] [Google Scholar]
- 68.Giske CG. Contemporary resistance trends and mechanisms for the old antibiotics colistin, temocillin, fosfomycin, mecillinam and nitrofurantoin. Clin Microbiol Infect. 2015;21(10):899–905. doi: 10.1016/j.cmi.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 69.Tijet N, Boyd D, Patel SN, Mulvey MR, Melano RG. Evaluation of the Carba NP test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemotherapy. 2013;57(9):4578–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osterblad M, Hakanen AJ, Jalava J. Evaluation of the Carba NP test for carbapenemase detection. Antimicrob Agents Chemother. 2014;58(12):7553–6. doi: 10.1128/AAC.02761-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oueslati S, Nordmann P, Poirel L. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother. 2015;70(4):1059–63. doi: 10.1093/jac/dku524 [DOI] [PubMed] [Google Scholar]
- 72.Literacka E, Herda M, Baraniak A, Zabicka D, Hryniewicz W, Skoczynska A, et al. Evaluation of the Carba NP test for carbapenemase detection in Enterobacteriaceae, Pseudomonas spp. and Acinetobacter spp., and its practical use in the routine work of a national reference laboratory for susceptibility testing. Eur J Clin Microbiol Infect Dis. 2017;36(11):2281–87. doi: 10.1007/s10096-017-3062-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang TD, Poirel L, Bogaerts P, Berhin C, Nordmann P, Glupczynski Y. Temocillin and piperacillin/tazobactam resistance by disc diffusion as antimicrobial surrogate markers for the detection of carbapenemase-producing Enterobacteriaceae in geographical areas with a high prevalence of OXA-48 producers. J Antimicrob Chemother. 2014;69(2):445–50. doi: 10.1093/jac/dkt367 [DOI] [PubMed] [Google Scholar]
- 74.NORM/NORM-VET. NORM/NORM-VET 2015. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. Tromsø/Oslo: 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Whole genome sequence data have been deposited at the National Center for Biotechnology Information (NCBI) under BioProject PRJNA295003. Original data regarding patient details have been aggregated to secure anonymity as approved by the Regional Ethical Committee and the Data Protection Officer at the University Hospital of North Norway. Complete data are available after consideration by the Regional Ethical Committee (rek-nord@asp.uit.no)/Data Protection Officer (personvernombudet@unn.no) for researchers who meet the criteria for access to confidential data. All other data contained within the paper and Supporting Information.