Abstract
Urinary tract infections (UTIs) are common nosocomial infections. This study evaluated the prevalence, pathogens, antibiotic resistances, clinical outcomes, and hospitalization costs associated with complicated UTIs in southern China, and risk factors delaying patient discharge. We retrospectively reviewed electronic medical records of 4,284 (61.4% women) complicated UTI-related hospitalizations from 2008 to 2013. Average patient age was 61.1 years and median hospital stay was 11 days. Pathogens were isolated from 1,071 urine and 148 blood specimens. Gram-negative bacteria were the most frequent and included Escherichia coli (48.2%), Klebsiella pneumoniae (9.5%), Pseudomonas aeruginosa (4.9%), and Proteus mirabilis (4.6%), while Enterococcus spp. (14.4%) was the most common Gram-positive bacteria causing UTIs. Both E. coli and K. pneumoniae showed high resistance rates (>45%) to wide-spectrum penicillins, cephalosporins, aztreonam, and ciprofloxacin. Resistances to beta-lactamase inhibitor/beta-lactam antibiotic combination were relatively lower. Imipenem, meropenem, and amikacin had the greatest activity against E.coli and K. pneumoniae. Recurrent infection was a risk factor for mortality. Age, sex, previous surgery, diabetes, and renal insufficiency were significant risk factors for delayed discharge (P<0.01). Response to initial treatment was associated with a lower cost. Initial empiric use of antibiotics least associated with resistance may reduce costs and medical resource usage.
Keywords: urinary tract infection, Escherichia coli, antibiotic resistance, recurrent infection, length of stay, hospitalization cost
Introduction
Urinary tract infections (UTIs) are the most common bacterial infections and a frequent nosocomial infection.1 Approximately 250 million individuals experience a UTI each year.2,3 Around 40%–50% of women experience at least one UTI during their lifetime and an estimated one in three women would experience a UTI by the age of 24 years,4,5 with 15%–25% of these women developing recurrent infection.6–8 Community-acquired infections with Escherichia coli and other Enterobacteriaceae occur in ~75% of the patients.9 Cases of UTIs can be classified as uncomplicated or complicated.10 Uncomplicated UTIs occur in sexually active healthy women with structurally and functionally normal urinary tracts. However, complicated UTIs (cUTIs) are associated with conditions that prolong the need for treatment or increase the likelihood of therapeutic failure. These conditions include abnormalities in the urinary tract that impede urine flow, foreign bodies (eg, catheters or stones), infections with multidrug-resistant pathogens, and UTIs in male patients. Although uncomplicated infections in the community can usually be treated via brief antibiotic treatment, the cUTIs usually require longer and more intensive antibiotic treatment. The initial treatment for UTIs is generally empirical and is based on the known antimicrobial resistance patterns of urinary pathogens.11 However, the prevalence of antimicrobial resistance among urinary pathogens has been increasing, due to the increased and inappropriate prescription of antibiotics.12,13 Antibiotic resistance is a growing concern in China,14 where clinical isolates of E. coli have exhibited high rates of resistance to amoxicillin with clavulanic acid (20.6%–27.9%), ciprofloxacin (64.7%–74%), and piperacillin (71.1%–80.1%). Nevertheless, the distributions of urinary pathogens and their susceptibility to antibiotics exhibit regional variations, and there is a need for locally derived knowledge regarding antibiotic resistance patterns throughout China.15,16 The present study evaluated cases of cUTIs from southern China during 2008–2013 in order to evaluate the pathogens and their antibiotic resistance patterns, antibacterial treatment, and clinic outcomes, as well as the risk factors for delay to discharge, to provide useful information for regional clinicians who manage patients with cUTIs.
Methods
Study design
This 6-year retrospective, incidence-based, observational study was performed using electronic medical record (EMR) data from patients who were hospitalized at four hospitals (Dade Road General Hospital, Fangcun, University City, and Ersha Island) that belong to a single health care conglomerate (the Guangdong Provincial Hospital of Chinese Medicine).
The protocol was reviewed and approved by the ethical committee of the Guangdong Provincial Hospital of Chinese Medicine. The requirement for informed consent was waived, due to the retrospective design and the fact that all patient data were anonymized and deidentified prior to the analysis.
Patients
Patients were selected by searching the EMRs for hospitalized cases (January 1, 2008 to December 31, 2013) with discharge diagnoses of cUTIs, and the study cohort was further validated by the doctors. The data that we collected included demographic information (age, sex, admission and discharge dates, and marital status), comorbidities, lifestyle factors (smoking and alcoholism), discharge diagnoses, primary surgical procedures, unplanned additional surgeries, laboratory and microbiology results, antibiotic usage, change in antibiotics, length of hospital stay, discharge status (alive or dead), admission in hospital ward, in-hospital transfers (to other wards or the intensive care unit [ICU]), and hospitalization cost.
Pathogens and antibiotic susceptibility
Bacterial species was identified according to their unique activities, like physiological reactions and metabolites. The pathogens and their antibiotic susceptibilities were identified using Gram staining, microscopic observation of their morphotypes, characteristics in culture medium, and other biochemical reactions that are specified by the Clinical and Laboratory Standard Institute (CLSI) procedures.17 Urine and blood sample collection, and isolation and identification of uropathogens were performed. In brief, the urine samples with suspected infection were collected and subcultured with 5% sheep blood medium and McConquet plate medium for 18–24 h at 35°C±0.5°C. On the second day, cytobacteriological examination using Gram staining and microscopy was performed for any clinical signs of urine infection. Urine samples that contained no more than two types of bacteria were selected, and the cell count threshold was set to be more than 104 and less than 105 colony-forming units (CFU)/mL (>104 CFU/mL for Gram-positive bacteria and >105 CFU/mL for Gram-negative bacteria). The bacterial identification and susceptibility analysis were conducted on the selected samples using an automatic system (VITEK 2 system; bioMérieux, Marcy l’Etoile, France). Antibiotic resistance testing was performed using a susceptibility panel according to the CLSI procedures, which included penicillins (ampicillin, piperacillin, and piperacillin/tazobactam), third-generation cephalosporins (ceftriaxone, cefoperazone, cefoperazone/sulbactam, ceftazidime, and cefotaxime), a fourth-generation cephalosporin (cefepime), a carbapenem (imipenem), a monobactam (aztreonam), a fluoroquinolone (ciprofloxacin), and aminoglycosides (amikacin and gentamicin). The empirical antibiotic treatment principle was based on the Antibacterial Drug Selection Guidelines from the Chinese Medical Association. We calculated the yearly resistance rate of a pathogen to a specific antibiotic if there were at least 10 tests.
Study outcomes
We only analyzed antibacterials (ATC code J01), and the treatments were considered modified if new antibiotics were administered to the patient at >3 days after admission. The study outcomes included patient characteristics, pathogen characteristics, antibiotic susceptibility, and clinical outcomes (hospitalization length, recurrence rate, and mortality rate). All analyses were descriptive in nature. First-line empirical antibiotic treatment was defined as effective if all isolated bacteria were sensitive to at least one of the antibiotics that were administered to an infection-positive patient. Alternatively, in cases without bacterial culture data, the empirical treatment was defined as effective when a patient did not change the administered antibiotics.
Cost analysis
The total cost during each hospitalization was defined as the sum of all costs for each type of medicine (Western or herbal medicine) that was received by the patient during the hospitalization. The costs of the examinations (laboratory and instrumental tests), treatment, diagnosis (including specialists’ consultancies), and bed fees were directly recorded, and were assessed by referring to the fees that were reimbursed to the service providers. Costs related to any primary surgical procedures were not included in the treatment cost, as they were considered independent of the choice of antibiotic treatment.
Statistical analysis
All descriptive analyses were performed using R software (version 3.1.1; R Foundation for Statistical Computing, Institute for Statistics and Mathematics; Vienna, Austria). Continuous data were expressed as mean ± standard deviation or 95% confidence interval. Categorical data were expressed as the number of events and percentages. Univariate statistical analyses were performed using the Wilcoxon rank-sum test to compare baseline characteristics with the treatment outcomes. Fisher’s exact test was used to assess differences in categorical variables. Time series analysis was used to evaluate trends in the resistance rates and mortality rates. We performed a univariate analysis of the variables of interest by constructing Kaplan–Meier survival curves, which were stratified according to the covariate variables, and used the log-rank test to evaluate the relationship of each variable with time to discharge (while accounting for in-hospital death as a competing event). Finally, we constructed a Cox proportional hazards model to evaluate the effect of multiple variables on delayed discharge.
Results
Patient characteristics
This study included 3,829 patients (90% married, 61.4% women) with a mean age of 61.1 years, who underwent 4,284 hospitalizations due to a diagnosis of cUTIs during the 6-year study period. Dade Road General Hospital accounted for 1,384 cases, Ersha Island accounted for 1,075 cases, Fangcun accounted for 955 cases, and University City accounted for 870 cases (Table 1). The prevalence of cUTIs in the older group was higher than in the younger group (Table 1) and significantly increased with age (chi-squared test, P<0.01). While there was a strong female predominance in younger populations (age ≤30 years) with 3:1 female to male ratio, the prevalence was similar between both sexes (data not shown). Comorbidities were observed in 2,345 (54.8%) of the hospitalized cases, which included kidney stones (23.32%), diabetes (20.2%), solid tumors (13.7%), and renal failure (11.5%). Prehospitalization surgery was observed in 1,838 cases (42.9%), compared to 1,182 cases (27.6%) of surgery during the hospitalizations for cUTIs. Only 3.34% of patients were prescribed antibacterials prior to their hospitalization (Table S1). Most patients were prescribed medications for comorbidities during their hospitalization (4,226 cases, 98.7%), which included antibacterial (3,944 cases, 92.1%), vasoprotectives (2,586 cases, 60.4%), and cardiac therapies (2,142 cases, 50.0%).
Table 1.
Demographics of patients with complicated UTI
| Characteristics | N |
|---|---|
| Total number of cases (patients) | 4,284 (3,829) |
| Dade Road General Hospital | 1,384 (1,274) |
| University City | 870 (794) |
| Ersha Island | 1,075 (987) |
| Fangcun | 955 (853) |
| Sex: female, n (%) | 2,631 (61.41) |
| Marriage: married, n (%) | 3,851 (89.89) |
| Age, years, mean (±SD) [median] | 61.11 (20.09) [65] |
| ≤30, n (%) | 451 (10.5) |
| 30–60, n (%) | 1,341 (31.3) |
| ≥60, n (%) | 2,403 (56.1) |
| Smoking status, n (%) | |
| Smoker | 743 (17.34) |
| Non-smoker | 2,087 (48.72) |
| Unknown/missing | 1,454 (33.94) |
| Drinking status, n (%) | |
| Drinker | 320 (7.47) |
| Non-drinker | 2,361 (55.11) |
| Unknown/missing | 1,603 (37.42) |
| Comorbidities, n (%) | 2,345 (54.75) |
| Kidney stone | 999 (23.32) |
| Diabetes | 865 (20.19) |
| Solid tumor | 585 (13.66) |
| Renal failure | 494 (11.53) |
| Heart failure | 252 (5.89) |
| Autoimmune disease | 77 (1.80) |
| Liver failure | 57 (1.33) |
| Blood tumor | 14 (0.33) |
| HIV infection | 1 (0.023) |
| Surgical treatment during hospitalization, n (%) | 1,182 (27.59) |
| Surgical treatment prior to hospitalization, n (%) | 1,838 (42.90) |
Abbreviation: UTI, urinary tract infection.
Pathogens in cUTI
Among the 4,284 hospitalized cases, pathogens were identified in 1,142 cases. The urine testing reports were available for 1,071 cases and were positive for at least one microorganism, while 148 cases had blood culture reports and 77 cases had both urine test and blood culture reports. The most commonly identified uropathogens were Gram-negative bacteria, which included E. coli (551 cases, 48.2%), Klebsiella pneumoniae (K. pneumoniae) (108 cases, 9.5%), Pseudomonas aeruginosa (56 cases, 4.9%), Proteus mirabilis (52 cases, 4.6%), Enterobacter cloacae (22 cases, 1.9%), and Acinetobacter baumannii (21 cases, 1.8%). Enterococcus spp. (especially Enterococcus faecalis and Enterococcus faecium) were the most commonly identified Gram-positive bacteria, which accounted for 14.4% (165 cases) (Figure 1). Candida spp. (12.5%) and Mycoplasma spp. (3.9%) also existed in a few cUTI cases. The pathogens detected in the urine and blood samples are presented in Table 2. The most frequently identified pathogens (>4%) from the two sample types were similar. Staphylococcus capitis was identified only in blood culture while E. cloacae was observed only in urine test.
Figure 1.
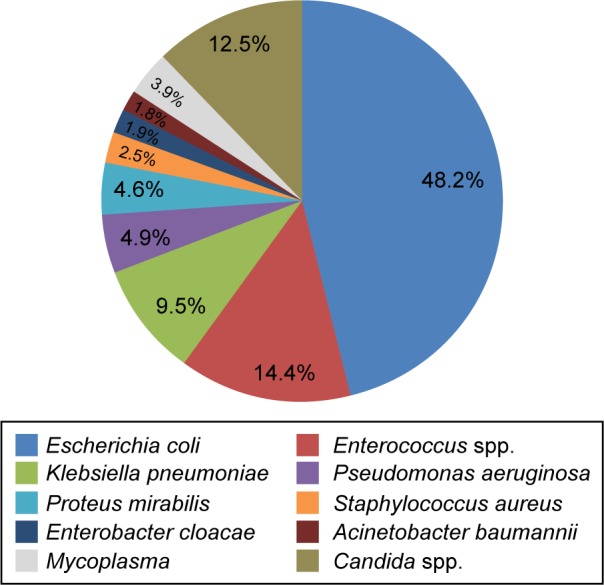
Most common pathogens detected in cUTI patients.
Abbreviation: cUTI, complicated urinary tract infection.
Table 2.
Frequency of pathogens isolated from urine and blood samples
| Pathogen | Isolated in urine sample, n (%) Na=1,071 | Isolated in blood sample, n (%) Na=148 |
|---|---|---|
| Gram-positive bacteria | 206 (19.23) | 41 (27.70%) |
| Staphylococcaceae | ||
| Staphylococcus aureus | 24 (2.24) | 8 (5.41) |
| Streptococcus agalactiae | 11 (1.03) | 0* (0.00) |
| Staphylococcus haemolyticus | 8 (0.75) | 5 (3.38) |
| Staphylococcus epidermidis | 6 (0.56) | 5 (3.38) |
| Staphylococcus hominis | 2 (0.19) | 4 (2.70) |
| Staphylococcus capitis | 0* (0.00) | 6 (4.05) |
| Enterococcaceae | ||
| Enterococcus faecalis | 86 (8.03) | 5 (3.38) |
| Enterococcus faecium | 62 (5.79) | 8 (5.41) |
| Enterococcus gallinarum | 5 (0.47) | 1 (0.68) |
| Gram-negative bacteria | 784 (73.20) | 112 (75.68) |
| Enterobacteriaceae | ||
| Escherichia coli | 505 (47.15) | 77 (52.03) |
| Klebsiella pneumoniae | 99 (9.24) | 11 (7.43) |
| Proteus mirabilis | 49 (4.58) | 4 (2.70) |
| Enterobacter cloacae | 22 (2.05) | 0* (0.00) |
| Morganella morganii | 7 (0.65) | 0* (0.00) |
| Proteus vulgaris | 9 (0.84) | 0* (0.00) |
| Citrobacter freundii | 7 (0.65) | 0* (0.00) |
| Enterobacter aerogenes | 5 (0.47) | 0* (0.00) |
| Moraxellaceae | ||
| Acinetobacter baumannii | 15 (1.40) | 7 (4.73) |
| Pseudomonadaceae | ||
| Pseudomonas aeruginosa | 54 (5.04) | 4 (2.70) |
| Pesudomonas pyocyaneum | 16 (1.49) | 3 (2.03) |
| Burkholderiaceae | ||
| Burkholderia cepacia | 8 (0.75) | 3 (2.03) |
| Fungus | 139 (12.98) | 8 (5.41) |
| Candida spp. | 138 (12.89) | 8 (5.41) |
Notes:
N represents the total number of cases detected with pathogens in urine or blood samples; 0* represents pathogen not found in the specified sample.
Among the 1,071 cases with urine test results, 131 cases (12.2%) exhibited ≥2 microorganisms in urine. Among the 148 cases with blood test results, 12 cases (9.5%) exhibited ≥2 microorganisms in blood. E. coli and K. pneumoniae were the two most common Gram-negative bacteria that were isolated from urine or blood samples during the study period, and the incidence trends for these bacteria are shown in Figure S1. The incidence of E. coli in both urine and blood samples exhibited stable trends, whereas the incidence of K. pneumoniae in blood samples exhibited a steady decrease (P<0.01).
Antibiotic resistance and treatment
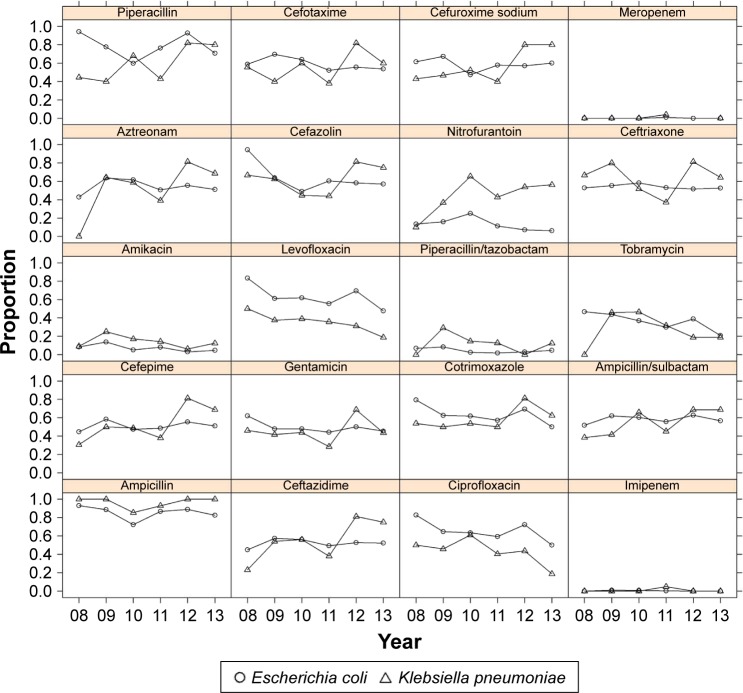
The resistance patterns of epidemic bacteria were evaluated over time (Figures 2 and 3). Both E. coli and K. pneumoniae isolates exhibited similar high average rates of resistance (56%–94%) to wide-spectrum penicillins (ampicillin and piperacillin), followed by (≥45% resistance) cephalosporins (cefazolin, ceftriaxone, ceftazidime, and cefepime), aztreonam, and ciprofloxacin. However, these isolates exhibited sustained low rates of resistance to imipenem, meropenem, and amikacin (Table 3). It was interesting that the rates of resistance to beta-lactam antibiotics (piperacillin/tazobactam, ampicillin/sulbactam) with peptidoglycan cross-linker blockers were obviously lower (Figure 2). These findings suggest that wide-spectrum penicillins or cephalosporins should be combined with beta-lactamase inhibitors to decrease resistance, and that imipenem or meropenem or amikacin should be used for the initial empirical treatment of cUTIs. No significant changes in the resistance rates to most antibiotics were observed over the 6-year study period except that the resistance of K. pneumoniae to levofloxacin was decreasing with year (time series analysis, P=0.007).
Figure 2.
Antibiotic resistance patterns of top two frequently isolated pathogens (Escherichia coli and Klebsiella pneumonia) from 2008 to 2013.
Figure 3.
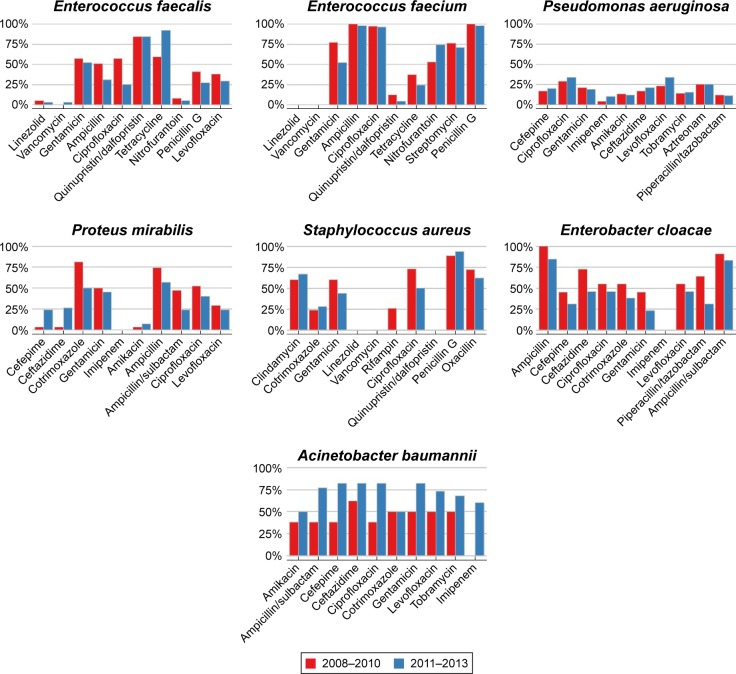
Antibiotic resistance patterns of frequently isolated pathogens in two periods: 2008–2010 vs 2011–2013.
Note: The pathogens isolated were Enterococcus faecalis, Enterococcus faecium, Pseudomonas aeruginosa, Proteus mirabilis, Staphylococcus aureus, Enterobacter cloacae, and Acinetobacter baumannii.
Table 3.
Average rates of resistance of E. coli and K. pneumoniae to antibiotics during 2008–2013
| Antibiotics | Rates of resistance (%)
|
|
|---|---|---|
| E. coli | K. pneumoniae | |
| Ampicillin | 82.38 | 94.1 |
| Ampicillin/sulbactam | 58.2 | 54.6 |
| Piperacillin | 70.93 | 55.9 |
| Piperacillin/tazobactam | 3.3 | 13.4 |
| Ciprofloxacin | 61.66 | 45.7 |
| Cotrimoxazole | 59.83 | 55.9 |
| Cefotaxime | 58.98 | 52.1 |
| Levofloxacin | 58.56 | 35.2 |
| Cefazolin | 57.72 | 56.7 |
| Aztreonam | 55.54 | 56.2 |
| Cefuroxime sodium | 54.87 | 51.8 |
| Ceftriaxone | 54.48 | 58.0 |
| Ceftazidime | 52.73 | 52.6 |
| Cefepime | 49.81 | 50.0 |
| Gentamicin | 46.83 | 42.1 |
| Tobramycin | 33.38 | 34.5 |
| Nitrofurantoin | 14.67 | 47.1 |
| Amikacin | 7.3 | 15.3 |
| Imipenem | 0.5 | 1.3 |
| Meropenem | 0.25 | 1.4 |
Abbreviations: E. coli, Escherichia coli; K. pneumoniae, Klebsiella pneumoniae.
Gram-negative P. aeruginosa exhibited marginally low rates (<20%) of resistance to most of the tested antibiotics, while E. cloacae exhibited much higher rates of resistance to most of the antibiotics that were tested, with the exception of imipenem (0%). P. mirabilis showed high sensitivity to amikacin (3%–7%), as well as to imipenem (0%). It was noteworthy that the resistance rates of A. baumannii to nearly all antibiotics which were tested increased (2011–2013 vs 2008–2010) and all the resistance rates were ≥50% in 2011–2013 (Figure 3). The Gram-positive bacteria such as E. faecalis, E. faecium, and S. aureus were most sensitive to linezolid and vancomycin (0%–4.7% were resistant).
The antibiotics that were used to treat cUTIs and the 20 most frequently prescribed first-line antibiotics were also reviewed (Tables S2 and S3). Third-generation cephalosporins (eg, cefixime, cefoperazone/tazobactam, cefoperazone/sulbactam, and ceftriaxone) and fluoroquinolones (mainly levofloxacin) were empirically administered as the first-line systemic treatment in 1,415 cases (36.10%) and 1,087 cases (28.35%), respectively. Third-generation cephalosporins were used to treat 60.07% of all cases (2,303 cases) and fluoroquinolones were used to treat 35.55% of all cases (1,363 cases). This treatment pattern may explain the high rates of resistance (>35%) of E. coli and K. pneumoniae to levofloxacin and many third-generation cephalosporins.
Clinical outcomes and economic burden
The median hospital stay for all patients with cUTIs was 11 days. During the study period, 8.15% of the patients experienced recurrent UTI infections, and the 30-day and 3-month readmission rates for these recurrent cases were 33.97% and 56.73%, respectively. A change in the medication was required in 1,790 (41.78%) of the cases (Table 4). The overall mortality rate was 2.41% compared to 0.61% for deaths caused by only UTI, and these rates exhibited significant decrease year-over-year. Patients with recurrent infections exhibited a significantly higher mortality rate, compared to the overall mortality rate (4.49% vs 2.41%, P<0.05). Patients who required a change in their antibiotic treatments incurred significantly greater treatment-related costs compared to patients who did not require a change in treatment (P<0.01) (Table 5).
Table 4.
Clinic outcomes of UTI
| Clinical outcome | Hospitalized cases with UTI |
|---|---|
| Length of stay (days), mean (±SD) [median] | 13.91 (19.31) [11] |
| ICU admission rate, n (%) | 213 (5.56) |
| Medication modified, n (%) | 1,790 (41.78) |
| Discharged from hospital, n (%) | 4,178 (97.53) |
| Mortality, n (%) | 106 (2.47) |
| Mortality (caused by UTI infection), n (%) | 26 (0.61) |
| Patients with recurrent infection, n (%) | 312 patients (8.15) |
| Mortality for patients with recurrent infection | 14 (4.49) |
| Recurrence interval (days), n patients (%) | 312 patients |
| ≤30 | 106 (33.97) |
| 30–90 | 71 (22.76) |
Abbreviations: UTI, urinary tract infection; ICU, intensive care unit.
Table 5.
Economic burden of UTI
| Cost category, US$, mean|median [range] | All cases N=4,238 | Cases with treatment modification N=1,779 | Cases with no treatment modification N=2,459 | P-valuea |
|---|---|---|---|---|
| Total cost | 2,328.28|1,440.61 [0.48–98,354.64] | 3,450.94|2,095.1 [0.73–98,354.64] | 1,516.07|1,143.72 [0.48–23,172.98] | <0.001 |
| Western medicine | 710.31|359.05 [0–40,267.45] | 1,146.61|549.65 [0–40,267.45] | 394.66|250.35 [0–6,205.73] | <0.001 |
| Herbs | 184.77|87.8 [0–6,350.98] | 224.88|107.51 [0–6,350.98] | 155.75|73.5 [0–3,031.23] | <0.001 |
| Examination | 511.12|353.87 [0–17,957.15] | 700.88|434.32 [0–17,957.15] | 373.84|308.55 [0–3,091.9] | <0.001 |
| Treatment procedure | 770.24|313.24 [0–36,220.32] | 1,176.65|611.11 [0–36,220.32] | 476.21|197.32 [0–14,238.16] | <0.001 |
| Bed | 145.65|96.77 [0–3,306.45] | 193.98|124.19 [0–3,306.45] | 110.68|79.03 [0–3,306.45] | <0.001 |
| Diagnosis | 6.2|4.84 [0–126.77] | 7.95|6.29 [0–126.77] | 4.94|3.87 [0–39.68] | <0.001 |
Note:
P-value was calculated using Wilcoxon rank-sum test.
Abbreviation: UTI, urinary tract infection.
Risk factors for prolonged hospitalization
A patient’s length of hospitalization is related to their medical resource usage and incurred costs. Therefore, we analyzed the risk factors for a delayed discharge among adult patients with a UTI. A stratified Kaplan–Meier survival curve was created for each variable, and the log-rank test was used to determine if there was a significant unadjusted relationship between the predictor variable and the time to discharge. The time to discharge in days was plotted against the survival distribution function for the different age groups, which revealed an increase with age (Figure S2). Age, sex, marriage, previous surgery, and comorbidities (diabetes or renal insufficiency) exhibited a statistically significant unadjusted relationship with a delayed discharge (Table S4). After further analysis of the variables’ interactions, we found that marriage status was correlated with age (R =0.43, P<0.001). Therefore, our Cox proportional hazards model did not include marriage status. This model revealed that age, sex, prehospitalization surgery, diabetes, and renal insufficiency were significantly associated with the time to discharge, after controlling for the other variables in the model. Compared to female patients, male patients were more likely to experience a prolonged hospitalization (hazard ratio: 1.12, 95% confidence interval: 1.05–1.20) (Table 6). Old age, previous surgery, diabetes, and renal insufficiency were risk factors for a delayed discharge among patients who were hospitalized for a UTI.
Table 6.
Relative risk of delay to discharge as determined by Cox models
| Stratified variable (study group|control group) | Hazard ratio* | 95% CI | P-value |
|---|---|---|---|
| Sex: male|female | 1.12 | 1.06–1.20 | <0.001 |
| Age (years): (30–90)|(18–30) | 1.42 | 1.26–1.60 | <0.001 |
| Age (years): ≥60|(18–30) | 2.08 | 1.85–2.34 | <0.001 |
| Diabetes: true|false | 1.17 | 1.08–1.27 | <0.001 |
| Kidney stone: yes|no | 0.98 | 0.91–1.06 | 0.670 |
| Renal insufficiency: true|false | 1.15 | 1.04–1.27 | 0.005 |
| Surgery history: true|false | 1.16 | 1.07–1.26 | <0.001 |
Notes:
Hazard ratio (HR) is the ratio of the hazard rates of length of stay for the study group compared to control group; HR >1 indicates longer delay to discharge while HR <1 indicates relatively short time of stay in hospital.
Discussion
UTIs are one of the most common infectious diseases among women of all ages, and affect ~10% of US women annually.4 It is well established that the prevalence of UTI increases with age, and this increase is seen in both sexes.18 Similarly, we found that 61.4% of the cUTIs in the present study were diagnosed in women, especially 75% in young patients. The average age of our patient cohort was 61 years, and the prevalence of UTI significantly increased with age. The pathogenic organisms were identified in 26.65% of the evaluated patients and this is a higher rate in comparison to similar Western studies.2,3,9,12,19 Gram-negative bacteria were the most commonly isolated organisms in the present study, and previous studies have reported similar findings.9,12,19 E. coli was the most common uropathogen, and was followed by Gram-positive Enterococcus spp., Candida spp., Gram-negative K. pneumoniae, P. aeruginosa, and P. mirabilis.
Current clinical treatments are guided by published recommendations for the diagnosis and treatment of UTIs in adults.20–23 In the absence of genitourinary symptoms, the infection is not treated except in cases of pregnancy or surgical manipulation of the urinary tract, although clinicians and patients have expressed concerns that delayed treatment will lead to adverse outcomes and increased morbidity.
Despite the widespread availability of antimicrobial agents, UTIs have become increasingly difficult to treat, due to increasing resistance to antimicrobial agents. Furthermore, patients with cUTIs are more likely to experience treatment failure and serious complications, since they are often caused by Gram-negative bacilli resistant to multiple antimicrobial drugs. In the patients who were examined, we observed high rates of resistance among the most frequently isolated Gram-negative rods. However, these bacteria were effectively eradicated using a peptidoglycan cross-linking blocker with a beta-lactamase inhibitor, carbapenems, or amikacin. Furthermore, most of the resistance that we observed was due to the expression of chromosomal AmpC β-lactamase, which is induced after exposure to β-lactams (a structural component of cephalosporins). These enzymes are resistant to inhibition using clavulanic acid, which indicates that different beta-lactamase inhibitors should be added to cephalosporins or piperacillin for the empirical management of UTIs. In contrast, carbapenems are broad-spectrum agents that can be used as empiric therapies for severe sepsis due to bacteria that produce extended spectrum β-lactamase. Although carbapenemase-producing E. coli and K. pneumoniae strains have been reported, they remain uncommon in China.24 Thus, antibiotics without beta-lactamase resistance should probably be reserved for the treatment of organisms whose sensitivity to those agents has been demonstrated with testing.
Surveillance studies of pathogens’ resistance to frequently used antibiotics have documented a decrease in their activity over time.25 Unfortunately, the extensive and inappropriate use of antimicrobial agents remains the primary cause of antibiotic-resistant uropathogens. Most bacterial isolates that we examined were resistant to two or more drugs, and these findings are similar to those in other geographic regions within China.26 For example, we found that E. coli was the predominant cause of cUTIs, with high rates of resistance to ampicillin, piperacillin, many third-generation cephalosporins, ciprofloxacin, and gentamycin. Unfortunately, multidrug resistance is an increasing concern in China, and this issue is usually attributed to the increased use of broad-spectrum antibiotics in the community. Therefore, programs that target inappropriate use are being set up, in order to decrease the prevalence of drug-resistant organisms, and additional actions have been suggested to address this problem. For example, the implementation of nationwide antimicrobial surveillance and in vitro susceptibility testing would promote better awareness regarding drug resistance and facilitate more rapid treatment with appropriate antibiotics. Furthermore, strict adherence to antibiotic use guidelines will inhibit the spread of drug-resistant microbes. Moreover, empirical treatment is typically used for bacteria with a resistance level of ≤10%, although our patients exhibited a resistance level of >10%, which indicates that empirical treatment alone is no longer appropriate. Therefore, sensitivity studies are needed to identify appropriate treatments for future use.
Our study also revealed the risk factors for a delayed discharge among patients with cUTIs. The results indicate that old age, previous surgery, diabetes, and renal insufficiency were risk factors for prolonged treatment and hospitalization. Therefore, it may be useful for helping clinicians in southern China to identify patients who need to get timely medical treatment and may require additional care.
To our best knowledge, this was the first study to investigate the clinical outcomes and economic costs among patients with cUTIs in southern China. Our results indicate that empirical antibiotic treatment exerted a large effect on clinical outcomes and treatment cost, as failure of the initial empirical treatment was the strongest independent predictor of a prolonged hospitalization and higher treatment costs. Furthermore, patients who failed the initial treatment required prolonged antibiotic treatment and hospitalization, compared to patients who were successfully treated using the initial treatment. Therefore, careful consideration is critical when selecting the initial treatment for cUTIs to optimize the clinical and economic outcomes. Moreover, our epidemiological data and profiles of local drug resistance trends can help physicians in southern China select appropriate antibiotic therapies for cases of cUTIs in this region.
This study also included several limitations that warrant consideration. First, only a small number of E. cloacae and S. aureus specimens were available for review, and these findings may not be representative of other patient populations. Second, the costs of the primary surgical procedures were not included in our analysis, as we assumed that they were independent of the choice of antibiotic. Thus, if these procedures were related to the choice of antibiotic, this relationship may have affected our cost-related findings. Third, the patients had been hospitalized and treated for cUTIs, and our findings may not be representative of patients who were not hospitalized or who had simple UTIs.
Conclusion
Multidrug resistance is an increasing concern when treating patients with cUTIs in southern China. However, no official guidelines are available to address this concern. Therefore, selection of an appropriate initial antibiotic treatment for cUTIs should be guided by a careful assessment of the patient’s characteristics (age, sex, and comorbidities).
Acknowledgments
This study was funded by AstraZeneca. The design and conduct of the study, as well as analysis of the study data and opinions, conclusions, and interpretation of the data, are the responsibility of the authors.
Footnotes
Disclosure
JW, YC, WG, ZS, and HY are employees of AstraZeneca. ZW and XL received institutional/research grant funding from AstraZeneca for the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Gastmeier P, Kampf G, Wischnewski N, et al. Prevalence of nosocomial infections in representative German hospitals. J Hosp Infect. 2007;38(1):37–49. doi: 10.1016/s0195-6701(98)90173-6. [DOI] [PubMed] [Google Scholar]
- 2.Ronald AR, Nicolle LE, Stamm E, et al. Urinary tract infection in adults: research priorities and strategies. Int J Antimicrob Agents. 2001;17(4):343–348. doi: 10.1016/s0924-8579(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 3.Barisic Z, Babic-Erceg A, Borzic E, et al. Urinary tract infections in South Croatia: aetiology and antimicrobial resistance. Int J Antimicrob Agents. 2003;22(Suppl 2):61–64. doi: 10.1016/s0924-8579(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B, Barlow R, D’Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 5.Engel JD, Schaeffer AJ. Evaluation of and antimicrobial therapy for recurrent urinary tract infections in women. Urol Clin North Am. 1998;25(4):685–701. x. doi: 10.1016/s0094-0143(05)70057-4. [DOI] [PubMed] [Google Scholar]
- 6.Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259–268. doi: 10.1016/s0924-8579(00)00350-2. [DOI] [PubMed] [Google Scholar]
- 7.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton A. Prevention of recurrent urinary-tract infections in women. Lancet. 1999;353(9146):7–8. doi: 10.1016/S0140-6736(05)74875-3. [DOI] [PubMed] [Google Scholar]
- 9.Beyene G, Tsegaye W. Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in Jimma University specialized hospital, southwest Ethiopia. Ethiop J Health Sci. 2011;21(2):141–146. doi: 10.4314/ejhs.v21i2.69055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamm WE, Hooton TM. Management of urinary tract infections in adults. New Eng J Med. 1993;329(18):1328–1334. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- 11.Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;38:1150–1158. doi: 10.1086/383029. [DOI] [PubMed] [Google Scholar]
- 12.Bonadio M, Meini M, Spitaleri P, Gigli C. Current microbiological and clinical aspects of urinary tract infections. Eur Urol. 2001;40(4):439–444. doi: 10.1159/000049813. discussion 445. [DOI] [PubMed] [Google Scholar]
- 13.Grude N, Tveten Y, Kristiansen BE. Urinary tract infections in Norway: bacterial etiology and susceptibility. A retrospective study of clinical isolates. Clin Microbiol Infect. 2001;7(10):543–547. [PubMed] [Google Scholar]
- 14.Yezli S, Li H. Antibiotic resistance amongst healthcare-associated pathogens in China. Int J Antimicrob Agents. 2012;40(5):389–397. doi: 10.1016/j.ijantimicag.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathai D, Jones RN, Pfaller MA, et al. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1,510 hospitalized patients: a report from the SENTRY Antimicrobial Surveillance Program (North America) Diagn Microbiol Infect Dis. 2001;40(3):129–136. doi: 10.1016/s0732-8893(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 16.Farrell DJ, Morrissey I, De Rubeis D, et al. A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J Infect. 2003;46(2):94–100. doi: 10.1053/jinf.2002.1091. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Second Informational Supplement. Wayne, PA: CLSI; 2012. (CLSI Document M100-S22). [Google Scholar]
- 18.Cove-Smith A, Almond M. Management of urinary tract infections in the elderly. Trends Urol Gynaecol Sexual Health. 2007;12:31–34. [Google Scholar]
- 19.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists ACOG Practice Bulletin No. 91: treatment of urinary tract infections in non-pregnant women. Obstet Gynecol. 2008;111:785–794. doi: 10.1097/AOG.0b013e318169f6ef. [DOI] [PubMed] [Google Scholar]
- 21.Epp A, Larochelle A, Lovatsis D, et al. Recurrent urinary tract infection. J Obstet Gynaecol Can. 2010;32(11):1082–1101. doi: 10.1016/S1701-2163(16)34717-X. [DOI] [PubMed] [Google Scholar]
- 22.Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J. 2011;5(5):316–322. doi: 10.5489/cuaj.11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 24.Lou Z, Qi Y, Qian X, et al. Emergence of Klebsiella pneumoniae carbapenemase-producing Escherichia coli sequence type 131 in Hangzhou, China. Chinese Medical J. 2014;127(3):528–531. [PubMed] [Google Scholar]
- 25.Masterton R. The importance and future of antimicrobial surveillance studies. Clin Infect Dis. 2008;47(Suppl 1):S21–S31. doi: 10.1086/590063. [DOI] [PubMed] [Google Scholar]
- 26.Qiao LD, Chen S, Yang Y, et al. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ Open. 2013;3(12):e004152. doi: 10.1136/bmjopen-2013-004152. [DOI] [PMC free article] [PubMed] [Google Scholar]