Abstract
There is a growing need for new translational animal models designed to capture complex behavioral phenotypes implicated in addiction and other neuropsychiatric conditions. For example, a complete understanding of the effects of commonly abused drugs, as well as, candidate medications, requires assessments of their effects on learning, memory, attention, and other cognition-related behavior. Modern touch-sensitive technology provides an extremely flexible means to expose an experimental subject to a variety of complex behavioral tasks designed to assay dimensions of cognitive function before, during, and after drug administration. In addition to tailored variants of gold standard cognitive assessments, touchscreen chambers offer the ability to develop novel tasks based upon the researcher’s needs. This methods perspective presents 1) a brief review of previous touchscreen-based animal studies, 2) a primer on the construction of a touch-sensitive experimental chamber, and 3) data from a proof-of-concept study examining cross-species continuity in performance across a diverse assortment of animal subjects (rats, marmosets, squirrel monkeys, rhesus macaques) using the repeated acquisition task – a modern variant of a traditional animal model of learning. Taken together, the procedures and data discussed in this review illustrate the point that contemporary touchscreen methodology can be tailored to desired experimental goals and adapted to provide formal similarity in cognition-related tasks across experimental species. Moreover, touchscreen methodology allows for the development of new translational models that emerge through laboratory and clinical discovery to capture important dimensions of complex behavior and cognitive function.
Keywords: Touchscreen, learning, rat, marmoset, squirrel monkey, rhesus macaque
INTRODUCTION
The use of self-administration, drug discrimination, and other procedures employing schedule-controlled performance has provided a wealth of information on the behavioral effects of psychoactive drugs. These operant-based approaches have been refined over the past five decades to provide rigorous and highly profitable analyses of pharmacological and environmental determinants of drug action (reviewed in Ator and Griffiths, 2003; Glennon and Young, 2011; Schindler et al., 2002). More recently, the importance of also understanding how psychoactive drugs, including both commonly abused drugs and therapeutic medications, modify other complex behavioral processes has been increasingly recognized. In the case of abused drugs, determining the extent to which they have adverse effects on learning, memory, attention, and other cognition-related behavior can provide a more complete profile of drug action and potential harm than conventional indices of abuse liability alone. For example, a number of reports have documented deleterious effects of monoaminergic stimulants such as methamphetamine on aspects of executive function such as cognitive flexibility (e.g., Groman et al., 2012; 2013; Izquierdo and Jentsch 2012; Kangas and Bergman, 2016). It would seem to be of clear importance to evaluate newly emerging types of drugs of abuse with monoaminergic mechanisms of action (e.g., bath salts) for their effects on such endpoints. In the case of candidate therapeutics, demonstrating inconsequential effects on cognition-related endpoints following administration of doses known to have medicinal value can provide important preclinical indications of the safety of a pharmacotherapy. Thus, in view of the widely-reported effects of cannabis on memory processes (Ranganathan and D’Souza, 2006; Solowij and Battisti, 2008), an examination of both the short-term and long-term effects of novel cannabinergic candidate medications on different facets of memory function can provide important preclinical indications of the likelihood and, perhaps, severity of such adverse events.
Touch-sensitive technology provides a means to address the above-described need by permitting the exposure of laboratory animals and human subjects to a battery of similar complex behavioral tasks that are designed to assay multiple dimensions of cognitive function before, during, and after drug administration. The commercially available Cambridge Neurological Test Automated Battery (CANTAB) was an early and important example of a touchscreen-based apparatus designed to capture these complex cognition-related behavioral endpoints. First developed for tasks in human subjects to assess cognitive deficits implicated in a variety of psychiatric conditions including schizophrenia, depression, and dementia, CANTAB remains a central diagnostic tool for clinical researchers and practitioners (Luciana, 2003; Robbins et al., 1994; Sahakian and Owen, 1992). Simplified touchscreen-based analogs of some of the same tasks were subsequently developed and made commercially available for behavioral and pharmacological studies in nonhuman primate subjects (Monkey CANTAB, Lafayette Instrument Company, Lafayette, IN). It is useful to consider how this technology could be applied. For example, Weed et al. (1999) summarized in rhesus macaques the acquisition and long-term performance of a variety of touchscreen-based models of memory (self-ordered spatial search and delayed non-matching to sample), attention and learning (intra-dimensional/extra-dimensional shift), fine motor performance (bimanual motor task and reaction time), and motivation (progressive ratio). Notwithstanding some individual differences in task acquisition, monkeys were readily able to perform multiple tasks with high levels of accuracy across extended periods of time. Importantly, the use of CANTAB technology allowed the researchers to convincingly identify a general correlation between task difficulty and performance sensitivity to parametric manipulations. As they notes, in addition to demonstrating the feasibility of these touchscreen-based analogs of human neuropsychological tests, the data provided normative information for within-subject comparison with results from acute and chronic drug treatment or other neurotoxic challenges, in much the same way as human CANTAB researchers can juxtapose between-subject performance from healthy controls and subjects with psychiatric conditions. Accordingly, Weed et al. (2004) went on to study macaques infected with simian immunodeficiency virus (SIV) in an animal model of AIDS, showing that, as with HIV-infected AIDS patients, tasks thought to involve frontostriatal dopaminergic functioning (i.e., self-ordered spatial search, bimanual motor task, and reaction time) were particularly vulnerable. These studies formed an elegant illustration of the great value of touchscreen technology, providing supportive evidence of homology between human and nonhuman primate behavioral test batteries (see also Nagahara et al., 2010) and, also, SIV and HIV infection models.
Other researchers have effectively used the CANTAB system for much smaller and evolutionarily distant monkey subjects. For example, Spinelli et al. (2004) evaluated the ability of marmosets to engage in CANTAB-based tasks, including the five-choice serial reaction time task (5-CSRT), stimulus discrimination and reversal task, delayed match-to-position task, and progressive ratio task. Results from that work show that marmosets were able to master the tasks presented and that performance was comparable to that observed with rhesus macaques in some tasks (e.g., 5-CSRT), but not in others (e.g., some variants of the delayed match-to-position task). In addition, marmosets needed to be trained and tested on tasks individually rather than, as was common in human and macaque studies, in a test battery during a single daily session. Nevertheless, this empirical validation of CANTAB in the marmoset identified suitable species-specific conditions to conduct subsequent analysis of muscarinic, nicotinic, and glutamatergic drugs on attention and working memory (Spinelli et al., 2005, 2006). Importantly, recent developments in precision gene editing have promoted the marmoset as an experimental subject of considerable translational value (Belmonte et al. 2015; Kishi et al. 2014; Sasaki et al. 2009). Thus, the elucidation of normative marmoset behavior will be critical to understanding the specificity of such genetic modifications on cognitive function and other organized complex behavior. This is an undertaking for which touchscreen chamber methodology is especially well-suited because, as stressed above, a variety of cognition-related procedures can be established with methodology that is both formally and functionally similar across nonhuman primate species and humans.
More recently, following optimization and assessment of task competency, smaller CANTAB-like touchscreen chambers and task software for rodent subjects have been developed and made commercially available (Bussey-Saksida Rat Touchscreen Chamber, Lafayette Instrument Company, Lafayette, IN). For example, Bussey et al. (2008) used this apparatus in a series of studies to optimize stimulus size and trial requirements, and to examine sex and strain differences, and discriminative capabilities of photographic stimuli. These and other methodological and pharmacological efforts in optimization and standardization (e.g., Cook et al., 2004; Mohler et al., 2015; Talpos et al., 2012) have verified the ability to assess analog task performance in rodents. However, accommodations in variables to reduce task complexity, as well as, careful consideration of visual stimulus dimensions, are paramount in rodent subjects. Nevertheless, these simplified cognitive tasks for rodents are becoming increasingly prevalent in translational neuropsychiatric research (reviewed in Bussey et al., 2012; Hvoslef-Eide et al., 2016).
The primary advantages of utilizing the CANTAB or other commercially available prefabricated systems include easy plug-and-play installation and a selection of empirically validated cognitive tasks that allow experimental data to be directly compared to those from other laboratories. This standardization has obvious advantages, especially in view of recent emphases on reproducibility within the scientific community (Open Science Collaboration, 2015). However, such off-the-shelf systems also have a few notable disadvantages. First, although the available tasks include several highly translational cognitive assessments, the base code to program the tasks is proprietary. Thus, the researcher’s ability to tailor experimental parameters is limited. Second, newly-developed tasks cannot be introduced into the battery, unless they are distributed by the manufacturer. Finally, the costs for patented touchscreen chamber hardware and licensed software may be severely prohibitive for smaller laboratories, restricting their ability to contribute to this important area of research.
An alternative approach has developed recently by focusing on the use of generic nonproprietary touch-sensitive hardware operated by researcher-composed code. This second generation touchscreen methodology allows for an experimental interface with maximal control over all parameters of traditional cognitive tasks and, also, the possibility of creating novel translational tasks (e.g., Daniel and Katz, 2016; Hutsell and Banks, 2015; Kangas and Bergman, 2012; Rice et al., 2017). The advantages of this approach are readily apparent, for example, in work to identify and capture key features of novel behavioral phenotypes. It is worth noting that the stalled development of innovative psychotherapeutics has been attributed, at least in part, to the inadequacy of current animal models (Nestler and Hyman, 2010). Thus, the growing appreciation of the need for information on how psychoactive drugs alter cognitive processes has been accompanied by a corresponding awareness of the need for new translational animal models to capture features of such neurobehavioral endpoints. Touchscreen-based apparatuses are well suited to address this need because they can be designed to allow a broad-based, yet dynamic, interface capable of methodological possibilities limited only by the experimenter’s imagination and programming abilities. Flexible touchscreen-based systems in which novel procedures can be creatively developed and optimized for use in both laboratory animals and humans, can greatly improve the evaluation of new psychotherapeutic drugs and the translational significance of those preclinical data. In addition, since non-proprietary touchscreen hardware and researcher-composed programs can be designed for a variety of chamber configurations, cognitive tasks may be shared across laboratories in an open source manner if researchers and their collaborators are so inclined. Finally, as touch-sensitive technology continues to improve and costs continue to fall, the budget-minded researcher may find the construction of a touchscreen chamber to be a fraction of the cost of prefabricated behavioral testing chambers equipped with a few lights and levers. This economy can be further maximized by designing the touchscreen chamber to accommodate a wide assortment of complex cognitive tasks, as well as operant assays that are currently widely used in behavioral pharmacology (e.g., self-administration, drug discrimination, schedule-controlled performance, etc.).
A PRIMER IN TOUCHSCREEN CHAMBER CONSTRUCTION
The general outline provided below is designed to be a brief primer for researchers interested in constructing a touch-sensitive operant chamber that can be tailored to individual experimental needs1. (Additional chamber construction details can be requested from the corresponding author.) Each chamber can be powered by a standard desktop or laptop computer. The touchscreen is connected through the video port and serves as the monitor for the computer. Screen touches (responses) are usually programmed to emulate mouse clicks. Using any commercially available or open source object-oriented programming software (e.g., E-Prime, MATLAB, Python, Visual Basic), a variety of touch-contingent and non-contingent visual and auditory stimuli can be scheduled. Touchscreens come in a variety of sizes and can be affixed to (or entirely comprise) the inside wall of a handmade cubicle or a prefabricated, but otherwise unequipped, commercially-available experimental chamber.
The Touchscreen (Multipurpose Intelligence Panel)
One may think of the touchscreen interface as a collection of programmable inputs and outputs. Inputs (touches) are detected on the screen, depending on the model, by either electric impulses via skin transduction or infrared beam breaks that are detected as x,y coordinates. Operanda (e.g., virtual levers) can be constructed via programming code, presented on the screen in a variety of positions, and made functional upon touch. Multiple operanda can be presented, singly or concurrently, in arrangements that are fixed or moving, and protracted and retracted instantly. Outputs also can be arranged on the screen visually. A near-infinite number of visually distinct stimuli including a spectrum of color, icons, photographs, and video clips can be presented on the screen in an assortment of stationary or dynamic arrangements. Importantly, inputs and outputs can be designed to be one and the same by programming discriminative visual stimuli that, when touched, serve as operanda producing consequent events. In addition to traditionally-conceived inputs and outputs, otherwise inactive background screen luminance and color can be purposed to serve two important functions. First, background screen luminance can provide general chamber illumination, eliminating the need for houselights and, by keeping all visual stimuli streamlined into the same source, further reduce required hardware. Second, multiple cognitive tasks can be presented to the well-trained subject in a single uninterrupted session by using distinct task-associated background screen colors to serve as contextual stimuli that signal which task is currently active. This is especially valuable in pharmacological research because the ability to assess multiple endpoints in the same session can provide a highly powerful within-subject evaluation of a drug’s relative potency on different dimensions of cognitive function (cf. Kangas et al., 2016b).
The Speaker (Auditory Feedback)
Adding a speaker to the chamber through the computer’s audio port provides the ability to program and present audio feedback in response to experimental events. For example, audible clicks marking responses to active virtual lever inputs can assist in refining operant response topography and improving stimulus control (Hake and Azrin, 1969). In addition, auditory stimuli that accompany the delivery of primary reinforcers can, if desired, also be arranged to serve as conditioned reinforcers (Kelleher and Gollub, 1962).
Non-Touchscreen Outputs (Delivery of Unconditioned Consequences)
Aside from the touchscreen, one or more additional outputs are typically required for the ability to administer primary reinforcers that may be either appetitive (food, drug) or noxious (shock). This delivery can be controlled using the computer’s USB or parallel ports. For example, programming the address of a parallel pin to deliver a pulse, contingent upon an event (e.g., a response or the end of a programmed interval), can be arranged to operate a pellet dispenser, syringe pump for i.v. drug infusions, or shock delivery to an electric floor grid.
Repeated Acquisition Task: A Case Study of Advantages
There are two fundamental programmatic advantages of designing an operant-based cognitive task in a touchscreen-equipped chamber. First, as discussed above, touchscreens have the capacity to present a near-infinite number of visually distinct stimuli to the subject. This makes it possible to engage the same subject in a variety of tasks without the concern of task generalization in a more conventional apparatus with the possibility of only a few colored stimulus lights. Second, having operanda (e.g., virtual levers) that do not have to remain in fixed positions allows the experimenter to avoid position biases, which otherwise are ubiquitous in operant research. For example, subjects commonly exhibit lever side biases in typical 2-lever operant conditioning chambers that cannot be accounted for by programmed contingencies (Mackay, 1991). Although correction procedures can reduce these biases in stimulus control (Kangas and Branch, 2008), the ability to present visually distinct operanda in varying screen locations across choice trials can preemptively avert this problem (Kangas and Bergman, 2012).
As discussed above, touch-sensitive chambers have been effectively used with a variety of laboratory animal species. A recent series of behavioral studies with rats and three diverse nonhuman primate species (marmoset, squirrel monkey, rhesus macaque) using the repeated acquisition task illustrates the programmatic strengths highlighted above. Originally developed by Harlow (1949), the repeated acquisition task is an animal model thought to capture some fundamental features of visual discrimination learning. In the original arrangement, an experimenter manually presented a nonhuman primate subject with two visually-distinct and concurrently-available objects. Displacing one of the objects (S+) would reveal a food well, displacing the other (S-) would reveal nothing. Through trial-and-error, the subject would learn to respond exclusively to S+. Following discrimination mastery, the experimenter would present another novel pair of stimuli (i.e., repeated acquisition). The fundamental discovery of these early capstone studies was not simply that the subject had the ability to learn numerous visual discriminations, but rather that the rate of acquisition increased in an orderly manner across successive problems mastered. Harlow termed this phenomenon learning set (or learn-to-learn). An automated version of this task designed for touchscreen chambers was recently developed to examine some basic parameters of this animal model of learning (Kangas and Bergman, 2012; 2014; Kangas et al., 2016a), and to refine its ability to serve as a component of a larger battery of cognitive tasks for the examination of both commonly abused drugs and candidate medications (e.g., Kangas and Bergman, 2016; Kangas et al., 2016b).
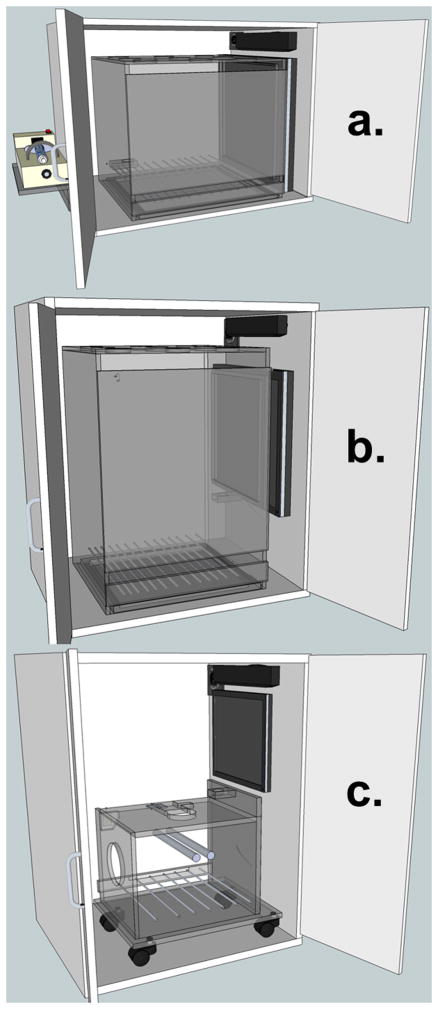
To evaluate cross-species continuity of the learning set phenomenon using this touchscreen variant of the repeated acquisition task, four commonly used animal subjects were examined. Four adult male rats (Long-Evans strain), four adult male marmosets (Callithrix jacchus), four adult male squirrel monkeys (Saimiri sciureus), and four juvenile female rhesus macaques (Macaca mulatta) were studied in sound- and light-attenuating touchscreen chambers with specifications that varied in accordance with the size of the subject. All chambers were equipped with a 17″ touch-sensitive screen (1739L, ELO TouchSystems, Menlo Park, CA) and a speaker bar (NQ576AT, Hewlett-Packard, Palo Alto, CA) mounted above to provide audible feedback. Specifications for the marmoset chamber can be found in Kangas et al. (2016a). Rats were studied in chambers with identical specifications to the marmoset chambers (see also Fig. 1A). Schematics and photographs of the squirrel monkey chamber can be found in Kangas and Bergman (2012; see also Fig. 1B). All subjects except rhesus macaques were unrestrained in their respective experimental chambers during experimental sessions. The rhesus macaques were seated in a primate chair that, during experimental sessions, was positioned inside a sound- and light-attenuating enclosure measuring 150×75×85 cm; the touchscreen was mounted to the inside right wall of the enclosure (see also Fig 1C). Sweetened condensed milk (30% milk/70% water) served as the consequence to maintain behavior for all subjects. An infusion pump (PHM-100, Med Associates, St. Albans, VT) outside the enclosure was used to deliver precise quantities of milk into an accessible well in volumes of 0.1 mL for rats, 0.15 mL for marmosets and squirrel monkeys, and 0.3 mL for rhesus macaques. All experimental events and data collection were programmed in E-Prime Professional 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA).
Figure 1.
Touchscreen chamber schematics for rats and marmosets (a), unrestrained squirrel monkeys (b), and chaired rhesus macaques (c).
Response shaping techniques were used to first train all subjects to touch the screen with a paw (see Kangas and Bergman, 2012, for touch training protocol details). After reliable touch responding was observed, subjects were exposed to the repeated acquisition task. Each session began with concurrent presentation of two novel 7×7 cm digital photographs. For nonhuman primate subjects, each stimulus was positioned in a different quasi-randomly selected quadrant of the screen. For rat subjects, each stimulus was presented quasi-randomly either left or right of center, 10 cm above the floor bars, requiring the subject to rear to reach the stimulus with its paw. For nonhuman primate subjects, a touch response on one stimulus (S+) produced an audible feedback click, initiated milk delivery paired with an 880 ms yellow screen flash and a 440-hz tone, followed by a 10-s inter-trial interval (ITI) blackout; a touch response on the other stimulus (S-) produced an audible feedback click and immediately initiated the 10-s ITI blackout. For rat subjects, a fixed-ratio 3 (FR3) was first required on a stimulus to produce the S+/S− consequences describe above1. (In pilot studies, some rats appeared to inadvertently touch a stimulus when rearing. Therefore, the FR3 response requirement was programmed to avoid counting premature responses.) The same two stimuli were presented during each of 200 trials comprising each session. The primary dependent variable was the number of trials to acquire the discrimination; the criterion for mastery was responses on the S+ stimulus in nine of ten consecutive trials (i.e., ≥90% correct). If mastery was achieved, subjects were presented with a new S+/S− pair during the next session. If the subject failed to master the discrimination within the 200-trial session, the same stimuli were presented during the next day’s 200-trial session. Photographs for each session were selected from a laboratory bank of >10,000 images. Thus, the subject was required to repeatedly learn new S+/S− discriminations based on distinguishing features of two visual stimuli that had not been previously viewed. Sessions were conducted until 30 discriminations were mastered.
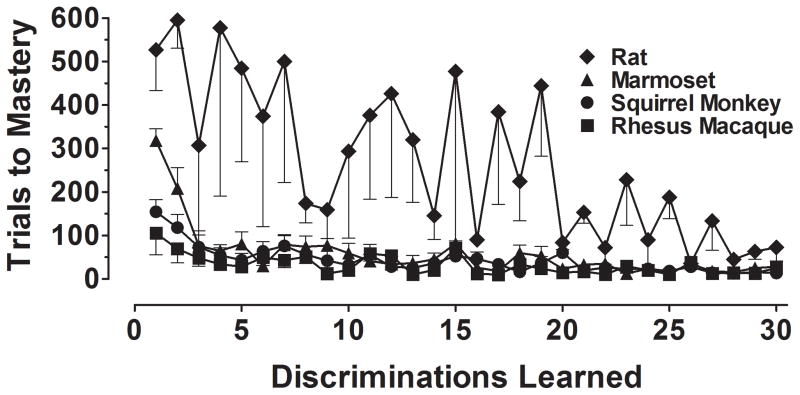
Figure 2 presents group averages for the number of trials (±S.E.M.) required to acquire each of the first 30 novel discriminations under the repeated acquisition task in rats (diamonds), marmosets (triangles), squirrel monkeys (circles), and rhesus macaques (squares). No consistent position or side biases were observed in any of the species tested (data not shown). In general, the rate of acquisition in all species increased across successive discriminations until subjects acquired the discrimination quickly each session. This was evident in the number of trials to reach mastery, which decreased steadily across the set of 30 discriminations.
Figure 2.
Mean number of trials (±S.E.M.) to master (≥90% correct) the first 30 novel visual discriminations during repeated acquisition in rats (diamonds), marmosets (triangles), squirrel monkeys (circles), and rhesus macaques (squares), n=4/group.
In rats, both the number of trials to mastery required and between-subject variability in performance were markedly higher than observed in the 3 nonhuman primate species. Acquisition rates were beginning to approximate nonhuman primates levels following 25 mastered discriminations; however, given that sessions were comprised of 200 trials for all species tested, this took on average approximately 30 additional sessions relative to the nonhuman primates. Although the performance of rats was considerably below that of the nonhuman primate subjects tested, it is nevertheless remarkable that subjects with well-recognized constraints in visual function (see Jacobs et al., 2001) were able to repeatedly learn visual discriminations and, like nonhuman primates with excellent visual function, do so at an increasing rate of acquisition. The precise determining factors contributing to the development of this performance are unclear; however, it is likely the case that the ability to present large, bright, salient stimuli on the touchscreen is primarily responsible for the effective repeated acquisition of visual discriminations in the rat.
Juxtaposing performance in the 3 nonhuman primate species reveals an orderly ranking in acquisition rate, most apparent in the number of trials required to master the first 10 discriminations (rhesus macaque < squirrel monkey < marmoset). Interestingly, this performance ranking corresponds to their evolutionary distance to humans. However, the meaningfulness of this observation is uncertain because, following the mastery of approximately 10 discriminations, acquisition rate reached a plateau of approximately 15–25 trials to mastery, on average, and individual-subject performance became increasingly indistinguishable across the 3 nonhuman primate species.
CONCLUSION
As described above, CANTAB methodology provided a first generation of powerful touchscreen-based techniques for studying cognition and other complex behavior. More recently, touchscreen technology has advanced to the point that touchscreen-based systems can be constructed for a variety of research applications in different species. Although performances may differ based on dissimilar physiology, hardware specifications and behavioral programs can be tailored to accommodate such differences and maximize assay flexibility across species. The touchscreen data shown in Figure 2 provide an example of formally equivalent translational studies across species and furthermore suggest that, depending on experimental and pharmacological goals, this approach can be used as an indicator of model and species fitness. More generally, the present studies illustrate available and relatively inexpensive means by which traditional cognitive assessment can be modernized for laboratory research. Perhaps the most promising feature of modern touch-sensitive technology lies in the potential for designing completely novel animal models to capture important dimensions of complex behavior and cognitive function that emerge through laboratory and clinical discovery.
Acknowledgments
The authors have no conflicts of interest to report. The authors thank Michelle Doyle, David Jacobs, Michael Leonard, and Erica Porter for assistance conducting these studies. This research was supported by grants K01-DA035974 (BDK) and R01-DA035857 (JB) from the National Institute on Drug Abuse.
References
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70(3 Suppl):S55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Belmonte JC, Callaway EM, Churchland P, Caddick SJ, Feng G, Homanics GE, Lee KF, Leopold DA, Miller CT, Mitchell JF, Mitalipov S, Moutri AR, Movshon JA, Okano H, Reynolds JH, Ringach D, Sejnowski TJ, Silva AC, Strick PL, Wu J, Zhang F. Brains, Genes, and Primates. Neuron. 2015;86:617–631. doi: 10.1016/j.neuron.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, Saksida LM. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn Mem. 2008;15:516–523. doi: 10.1101/lm.987808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RG, Geller AI, Zhang GR, Gowda R. Touchscreen-enhanced visual learning in rats. Behav Res Methods Instrum Comput. 2004;36:101–6. doi: 10.3758/bf03195555. [DOI] [PubMed] [Google Scholar]
- Daniel TA, Katz JS. A negative stimulus movement effect in pigeons. Behav Processes. 2016;130:11–18. doi: 10.1016/j.beproc.2016.04.020. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, editors. Drug discrimination: Applications to medicinal chemistry and drug studies. Malden, MA: Wiley; 2011. [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD. Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32:5843–5852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Morales AM, Lee B, London ED, Jentsch JD. Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology (Berl) 2013;229:527–538. doi: 10.1007/s00213-013-3159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake DF, Azrin NH. A response-spacing effect: an absence of responding during response-feedback stimuli. J Exp Anal Behav. 1969;12:17–25. doi: 10.1901/jeab.1969.12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF. The formation of learning sets. Psychol Rev. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Hutsell BA, Banks ML. Effects of environmental and pharmacological manipulations on a novel delayed nonmatching-to-sample ‘working memory’ procedure in unrestrained rhesus monkeys. J Neurosci Methods. 2015;251:62–71. doi: 10.1016/j.jneumeth.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvoslef-Eide M, Nilsson SR, Saksida LM, Bussey TJ. Cognitive translation using the rodent touchscreen testing approach. Curr Top Behav Neurosci. 2016;28:423–47. doi: 10.1007/7854_2015_5007. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH, Fenwick JA, Williams GA. Cone-based vision of rats for ultraviolet and visible lights. J Exp Biol. 2001;204:2439–2446. doi: 10.1242/jeb.204.14.2439. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys. J Neurosci Methods. 2012;209:331–336. doi: 10.1016/j.jneumeth.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. Repeated acquisition and discrimination reversal in the squirrel monkey (Saimiri sciureus) Anim Cogn. 2014;17:221–228. doi: 10.1007/s10071-013-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. Effects of self-administered methamphetamine on discrimination learning and reversal in nonhuman primates. Psychopharmacology (Berl) 2016;233:373–380. doi: 10.1007/s00213-015-4107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Branch MN. Empirical validation of a procedure to correct position and stimulus biases in matching-to-sample. J Exp Anal Behav. 2008;90:103–112. doi: 10.1901/jeab.2008.90-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J, Coyle JT. Touchscreen assays of learning, response inhibition, and motivation in the marmoset (Callithrix jacchus) Anim Cogn. 2016a;19:673–677. doi: 10.1007/s10071-016-0959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, Bergman J. Comparisons of Δ9-Tetrahydrocannabinol and Anandamide on a Battery of Cognition-Related Behavior in Nonhuman Primates. J Pharmacol Exp Ther. 2016b;357:125–133. doi: 10.1124/jpet.115.228189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Gollub LR. A review of positive conditioned reinforcement. J Exp Anal Behav. 1962;5:543–597. doi: 10.1901/jeab.1962.5-s543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Sato K, Sasaki E, Okano H. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev Growth Differ. 2014;56:53–62. doi: 10.1111/dgd.12109. [DOI] [PubMed] [Google Scholar]
- Luciana M. Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB) J Child Psychol Psychiatry. 2003;44:649–663. doi: 10.1111/1469-7610.00152. [DOI] [PubMed] [Google Scholar]
- Mackay HA. Conditional stimulus control. In: Iversen IH, Lattal KA, editors. Techniques in the behavioral and neural sciences: Vol. 6. Experimental analysis of behavior. Amsterdam: Elsevier; 1991. pp. 301–350. [Google Scholar]
- Mohler EG, Ding Z, Rueter LE, Chapin D, Young D, Kozak R. Cross-site strain comparison of pharmacological deficits in the touchscreen visual discrimination test. Psychopharmacology (Berl) 2015;232:4033–41. doi: 10.1007/s00213-015-4012-0. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Bernot T, Tuszynski MH. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging. 2010;31:1020–1031. doi: 10.1016/j.neurobiolaging.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Open Science Collaboration. Estimating the reproducibility of psychological science. Science. 2015;349(6251):aac4716. doi: 10.1126/science.aac4716. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Rice NC, Makar JR, Myers TM. Sex and the stimulus-movement effect: Differences in acquisition of autoshaped responding in cynomolgus monkeys. Physiol Behav. 2017;171:40–49. doi: 10.1016/j.physbeh.2016.12.028. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 2002;163:327–44. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR. Performance of the marmoset monkey on computerized tasks of attention and working memory. Cognitive Brain Res. 2004;19:123–137. doi: 10.1016/j.cogbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Ballard T, Gatti-McArthur S, Richards GJ, Kapps M, Woltering T, Wichmann J, Stadler H, Feldon J, Pryce CR. Effects of the mGluR2/3 agonist LY354740 on computerized tasks of attention and working memory in marmoset monkeys. Psychopharmacology (Berl) 2005;179:292–302. doi: 10.1007/s00213-004-2126-x. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Ballard T, Feldon J, Higgins GA, Pryce CR. Enhancing effects of nicotine and impairing effects of scopolamine on distinct aspects of performance in computerized attention and working memory tasks in marmoset monkeys. Neuropharmacology. 2006;51:238–50. doi: 10.1016/j.neuropharm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Talpos JC, Fletcher AC, Circelli C, Tricklebank MD, Dix SL. The pharmacological sensitivity of a touchscreen-based visual discrimination task in the rat using simple and perceptually challenging stimuli. Psychopharmacology (Berl) 2012;221:437–49. doi: 10.1007/s00213-011-2590-z. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Weed MR, Gold LH, Polis I, Koob GF, Fox HS, Taffe MA. Impaired performance on a rhesus monkey neuropsychological testing battery following simian immunodeficiency virus infection. AIDS Res Hum Retroviruses. 2004;20:77–89. doi: 10.1089/088922204322749521. [DOI] [PubMed] [Google Scholar]