Abstract
We investigated the relationships between cerebral blood flow (CBF), cognitive and mobility decline in type 2 diabetes mellitus (T2DM) over a two-year period. Seventy-three participants (41 T2DM and 32 controls) were evaluated using volumetric CBF with arterial spin labeling (ASL) perfusion magnetic resonance imaging (pMRI) at baseline and at the two-year follow-up. Regions with significant CBF differences between T2DM participants and controls at baseline were detected using voxel-wise analysis. Correlation analysis was performed to investigate the association between regional CBF and cognitive or mobility performance over the two-year span. Compared to controls, participants with T2DM had decreased CBF in the resting state default mode, visual, and cerebellum networks. Greater decrease in longitudinal CBF values at these regions over a two-year span was associated with worse gait, memory and executive functions, and higher baseline insulin resistance and worse baseline cognitive performance. In T2DM, impairment of resting regional perfusion is closely related to worse cognitive and mobility performance. Insulin resistance may further contribute to regional perfusion deficit in T2DM.
Keywords: type 2 diabetes mellitus, cognitive impairment, cerebral blood flow, arterial spin labeling MRI, voxel-based analyses, insulin resistance
1. Introduction
Type 2 diabetes mellitus (T2DM) is associated with altered cerebral vasoreactivity (Novak et al. 2011; Chung et al. 2015; Last et al. 2007), cerebral atrophy (Franke et al. 2013; Novak et al. 2011; van Elderen et al. 2010), cognitive impairment (Chung et al. 2015; Wong, Scholey, and Howe 2014) and functional decline (Chung et al. 2015). T2DM-related endothelial dysfunction secondary to a chronic state of hyperglycemia, inflammation and insulin resistance (Kim et al. 2006; Starr et al. 2003; Brownlee 2005), has been associated with alterations in the blood brain barrier (Mogi and Horiuchi 2011; Starr et al. 2003), neuronal damage (Umegaki 2014), and arterial stiffness (Zhou, Zhang, and Lu 2014), thus negatively affecting cerebral metabolism and cerebral blood flow (CBF)(Roberts et al. 2014).
Arterial spin labeling (ASL) is a functional MRI technique capable of quantifying regional CBF, among the several neuroimaging techniques. ASL is a noninvasive technique that magnetically labels the water in the blood vessels (Detre et al. 1992; Williams et al. 1992). ASL has been recently used to evaluate CBF in T2DM patients, however the reports are contradictory. Some studies found that resting CBF is similar between T2DM patients and controls (Tiehuis et al. 2008; Rusinek et al. 2015; Novak et al. 2011), while others reported reduced CBF in patients with T2DM (Xia et al. 2015; Novak et al. 2006; Last et al. 2007; Nagamachi et al. 1994). The cross-sectional design of these studies however, did not allow to investigate the association between T2DM and changes in brain perfusion or structure and cognitive or mobility functions over time. Pseudo-continuous ASL (PCASL), with great labeling efficiency (Dai et al. 2008; Wu et al. 2007) and recommended for clinical applications (Alsop et al. 2014), was adopted for the measurement of whole-brain CBF maps. Moreover, PCASL has been shown to have excellent test-retest reliability for both young and elderly subjects (Xu et al. 2010), and hence can serve as a useful technique even for longitudinal studies.
In the current study, we used ASL CBF imaging to determine the effect of T2DM on CBF at baseline and the relationship between CBF and functional outcomes (cognition and gait) baseline and after the two-year follow-up. We hypothesized that 1) T2DM is associated with altered resting perfusion patterns, and 2) impaired CBF is associated with alterations in cognitive function and gait.
2. Methods
The current work is an analysis from our prospective study on cerebro-microvascular disease in elderly with T2DM. The study was conducted at the Syncope and Falls in the Elderly Laboratory (SAFE), Clinical Research Center (CRC) and MRI Center of Beth Israel Deaconess Medical Center (BIDMC) between August 2009 and July 2013. Participants were recruited from the greater Boston area using community advertisement. They were assigned to either the T2DM group or the non-diabetic control group.
2.1. Participants
A hundred and thirty-one participants, 50–85 years old, were enrolled in this two-year study. All participants signed written informed consent approved by the Committee on Clinical Investigations of BIDMC. Of 131 participants, seventy-three participants, 41 T2DM and 32 non-diabetic controls were eligible and included in baseline analysis, according to the inclusion criteria of the study. Of those, forty-two participants, 19 T2DM and 23 non-diabetic controls, who completed the two-year follow-up were included in the follow-up analyses.
Inclusion criteria for the T2DM group were: men and postmenopausal women of 50–85 years of age, diagnosed with T2DM and treated with oral agents and/or insulin for more than 5 years, with hypertension (blood pressure (BP) ≥140/90 mm Hg and/or treated for hypertension) or without hypertension (BP <140/90 mm Hg and no medical history of hypertension). Inclusion criteria for the control group were: men and postmenopausal women with normal fasting blood glucose and glycated hemoglobin A1c (HbA1c) matched with the diabetes group by age ±5 years, gender, and presence of hypertension. Exclusion criteria for both groups were: Type I diabetes mellitus, any unstable or acute medical condition, myocardial infarction or major surgery within 6 months, history of a major stroke, carotid stenosis >50% by medical history, Doppler ultrasound or by MR angiography, hemodynamically significant vascular disease, arrhythmias, liver or renal failure or transplant, severe hypertension (systolic BP >200 and/or diastolic BP >110 mm Hg or subjects taking 3 or more antihypertensive medications), seizure disorders, malignant tumors, current recreational drug or alcohol abuse, active smoking, morbid obesity (BMI >40), dementia (by history) or Mini-Mental State Examination (MMSE) score (≤24). MRI exclusion criteria included: incompatible metal implants, pacemakers, and claustrophobia.
Reasons for exclusion from baseline analyses included: withdrew consent (n=11), lost to follow-up (n=10), smoking (n=1), arrhythmias (n=4), cancer (n=2), MMSE score ≤24 (n=3), stroke/TIA (n=2), heart failure (n=1), MRI-exclusion criteria (n=1), renal failure (n=1), T2DM <5 years (n=3), poor glycemic and/or hypertension control (n=7), undetermined neurologic disorder (n=2), adverse event (n=1), and incomplete datasets (n=9). Reasons for exclusion after two-year follow-up period included: withdrew consent (n=5), lost to follow-up (n=25), and new diagnosis of dementia (n=1).
2.2. Experimental Protocol
Screening visit included: medical history review, completion of autonomic function questionnaires, physical and neurological evaluation, ECG and fasting laboratory measurements. After enrollment, participants came for an inpatient two-day baseline visit at the BIDMC CRC. On Day 1, participants had vital signs and anthropometric measurements taken, including height and weight, and a cognitive assessment battery testing. On Day 2, a fasting blood draw was obtained, a cognitive assessment battery testing and walking test were completed, and MRI scans were performed. The same protocol was completed at the two-year follow-up visit.
2.3. Cognitive Assessment
The cognitive assessment battery that was used is a standard battery of cognitive tests that evaluate specific domains of cognition and daily living activities. It consists of measures of learning and memory (Hopkins Verbal Learning Test-Revised (HVLT-R) (Shapiro et al. 1999) and Mini-Mental State Examination (MMSE) (Folstein, Folstein, and McHugh 1975)), measures of executive function (Verbal fluency (VF) (Benton and Hamsher 1989), Trail Making (TM) (Pugh et al. 2003), Clock Drawing (CD) (Grande et al. 2005)), and measures of attention (Digit Span (DS) (Wechsler 1987)). HVLT-R includes a Total Recall (HVLT: Total Recall, total number of list items learned across trials), Delayed Recall (HVLT: Delayed Recall, total number of list items recalled after the delay), Retention (HVLT: Retention, percentage of items from HVLT: Total Recall that are subsequently recalled on HVLT: Delayed Recall), and Recognition Discrimination index (HVLT: RDI, number of list items correctly identified among non-list items). MMSE assesses cognitive impairment. VF assesses phonemic and semantic fluency tasks. The phonemic fluency task requires the participant to generate as many words as possible beginning with a given letter (e.g., “S”) for one minute. The semantic fluency task requires the participant to generate items of a given semantic category (e.g., animals) for one minute. Dependent variables for the fluency measures include the number of items generated for all three phonemic trials (e.g., F, A, S; VF FAS Total) and the number of items generated for the semantic task (e.g., animals; VF: animals). A composite executive score was calculated from all three measures of executive functions (VF, TM, and CD). DS assesses immediate memory/attention.
2.4. Gait Assessment
Participants completed two 6-min walking tests on a 75 m course of an 80 m x 4 m indoor hallway. For the first test participants were instructed to walk for 6 min at their usual and comfortable pace (normal walk), while for the second dual-task test (DT) they were asked to perform the same while counting backwards. The time taken to complete each 75 m length and the total distance walked were recorded. No assistive devices were used for ambulation. The rate of perceived exertion (RPE) before and after each walking test was self-rated on a 10-point scale. Gait speed was calculated by dividing distance (m) by time (s).
2.5. Blood samples analysis
Serum/plasma glucose, insulin panels and hematocrit were measured at Lab Corp (Laboratory Corporation of America Holdings, Burlington, NC). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as the product of fasting glucose (md/dl) times insulin levels (mU/L) divided by 405 (Matthews et al. 1985).
2.6. MRI Acquisition
All participants (73 participants at baseline and 42 participants at the two-year follow-up) were scanned at the same 3-Tesla, GE HDxt scanner using a receive-only 8-channel head array coil and a body transmit coil. ASL images were obtained using the pseudo-continuous arterial spin labeling (PCASL) (Dai et al. 2008) with a 1.5 sec labeling and 1.5 sec post-labeling delay. Additional reference images for M0 values were obtained for absolute perfusion quantification. All ASL and reference images were acquired with a 3D stack of spirals RARE imaging sequence (TR = 5 s, FOV = 24 cm × 24 cm, slice thickness = 4mm, matrix size: 128×128×40, bandwidth = 62.5 kHz). T1 anatomical images were acquired with a 3 dimensional magnetization prepared rapid acquisition gradient echo (MP-RAGE) sequence (TR = 7.9 msec, TE = 3.2 msec, flip angle = 158, bandwidth = 32 kHz, coronal acquisition plane field of view = 24 × 19cm, in-plane resolution = 0.94mm, slices = 1.4mm, preparation time with repeated saturation at the beginning of the preparation period = 1100 msec, and adiabatic inversion pulse before imaging = 500 msec). An MRI scanner upgrade was performed between the baseline and two-year follow-up scans, and the global signals may be altered between the scans. Therefore, we chose to present the relative CBF for the longitudinal analysis to reduce the effects of global signal change between two years.
2.7. Image Processing
Quantitative CBF images were calculated for each participant as previously described (Alsop and Detre 1996; Buxton et al. 1998; Wang et al. 2002). CBF maps were normalized to the a priori gray matter (GM) template using the SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm/). T1 anatomical images served as intermediate images for CBF image normalization as they allow better alignment with the template. They were segmented by the “new segment” algorithm (Ashburner and Friston 2005; Klein et al. 2009), which output gray GM images as well as other images in the original image space. Subtraction images (between label and control) from PCASL acquisition were co-registered to the GM images from the segmentation and then the GM images were normalized to the GM template. The combined warping parameters from the co-registration and normalization were used to warp the quantitative CBF maps from each subject to the template space. Quantitative CBF maps were smoothed using a Gaussian kernel with full-width at half maximum (FWHM) of 8 mm. Global CBF was calculated as the average of the CBF values on the whole brain mask.
To control for the effects of potential differences in tissue volume between the two groups that could account for differences of CBF, the GM probability map for each participant were generated using T1 anatomical images from the segmentation of SPM8. The binary map of GM probability map (assign 1/0 to the voxels with GM probability greater/lower than 0.2) was transformed to the standard GM template using SPM spatial normalization with volume-preserving transformation. Total volume of GM was calculated and used as a covariate of CBF analysis for GM volume correction.
2.8. Statistical Analysis
Demographic and neuropsychological data were analyzed with MATLAB, version R2015a. All tests were 2-tailed and significance was set for p < 0.05. Normality was assessed with Shapiro-Wilk test. Differences in demographic and neuropsychological characteristics at baseline and two-year follow-up between the T2DM patients and the controls were compared using either two-sample t-tests or Mann-Whitney nonparametric U-tests for continuous variables and χ2 test for categorical variables.
2.8.1. Global CBF and CBF maps at baseline and two-year follow-up
Global CBF between the two groups was initially compared at baseline using two-sample t-test. Given that hematocrit and gender are important predictors of CBF (Liu et al. 2012; Henriksen et al. 2014; Henriksen et al. 2013), and hematocrit is significantly different between the two genders (p ≤ 0.001, in the current study), Pearson correlation was performed between each variable (hematocrit or gender) and CBF in order to determine which one is the most sensitive predictor of CBF. Hematocrit (r = −0.39, p ≤ 0.001) but not gender (r = 0.18, p = 0.14) was negatively correlated with CBF, thus hematocrit was used as a confounder in the subsequent analysis. Multiple linear regression analysis was performed with CBF as the dependent variable, group (T2DM/Control) as the independent variable and age, hematocrit and hypertension (presence/no presence) as confounding variables.
In order to examine CBF differences between the two groups across the whole brain volume, CBF map was modeled as a multiple linear regression on a voxel-by-voxel basis using SPM8. Age, hematocrit and hypertension were included as covariates. The voxel-level significance threshold was set for p < 0.005 while the cluster-level threshold was set for p < 0.05 in order to minimize any false positive findings because of the multiple comparisons.
Due to the potentially increased family-wise error (FWE) rates from the SPM cluster-level analysis (Woo, Krishnan, and Wager 2014; Eklund, Nichols, and Knutsson 2016), we verified the statistical results using Statistical nonParametric Mapping (SnPM, http://www.sph.umich.edu/ni-stat/SnPM/). The non-parametric approach was shown to be robust with the nominated false positive rate of 5% (Eklund, Nichols, and Knutsson 2016). In the SnPM analysis, CBF map was modeled as a multiple linear regression on a voxel-by-voxel basis. Age, hematocrit and hypertension were included as covariates same as those in the SPM analysis. One-thousand random permutations were performed on the disease state (either T2DM or control). For each permutation, a voxel-level p-value threshold of 0.005 (same as SPM analysis) was chosen to define clusters first and then the largest supra-threshold cluster size was calculated. Largest cluster sizes from all 1000 permutations were used to calculate the empirical distribution in order to correct for multiple comparisons among voxels. The cutoff cluster size with FWE of 5% was derived. The T2DM affected regions were used for further region-based analysis. Based on the T2DM affected regions at baseline, post-hoc brain network-based analysis was also performed to identify the involvement of brain networks in T2DM. CBF value at each identified brain network was calculated as the mean CBF over the corresponding brain network mask. The brain network masks were generated from our recent ASL resting state network study (Dai et al. 2016).
Global CBF values and CBF maps between the T2DM group and the control group were compared at the two-year follow-up using the same approach as described at baseline. Regional and network CBF values at the regions and networks affected by T2DM (derived from baseline) were calculated at the follow-up, and compared using two-sample t-tests and multiple linear regression with the same confounding variables as in the global CBF comparisons at baseline.
2.8.2. Post-hoc correlation analysis of regional and network CBF at baseline
In order to investigate the relationship between CBF in the brain regions and networks affected by T2DM and cognitive and mobility performance, post-hoc correlation analysis was performed. The partial correlation coefficients between the regional CBF values and cognitive or mobility performance scores were examined with age, hematocrit and hypertension being used as covariates. Since the difference in GM volume between T2DM and control groups could potentially account for the CBF differences between the two groups, GM volume was further included in the partial correlation analysis as an additional covariate.
2.8.3. Longitudinal change of global CBF and CBF maps over a two-year period
Longitudinal change of global CBF was compared between the T2DM and control groups using a multiple linear regression with the same confounding variables as in the global CBF comparison at baseline. Longitudinal change of CBF maps was also compared between the two groups using the same SPM8 voxel-by-voxel analysis as in the comparison of CBF maps at baseline. For the longitudinal change of CBF maps, we used the relative CBF maps (calculated as the ratio of CBF maps to the associated global CBF value) at baseline and two-year follow-up to reduce the effect of of potentially altered global CBF over a two-year period. Longitudinal regional and network CBF change at the T2DM affected regions and networks (derived from the baseline study) was also compared using the same tests as in the baseline regional CBF comparison. The relative regional CBF values (regional CBF values divided by the corresponding global CBF values) were also used in the calculation of longitudinal regional CBF change.
2.8.4. Correlation of longitudinal CBF change with longitudinal variable change/baseline variable
The longitudinal correlation analysis was performed only for the T2DM affected regions and networks. In order to investigate whether the longitudinal CBF change is related to the longitudinal change of cognitive and mobility performance, post-hoc correlation analysis was performed. Partial correlation coefficients were calculated between CBF change and the baseline variables (including cognitive, mobility and disease severity variables) in order to investigate which baseline variables can predict the longitudinal CBF change. Age, hematocrit, hypertension, GM volume and education years were used as covariates. Education was included as an additional covariate because it was significantly different between the T2DM and control groups at two-year follow-up (p=0.012).
3. Results
Table 1 summarizes participants’ demographic and clinical characteristics, gait results and cognitive scores at baseline and at the two-year follow up. At baseline, no differences between age, gender, education, and hematocrit values were found between the two groups. T2DM group had higher prevalence of hypertension (p≤0.001), body mass index (BMI) (p=0.007), fasting glucose (p≤0.001), HbA1c (p≤0.001), insulin level (p=0.003) and HOMA-IR (p≤0.001) compared to controls. Furthermore, participants with T2DM had worse performance on learning, memory, executive, attention and mobility functions, including HVLT: Total Recall, HVLT: Delayed Recall, HVLT: RDI, VF: FAS total, VF: Animal, CD, DS, Composite Executive Score, Gait Speed (Normal), and Gait Speed (DT) (p < 0.05), as compared to controls. Similar values between groups were found for MMSE, HVLT: Retention, Trail Making, RPE before Gait (Normal), RPE after Gait (Normal), RPE before Gait (DT), and RPE after Gait (DT).
Table 1.
Demographic, clinical ratings, postural control and cognitive scores at baseline and follow-up.
| Baseline | Two-year follow-up | |||||
|---|---|---|---|---|---|---|
| T2DM (n=41) | Controls (n=32) | P-value | T2DM (n=19) | Controls (n=23) | P-value | |
| Age (years) | 65.51± 8.30 | 67.28± 10.08 | NS | 66.94± 8.18 | 67.09 ± 9.29 | NS |
| Gender (females, N, %) | 22 (54%) | 16 (50%) | NS | 12 (52%) | 12 (63%) | NS |
| Education (years) | 15.35 ± 3.78 | 16.05 ± 2.98 | NS | 14.03 ± 2.93 | 16.35± 2.81 | 0.012 |
| Hematocrit (%) | 38.83 ± 3.70 | 39.54 ± 3.89 | NS | 38.58± 3.21 | 38.71± 3.56 | NS |
| Hypertension (N, %) | 32 (78%) | 7 (22%) | ≤ 0.001 | 15 (79%) | 5 (22%) | ≤ 0.001 |
| Diabetes Duration (years) | 9.93 ± 7.91 | _ | _ | 9.63 ± 6.91 | _ | _ |
| BMI (kg/m2) | 29.12 ± 6.77 | 25.17 ± 6.68 | 0.007 | 29.46 ± 5.43 | 24.05 ± 3.08 | ≤ 0.001 |
| Insulin (uIU/ml) | 13.61 ± 13.30 | 6.26 ± 3.77 | 0.003 | 15.19 ± 17.55 | 6.06 ± 4.30 | 0.020 |
| Fasting Glucose (mg/dl) | 119.70 ± 36.78 | 89.94 ± 10.22 | ≤ 0.001 | 112.69 ± 31.81 | 91.59 ± 8.92 | 0.005 |
| HOMA-IR* | 3.87 ± 3.42 | 1.40 ± 0.83 | ≤ 0.001 | 4.35 ± 4.67 | 1.33 ± 0.92 | ≤ 0.001 |
| HbA1c (%) | 7.34 ± 1.25 | 5.72 ± 0.30 | ≤ 0.001 | 7.82 ± 1.79 | 5.64 ± 0.33 | ≤ 0.001 |
| Gait Speed (Normal) (m/s) | 1.03 ± 0.15 | 1.16 ± 0.13 | ≤ 0.001 | 1.04 ± 0.14 | 1.18 ± 0.13 | 0.004 |
| Gait Speed (DT) (m/s) | 0.92 ± 0.19 | 1.01 ± 0.19 | 0.049 | 0.97 ± 0.19 | 1.02 ± 0.21 | NS |
| Gait (Normal) RPE Start | 0.89 ± 1.56 | 0.28 ± 0.63 | NS | 0.58 ± 1.02 | 0.22 ± 0.42 | NS |
| Gait (Normal) RPE End | 2.53 ± 2.02 | 1.81 ± 1.35 | NS | 2.21 ± 1.55 | 1.64 ± 1.22 | NS |
| Gait (DT) RPE Start | 1.54 ± 1.88 | 0.55 ± 0.74 | NS | 1.06 ± 1.26 | 0.43 ± 0.68 | NS |
| Gait (DT) RPE End | 2.68 ± 2.00 | 2.33 ± 1.52 | NS | 2.84 ± 2.14 | 2.36 ± 1.56 | NS |
| MMSE | 28.59 ± 1.52 | 28.94 ± 1.56 | NS | 28.76 ± 1.35 | 28.83 ± 1.70 | NS |
| HVLT: Total Recall | 12.61 ± 13.04 | 30.40 ± 5.12 | ≤ 0.001 | 24.26 ± 6.07 | 27.96 ± 5.50 | 0.045 |
| HVLT: Delayed Recall | 43.03 ± 12.98 | 52.16 ± 10.86 | 0.002 | 42.47 ± 15.42 | 53.48 ± 11.70 | 0.012 |
| HVLT: Retention | 81.87 ± 16.79 | 87.20 ± 16.66 | NS | 79.34 ± 17.95 | 84.63 ± 15.90 | NS |
| HVLT: RDI | 44.17 ± 12.28 | 50.79 ± 8.38 | 0.023 | 42.79 ± 13.29 | 50.52 ± 10.69 | 0.043 |
| VF: FAS Total | 35.80 ± 10.79 | 45.38 ± 12.25 | ≤ 0.001 | 38.32 ± 13.47 | 49.57 ± 12.79 | 0.009 |
| VF: Animal | 4.39 ± 1.38 | 9.72 ± 3.14 | ≤ 0.001 | 8.37 ± 3.30 | 11.48 ± 3.22 | 0.004 |
| Trail Making | 47.92 ± 11.37 | 50.60 ± 9.29 | NS | 50.89 ± 12.38 | 49.13 ± 8.44 | NS |
| Clock Drawing | 6.71 ± 1.29 | 7.31 ± 0.82 | 0.027 | 6.68 ± 1.11 | 7.43 ± 0.73 | 0.016 |
| Digit Span | 50.05 ± 10.37 | 57.54 ± 12.10 | 0.008 | 47.83 ± 10.67 | 57.70 ± 8.90 | 0.004 |
| Composite Executive score | 47.13 ± 8.09 | 51.87 ± 7.54 | 0.007 | 50.39 ± 8.51 | 51.33 ± 7.37 | NS |
Type 2 Diabetes Mellitus (T2DM); Body Mass Index (BMI); Homeostatic Model Assessment of Insulin Resistance (HOMA-IR); Hemoglobin A1C (HbA1c); Dual Task (DT); Mini-Mental State Examination (MMSE); Hopkins Verbal Learning Test (HVLT) (Shapiro et al. 1999); Recognition Discrimination index (RDI).
HOMA:IR was derived to index Insulin resistance by the homeostasis model (Matthews et al. 1985).
At the two-year follow-up, the significant differences between the T2DM and control groups remained similar in demographics, clinical characteristics, gait results and cognitive scores. The markedly reduced number of participants rendered Gait Speed (DT) and Composite Executive Score results insignificant, while the years of education became significant.
3.1. Global CBF at baseline
At baseline, the T2DM group had lower global CBF (29.83± 9.78 ml/100 g.min) as compared to controls (36.91± 11.77 ml/100g.min), after adjusting for age, hematocrit and hypertension diagnosis (yes/no) (p=0.027). Greater global CBF was associated with the presence of hypertension (r = 0.26, p = 0.028) and lower hematocrit levels (r = −0.43, p = 0.0001), but only marginally associated with younger age (r = −0.21, p = 0.086). The difference between the two groups was not observed on the unadjusted two-sample t-test comparison.
3.2. CBF maps at baseline
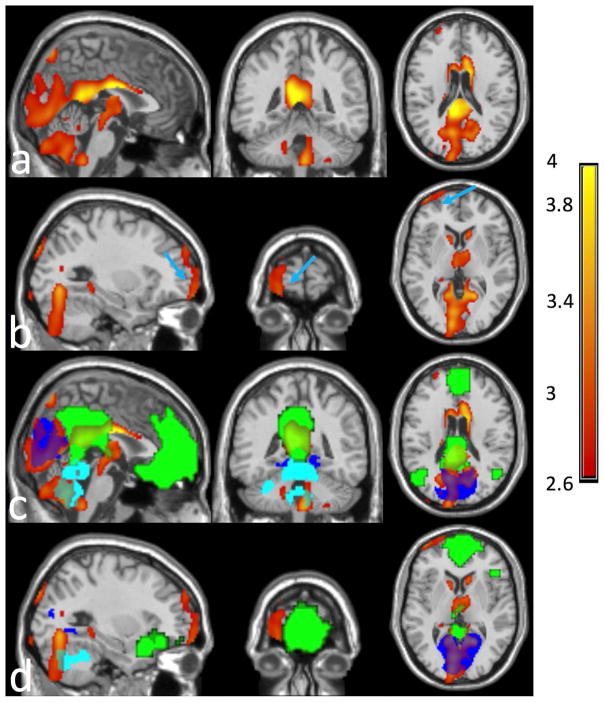
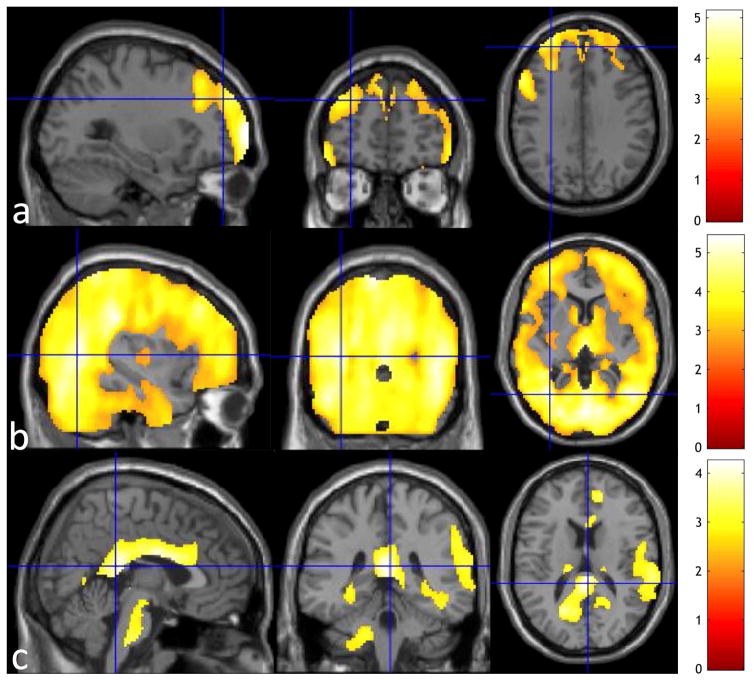
Compared to controls, T2DM patients had significantly lower CBF using SPM analysis after adjusting for age, hematocrit and hypertension in two clusters: one large posterior cluster primarily in the occipital, cerebellum, posterior cingulate, precuneus, thalamus, parietotemporal, basal ganglia regions, and one anterior cluster in the ventromedial, ventrolateral and orbitofrontal regions (Fig. 1a and Fig. 1b). Cluster statistics of the two clusters are shown in Table 2 with anatomical regions containing more than 1% of each cluster size or more than 30% of the entire anatomical regions listed. The cutoff cluster size with FWE of 5% from SnPM was 834 voxels. The posterior and anterior cluster sizes (shown in Table 2) exceeded the cutoff cluster size threshold, supporting the CBF deficits of T2DM at the posterior and anterior clusters derived from SPM analysis. The posterior cluster was in the posterior regions of DMN, medial visual, and cerebellum networks (Fig. 1c), and the anterior cluster was located close to but not exactly in the prefrontal region of the default mode network (DMN) (Fig. 1d). CBF maps were significantly correlated with age, hematocrit and hypertension (Fig. 2). Older participants showed significantly decreased CBF in the prefrontal regions (Fig. 2a). Higher hematocrit was correlated with significantly decreased CBF in extensive areas covering almost all cerebrum, including all frontal, parietal, occipital, temporal regions (Fig. 2b). Hypertensive participants showed significantly increased CBF in the posterior cingulate, anterior cingulate, and prefrontal regions (Fig. 2c).
Fig. 1.
Compared to controls, T2DM patients exhibited significantly reduced Cerebral Blood Flow (CBF) in (a) a posterior cluster, including occipital, cerebellum, posterior cingulate, precuneus, cuneus, thalamus, basal ganglia regions, and (b) an anterior cluster (pointed by light blue arrows), including the ventromedial, ventrolateral and orbitofrontal regions. Cerebrospinal fluid (CSF) was masked out from the clusters for better visibility. The posterior cluster exhibited (c) large overlap with the visual network (in blue color), cerebellum network (in cyan color), and the posterior part of the default mode network (in green color), while the anterior cluster was close to (but not exactly in) the anterior part of the default mode network (in green color). The overlaps between the clusters (posterior cluster in (a) and anterior cluster in (b)) and the brain networks can be seen through the colored brain networks.
Table 2.
Clusters with significant CBF decrease in T2DM relative to age-matched controls
| N Voxels | Peak-t | Peak-t MNI coordinates | Anatomical Locations | % Cluster | % Region | |
|---|---|---|---|---|---|---|
|
| ||||||
| Posterior cluster | 32240 | 4.63 | −2, −8, 24 | Cerebellum | ||
| Cerebelum_Crus1_R | 4.61 | 56.16 | ||||
| Cerebelum_Crus1_L | 2.05 | 25.39 | ||||
| Cerebelum_Crus2_R | 4.63 | 70.48 | ||||
| Cerebelum_Crus2_L | 2.68 | 45.62 | ||||
| Cerebelum_4_5_R | 0.92 | 34.26 | ||||
| Cerebelum_6_R | 3.68 | 66.07 | ||||
| Cerebelum_6_L | 1.20 | 22.90 | ||||
| Cerebelum_7b_R | 1.17 | 70.60 | ||||
| Cerebelum_7b_L | 0.72 | 39.83 | ||||
| Cerebelum_8_R | 4.18 | 58.41 | ||||
| Cerebelum_9_R | 1.72 | 68.92 | ||||
| Vermis_4_5 | 1.16 | 56.39 | ||||
| Vermis_6 | 0.41 | 35.58 | ||||
| Vermis_7 | 0.31 | 52.06 | ||||
| Vermis_8 | 0.59 | 77.78 | ||||
| Vermis_9 | 0.43 | 80.46 | ||||
| Vermis_10 | 0.11 | 31.25 | ||||
| Basal Ganglia | ||||||
| Caudate_R | 1.40 | 45.47 | ||||
| Caudate_L | 0.97 | 32.43 | ||||
| Thalamus_R | 1.44 | 43.80 | ||||
| Thalamus_L | 1.32 | 38.82 | ||||
| Occipital Lobe | ||||||
| Calcarine_R (Visual) | 2.40 | 41.64 | ||||
| Calcarine_L (Visual) | 6.18 | 88.22 | ||||
| Cuneus_R | 0.93 | 31.00 | ||||
| Cuneus_L | 3.91 | 82.63 | ||||
| Fusiform_L | 1.28 | 17.84 | ||||
| Lingual_R | 4.22 | 59.09 | ||||
| Lingual_L | 3.61 | 55.56 | ||||
| Occipital_Sup_L | 2.33 | 54.90 | ||||
| Limbic System | ||||||
| Cingulum_Post_R | 0.85 | 82.09 | ||||
| Cingulum_Post_L | 1.37 | 95.46 | ||||
| Parietal Lobe | ||||||
| Precuneus_R | 1.59 | 15.71 | ||||
| Precuneus_L | 3.08 | 28.17 | ||||
|
| ||||||
| Anterior cluster | 1068 | 3.78 | −44, 60, −10 | Frontal Lobe | ||
| Frontal_Sup_L | 17.51 | 5.20 | ||||
| Frontal_Inf_Orb_L | 1.40 | 0.89 | ||||
| Frontal_Mid_L | 4.49 | 0.99 | ||||
| Frontal_Mid_Orb_L | 12.55 | 15.09 | ||||
| Frontal_Sup_Orb_L | 2.25 | 2.49 | ||||
%Cluster indicates the percentage of each cluster that falls within the defined region, %Region indicates the percentage of each defined region that falls within the cluster. The listed anatomical regions are either “%Cluster” > 1% or “%Region” > 30%.
Fig. 2.
The regions where Cerebral Blood Flow (CBF) is significantly associated with (a) age, (b) hematocrit, and (c) hypertension is overlaid on the anatomical brain. CSF was masked out from the regions for better visibility. CBF is reduced significantly in the prefrontal regions as people age. Subjects with higher hematocrit showed significantly decreased CBF in very extensive areas covering almost all cerebrum, including all frontal, parietal, occipital and temporal regions. Hypertensive subjects showed significantly increased CBF in the posterior cingulate, anterior cingulate, and prefrontal regions.
3.3. Post-hoc correlation analysis of regional and network CBF at baseline
Post-hoc correlation analysis for all participants showed significant correlation between CBF and plasma insulin, HOMA-IR, gait speed, and VF performance after correcting for age, hematocrit, and hypertension. At the posterior cluster, an increase of CBF was associated with lower insulin levels (r = −0.35, p = 0.0026) and HOMA-IR (r = −0.35, p = 0.0036), higher Gait Speed (DT) (r = 0.40, p = 0.0013), and higher VF: Animal score (r = 0.43, p = 0.0002). At the anterior cluster, an increase of CBF was associated with lower insulin levels (r = −0.28, p = .019), lower HOMA-IR (r = −0.30, p = 0.011), higher Gait Speed (DT) (r = 0.26, p = 0.040), and higher VF: Animal score (r = 0.44, p = 0.0002). No significant associations were observed for the other variables reflecting T2DM severity (fasting glucose and HbA1c), cognitive performance (MMSE, HVLT-R, VF: FAS total, and DS), and gait measures.
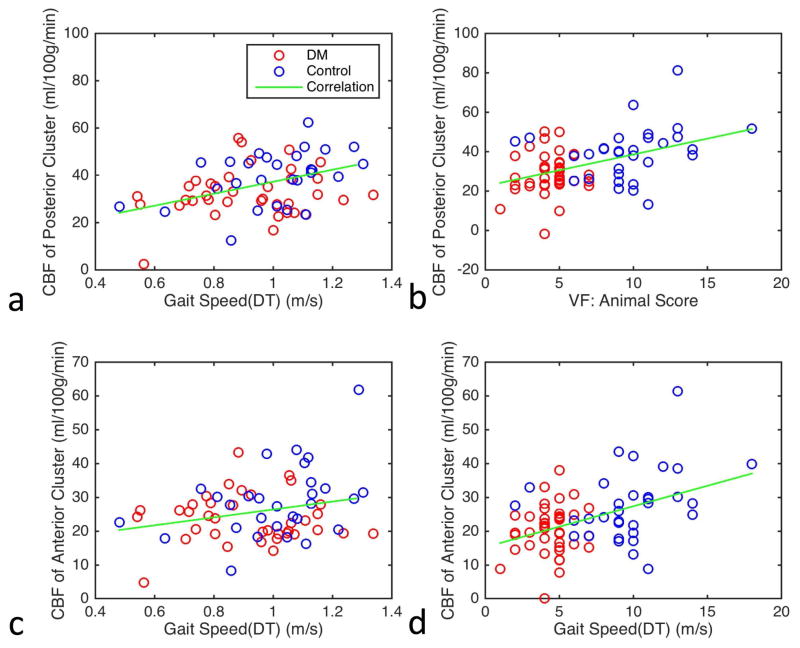
The post-hoc correlation analysis for the whole cohort was further corrected for GM volume. With the additional correction, the posterior cluster remained negatively associated with insulin levels (r = −0.32, p = 0.0077) and HOMA-IR (r = −0.30, p = 0.012), and positively associated with Gait Speed (DT) (r = 0.38, p = 0.0025) (Fig. 3a), and VF: Animal score (r = 0.39, p = 0.0009) (Fig. 3b). The anterior cluster was negatively correlated with insulin levels (r = −0.24, p = 0.049) and HOMA-IR (r = −0.26, p = 0.034), and positively with Gait Speed (DT) (r = 0.27, p = 0.037) (Fig. 3c) and VF: Animal score (r = 0.40, p = 0.0007) (Fig. 3d).
Fig. 3.
Association of baseline absolute Cerebral Blood Flow (CBF) with (a) Gait Speed Dual-Task (DT) and (b) Verbal Fluency (VF): Animal score at the posterior cluster, and (c) Gait Speed (DT) and (d) VF: Animal score at the anterior cluster after correcting for age, hematocrit, hypertension, and gray matter volume.
In the final regression model (corrected for age, hematocrit, hypertension, and GM volume), CBF was negatively associated at both the posterior and anterior clusters with insulin levels (r = −0.42, p = 0.010 and r = −0.36, p = 0.027, respectively) and/or HOMA-IR (r = −0.35, p = 0.038 and r = −0.33, p = 0.046, respectively) in patients with T2DM. In the control group, CBF was not significantly associated with any of the above mentioned variables either at the posterior or the anterior cluster. The regional association of baseline CBF with diabetes disease variable, cognitive performance, and mobility performance are summarized in Table 3.
Table 3.
Regional association of baseline Cerebral Blood Flow (CBF) and longitudinal CBF change with diabetes related variables, cognitive and mobility measures
| Posterior regions | Anterior Regions | |
|---|---|---|
| Baseline CBF | Baseline Insulin* (r = −0.32, p = 0.0077) | Baseline Insulin* (r = −0.24, p = 0.049) |
| Baseline HOMA:IR* (r = −0.30, p = 0.012) | Baseline HOMA-IR* (r = −0.26, p = 0.034) | |
| Baseline Gait Speed (DT) (r = 0.38, p = 0.0025) | Baseline Gait Speed (DT) (r = 0.27, p = 0.037) | |
| Baseline VF: Animal (r = 0.39, p = 0.0009) | Baseline VF: Animal (r = 0.40, p = 0.0007) | |
| Longitudinal CBF Change | Change of HVLT: Total Recall (r = 0.42, p = 0.0095) | Change of HVLT: Total Recall (r = 0.36, p = 0.029) |
| Longitudinal CBF Change | Baseline HOMA:IR (r = −0.36, p = 0.031) | Baseline HOMA-IR (r = −0.45, p = 0.0063) |
| Baseline HVLT: Retention* (r = 0.38, p = 0.022) |
Correlation coefficient and p value for each regional association in the entire study cohort is shown in brackets.
represents significant association in T2DM group
Insulin levels were outside normal range for four participants and these values drove the significant associations observed between baseline CBF and insulin levels, and baseline CBF and HOMA-IR (data not shown). After excluding these participants from the analysis, no significant association was observed between baseline CBF and insulin levels, and baseline CBF and HOMA-IR. More subjects are needed to confirm any association between baseline CBF and insulin/HOMA-IR.
After adjusting for age, hematocrit, hypertension and GM volume, T2DM patients had significantly lower network CBF in DMN (p = 0.016), medial visual network (p = 0.0063), and cerebellum network (p = 0.016) when compared to controls. For all participants, network CBF values at DMN, visual and cerebellum networks were associated with insulin levels (r = −0.31, p = 0.0098; r = −0.31, p = 0.0094; and r = −0.30, p = 0.012 respectively), HOMA-IR (r = −0.29, p = 0.019; r = −0.30, p = 0.013; and r = −0.29, p = 0.018 respectively), Gait Speed (DT) (r = 0.32, p = 0.012; r = 0.37, p = 0.0029; and r = 0.32, p = 0.011 respectively) and VF: Animal score (r = 0.33, p = 0.0054; r = 0.36, p = 0.0027; and r = 0.32, p = 0.0072 respectively). In T2DM participants, network CBF values were negatively associated at DMN, visual and cerebellum networks with insulin levels (r = −0.43, p = 0.0079; r = −0.40, p = 0.015; and r = −0.40, p = 0.014 respectively) and HOMA-IR (r = −0.36, p = 0.033; r = −0.35, p = 0.038; and r = −0.35, p = 0.038 respectively). In the control group, network CBF values were not significantly associated with insulin levels and HOMA-IR at either brain network. However, similar to region-based analysis, due to insulin levels outside normal range for four participants, more subjects are needed to confirm any association between baseline network CBF and insulin/HOMA-IR.
3.4. CBF at two-year follow-up and longitudinal CBF change after two years
At the two-year follow-up, we did not observe the significant CBF differences between T2DM patients and controls (p > 0.05) at the global level, voxel-by-voxel level and regional level (for both posterior and anterior regions), after adjusting for age, hematocrit and hypertension. Longitudinal CBF change from baseline to two-year follow-up was also not significant at the global, voxel-by-voxel and/or regional level between the two groups, after adjusting for age, hematocrit and hypertension.
3.5. Correlation of longitudinal CBF change with cognitive and gait changes
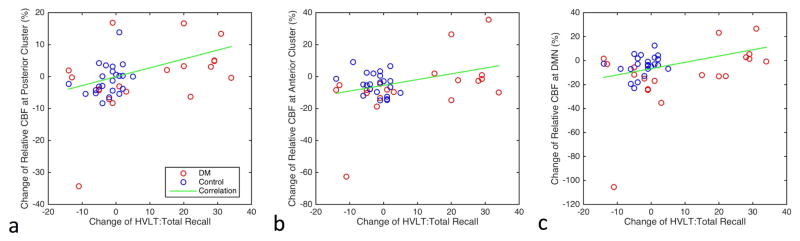
For all participants, CBF change at the two-year follow-up was correlated with the longitudinal change in HVLT: Total Recall at the posterior cluster (r = 0.42, p = 0.0095) (Fig. 4a), at the anterior cluster (r = 0.36, p = 0.029) (Fig. 4b), and at the DMN (r = 0.35, p = 0.038) (Fig. 4c); One subject experienced very large longitudinal CBF decrease at the posterior cluster, anterior cluster and DMN (Figure 4). After removing the subject, the longitudinal CBF change remained significantly associated with the longitudinal change in HVLT: Total Recall at the posterior cluster, anterior cluster and DMN (r = 0.42, p = 0.011; r = 0.36, p = 0.032; and r = 0.35, p = 0.038). CBF change at the two-year follow-up was not correlated with either cognitive and gait changes at the visual and cerebellum networks.
Fig. 4.
Greater longitudinal decline in Cerebral Blood Flow (CBF) at the two-year follow-up is associated with less Total Recall in HVLT (a) at the posterior cluster, (b) at the anterior cluster, and (c) at the default mode network (DMN).
In T2DM participants, no significant association was found between CBF change and the change in HVLT: Total Recall either at either cluster or brain network. The regional association of longitudinal CBF change with longitudinal change of diabetes disease variable, cognitive performance, and mobility performance are summarized (Table 3).
3.6. Correlation of longitudinal CBF change with baseline HOMA_IR, Cognition
For all participants, the longitudinal CBF decrease at the posterior cluster was significantly associated with greater baseline HOMA-IR values (r = −0.36, p = 0.031). Similarly, the longitudinal CBF decrease at the anterior cluster and DMN was significantly associated with larger baseline HOMA-IR values (r = −0.45, p = 0.0063 and r = −0.40, p = 0.016) and smaller baseline HVLT: Retention (r = 0.38, p = 0.022 and r = 0.34, p = 0.039) (Fig. 5). One subject experienced very large longitudinal CBF decrease at the anterior cluster and DMN (Figure 5). After removing the subject, the longitudinal CBF decrease remained significantly associated with smaller baseline HVLT: Retention at the anterior cluster and DMN (r = 0.38, p = 0.026 and r = 0.34, p = 0.045). The association of longitudinal CBF change with HOMA-IR was mainly influenced by the values of three participants that were very high (range: 12–16, while the values from all other subject are in the range of 0–5) (data not shown). After excluding the three subjects from the analysis, the association was not significant any more. The relatively lack of subjects with HOMA-IR in the intermediate range (e.g. 5–12) may have contributed to a masked potential association between HOMA-IR and longitudinal CBF change.
Fig. 5.
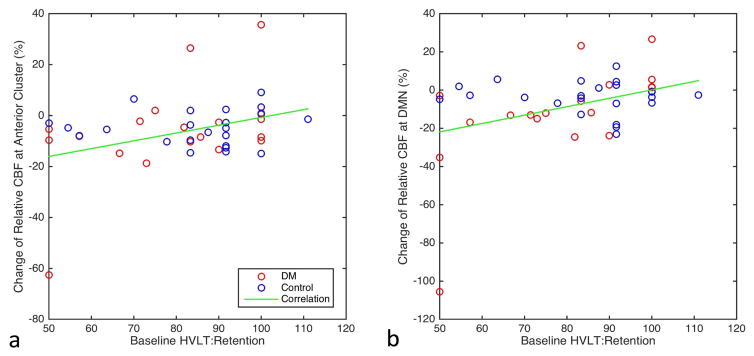
Greater longitudinal decline in Cerebral Blood Flow (CBF) after two-year follow-up (a) at the anterior cluster and (b) at the default mode network (DMN) is associated with reduced baseline Hopkins Verbal Learning Test (HVLT): retention.
For T2DM participants, the longitudinal CBF change at the anterior cluster and DMN was correlated with the baseline HVLT: Retention (r = 0.62, p = 0.024 and r = 0.60, p = 0.024 respectively), while for the control participants, it was not (r = −0.05, p = 0.81 and r = −0.035, p = 0.89 respectively). CBF change at the posterior cluster, visual network and cerebellum network was not correlated with any baseline variables in either group. The regional association of the longitudinal CBF change with baseline diabetes disease variable, cognitive performance, and mobility performance are also summarized in Table 3.
4. Discussion
The results of our study has shown that T2DM is associated with impairment of resting CBF in posterior and anterior regions and increased HOMA-IR, as compared to controls matched for age, hypertension and hematocrit. The pattern of resting CBF impairment is associated with functional (mobility and executive) performance. T2DM patients exhibit significantly reduced CBF in the medial visual, cerebellum, and DMN, which include one posterior cluster in the occipital, cerebellum, posterior cingulate, precuneus, thalamus, parietotemporal, basal ganglia regions, and one anterior cluster in the prefrontal region of the DMN. Reduced CBF was strongly correlated with higher hematocrit globally. These findings are independent of the presence of hypertension and age. In addition, reduced CBF was also associated with baseline memory performance and insulin resistance, and with compromised gait, lower memory and executive function scores at the two-year follow-up.
The association of T2DM with CBF has been investigated in several studies, using a variety of techniques, including positron emission tomography (PET), single-photon emission computed tomography (SPECT), and ASL. The results did not reach an agreement yet. Some studies have reported decreased CBF in T2DM (Xia et al. 2015; Novak et al. 2006; Last et al. 2007; Nagamachi et al. 1994), while others did not observe CBF alteration in T2DM (Tiehuis et al. 2008; Rusinek et al. 2015; Novak et al. 2011). Most of the studies reporting no CBF alteration in T2DM typically used large regions of interest (ROI) and/or did not adequately account for the potential risk factors. The ROI were chosen as the entire brain, whole GM, or several large cortical regions. Regarding the risk factors, some studies did not account for any hypertension effect, although hypertension has been reported more frequent in T2DM than in non-diabetic populations (Colosia, Palencia, and Khan 2013). The higher frequency of hypertension in T2DM has been observed in the current study. Some studies did not have similar gender distributions between T2DM and non-diabetic control groups, but female subjects had higher CBF than male subjects (Pirson, Vander Borght, and Van Laere 2006; Gur and Gur 1990), which may obscure the CBF difference between the groups. Some studies also did not account for brain volume difference, which has been shown to largely explain reduced CBF (Sabri et al. 2000).
We observed decreased CBF in T2DM, mainly in visual, cerebellum and DMN regions after correcting for age, hematocrit (or gender), hypertension, and GM volume. This is consistent with our previous findings that T2DM reduces CBF velocity and regional cerebral vasoreactivity of CO2 challenges in the parietal and occipital regions (Novak et al. 2006). Our findings are consistent with a recent T2DM ASL-CBF study (Xia et al. 2015). They reported that the main effects of T2DM were primarily in visual and DMN regions. We found an additional cerebellum region affected by T2DM. Presumably, the additional regions observed may emerge from the more sensitive ASL MRI sequence for our CBF measurements. The PCASL sequence that was used in our study has been recommended as the most effective noninvasive CBF measurement in clinical practice (Alsop et al. 2014). More importantly, we have shown that HOMA-IR is a potential predictor of CBF decline at the two-year follow-up period.
We observed that age, hematocrit, and hypertension alter CBF in different regions. This indicates that correction for these factors is crucial, especially when comparing CBF in different brain regions. Older age and higher hematocrit were associated with decreased CBF in the current study while hypertension was associated with regionally increased CBF. In fact, the inverse relationship between hematocrit and global CBF has been noted in earlier studies using microsphere techniques (Hudak et al. 1986; Thomas et al. 1977; Massik et al. 1987) and Xe-133 CT methods (Kusunoki et al. 1981). We have shown the inverse correlation between hematocrit and almost entire brain cerebrum, which is in line with the earlier results. The underlying reasons for the relationship are not clear from our study alone. The possible explanations could include two perspectives: (1) low hematocrit leads to reduced oxygen supply, and thus results in increased blood flow because of brain autoregulation; (2) low hematocrit leads to reduced viscosity, and further increases blood flow velocity. We have observed significantly higher CBF in female participants compared to male participants (results not shown), which could be resulted from the lower hematocrit in women than men. We have found that hypertension is associated with increased CBF both globally and locally. Noticing that most hypertensive participants in our study were under antihypertensive treatment, the findings are in agreement with the literature. Reduction of BP in older populations has been associated with increased CBF and blood flow velocity (Lipsitz et al. 2005).
In the T2DM group, impaired CBF at the baseline was associated with higher insulin level and HOMA-IR, but not with worse performance in mobility and executive function. Interestingly, greater CBF was correlated with faster walking and better executive function (VF: animal scores) in the entire study cohort. These results may be due to the range of variables and/or the small sample size of mobility and executive function in each group. We found correlation between CBF and mobility function, and between CBF and executive function, once we included the entire population in the analyses (with broader range and/or the larger sample size. However, whether significant association was found with T2DM group specifically, it’s important to point out that reduced CBF may exacerbate the deficits in executive function and the mobility function in older adults.
The T2DM-specific pattern of reduced CBF (in posterior and anterior regions) is very similar to the pattern observed in patients with MCI and Alzheimer’s disease (AD) (Li et al. 2016; Mevel et al. 2011). Moreover, in the current study the T2DM-specific pattern of reduction in CBF was associated with insulin resistance, mobility function, and executive function. The association was independent of age, hematocrit (or gender), hypertension, and brain volume. Note that the severity of AD is mostly assessed based on status of cognitive and executive function (especially categorical VF test such as name of animals). Our participants received careful neuropsychological assessment and were not cognitively impaired according to the current criteria for MCI. These results could indicate that insulin resistance may be a marker of AD that is associated with reduced CBF, and cognitive and executive impairment before the onset of MCI. These findings suggest that the amelioration of insulin resistance in people in high risk of developing T2DM (e.g. impaired glucose metabolism), could be a way to prevent them from developing cognitive or mobility impairment.
The longitudinal design of the study allows us to estimate the relationships among the studied variables, and shed light on the potential causal effect of insulin resistance on T2DM progression and severity. We observed that the longitudinal CBF change was associated with baseline HOMA-IR in the entire cohort, but not in the T2DM and control groups separately. This might be attributed to a reduced power to detect such associations in the separate groups, due to the reduced sample size at the two-year follow-up. These results indicate that insulin resistance in T2DM is related to longitudinal CBF change. Our findings suggest that the longitudinal change in CBF might represent modulation of brain function by insulin resistance.
No difference in CBF at the two-year follow-up was observed between the T2DM patients and controls, possibly due to the increased variance in T2DM group secondary to the reduced sample size. However, we observed that the longitudinal CBF decrease was associated with the decline in memory (HVLT: total recall) function. This demonstrates that CBF decrease can be indicative of cognitive decline as T2DM progresses. We have identified that the baseline HOMA-IR can predict longitudinal decrease of CBF. Taken together, these results suggest that controlling insulin resistance may potentially prevent further cognitive decline in T2DM and that insulin resistance may be a common pathway between T2DM, AD and other dementias.
Our study shed light on the adverse effects of insulin resistance to the longitudinal CBF change in T2DM. The reduced sample size in the two-year follow-up, and the reduced power associated with this might have affected our ability to detect existing associations between T2DM, CBF and cognitive/mobility performance or the changes between these variables at the follow-up period; therefore, the overall impact of diabetes on CBF and cognitive/mobility performance may be even greater. Interestingly, we also provide evidence that T2DM is associated with a pattern of reduced CBF in the DMN, visual, and cerebellum networks, and that the pattern of resting CBF impairment is closely related to the decline of memory, executive, and mobility functions. However, resting CBF was not found associating with either fasting blood glucose or HbA1c. The pathways by which insulin resistance may affect brain perfusion and cognition is independent of glycemic control and require further investigation. A study with larger sample size and longer follow-up is warranted to confirm the association of HOMA-IR and longitudinal CBF decrease in T2DM.
T2DM had decreased CBF in the default mode, visual, and cerebellum networks.
CBF decline is closely related to worse cognitive and mobility performance.
CBF change over two years was associated with baseline disease severity.
Insulin resistance may contribute to CBF deficits in T2DM.
Acknowledgments
The study was supported by NIH-NIA 1R01-AG0287601A2, NIH-NIDDK 5R21 DK084463, NIH-NIDDK-1R01DK13902-01A1, American Diabetes Association, Clinical 1-03-CR-23 and 1-06-CR-25 to Dr. Vera Novak. The project described was supported by Grant Number UL1 RR025758- Harvard Clinical and Translational Science Center and M01-RR-01032, from the National Center for Research Resources.
Abbreviations
- Perfusion Pattern in Type 2 Diabetes
Footnotes
Disclosure statement
The authors do not have any conflicts of interest. Appropriate approval and procedures were used concerning human subjects.
-
Conflicts of interest
- we do not have any actual or potential conflicts of interest.
- No author’s institution has contracts relating to this research through which it or any other organization may stand to gain financially now or in the future.
-
Financial support related to the manuscript being submitted
- Dr. Freddy J Alfaro is supported by 1R01DK3902-01A1.
- Dr. Anna Gavrieli is supported by 1R01DK3902-01A1.
- Dr. Vera Novak was funded by NIH-NIA 1R01- AG0287601A2, NIH-NIDDK 5R21 DK084463, American Diabetes Association, Clinical 1-03-CR-23 and 1-06-CR-25, and by 1R01DK3902-01A1.
- The data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
- Appropriate approval and procedures were used concerning human subjects.
- We have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of Human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–49. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, Macintosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2014 doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual Aphasia Examination. Manual of instructions. 2. AJA Associates; Iowa City: 1989. [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383–96. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- Chung CC, Pimentel D, Jor’dan AJ, Hao Y, Milberg W, Novak V. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology. 2015;85:450–8. doi: 10.1212/WNL.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327–38. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–97. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Varma G, Scheidegger R, Alsop DC. Quantifying fluctuations of resting state networks using arterial spin labeling perfusion MRI. J Cereb Blood Flow Metab. 2016;36:463–73. doi: 10.1177/0271678X15615339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franke K, Gaser C, Manor B, Novak V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci. 2013;5:90. doi: 10.3389/fnagi.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande LJ, Milberg W, Rudolph J, Gaziano M, McGlinchey R. A timely screening for executive functions and memory. Journal of the International Neuropsychological Society. 2005;11:31. [Google Scholar]
- Gur RE, Gur RC. Gender differences in regional cerebral blood flow. Schizophr Bull. 1990;16:247–54. doi: 10.1093/schbul/16.2.247. [DOI] [PubMed] [Google Scholar]
- Henriksen OM, Jensen LT, Krabbe K, Guldberg P, Teerlink T, Rostrup E. Resting brain perfusion and selected vascular risk factors in healthy elderly subjects. PLoS One. 2014;9:e97363. doi: 10.1371/journal.pone.0097363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen OM, Kruuse C, Olesen J, Jensen LT, Larsson HB, Birk S, Hansen JM, Wienecke T, Rostrup E. Sources of variability of resting cerebral blood flow in healthy subjects: a study using (1)(3)(3)Xe SPECT measurements. J Cereb Blood Flow Metab. 2013;33:787–92. doi: 10.1038/jcbfm.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak ML, Koehler RC, Rosenberg AA, Traystman RJ, Jones MD., Jr Effect of hematocrit on cerebral blood flow. Am J Physiol. 1986;251:H63–70. doi: 10.1152/ajpheart.1986.251.1.H63. [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunoki M, Kimura K, Nakamura M, Isaka Y, Yoneda S, Abe H. Effects of hematocrit variations on cerebral blood flow and oxygen transport in ischemic cerebrovascular disease. J Cereb Blood Flow Metab. 1981;1:413–7. doi: 10.1038/jcbfm.1981.45. [DOI] [PubMed] [Google Scholar]
- Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–9. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang X, Li Y, Sun Y, Sheng C, Li H, Li X, Yu Y, Chen G, Hu X, Jing B, Wang D, Li K, Jessen F, Xia M, Han Y. Abnormal Resting-State Functional Connectivity Strength in Mild Cognitive Impairment and Its Conversion to Alzheimer’s Disease. Neural Plast. 2016;2016:4680972. doi: 10.1155/2016/4680972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitz LA, Gagnon M, Vyas M, Iloputaife I, Kiely DK, Sorond F, Serrador J, Cheng DM, Babikian V, Cupples LA. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–21. doi: 10.1161/01.HYP.0000153094.09615.11. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, Schuff N. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2012;68:912–22. doi: 10.1002/mrm.23286. [DOI] [PubMed] [Google Scholar]
- Massik J, Tang YL, Hudak ML, Koehler RC, Traystman RJ, Jones MD., Jr Effect of hematocrit on cerebral blood flow with induced polycythemia. J Appl Physiol (1985) 1987;62:1090–6. doi: 10.1152/jappl.1987.62.3.1090. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mevel K, Chetelat G, Eustache F, Desgranges B. The default mode network in healthy aging and Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:535816. doi: 10.4061/2011/535816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Horiuchi M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ J. 2011;75:1042–8. doi: 10.1253/circj.cj-11-0121. [DOI] [PubMed] [Google Scholar]
- Nagamachi S, Nishikawa T, Ono S, Ageta M, Matsuo T, Jinnouchi S, Hoshi H, Ohnishi T, Futami S, Watanabe K. Regional cerebral blood flow in diabetic patients: evaluation by N-isopropyl-123I-IMP with SPECT. Nucl Med Commun. 1994;15:455–60. doi: 10.1097/00006231-199406000-00010. [DOI] [PubMed] [Google Scholar]
- Novak V, Last D, Alsop DC, Abduljalil AM, Hu K, Lepicovsky L, Cavallerano J, Lipsitz LA. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006;29:1529–34. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak V, Zhao P, Manor B, Sejdic E, Alsop D, Abduljalil A, Roberson PK, Munshi M, Novak P. Adhesion molecules, altered vasoreactivity, and brain atrophy in type 2 diabetes. Diabetes Care. 2011;34:2438–41. doi: 10.2337/dc11-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirson AS, Vander Borght T, Van Laere K. Age and gender effects on normal regional cerebral blood flow. AJNR Am J Neuroradiol. 2006;27:1161–2. author reply 62–3. [PMC free article] [PubMed] [Google Scholar]
- Pugh KG, Kiely DK, Milberg WP, Lipsitz LA. Selective impairment of frontal-executive cognitive function in african americans with cardiovascular risk factors. J Am Geriatr Soc. 2003;51:1439–44. doi: 10.1046/j.1532-5415.2003.51463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Cha RH, Mielke MM, Pankratz VS, Boeve BF, Kantarci K, Geda YE, Jack CR, Jr, Petersen RC, Lowe VJ. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med. 2014;55:759–64. doi: 10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinek H, Ha J, Yau PL, Storey P, Tirsi A, Tsui WH, Frosch O, Azova S, Convit A. Cerebral perfusion in insulin resistance and type 2 diabetes. J Cereb Blood Flow Metab. 2015;35:95–102. doi: 10.1038/jcbfm.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O, Hellwig D, Schreckenberger M, Schneider R, Kaiser HJ, Wagenknecht G, Mull M, Buell U. Influence of diabetes mellitus on regional cerebral glucose metabolism and regional cerebral blood flow. Nucl Med Commun. 2000;21:19–29. doi: 10.1097/00006231-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13:348–58. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–6. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Marshall J, Russell RW, Wetherley-Mein G, du Boulay GH, Pearson TC, Symon L, Zilkha E. Effect of haematocrit on cerebral blood-flow in man. Lancet. 1977;2:941–3. doi: 10.1016/s0140-6736(77)90885-6. [DOI] [PubMed] [Google Scholar]
- Tiehuis AM, Vincken KL, van den Berg E, Hendrikse J, Manschot SM, Mali WP, Kappelle LJ, Biessels GJ. Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia. 2008;51:1321–6. doi: 10.1007/s00125-008-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umegaki H. Type 2 diabetes as a risk factor for cognitive impairment: current insights. Clin Interv Aging. 2014;9:1011–9. doi: 10.2147/CIA.S48926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, Bollen EL, Middelkoop HA, van Buchem MA, van der Grond J. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75:997–1002. doi: 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- Wang J, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, Detre JA. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med. 2002;48:242–54. doi: 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised (manual) Psychological Corporation; New York: 1987. [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA. 1992;89:212–16. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RH, Scholey A, Howe PR. Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus--a review with implications for future intervention studies. Curr Diab Rep. 2014;14:547. doi: 10.1007/s11892-014-0547-4. [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–9. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–7. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- Xia W, Rao H, Spaeth AM, Huang R, Tian S, Cai R, Sun J, Wang S. Blood Pressure is Associated With Cerebral Blood Flow Alterations in Patients With T2DM as Revealed by Perfusion Functional MRI. Medicine. 2015;94:e2231. doi: 10.1097/MD.0000000000002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, Christian BT, Oakes TR, Johnson SC. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR Biomed. 2010;23:286–93. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhang X, Lu J. Progress on diabetic cerebrovascular diseases. Bosn J Basic Med Sci. 2014;14:185–90. doi: 10.17305/bjbms.2014.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]