Abstract
The authors describe a magnetized carbon nanotube (MCNT)-based lateral flow strip biosensor for visual detection of proteins directly in whole blood avoiding complex purification and sample pre-treatments. MCNT were synthesized by coating Fe3O4 nanoparticles on the shortened multiwalled carbon nanotube (CNT) surface via co-precipitation of ferric and ferrous ions within a dispersion of shorten multiwalled CNTs. The antibody-modified MCNTs were used to capture target protein in whole blood; the formed MCNT-antibody-target protein complexes were applied to the lateral flow strip biosensor, in which a capture antibody was immobilized on the test zone of the biosensor. The captured MCNTs on the test zone and control zone were producing characteristic brown/black bands, and this enabled target protein to be visually detected. Quantification was accomplished by reading the intensities of the bands with a portable strip reader. Rabbit IgG was used as a model target to demonstrate the proof-of-concept. After systematic optimizations of assay parameters, the detection limit of the assay in whole blood was determined to be 10 ng mL-1 (S/N=3) with a linear dynamic range of 10 to 200 ng mL-1. This study provides a rapid and low-cost approach for detecting proteins in blood, showing great promise for clinical application and biomedical diagnosis, particularly in limited resource settings.
Keywords: Magnetized carbon nanotube, lateral flow, biosensor, protein, blood
Graphical abstract
Combining the superpara-magnetism of Fe3O4 nanoparticles and the outstanding mechanical properties of carbon nanotubes, magnetized carbon nanotube-based lateral flow strip biosensor was first used for visual detection of proteins directly in whole blood avoiding complex purification and sample pretreatment.
1. Introduction
Protein, involved in a variety of life events, plays critical roles in metabolism [1]. To sensitively detect proteins is of enormous interest for not only basic discovery research but also a broad range of applications, such as biology, disease diagnosis, food safety, and environmental analysis [2,3]. However, detecting proteins in physiological fluid samples, particularly in blood, is still a great challenge because of problems such as biofouling and nonspecific binding, and resulting need to use sample purification greatly reduces the clinical applications [4]. Traditional techniques to quantify the protein concentrations in blood include Radioimmunoassay [5,6], Western Blot [7,8], agarose and polyacrylamide gel electrophoresis [9], Enzyme-linked Immunosorbent Assay (ELISA) [10-13] and Mass Spectrometry (MS) [14-15]. However, these technologies are limited to laboratory use because they rely on sample purifications and sophisticated instruments, are time and labour intensive and expensive, and require highly trained personals. Numerous immunoassays and immunosensors in connection with different transducers (electrochemical, optical, acoustic, piezoelectric, etc.) have been reported to detect proteins in blood [16-23]. Although many of them have been applied at the laboratory research level, they have not been applied in-field or point-of-care detection because of the relatively long assay time or multiple washing and separation steps.
Lateral flow immunoassay (LFI), also known as immunochromatographic assay or lateral flow strip biosensor (LFSB), is a solid-phase immunoassay incorporating the technology of thin layer chromatography and immune recognition reaction. LFI is one of rapid, cost-effectiveness and portable detection techniques. LFI has been used for point-of-care or in-field screening of infectious diseases, drugs of abuse, and pregnancy [24-30]. Gold nanoparticles (GNPs), carbon nanoparticles, quantum dots (QDs) and Fe3O4 nanoparticles have been used as immunochromatographic labels among which GNPs are the most widely used due to their unique optical properties (plasma absorption) and easy surface modification [31-36]. However, GNP-based LFIs are not able to detect proteins with low concentrations in whole blood due to its low sensitivities and colour interference. Fluorescent and magnetic LFIs have attracted considerable interest because of their high sensitivities and anti-interferences. Gerd et al. reported a lateral flow immunoassay using europium (III) chelate microparticles and time-resolved fluorescence for eosinophils and neutrophils in whole blood [37]. Own to unique magnetic separation properties, magnetic microparticles and nanoparticles have been used as immunochromatographic labels for the detection of analytes in food matrixes [35, 38]. However, fluorescent and magnetic LFIs still require expensive or complex readers. Therefore, there is still a great challenge to develop inexpensive, rapid and easy-to-use technologies for protein detection in whole blood.
Since their discovery by Iijima, carbon nanotubes (CNTs) have been used to construct various chemical sensors and biosensors because of their unique physical, chemical and electronical properties [39,40]. Most of the CNT-based biosensors and bioassays still suffered from tedious assay time, multiple washing steps and the requirement of trained personnel [41-44]. Our group and others have reported CNT-based lateral flow nucleic acid biosensors for visual detection of DNA and Hg2+ [45, 46]. In this work, we synthesized a magnetized carbon nanotube (MCNT) by coating Fe3O4 nanoparticles on the shortened multiwall CNT surface via co-precipitation of ferric and ferrous ions within a dispersion of shorten multiwall CNTs [47, 48]. The MCNT was used as an immunochromatographic label for visual detection of proteins in whole blood. Rabbit IgG (Immunoglobulin G) was used as a model target to demonstrate the proof-of-concept. Rabbit IgG was first detected in buffer, the detection limit was 0.5 ng mL−1 under the optimized experimental condition, which is two times lower than that of the GNP-based LFSB and magnetic nanoparticle (MNP)-based LFSB, and forty times lower than that of the latex-based LFSB. In addition, MCNT-antibody conjugates were more stable compared with GNP-antibody conjugates because the latter was easy to aggregate during the preparation and test. The optimized MCNT-based LFSB was applied to detect rabbit IgG in whole blood with the detection limit of 10 ng mL-1.
2. Experimental
2.1. Apparatus
Nucleic Acid Extraction MCB 1200 (Sigris Research, Inc, Brea, California) was used to separate magnetic nanoparticles from solutions. A Hitachi SU8010 field scanning-electron microscope (SEM; Tokyo, Japan) was used for images taking of the nanoparticles. Fourier transform infrared (FT-IR) spectroscopy was measured by using a Nicolet iS10 FT-IR Spectrometer (Thermo Scientific, Rockford, IL) with attenuated total reflection (ATR) attachment. UV-vis absorption spectra were measured with a UV-1800 Spectrophotometer (Shimadzu, Japan). The Biojet BJQ 3000 dispenser, Clamshell Laminator, and the Guillotine cutting module CM 4000 purchased from Biodot LTD (Irvine, CA) were used to prepare lateral flow strip biosensors. A portable strip reader DT1030 (Shanghai Goldbio Tech. Co.; Shanghai, China) was used for signal recording. Nikon COOLPIX S4200 camera (Nikon, Japan) was used to take the photo images of lateral flow strip biosensors.
2.2. Reagents
Multiwalled carbon nanotubes (MWCNTs, SN2302, purity>95%) were purchased from Nanomaterial Store (Fremont, CA), FeCl2·4H2O (purity>99%) was purchased from Acros Organics BVBA (Geel, Belgium), MWCNTs (659258, purity>95%), carboxylated MWCNTs (755125, purity>95%), ammonia hydroxide, FeCl3·6H2O, N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (sulfo-NHS), 2-(4-Morpholino) ethanesulfonic acid (MES), streptavidin, sucrose, Tween 20, bovine serum albumin (BSA) and phosphate buffer saline (0.01 M PBS, pH 7.4) were purchased from Sigma-Aldrich (St. Louis, MO). Glass fibers (GFCP000800), cellulose fibers (CFSP001700), nitrocellulose membranes (HF090MC100, HFB18004 and HFB24004) and laminated cards (HF000MC100) were purchased from Millipore (Billerica, MA). Rabbit IgG, goat anti-rabbit IgG (Ab1) and donkey anti-goat IgG (Ab2) were purchased from ThermoFisher Scientific (Rockford, IL).
All the chemicals used in this study were analytical reagent grade. Solutions were prepared with ultrapure (Z18 MΩ) water from Millipore Milli-Q water purification system (Billerica, MA).
2.3. Preparation of magnetized carbon nanotubes and magnetic nanoparticles
2.3.1. Preparation of magnetized carbon nanotubes (MCNTs)
Ten milligrams of MWCNTs were treated with 4.8 mL H2SO4 and 1.6 mL HNO3 under vigorous ultra-sonication for 6 h. The shortened CNTs was centrifuged, washed with water several times until the solution was neutral and suspended in 10 mL water for further use. The synthesis of MCNTs were inspired by co-precipitation method [48]. Certain amount of shortened CNTs, 0.04054 g FeCl3·6H2O and 0.01491 g FeCl2·4H2O were dissolved and mixed in 10 mL of deionized water. The mixture was sonicated and stirred vigorously, and ammonia water was added dropwise under vigorous stirring till the pH value reached to 10, and kept stirring for 30 min. The brown MCNT were collected with an external magnet, washed with water for three times, and suspended in 10 mL water for further use.
2.3.2. Preparation of carboxylated magnetic nanoparticle (MNPs)
MNPs were synthesized according to the hydrothermal method with slight modification [50]. Briefly, 0.6 g FeCl3·6H2O and 1.5 g NaAc was dissolved in 20 mL ethylene glycol. After vigorous stirring for 30 min, the mixture was reacted at 200 °C in a sealed autoclave tube for 16 h. The products were washed several times with ultrapure water, and suspended in 20 mL water for further use.
2.4. Preparation of MCNT-Ab1 and MNP-Ab1 conjugates
The conjugates were prepared according to the reported method with slight modifications [45]. Two hundred and fifty microliters of MCNTs or one hundred microliters of MNPs was mixed with 4.8 mg EDC and 2.7 mg sulfo-NHS in 0.5 mL MES buffer (0.1 M, pH 4.7). After shaking 15 min at room temperature, activated MCNTs or MNPs was separated by applying an external magnet. Supernatant was discarded and the pellet was re-suspended in PBS buffer. The above process was repeated three times to remove the extra reagents. Then certain amount of anti-rabbit IgG (Ab1) was added to the activated MCNTs or MNPs, and the mixture was incubated overnight at 4 °C. This mixture was washed three times with the procedure described in the activation process. Supernatant was discarded and the pellet collected in the final washing step was re-suspended in 0.5 mL eluent buffer (20 mM Na3PO4·12H2O, 5% BSA, 10% sucrose, and 0.25% Tween-20). The conjugate solutions were stored at 4 °C.
2.5. Preparation of lateral flow strip biosensor (LFSB)
The LFSB was made up of three components: sample application pad, nitrocellulose membrane and absorbent pad. Untreated glass fibers (GFCP000800) (23 mm×30 cm) were used as the sample application pad. Capture antibody (Ab1) and secondary antibody (Ab2) solutions were dispensed onto nitrocellulose membrane at 1 cm s-1 speed to form the test and control zones with the aid of Biojet BJQ 3000 dispenser. Distance between the test and control zones was about 5 mm. Then the membrane was dried at 37 °C for 1 h and kept it at 4 °C. Clamshell laminator was used to assemble all the three components on a plastic adhesive backing (60 mm × 30 cm) and each part overlapped 2 mm to ensure the solution migration along the strip. Finally, the strips with 3 mm width were cut by the Guillotin cutting module CM 4000 and stored them at 4 °C before further use.
2.6. Assay procedure
2.6.1 Detection of rabbit IgG in running buffer
Different concentrations of target IgG and the as-prepared conjugates were mixed in one hundred microliters of running buffer (PBS + 1% BSA + 0.03% Tween solution). After gentle vortex, the LFSB was dipped into the sample solution and the solution migrated toward absorption pad. Ten minutes later, another 60 μL of running buffer was added to wash the LFSB. The test and control zones were evaluated visually within 20 min. For quantitative measurements, the optical intensities of the test and control zones were recorded with a portable strip reader. The images of the LFSBs were obtained with Nikon COOLPIX S4200 camera (Nikon, Japan).
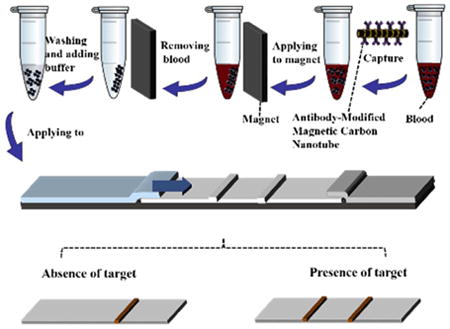
2.6.2 Detection of rabbit IgG in whole blood
Briefly, 20 μL of human blood spiked with certain concentration of rabbit IgG was mixed with 80 μL of PBS buffer and 5 μL of MCNT-Ab1 conjugates. The mixture was incubated 10 min under general shaking. After applying an external magnet 2 min, the MCNT-Ab1-IgG complexes were separated from the whole blood, washed twice with PBS and re-suspended to the running buffer before applying to the sample pad of the LFSB. Ten minutes later, another 60 μL of running buffer was added to wash the strip. The test and control zones were evaluated visually within 20 min.
3. Results and discussion
3.1. Preparation and Characterization of MCNT
Fig. 1A and 1B presents the typical SEM images of shortened CNTs and MCNTs. One can see the MCNTs have a length of 3 to 5 μm (Fig. 1B). Magnetic particles were coated either on the surface or the ends of CNTs. Fig. 1C shows Fourier transform infrared (FTIR) spectra of the unshorten CNTs (a), the shortened CNTs (b) and the MCNTs (c). The peaks of the shortened CNTs and MCNTs at 1800 and 1682 cm-1 indicated that the pretreatment in mixed acids generated carbonyl groups on the CNT surface, which can be utilized for co-precipitation of ferric and ferrous ions on CNT surface, and antibody immobilization. A peak observed around 563 cm-1 in MCNT is attributed to the Fe–O stretching mode of the tetrahedral and octahedral sites [49]. As expected, the magnetized CNTs exhibited superparamagnetic property at room temperature, which can be separated from its solution after applying an external magnet within 40 seconds (Fig. 1D). The extinction coefficient of MCNT at the wavelength of 633 nm was estimated to be 5.25 L g-1 cm-1 (Fig. S1).
Fig. 1.
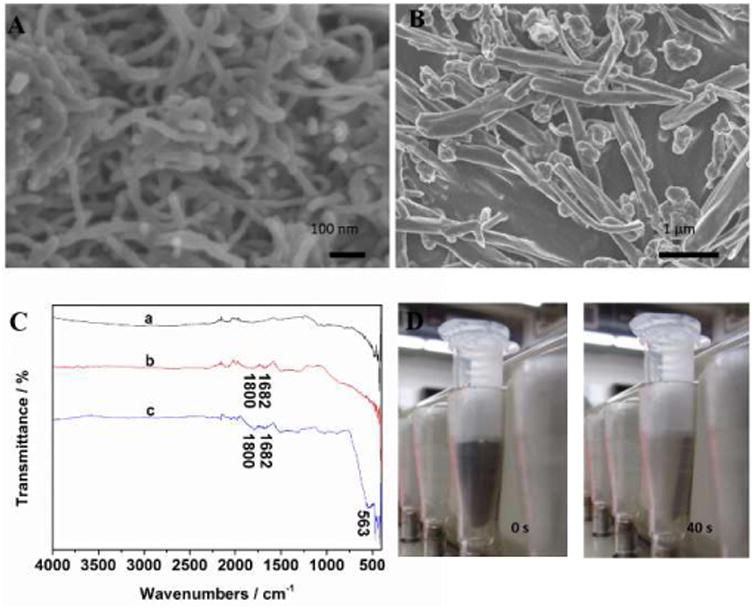
(A) SEM image of shortened multiwalled CNTs; (B) SEM image of magnetized CNTs; (C) FTIR spectra of unshortened multiwalled CNTs (a), shortened multiwalled CNTs (b) and magnetized CNTs (c). (D) Photo images of magnetized CNT suspension after applying an external magnet at the time of 0 s (left) and 40 s (right).
3.2. Principle of MCNT-based LFSB
The MCNTs were then functionalized with anti-IgG antibody (Ab1) by carbodiimide crosslinker chemistry via diimide-activated amidation between the carboxylic acid groups on the CNT surface and amino groups of the antibody (Fig. 2A). The formed MCNT-Ab1 conjugates were used as probes to capture target protein (rabbit IgG) in blood (Fig. 2B). After magnetic separation, the MCNT-Ab1-IgG complexes were suspended in a running buffer and applied to LFSB (Fig. 2C). In the presence of target IgG, the MCNT-Ab1-IgG complexes will be captured by the antibody pre-immobilized on the test zone. The accumulation of MCNT on the test zone produced a distinct brown band, whose intensity was proportional to the concentration of target IgG (Fig. 2D). The excess of MCNT-Ab1 conjugates continued to migrate along the strip and were captured by the second antibodies on the control zone to form the second characteristic brown band. In the absence of target IgG, only one band on the control zone was obtained, indicating the LFSB worked properly. Qualitative analysis was realized by observing the colour change of the test zone with the unaided eye, and quantitative detection was obtained by reading the greyscale of the brown band on the test zone with the aid of a portable strip reader. It should be noted that the MCNTs did not move well on cellulose fiber. Therefore, glass fiber instead of cellulose fiber used in traditional LFSB fabrication was used as the sample pad (Support Information).
Fig. 2.
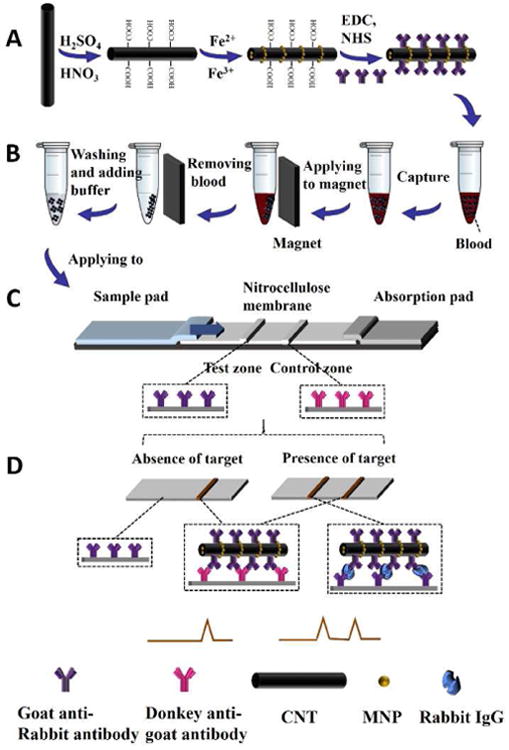
(A) schematic representation of preparation of MCNT-Ab1 conjugates; (B) schematic representation of steps to capture rabbit IgG in blood; (C) schematic representation of the configuration of the lateral flow strip biosensor; (D) measurement principle of the MCNT-based lateral flow strip biosensor.
The concept of using the MCNT as immunochromatographic label for visual detection of protein and the optimizations of experimental parameters were first studied with sample solutions prepared with pure buffer solution without blood. Fig. 3A presents the typical photo images of LFSBs after measuring the sample solutions containing 0 and 10 ng mL−1 target IgG. There was no test band observed in the absence of target IgG while a distinct, brown band appeared in the presence of 10 ng mL−1 IgG. To compare the analytical performances of the MCNT-based LFSB and the reported magnetic nanoparticle (MNP)-based LFSB, the responses of the sample solutions at three concentration levels (0, 1, and 5 ng mL−1 IgG) were tested (Fig. 3B). When rabbit IgG was absent in the sample solutions, neither of the LFSBs showed a response on the test zones (Fig. 3B-a). In the presence of 1 and 5 ng mL−1 rabbit IgG (Fig. 3B-b and Fig. 3B-c), the responses of the MCNT-based LFSBs (left) were higher than that of the MNP-based LFSBs (right). Fig. 3C displayed the corresponding optical responses of the MCNT- and MNP-based LFSBs and it was found that the sensitivity of MCNT-based LFSB was five-times higher than that of MNP-based LFSB. The high sensitivity of the MCNT-based LFSB would be ascribed to the big surface area of CNTs.
Fig. 3.
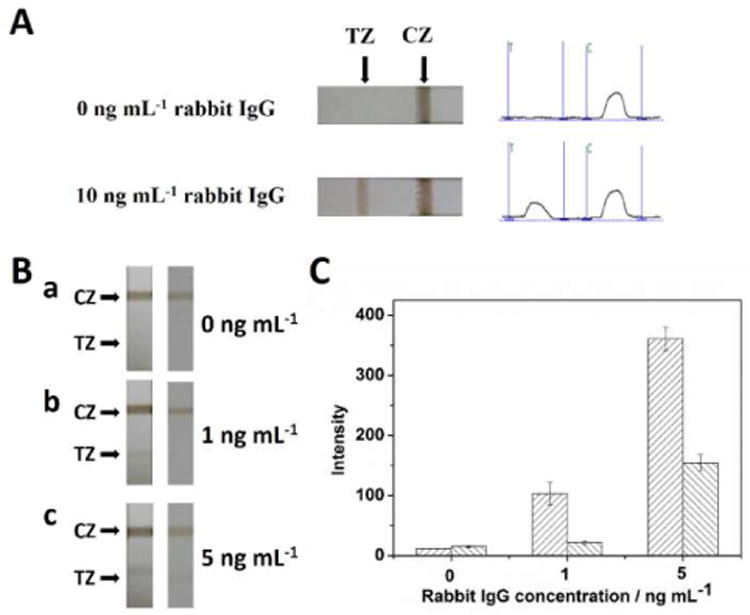
(A) Typical images of MCNT-based LFB in the absence and presence of 10 ng mL−1 target IgG; (B) Photo images of the MCNT-based LFSBs (left) and the magnetic nanoparticle-based LFSBs (right) in the presence of different concentrations of rabbit IgG: (a) 0 ng mL−1, (b) 1 ng mL−1, and (c) 5 ng mL−1; (C) corresponding intensity of the peak areas of the test zones of (B). CZ: control zone; TZ: test zone.
3.3 Optimization of Experimental Parameters
To obtain the best performance of MCNT-based LFSB, the following experimental parameters were optimized: (a) CNT resources and CNT amount used to prepare the MCNTs; (b) running buffers; (c) types of nitrocellulose membranes; (d) antibody amount for preparing MCNT-antibody conjugates; (e) concentration of capture antibody on the test zone; (f) the volume of conjugates per assay. Respective data and Figures (Fig. S2 and Fig. S3) are given in the Electronic Supporting Information. We found the following experimental conditions to give best results: (a) using 10 mg SN2302 multiwalled CNT (Nanomaterial Store) to prepare MCNT; (b) using PBS + 1% BSA + 0.03% Tween solution as running buffer; (c) using HF090MC100 membrane (Millipore) to prepare LFSB; (d) using 20 μg of detection antibody to prepare MCNT-antibody conjugates (The immobilized Ab1 on MCNT surface was around 80 ng per 1 μg MCNT); (e) dispensing 1.6 mg mL-1 of capture antibody on the test zone; (f) using 2.5 μL MCNT-Ab1 per assay.
3.4. Analytical performance
Under the optimal experimental conditions, the performance of the MCNT-based LFSB was examined with sample solutions containing different concentrations of target IgG. Fig. 4A presents the typical photo images and corresponding optical responses in the presence of 0 to 10 ng mL−1 rabbit IgG. The photo images recorded by a digital camera would be used for visual judgement. The peak areas in the left column would be used for quantitative detection. One can see that no band was observed on test zone in the absence of rabbit IgG (control), indicating negligible nonspecific adsorption. The test band can be still clearly observed in the presence of 0.5 ng mL−1 rabbit IgG, which was used as the visual detection limit of rabbit IgG without instrumentation. The intensity of the test zone increased with the increase of the IgG concentration and arrived at a plateau at 100 ng mL−1 (Fig. 4B). As shown in the inset of Fig. 4B, the corresponding calibration plots of the greyscales of test bands (peak area) had a linear correlation with the rabbit IgG concentration range over 0.5–10 ng mL−1. The limit of detection (based on S/N=3) (LOD) was calculated to be 0.34 ng mL−1 (∼2.3× 10−12 mol L−1). The LOD is two times lower than that of the GNP-based LFSB and forty times lower than that of the latex-based LFSB [51,52]. Moreover, the use of MCNT labels avoided the aggregation of conjugates, sample pretreatment and purification, which were often met in the traditional protein test in blood with GNP-based LFSB.
Fig. 4.
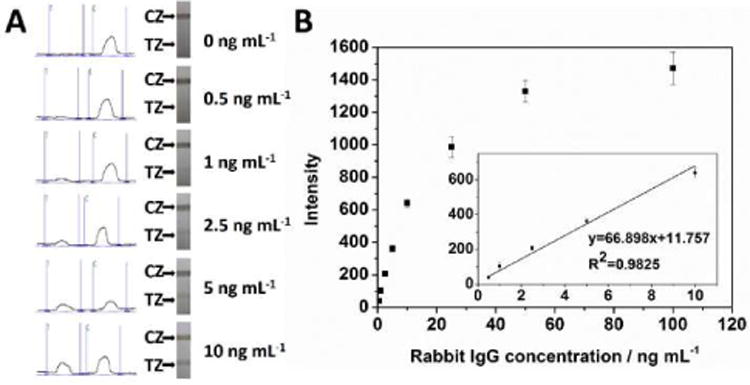
Typical optical responses and photo images of the LFSBs with an increasing rabbit IgG concentration (0.5 to 10 ng mL-1); (B) calibration curve of the LFSB. The inset shows the linear response for rabbit IgG. Each data point represents the average value obtained from three different measurements. Assay time: 20 min. CZ: control zone; TZ: test zone.
3.5. Reproducibility and specificity
The reproducibility of MCNT-based LFSB was studied by testing sample solutions containing 0, 1.0 and 5.0 ng mL-1 target IgG. Each concentration level was measured six times with six different LFSBs (Fig. S4). The corresponding RSD values were 7.72 %, 8.51% and 5.02 % respectively, demonstrating desirable analytical stability and reproducibility. The specificity of the MCNT-based LFSB was examined in the presence of an excess of non-target proteins (BSA, CEA, CA-19-9, mammoglobin). A high response was observed when 5.0 ng mL-1 IgG was tested, whereas the negligible signals were obtained from other proteins, indicating the excellent specificity of the MCNT-based LFSB (Fig. S5).
3.6. Detection of rabbit IgG in whole blood
The optimized MCNT-based LFSB was then applied to detect rabbit IgG in whole blood (Fig. 2). The volume of MCNT-Ab1 conjugates, the volume of blood used per assay, the capturing time of MCNT-Ab1 in blood and magnetic separation time were optimized to obtain the best results (Fig. S6). Briefly, 20 μL of human blood spiked with certain concentration of rabbit IgG was mixed with 80 μL of PBS buffer and 5 μL of MCNT-Ab1 conjugates. The mixture was incubated 10 min under general shaking. After applying an external magnet 2 min, the MCNT-Ab1-IgG complex were separated from the whole blood, washed twice with PBS and re-suspended to the running buffer before applying to the sample pad of the LFSB. Fig. 5A presents the typical photo images of LFSBs in the presence of different concentrations of rabbit IgG in blood. No band was observed in the absence of the target IgG in blood and the intensities of the test bands increased with the increase of rabbit IgG concentration in blood. It has a linear range from 10 to 200 ng mL−1, and the visual limit detection was 10 ng mL-1 of rabbit IgG in blood (Fig. 5B).
Fig. 5.
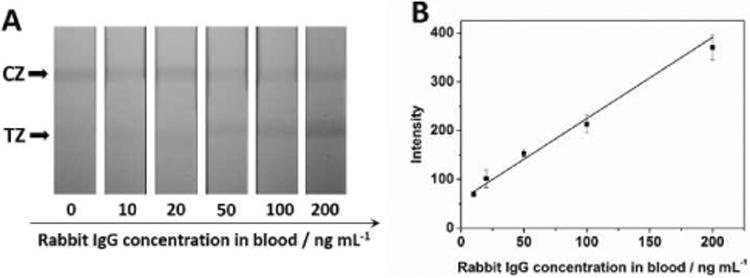
(A) typical photo images of the MCNT-based LFSB with an increasing rabbit IgG concentration in blood samples (10 to 200 ng mL-1); (B) corresponding calibration curve. Each data point represents the average value obtained from three different measurements. Running buffer: PBS+1% BSA+0.03% Tween; concentrations of rabbit IgG antibody on the test zone: 1.6 mg mL-1; concentration of rabbit IgG antibody on the conjugate: 20 μg; conjugates volume: 5 μL; blood stock sample: 20 μL; incubation time: 10 min; separate time: 2 min; assay time: 20 min. CZ: control zone; TZ: test zone.
4. Conclusions
In summary, we have developed a magnetized carbon nanotube-based lateral flow strip biosensor for visual detection of protein in whole blood. Combining the superparamagnetism of Fe3O4 nanoparticles and the outstanding mechanical properties of carbon nanotube, the antibody modified magnetized carbon nanotubes have three functions: (1) capturing target protein in whole blood; (2) magnetic separating the magnetized carbon nanotube-antibody-target protein complexes from the whole blood and reducing the matrix effect; (3) visualizing and quantifying the concentration of target protein on a lateral flow device. To the best of our knowledge, this is the first time of the successful application of magnetized carbon nanotube as immunochromatographic labels for visual detection of protein in whole blood without any complex purification or sample pre-treatments. This study provides a rapid and low-cost approach for detecting proteins in blood, showing great promise for clinical application and biomedical diagnosis, particularly in limited resource settings. Future work will aim to use the MCNT-based LFSB for detecting cancer protein biomarkers in blood.
Supplementary Material
Highlights.
Magnetized carbon nanotube is first used as an immunochromatographic label.
Visual detection of protein in whole blood avoiding complex purification and sample-pretreatment.
The method is rapid, simple, low-cost and portable.
Acknowledgments
This research was supported by the National Institute of Health, Centers of Biomedical Research Excellence (NIH, COBRE, Grant number: 1P20 GM109024). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors also would like to thank the NSFC (51373023, 21171019), Beijing Natural Science Foundation (2172039, 2122038), and the Fundamental Research Funds for the Central Universities and NCET-11-0584.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van der Vaart A, Bursulaya BD, Brooks CL, Merz KM. Are many-body effects important in protein folding. J Phys Chem B. 2000;104:9554–9563. [Google Scholar]
- 2.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 3.Csordas A, Gerdon AE, Adams JD, Qian J, Oh SS, Xiao Y, Soh HT. Detection of proteins in serum by micromagnetic aptamer PCR (MAP) technology. Angew Chem, Int Ed. 2010;49:355–358. doi: 10.1002/anie.200904846. [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Huang YY, Liu X, Zhang X, Ferrari M, Qin L. Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol. 2014;32:132–139. doi: 10.1016/j.tibtech.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willard JM, White DR, Wesson CA, Stellflug J, Sasser RG. Detection of fetal twins in sheep using a radioimmunoassay for pregnancy-specific protein B. J Anim Sci. 1995;73:960–966. doi: 10.2527/1995.734960x. [DOI] [PubMed] [Google Scholar]
- 6.Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621–1628. doi: 10.1002/1097-0142(197710)40:4<1621::aid-cncr2820400435>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann T. Analysis of heterogeneous beta A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive western blot assay. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- 8.Shacter E, Williams JA, Lim M, Levine RL. Differential susceptibility of plasma proteins to oxidative modification: Examination by western blot immunoassay. Free Radical Bio Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 9.Cao P, Moini M. Analysis of peptides, proteins, protein digests, and whole human blood by capillary electrophoresis/electrospray ionization-mass spectrometry using an in-capillary electrode sheathless interface. J Am Soc Mass Spectr. 1998;9:1081–1088. doi: 10.1016/S1044-0305(98)00081-6. [DOI] [PubMed] [Google Scholar]
- 10.Heeschen C, Goldmann BU, Langenbrink L, Matschuck G, Hamm CW. Evaluation of a rapid whole blood ELISA for quantification of troponin I in patients with acute chest pain. Clin Chem. 1999;45:1789–1796. [PubMed] [Google Scholar]
- 11.Lee BS, Lee JN, Park JM, Lee JG, Kim S, Cho YK, Ko C. A fully automated immunoassay from whole blood on a disc. Lab Chip. 2009;9:1548–1555. doi: 10.1039/b820321k. [DOI] [PubMed] [Google Scholar]
- 12.Queipo-Ortuno MI, Colmenero JD, Baeza G, Morata P. Comparison between LightCycler Real-Time Polymerase Chain Reaction (PCR) assay with serum and PCR-enzyme-linked immunosorbent assay with whole blood samples for the diagnosis of human brucellosis. Clin Infect Dis. 2005;40:260–264. doi: 10.1086/426818. [DOI] [PubMed] [Google Scholar]
- 13.Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-Linked Immunosorbent Assay Specific to Dengue Virus Type 1 Nonstructural Protein NS1 Reveals Circulation of the Antigen in the Blood during the Acute Phase of Disease in Patients Experiencing Primary or Secondary Infections. J Clin Microbiol. 2002;40:376–381. doi: 10.1128/JCM.40.2.376-381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adkins JN. Toward a human blood serum proteome analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics. 2002;1:947–955. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- 15.Espy RD, Teunissen SF, Manicke NE, Ren Y, Ouyang Z, van Asten A, Cooks RG. Paper spray and extraction spray mass spectrometry for the direct and simultaneous quantification of eight drugs of abuse in whole blood. Anal Chem. 2014;86:7712–7718. doi: 10.1021/ac5016408. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Yan Y, Yan F, Ju H. Electric field-driven strategy for multiplexed detection of protein biomarkers using a disposable reagentless electrochemical immunosensor array. Anal Chem. 2008;80:6072–6077. doi: 10.1021/ac800905k. [DOI] [PubMed] [Google Scholar]
- 17.Chikkaveeraiah BV, Bhirde AA, Morgan NY, Eden HS, Chen X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano. 2012;6:6546–6561. doi: 10.1021/nn3023969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W, Chang CL, Chan BD, Jalal SI, Matei DE, Low PS, Savran CA. Concurrent Detection of Cellular and Molecular Cancer Markers Using an Immunomagnetic Flow System. Anal Chem. 2015;87:10205–10212. doi: 10.1021/acs.analchem.5b02215. [DOI] [PubMed] [Google Scholar]
- 19.Chen HH, Wu CH, Tsai ML, Huang YJ, Chen SH. Detection of total and A1c-glycosylated hemoglobin in human whole blood using sandwich immunoassays on polydimethylsiloxane-based antibody microarrays. Anal Chem. 2012;84:8635–8641. doi: 10.1021/ac301756d. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Sunkara V, Kim TH, Hwang H, Cho YK. Lab-on-a-disc for fully integrated multiplex immunoassays. Anal Chem. 2012;84:2133–2140. doi: 10.1021/ac203163u. [DOI] [PubMed] [Google Scholar]
- 21.Meyer MH, Hartmann M, Keusgen M. SPR-based immunosensor for the CRP detection--a new method to detect a well known protein. Biosens Bioelectron. 2006;21:1987–1990. doi: 10.1016/j.bios.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Gan N, Li T, Zhou H, Li X, Cao Y, Wang L, Sang W, Hu F. Ultratrace detection of C-reactive protein by a piezoelectric immunosensor based on Fe3O4@SiO2 magnetic capture nanoprobes and HRP-antibody co-immobilized nano gold as signal tags. Sensor Actuat B-Chem. 2013;178:494–500. [Google Scholar]
- 23.Kriz K, Ibraimi F, Lu M, Hansson LO, Kriz D. Detection of C-reactive protein utilizing magnetic permeability detection based immunoassays. Anal Chem. 2005;77:5920–5924. doi: 10.1021/ac0508649. [DOI] [PubMed] [Google Scholar]
- 24.Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 25.Wang YK, Yan YX, Ji WH, Wang HA, Li SQ, Zou Q, Sun JH. Rapid simultaneous quantification of zearalenone and fumonisin B1 in corn and wheat by lateral flow dual immunoassay. J Agr Food Chem. 2013;61:5031–5036. doi: 10.1021/jf400803q. [DOI] [PubMed] [Google Scholar]
- 26.Paterson AS, Raja B, Garvey G, Kolhatkar A, Hagstrom AE, Kourentzi K, Lee TR, Willson RC. Persistent luminescence strontium aluminate nanoparticles as reporters in lateral flow assays. Anal Chem. 2014;86:9481–9488. doi: 10.1021/ac5012624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zangheri M, Di Nardo F, Anfossi L, Giovannoli C, Baggiani C, Roda A, Mirasoli M. A multiplex chemiluminescent biosensor for type B-fumonisins, aflatoxin B1 quantitative detection in maize flour. Analyst. 2015;140:358–365. doi: 10.1039/c4an01613k. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Wang Y, Wang J, Tang Z, Pounds JG, Lin Y. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal Chem. 2010;82:7008–7014. doi: 10.1021/ac101405a. [DOI] [PubMed] [Google Scholar]
- 29.Du D, Wang J, Wang L, Lu D, Lin Y. Integrated lateral flow test strip with electrochemical sensor for quantification of phosphorylated cholinesterase: biomarker of exposure to organophosphorus agents. Anal Chem. 2012;84:1380–1385. doi: 10.1021/ac202391w. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Jia Q, Yang C, Qiao R, Jing L, Wang L, Xu C, Gao M. Lateral flow immunochromatographic assay for sensitive pesticide detection by using Fe3O4 nanoparticle aggregates as color reagents. Anal Chem. 2011;83:6778–6784. doi: 10.1021/ac201462d. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Mao X, Zeng Q, Wang S, Kawde AN, Liu G. Aptamer-functionalized gold nanoparticles as probes in a dry-reagent strip biosensor for protein analysis. Anal Chem. 2009;81:669–675. doi: 10.1021/ac8020592. [DOI] [PubMed] [Google Scholar]
- 32.Lopez Marzo AM, Pons J, Blake DA, Merkoci A. High sensitive gold-nanoparticle based lateral flow Immunodevice for Cd2+ detection in drinking waters. Biosens Bioelectron. 2013;47:190–198. doi: 10.1016/j.bios.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Mao X, Ma Y, Zhang A, Zhang L, Zeng L, Liu G. Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Anal Chem. 2009;81:1660–1668. doi: 10.1021/ac8024653. [DOI] [PubMed] [Google Scholar]
- 34.Suarez-Pantaleon C, Wichers J, Abad-Somovilla A, van Amerongen A, Abad-Fuentes A. Development of an immunochromatographic assay based on carbon nanoparticles for the determination of the phytoregulator forchlorfenuron. Biosens Bioelectro. 2013;42:170–176. doi: 10.1016/j.bios.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Lu X, Liang X, Dong J, Fang Z, Zeng L. Lateral flow biosensor for multiplex detection of nitrofuran metabolites based on functionalized magnetic beads. Anal Bioanal Chem. 2016;408:6703–6709. doi: 10.1007/s00216-016-9787-2. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Wang W, Wu T, Xu LP, Wen Y, Zhang X. A three-line lateral flow biosensor for logic detection of microRNA based on Y-shaped junction DNA and target recycling amplification. Anal Bioanal Chem. 2016;408:8195–8202. doi: 10.1007/s00216-016-9925-x. [DOI] [PubMed] [Google Scholar]
- 37.Rundstrom G, Jonsson A, Martensson O, Mendel-Hartvig I, Venge P. Lateral flow immunoassay using Europium (III) chelate microparticles and time-resolved fluorescence for eosinophils and neutrophils in whole blood. Clin Chem. 2007;53:342–348. doi: 10.1373/clinchem.2006.074021. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Tian B, Zhang Z, Wang X, Fleming J, Bi L, Yang R, Zhang X. Detection of Bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “Road Closure”. Biosens Bioelectron. 2015;67:608–614. doi: 10.1016/j.bios.2014.09.067. [DOI] [PubMed] [Google Scholar]
- 39.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 40.Park S, Vosguerichian M, Bao Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale. 2013;5:1727–1752. doi: 10.1039/c3nr33560g. [DOI] [PubMed] [Google Scholar]
- 41.Lin Y, Lu F, Tu Y, Ren Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004;4:191–195. [Google Scholar]
- 42.Sotiropoulou S, Chaniotakis NA. Carbon nanotube array-based biosensor. Anal Bioanal Chem. 2003;375:103–105. doi: 10.1007/s00216-002-1617-z. [DOI] [PubMed] [Google Scholar]
- 43.Kruss S, Landry MP, Vander Ende E, Lima BM, Reuel NF, Zhang J, Nelson J, Mu B, Hilmer A, Strano M. Neurotransmitter detection using corona phase molecular recognition on fluorescent single-walled carbon nanotube sensors. J Am Chem Soc. 2014;136:713–724. doi: 10.1021/ja410433b. [DOI] [PubMed] [Google Scholar]
- 44.Li F, Peng J, Wang J, Tang H, Tan L, Xie Q, Yao S. Carbon nanotube-based label-free electrochemical biosensor for sensitive detection of miRNA-24. Biosens Bioelectron. 2014;54:158–164. doi: 10.1016/j.bios.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 45.Qiu W, Xu H, Takalkar S, Gurung AS, Liu B, Zheng Y, Guo Z, Baloda M, Baryeh K, Liu G. Carbon nanotube-based lateral flow biosensor for sensitive and rapid detection of DNA sequence. Biosens Bioelectron. 2015;64:367–372. doi: 10.1016/j.bios.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Yao L, Teng J, Zhu M, Zheng L, Zhong Y, Liu G, Xue F, Chen W. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens Bioelectron. 2016;85:331–336. doi: 10.1016/j.bios.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Wang J, Xie D, Chen G. Polyaniline-coated Fe3O4 nanoparticle-carbon-nanotube composite and its application in electrochemical biosensing. Small. 2008;4:462–466. doi: 10.1002/smll.200701018. [DOI] [PubMed] [Google Scholar]
- 48.Cao MS, Yang J, Song WL, Zhang DQ, Wen B, Jin HB, Hou ZL, Yuan J. Ferroferric oxide/multiwalled carbon nanotube vs polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl Mater Inter. 2012;4:6949–6956. doi: 10.1021/am3021069. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Zhao Z, Qu J, Wang Z, Qiu J. Fabrication and characterization of magnetic Fe3O4–CNT composites. J Phys Chem Solids. 2010;71:673–676. [Google Scholar]
- 50.Duan D, Fan K, Zhang D, Zhang J, Tan S, Liang M, Liu Y, Zhang P, Liu W, Qiu X, Kobinger GP, Gao GF, Yan X. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens Bioelectron. 2015;74:134–141. doi: 10.1016/j.bios.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 51.Kawde A, Mao X, Xu H, Zeng Q, He Y, Liu G. Moving enzyme-linked immunosorbent assay to the point-of-care dry-reagent strip biosensors. Am J Biomed Sci. 2010;2:23–32. [Google Scholar]
- 52.Birnbaum S. Udén, Latex-based thin-layer immunoaffinity chromatography for quantitation of protein analytes. Anal Biochem. 1992;206:168–171. doi: 10.1016/s0003-2697(05)80028-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


