SUMMARY
As an important regulator of macrophage cholesterol efflux and HDL biogenesis, miR-33 is a promising target for treatment of atherosclerosis, and numerous studies demonstrate that inhibition of miR-33 increases HDL levels and reduces plaque burden. However, important questions remain about how miR-33 impacts atherogenesis, including whether this protection is primarily due to direct effects on plaque macrophages, or regulation of lipid metabolism in the liver. We demonstrate that miR-33 deficiency in Ldlr−/− mice promotes obesity, insulin-resistance and hyperlipidemia, but does not impact plaque development. We further assess how loss of miR-33 or addition of miR-33b in macrophages and other hematopoietic cells impact atherogenesis. Macrophage-specific loss of miR-33 decreases lipid accumulation and inflammation under hyperlipidemic conditions, leading to reduced plaque burden. Therefore, the pro-atherogenic effects observed in miR-33 deficient mice are likely counterbalanced by protective effects in macrophages, which may be the primary mechanism through which anti-miR-33 therapies reduce atherosclerosis.
Graphical abstract

INTRODUCTION
MicroRNAs (miRNAs) have been demonstrated to play a key role in the control of mRNA translation and degradation, comprising an entirely new level of posttranscriptional regulation that is involved in nearly all biological processes (Ambros, 2004; Bartel, 2009; Fernandez-Hernando et al., 2013; Fernandez-Hernando et al., 2011). Targeting miRNAs is a promising therapeutic approach, as they are dysregulated during and involved in the development of many different disease states (Barwari et al., 2016; Poller et al., 2017). Recent work has elucidated the role of miRNAs in the development of numerous metabolic conditions including obesity, Type II diabetes and cardiovascular disease, which are responsible for the majority of chronic illnesses and deaths in the United States and other developed countries. Many factors including insulin signaling, inflammation, autophagy, mitochondrial function, and lipid metabolism are involved in the development of these cardiometabolic diseases, and previous work suggests a role for miR-33 in regulating all of these critical processes (Allen et al., 2012; Davalos et al., 2011; Goedeke et al., 2013; Karunakaran et al., 2015; Marquart et al., 2010; Ouimet et al., 2017; Ouimet et al., 2015; Rayner et al., 2010).
The miR-33 miRNA family, which consists of miR-33a and miR-33b, is one of the most well characterized examples of miRNA mediated regulation of lipid metabolism (Marquart et al., 2010; Najafi-Shoushtari et al., 2010; Rayner et al., 2010). miR-33a and miR-33b are intronic miRNAs encoded within the SREBP-2 and SREBP-1 genes respectively (Marquart et al., 2010; Najafi-Shoushtari et al., 2010; Rayner et al., 2010). SREBP-1 and SREBP-2 are two of the most important transcription factors involved in the regulation of fatty acid and cholesterol metabolism respectively (Horton et al., 2002). As such, the discovery of miRNAs within intronic regions of the genes that encode these key transcription factors directed multiple groups to investigate whether miR-33a and miR-33b may also be involved in the regulation of lipid metabolism. This work led to the demonstration that miR-33a and miR-33b are co-transcribed along with SREBP-2 and SREBP-1, and work in concert with their host genes to help maintain proper lipid levels within the cell (Davalos et al., 2011; Marquart et al., 2010; Najafi-Shoushtari et al., 2010; Rayner et al., 2010). While the SREBP transcription factors prevent deleterious reductions in cellular lipid levels by promoting lipid synthesis and uptake, miR-33a and miR-33b help prevent further loss of lipids by targeting genes involved in cholesterol trafficking and efflux [Niemann Pick C (NPC1), ATP-Binding Casette A1 (ABCA1), and ATP-Binding Casette G1 (ABCG1) (Marquart et al., 2010; Najafi-Shoushtari et al., 2010; Rayner et al., 2010)], and fatty acid β-oxidation [carnitine palmitoyltransferase 1A (CPT1A), carnitine O-octanoyltransferase (CROT), and hydroxyacyl-CoA dehydrogenase/3-ketoacy-CoA thiolase/enoyl-CoA hydratase β subunit (HADHB) (Davalos et al., 2011; Gerin et al., 2010)]. In addition to regulating cellular cholesterol efflux, ABCA1 also controls the biogenesis of high-density lipoproteins (HDL) and a number of groups have demonstrated that in vivo antagonism or genetic deletion of miR-33 enhances hepatic ABCA1 expression and increases circulating HDL-cholesterol (HDL-C) (Marquart et al., 2010; Najafi-Shoushtari et al., 2010; Rayner et al., 2010). Further work has demonstrated an additional mechanism by which the miR-33 family prevents lipid depletion in the liver by targeting genes regulating bile acid synthesis and secretion [ATP-binding cassette subfamily B member 11 (ABCB11), amino phospholipid transporter class I type 8B member 1 (ATP8B1), and cytochrome P450 family 7, subfamily A, polypeptide 1 (CYP7A1) (Allen et al., 2012; Li et al., 2013)]. Together these studies established miR-33 is an important regulator of many facets of lipid metabolism, including several steps of the reverse cholesterol transport (RCT) pathway, the process by which cholesterol is returned to the liver for conversion into bile acids and removal via the feces (Lewis and Rader, 2005; Rader and Hovingh, 2014).
RCT is the only mechanism by which arterial macrophages (Mθs) can remove excess cholesterol, thereby reducing the formation of atherosclerotic plaques by limiting the accumulation of lipid-laden Mθs within the arterial wall (Rosenson et al., 2012). As such, miR-33 has been demonstrated to be an important factor in controlling the development of atherosclerosis, and a great deal of work has been done exploring the possible therapeutic value of inhibiting miR-33 during atherogenesis. Numerous reports have demonstrated that antagonism of miR-33 in mouse models of atherosclerosis can reduce plaque burden by either impeding the progression or promoting the regression of atherosclerotic plaques (Rayner et al., 2011b; Rotllan et al., 2013). However, other similar studies did not observe any beneficial effects of anti-miR-33 therapy on atherogenesis (Marquart et al., 2013). Additionally, some studies of long-term anti-miR-33 treatment have reported deleterious effects including increased circulating levels of triglycerides (TAGs) and the development of hepatic steatosis (Allen et al., 2014; Goedeke et al., 2014). Most of the beneficial effects of miR-33 on atherosclerosis have been attributed to its ability to increase circulating levels of HDL-C and/or promote Mθ cholesterol efflux (Rayner et al., 2011b; Rayner et al., 2010), although additional functions of miR-33 including regulation of mitochondrial function, autophagy and Mθ activation status may also contribute to these effects (Karunakaran et al., 2015; Ouimet et al., 2017; Ouimet et al., 2015). Importantly, studies performed in monkeys have demonstrated that antagonism of miR-33 can also increase circulating levels of HDL-C in non-human primates (Rayner et al., 2011a; Rottiers et al., 2013). These findings are especially relevant, as the intronic region of the SREBP-1 gene encoding miR-33b is disrupted in rodents and other small mammals. Because of the inability to study miR-33b using most conventional animal models, many questions still remain about its specific role in regulation of atherosclerosis and other metabolic disease.
In order to properly assess the possible therapeutic value of anti-miR-33 therapies, it will be necessary to determine whether increased HDL biogenesis or improved cholesterol efflux from plaque Mθs is primarily responsible for mediating the beneficial effects that have been reported. This question is especially relevant as recent clinical trials have demonstrated that other therapeutic approaches, though effective at raising circulating HDL-C levels, did not appear to have any impact on clinical outcomes in patients with cardiovascular disease. To begin to address this question, Horie and colleagues created mice deficient in miR-33 (Horie et al., 2010). By crossing these animals into the Apoe−/− mouse model of atherosclerosis they were able to demonstrate that whole body loss of miR-33 reduces atherosclerotic plaque size and lipid content (Horie et al., 2012). Additionally, bone marrow transplant from miR-33 deficient animals into Apoe−/− mice, to selectively remove miR-33 from Mθs and other hematopoietic cells, was found to reduce lipid content in atherosclerotic plaques but did not have a significant impact on plaque size (Horie et al., 2012). This study suggests that both increased circulating HDL-C levels and improved efflux of cholesterol from arterial Mθs may contribute to the ability of anti-miR-33 therapies to reduced atherosclerotic plaque burden.
While this work provides some insight into the potential impact of whole body vs Mθ specific antagonism of miR-33, the potential relevance of these findings is limited by the nature of the atherosclerotic mouse model that was used. Because both HDL-C levels and RCT are dramatically reduced in Apoe−/− mice, the elevated HDL-C levels seen in mice lacking miR-33 may be substantially more impactful. At the same time work done using this model may underestimate the impact of miR-33 on regulating Mθ cholesterol efflux, as ABCA1 and ABCG1, two of the most well established targets of miR-33, are primarily responsible for transport of cholesterol onto circulating HDL particles. Additionally, a later report by this same group has demonstrated that loss of miR-33 results in a predisposition to the development of obesity and insulin resistance (Horie et al., 2013), but the impact of these effects on atherosclerosis are unclear as these phenotypes were not reported in their earlier work in the Apoe−/−background.
In this work, we attempt to address many of the outstanding questions regarding how miR-33 regulates atherosclerotic plaque development. To do this we have generated a novel miR-33 knockout mouse model (miR-33−/−) and utilized these mice to assess how loss of miR-33 impacts atherosclerosis in the Ldlr−/− mouse model of atherosclerosis, in which HDL-C levels and RCT capacity are similar to wild-type (WT) mice. Using this model, we are able to demonstrate that miR-33 deficiency results in elevated expression of ABCA1 in both the liver and Mθs, and enhances the capacity of Mθs to efflux cholesterol in vivo leading to a dramatic reduction in the accumulation of cholesterol esters in miR-33−/−Mθs under hyperlipidemic conditions. Consistent with this, we observe that selective loss of miR-33 in Mθs and other hematopoietic cells results in a substantial reduction in both atherosclerotic plaque size, and lipid content. We further demonstrate that both the number of apoptotic cells and the size of the necrotic cores are reduced in plaques from mice transplanted with miR-33−/− bone marrow indicative of increased plaque stability. Alternatively, whole body loss of miR-33 in Ldlr−/− mice was found to result in increased body weight (BW), impaired insulin sensitivity, and a pro-atherogenic lipid profile. These effects appear have offset the beneficial effects of miR-33 within the atherosclerotic plaques, as no difference was found in the plaque size or lipid accumulation of Ldlr−/−miR-33−/− double knockout (DKO) mice compared to Ldlr−/− controls. We further assess how miR-33b in Mθs impacts atherosclerotic plaque formation using a newly generated mouse model in which the mouse Srebp-1 gene has been replaced with the human form, including the intronic region encoding miR-33b (miR-33bKI). Finally, we have performed in depth RNA sequencing under both normal and hyperlipidemic (Ldlr−/−) conditions to provide a more complete view of the impact ablation of miR-33 has on Mθ gene expression. Together these findings help clarify the role of miR-33 in the development of atherosclerosis and highlight both the beneficial effects of removing miR-33 in arterial Mθs to promote cholesterol efflux from within atherosclerotic plaques, and the deleterious effects that loss of miR-33 in other tissues may have on atherogenesis and other metabolic conditions.
RESULTS
Generation of miR-33 knockout mice
To address some of the primary unresolved questions surrounding how miR-33 regulates atherosclerosis, we utilized CRISPR/Cas9 technology to excise the intronic region of the Srebp-2 gene encoding miR-33, thus generating a new miR-33 knockout mouse model (miR-33−/−). This excision of roughly 200bp can be readily confirmed by PCR analysis and has been used to detect effective excision of the miR-33 sequence in all tissues examined (Figure 1A). Consistent with previous studies, Western blot analysis of the liver of miR-33−/− mice reveals increased expression of miR-33 target genes ABCA1 and HADHβ compared to control animals, while other miR-33 targets including CROT were unaffected (Figure 1B). Consistent with the observed increase in ABCA1 expression, plasma HDL-C levels were found to be elevated in miR-33−/− mice as visualized by FPLC fractionation (Figure 1C) and confirmed by quantification of circulating HDL-C levels (Figure 1D).
Figure 1. Global deletion of miR-33 in Ldlr−/− mice results in obesity, insulin resistance and dyslipidemia.
(A) Strategy used to generate miR-33−/− mice (upper panel). PCR analysis of tail DNA isolated from WT, miR-33+/− and miR-33−/− mice (middle panel). PCR analysis showing the deletion of miR-33 in the indicated tissues (bottom panel). (B) Representative Western Blot analysis of ABCA1, CROT and HADHB in liver samples isolated from WT and miR-33−/− mice. Relative protein levels were determined by band densitometry and are expressed in arbitrary units after correction for loading control Actin (n=3). (C) Lipoprotein profile analysis from pooled plasma of WT and miR-33−/− mice. (D) Plasma HDL-C levels from WT and miR-33−/− mice (n=9). (E) Body weight of Ldlr−/− and Ldlr−/−miR-33−/− (DKO) mice fed a Western diet (WD) for 10wks (n=9). (F) Insulin tolerance test (ITT) and (G) Glucose tolerance test (GTT) in Ldlr−/− and DKO mice fed a WD for 10wks (n=6–7). Inset represents the area under the curve (AUC). (H) Plasma glucose levels in fed and fasted 2-month-old Ldlr−/− and DKO mice (n=7). (I) Plasma insulin levels in fasted Ldlr−/− and DKO mice fed a WD for 12 wks. (J) Cholesterol and (K) TAG content of FPLC-fractionated lipoproteins from pooled plasma of Ldlr−/− and DKO mice fed a WD for 12wks (L) Western blot analysis (representative of two blots) of plasma APOB and APOA1 in the FPLC-fractionated lipoproteins in panel. Lanes 1–13 correspond to the following pooled fractions: 1 (28–30), 2 (31–33), 3 (34–36), 4 (37–39), 5 (40–42), 6 (43–45), 7 (46–48), 8 (49–51), 9 (52–54), 10 (55–57), 11 (58–60), 12 (61–63) and 13 (64–66). (M-O) Plasma TAGs (M), total cholesterol (N) and HDL-C (O) in the plasma of Ldlr−/− and DKO mice fed a WD for 12 wks (n=11–16). All data represent the mean ±SEM and * indicates P<0.05 comparing miR-33−/− with WT mice or DKO with LDLR−/− mice on the same diet.
Ldlr−/−miR-33−/− mice have increased body weight and impaired insulin sensitivity
Following initial characterization, miR-33−/− mice were bred into the LDLR deficient model of mouse atherosclerosis (Ldlr−/−) to generate Ldlr−/−miR-33−/− (DKO) mice. Similar to earlier work on miR-33 deficient mice fed a high fat diet (HFD), our DKO mice had significantly higher body weight (BW) compared to Ldlr−/− controls, which was exacerbated by feeding a pro-atherogenic Western diet [WD (1.25% cholesterol)] (Figure 1E). Additionally, following 10wks of WD feeding DKO animals showed reduced responsiveness to insulin during an insulin tolerance test (Figure 1F) indicating impaired insulin sensitivity. However, this was not sufficient to disrupt regulation of glucose homeostasis as neither glucose tolerance tests (Figure 1G) nor measurement of fed and fasted blood glucose levels (Figure 1H) revealed any difference between Ldlr−/− and DKO mice at this time. DKO mice also had higher levels of circulating insulin, suggesting that increased insulin production was able to compensate for the impaired insulin sensitivity (Figure 1I). FPLC fractionation of plasma from Ldlr−/− and DKO mice following 12wks of WD feeding showed that DKO mice had a substantial increase in the amount of cholesterol (Figure 1J) and triglycerides (TAGs) (Figure 1K) in fractions containing very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) particles. Furthermore, Western blot analysis on FPLC fractions from DKO mice further demonstrates increased expression of the apolipoproteins associated with these particles (Figure 1L). Finally, DKO mice also had elevated total levels of TAGs and cholesterol compared to Ldlr−/− controls, while HDL-C levels were not altered (Figure 1M–O). The similar HDL-C levels observed between Ldlr−/− and DKO mice may be due to the repression of SREBP-2 and miR-33 under hyperlipidemic conditions (Rotllan et al., 2013).
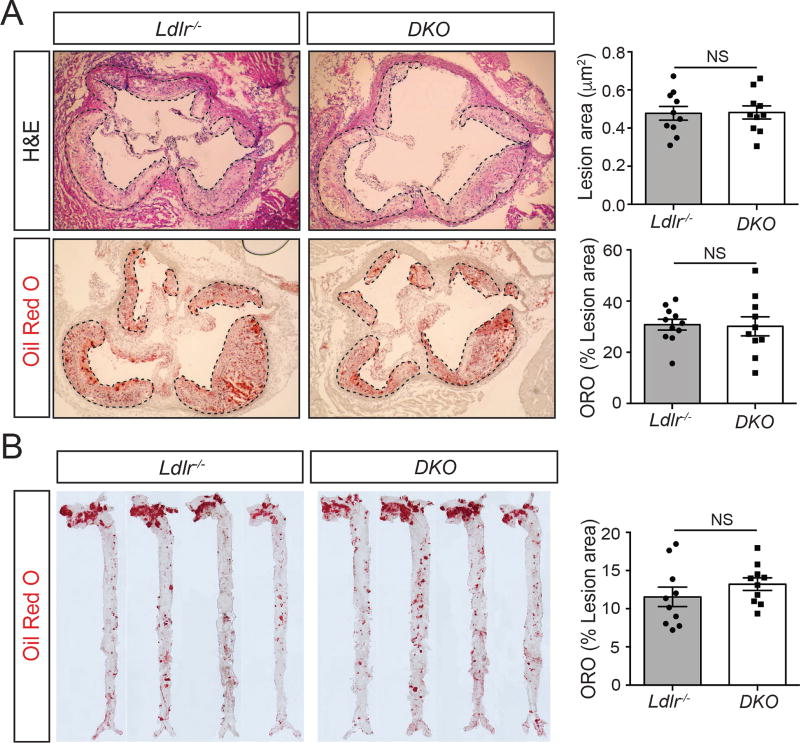
Loss of miR-33 in Ldlr−/− mice does not impact plaque size or morphology
Despite the increased BW, insulin resistance (IR), and pro-atherogenic lipid profile observed in DKO mice, histological analysis of progressive sections of the aortic root did not reveal any major differences between DKO mice and Ldlr−/− controls. No significant changes were observed in total plaque area or lipid accumulation, as indicated by Oil Red O (ORO) staining for neutral lipids (Figure 2A). Similarly, en face analysis of lipid accumulation in the aorta did not show any differences between DKO and Ldlr−/− mice (Figure 2B) and measurements of circulating blood leukocytes by FACS and HEMAVET did not reveal any significant differences between groups (Figure S1A and S1B). These findings are contrary to what has been previously observed in most studies in animals treated with anti-miR-33 therapies as well as miR-33 deficient Apoe−/− mice. While further work is needed to fully understand the mechanisms underlying the detrimental whole-body changes observed in DKO mice, these findings could suggest that the beneficial effects of removing miR-33 within arterial Mθs may offset these negative effects.
Figure 2. Germline deficiency of miR-33 does not influence the progression of atherosclerosis.
(A) Representative histological analysis of cross sections of the aortic sinus stained with hematoxylin and eosin (H&E; upper panels) or Oil-red O (ORO; bottom panels) of Ldlr−/− and Ldlr−/−miR-33−/− (DKO) mice fed a Western diet (WD) for 12 wks. Right panels are the mean lesion area and neutral lipid accumulation calculated from H&E and ORO aortic cross sections respectively. Each dot represents the mean of the quantification of ≥9 sections from an individual animal (n=10 animals per group). (B) Representative en face ORO staining of 4 aortas from Ldlr−/− and DKO mice fed a WD for 12 wks. Quantification of the ORO positive area is shown in the right panel and represents the mean ± SEM (n=10). All data represent the mean ±SEM and * indicates P<0.05 comparing DKO with Ldlr−/− mice on the same diet.
Hematopoietic specific loss of miR-33 impedes the development of atherosclerotic plaques
To directly address the question of how loss of miR-33 within the Mθs making up atherosclerotic plaques impacts atherogenesis, we performed bone marrow transplants (BMT) into lethally irradiated Ldlr−/− mice using bone marrow (BM) from either WT or miR-33−/− mice. Unlike comparisons between Ldlr−/− and DKO mice, animals transplanted with BM from miR-33−/− mice did not have any differences in plasma lipids, or lipoprotein profile (Figure S1C–S1F) and also do not show significant changes in levels of circulating leukocytes (Figures S2A and S2B). However, following 12 wks of WD feeding, atherosclerotic plaque burden was substantially reduced in mice transplanted with BM from miR-33−/−animals. Histological analysis of the aortic root revealed a significant reduction in both total plaque area and lipid accumulation (Figure 3A), and further analysis of en face preps of the aorta also demonstrated a significant decrease in lipid accumulation (Figure 3B). More detailed analysis of these plaques showed that the size of the necrotic core was also decreased in mice transplanted with miR-33−/− BM (Figure 3C). The percentage of plaque area that stained positive for the Mθ marker CD68 was similar to that of control animals, indicating that the proportion of the plaque made up of macrophages was not impacted by loss of miR-33. Alternatively, CD68/TUNEL co-staining was decreased in plaques from animals that received miR-33−/− BM, indicating that Mθ apoptosis may contribute to the elevated formation of necrotic cores in WT animals (Figure 3D). Furthermore, by digesting the aortas of WD fed mice and performing FACs analysis we were able to show a significant decrease in the number of both total and activated (Ly-6Chigh) Mθs in plaques from mice transplanted with miR-33−/− BM (Figure 3E). Together, these findings demonstrate that while hematopoietic loss of miR-33 does not alter the proportion of the plaque that is made up of macrophages there is an overall decrease in the number of macrophages within the arterial wall corresponding to the overall decrease in plaque size.
Figure 3. Hematopoietic miR-33 deficiency attenuates atherogenesis.
(A) Representative histological analysis of cross sections of the aortic sinus stained with hematoxylin and eosin (H&E; upper panels) or Oil-red O (ORO; lower panels) of Ldlr−/− mice transplanted with WT or miR-33−/− bone marrow (BM) fed for 12 wks on a Western diet (WD). Right panels are the mean lesion area and neutral lipid accumulation calculated from H&E or ORO aortic cross sections respectively. Each dot represents the mean of the quantification of ≥9 sections from individual animal (n=9–11 animals per group). (B) Representative en face ORO staining of aortas from Ldlr−/− transplanted with WT and miR-33−/− BM and fed a WD for 12 wks. Quantification of the ORO positive area is shown in the right panel and represents the mean ±SEM (n=10 per group). (C) Representative images of aortic root cross sections stained with hematoxylin and eosin (H&E) from Ldlr−/− mice transplanted with WT or miR-33−/− BM fed for 12 wks on WD. Images reveal how the necrotic core area was defined for quantification in each section. Dashed lines show the boundary of the developing necrotic core (NC). Quantification is shown in the right panel. Each dot represents the mean of the quantification of ≥9 sections from individual animal (n=9–11 animals per group). (D) Representative cross-sections analysis of macrophage (MO) content (CD68 positive cells; upper panels) and apoptotic cells (TUNEL positive cells; bottom panels) of the aortic root from Ldlr−/− mice transplanted with WT or miR-33−/− BM and fed a WD for 12 wks. Quantifications are the graphs on the right. Each dot represents the mean of the quantification of ≥9 sections from individual animal (n=9–11 animals per group). (E) Flow cytometry analysis of aortic Mθs and monocytes from Ldlr−/− mice transplanted with WT or miR-33−/− BM and fed for a WD for 12wks. Left, representative dot plots showing gating schemes. Right, quantification of total number of Mθs or Ly-6Chigh monocytes per aorta (n=6). All data represent the mean ±SEM and * indicates P<0.05 comparing Ldlr−/− mice transplanted with miR-33−/− BM to Ldlr−/− mice transplanted with WT BM on the same diet.
Generation of miR-33b knock-in mice
While these findings highlight the important role of Mθ miR-33 in the regulation of atherogenesis in mice, the situation is more complicated in humans due to the presence of a second miR-33 isoform, miR-33b. To determine whether miR-33b may also impact atherosclerosis, we generated a novel mouse model in which the entire mouse Srebp-1 gene has been replaced with the human sequence, including the region encoding miR-33b, under the control of the mouse promoter (miR-33bKI). Insertion of this construct can be confirmed by the presence of the neo cassette, which was later excised, or with specific primers demonstrating the presence of human Srebp-1 or the loss of mouse Srebp-1 (Figure S3A). Furthermore, the introduction of human SREBP-1 and miR-33b, and the loss of mouse Srebp-1 in homozygous miR-33bKI mice was confirmed by qPCR analysis (Figure S3B, S3C, and S3E). Importantly, this analysis also demonstrates that the expression of Srebp-2 and miR-33a was not altered by addition of miR-33b (Figure S3D and S3F). Expression of miR-33b was detected in a variety of tissues, among which the liver showed the highest expression levels (Figure S3G). This is consistent with what has previously been shown for miR-33b in human tissues (Najafi-Shoushtari et al., 2010). Human SREBP-1 also had high levels of expression in the liver, although it was also highly expressed in other tissues including the white adipose tissue (WAT) and kidney which had relatively low levels of miR-33b expression (Figure S3H).
Reconstitution with bone marrow from cells expressing miR-33b does not impact atherosclerotic plaque formation
Similar to BMT experiments with miR-33−/− mice, transplantation of Ldlr−/− mice with BM from miR-33bKI animals did not have any impact on lipid profiles or circulating blood leukocytes (Figure S4A–D). Unlike the loss of miR-33 however, introduction of Mθs expressing miR-33b also did not have any impact on total plaque size or lipid accumulation in either the aortic root or whole aorta (Figure S5A). These findings are likely due to the low levels of expression of miR-33b within Mθs compared to that of miR-33a. While miR-33b can be readily detected in Mθs from either miR-33bKI mice or derived from human peripheral blood mononuclear cells (PBMCs), its expression in these cells is dramatically lower than that of miR-33a (Figure S5B). Consistent with this, the expression of miR-33 target genes was not changed in Mθs from miR-33bKI mice either under basal conditions, following stimulation with an LXR ligand (T0901317), or loading with acetylated LDL (Ac-LDL) (Figure S5C). Unfortunately, assessment of how addition of miR-33b across the whole-body impacts atherosclerosis was not possible due to the sub-Mendelian birth rates, poor survival and reduced size of miR-33bKI mice.
Loss of miR-33 improves Mθ RCT in vivo
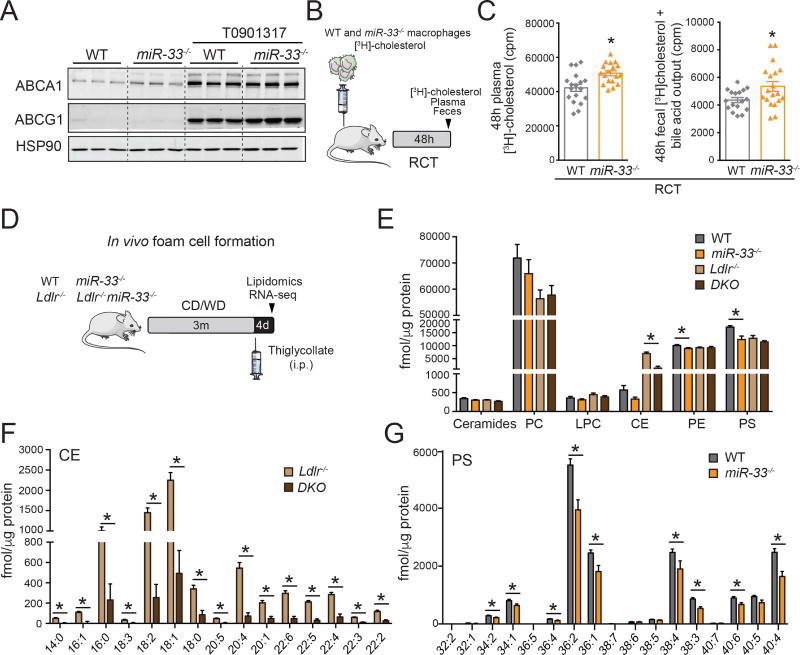
Although the metabolic differences observed in miR-33−/− mice did not impede overall assessment of atherogenesis, the altered BW, IR and lipid levels of DKO mice precludes any direct examination of how changes in HDL biogenesis contribute to these changes. As such, we next sought to better understand the mechanisms underlying the beneficial effects observed in BMT experiments with miR-33 deficient donors. Previous work has demonstrated that inhibition of miR-33 enhances Mθ cholesterol efflux (Marquart et al., 2010; Najafi-Shoushtari et al., 2010; Rayner et al., 2010). Consistent with this we find that the expression of ABCA1 and ABCG1 is increased in Mθs isolated from miR-33−/−mice (Figure 4A). Next, we determined whether the absence of miR-33 in Mθs enhances RCT. To this end, bone marrow derived Mθs (BMDM) from WT or miR-33−/− mice were differentiated in vitro, preloaded with [H3]-Cholesterol and injected into the peritoneal cavity of WT mice (Figure 4B). By quantifying the amount of [H3]-Cholesterol incorporated into the blood and feces of these animals over 48h, we are able to demonstrate that loss of miR-33 improves RCT from Mθs in vivo, resulting in a significant increase in radioactivity counts in both the plasma and feces (Figure 4C). These results represent the first demonstration that loss of miR-33 in Mθs accelerates RCT in vivo.
Figure 4. Absence of miR-33 in macrophages increases ABCA1 expression, enhances RCT and attenuates lipid accumulation in vivo.
(A) Representative Western blot analysis of ABCA1 and ABCG1 expression in peritoneal macrophages (Mθs) isolated from WT and miR-33−/− mice untreated and treated with T0901317. HSP90 is used as a loading control. (B) Schematic representation of the RCT experiment. WT and miR-33−/− bone marrow derived Mθs (BMDM) are loaded with [3H]-cholesterol and injected to WT mice. Exit of [3H]-cholesterol from Mθs is measured in the plasma and feces 48h after injection. (C) [3H]-cholesterol in plasma (left panel) and in feces (right panel) from WT mice injected subcutaneously with [3H]-cholesterol-labeled BMDM from WT or miR-33−/− mice. (n=18). (D) Schematic representation of the lipidomic and genomic analysis of Mθ foam cells isolated in vivo from Ldlr−/− and Ldlr−/−miR-33−/− (DKO) mice fed a chow diet (CD) or Western diet (WD) for 12wks. (E–G) Lipidomic analysis of WT, miR-33−/−, Ldlr−/−, and DKO Mθs. (E) Levels of ceramides, phosphatidylcholine (PC), lysophosphatidylcoline (LPC), cholesterol esters (CE), phosphatidylethanolamine (PE) and phosphatidylserine (PS). (F) Levels of CE species. (G) Levels of PS species (n=4–6). All data represent the mean ±SEM and * indicates P<0.05 comparing miR-33−/− with WT mice or DKO with Ldlr−/− mice on the same diet.
Mθs lacking miR-33 have reduced levels of cholesterol esters and phospholipids
To determine how this increased RCT capacity impacts the lipid content of miR-33−/− Mθs, we performed lipidomic analysis of thioglycollate-elicited peritoneal Mθs from WT, miR-33−/−, LDLR−/−, and DKO mice under normal (WT) or hypercholesterolemic (Ldlr−/−) conditions (Figure 4D). Consistent with our previous results, we observe a significant decrease in the accumulation of cholesterol esters (CE) in miR-33-deficient Mθs from Ldlr−/− mice (Figure 4E), including reductions in all CE subspecies (Figure 4F). Interestingly, in a WT background loss of miR-33 was also found to significantly reduce the amount of Mθ phosphatidylethanolamines (PE) and phosphatidylserines (PS) (Figure 4E and 4G). These lipid subspecies are important components of cellular membranes and are involved in a number of important cellular functions including apoptosis, migration and phagocytosis (Greenberg et al., 2006; Marino and Kroemer, 2013; Shiratsuchi et al., 1998).
Mθs deficient in miR-33 do not show differences in in vitro polarization or apoptosis, but do have increased efferocytosis capacity
To further determine what effects of miR-33 deficiency in Mθs may contribute to the observed differences in plaque formation, we performed a series of experiments to assess Mθ functions related to atherogenesis. Previous reports have suggested that miR-33 can directly regulate Mθ activation status and inflammatory response (Ouimet et al., 2015; Rayner et al., 2011b), and our own data show that the number of Ly-6Chi-activated Mθs (M1 like) is reduced in plaques from mice reconstituted with miR-33−/− BM. However, induction of Mθ polarization in vitro did not reveal any significant differences between WT and miR-33−/− Mθs (Figure S6A–S6G). Treatment with LPS/IFNγ stimulates BMDM toward a classical (M1 like) activation status, and induction of a number of inflammatory genes. While the induction of IL-6 and IL-1β is somewhat diminished in Mθs deficient in miR-33, these changes do not reach statistical significance (Figure S6A and S6B). Moreover, loss of miR-33 does not have any impact on the induction of other markers of classically activated Mθ, NOS2 or COX2, either at the gene expression or protein level (Figure S6C–S6E). Additionally, following treatment with IL-4, to stimulate an alternative (M2 like) activation status, we do not find any differences in the expression of markers of alternatively activated Mθs, ARG1 and FIZZ1 (Figure S6F and S6G). Together these findings suggest that the changes in Mθ accumulation and activation within the plaque may not be due to direct regulation of Mθ polarization by miR-33.
Similarly, we do not observe any differences in the induction of apoptosis in miR-33 deficient Mθs in response to cholesterol loading. Treatment of Mθs with Ac-LDL and an ACAT inhibitor (Sandoz) to induce excessive free cholesterol accumulation resulted in apoptosis in approximately 15% of WT cells, and was not significantly altered in Mθs lacking miR-33 (Figure S6H). While these in vitro experiments do not properly reflect conditions within the atherosclerotic plaque, they do suggest that factors other than reduced apoptosis may contribute to the lower numbers of TUNEL positive cells and decreased necrotic core size found in plaques from animals transplanted with miR-33 deficient BM. Consistent with this, we find that loss of miR-33 increases the ability of Mθs to engulf and incorporate fluorescently labeled apoptotic cells (Figure S6I). Interestingly, the expression of MERTK, a kinase involved in the regulation of efferocytosis, was found to be upregulated in miR-33−/− Mθs (Figure S6J). These findings are important, as efficient clearance of apoptotic cells limits prolonged inflammation and may contribute to the reduced recruitment and activation of Mθs lacking miR-33.
Lack of miR-33 in Mθs attenuates the expression of genes associated to inflammatory response in vivo
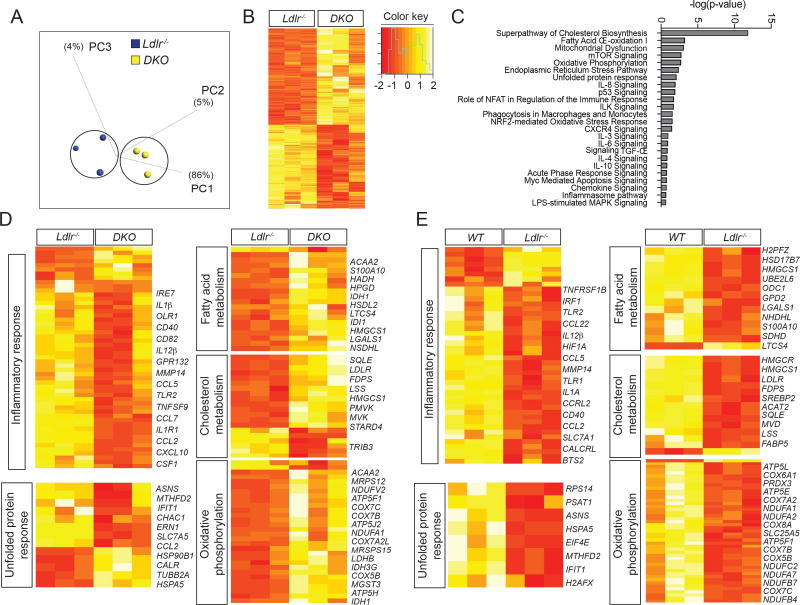
Similar to our lipidomic analysis, we have also performed an unbiased analysis of how gene expression levels are impacted by loss of miR-33 in Mθs in vivo under hyperlipidemic (Ldlr−/−) conditions. This approach, developed by Glass laboratory, allows the analysis of changes in lipid metabolism and gene expression during foam-cell formation in vivo (Li et al., 2004). RNA-seq analysis has revealed a number of pathways and specific genes that may be involved in mediating the effects of miR-33 on Mθ function and atherosclerotic plaque formation. Furthermore, comparison between WT and Ldlr−/− mice allows us to distinguish between those genes that are generally regulated in response to changes in lipid content and those that are more likely to be directly regulated by miR-33 (Figure 5). Among those factors found to be significantly altered in response to loss of miR-33 (Figure 5B), a number of important pathways were found to be overrepresented, including those involved in inflammation, fatty acid metabolism, cholesterol biosynthesis, and unfolded protein response (Figure 5C and 5D). These pathways and many of the genes within them are known to play an important role in the development of atherosclerotic plaques and may contribute either directly or indirectly to the beneficial effects observed in miR-33 deficient Mθs. Additional analysis of gene expression changes between WT and Ldlr−/− mice demonstrates that many of these same pathways are also impacted by hypercholesterolemia. Indeed, many of the metabolic pathways that are upregulated in DKO deficient macrophages compared to Ldlr−/−mice are downregulated in Ldlr−/− mice compared to WT (Figure 5E). This suggest that these changes may be largely due to differences in lipid content, while pathways related to stress response are downregulated in both comparisons, indicating that these changes are likely regulated by factors independent of lipid content. Indeed, these changes would be predicted to result in diminished inflammatory signaling through the NFκB and TLR4 mediated pathways resulting in a reduction in Mθ activation and polarization in vivo (Figure 6A and S7). Moreover, some of the specific changes in lipid sub-species that we have observed in miR-33−/− macrophages may also contribute to the observed changes in inflammatory response. Amongst the molecules that are known to interact with phosphatidylserine and phosphatidylethanolamine, many were found to be dysregulated by loss of miR-33 in our RNA-seq analysis. Indeed, the observed decreases in these two classes of lipids could be predicted to promote many of these changes in gene expression, and the overall changes in these genes is predicted to result in an overall decrease in inflammatory response (Figure 6B). While additional work will be needed to identify the specific miR-33 targets responsible for mediating many of these effects, these data provide an important resource for establishing how changes in lipid content and miR-33 levels impact global gene expression patterns in Mθs in vivo.
Figure 5. Lack of miR-33 in macrophages impacts genes involved in stress response and metabolic function in vivo.
(A) Principal component (PC) analysis of peritoneal macrophages (Mθs) from Ldlr−/− and Ldlr−/−miR-33−/−(DKO) mice (n=3) based on RNA-sequencing gene expression levels, color-coded according to the group of mice. (B) Heatmaps representing gene expression (rlog transformed values) of genes differentially expressed between Ldlr−/− and DKO Mθs. (C) Top canonical pathways involving differentially expressed molecules and related to atherosclerosis expressed as -log(p-value). (D) Heatmaps of pathways relevant to atherosclerosis that are altered in DKO mice compared to Ldlr−/−controls. (E) Heatmaps of pathways relevant to atherosclerosis that are altered in Ldlr−/− mice compared to WT controls.
Figure 6. Absence of miR-33 attenuates NFKB activation and innate immune response.
(A) NFKB signaling pathway analysis using Ingenuity Pathway Analisis (IPA) tool. Genes represented in green color are down-regulated in peritoneal macrophages (Mθs) isolated from Ldlr−/−miR-33−/− (DKO) fed a WD for 12 wks compared to Mθs obtained from WT mice. (B) Network of genes known to interact with phosphatidylserine and phosphatidylethanolamine whose expression is significantly altered in miR-33 deficient macrophages. Prediction analysis identifies genes in which inhibition (blue) or activation (red) is predicted based on changes in these lipid species. Overall changes in this network are predicted to inhibit inflammatory response.
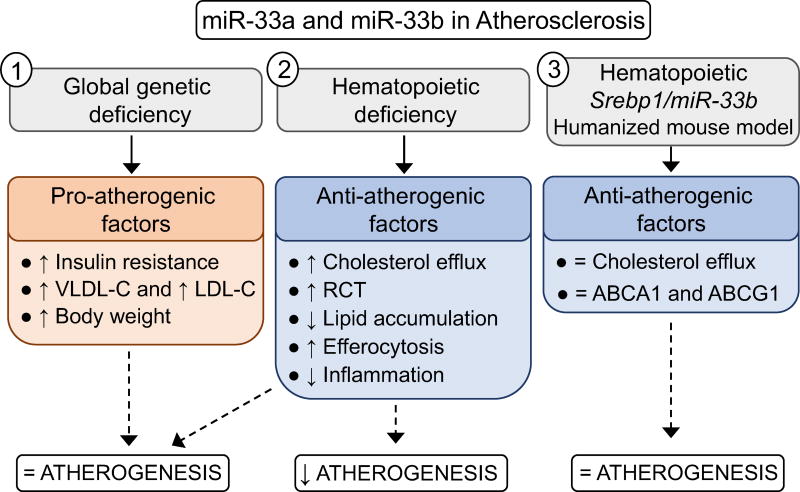
Overall this work demonstrates that loss of miR-33 selectively in hematopoietic cells is sufficient to reduce Mθ recruitment and lipid accumulation in atherosclerotic plaques. The beneficial effects however are offset by the pro-atherogenic factors that are found to be increased in response to whole body loss of miR-33, including increased body weight, IR and dyslipidemia. Alternatively, Mθ miR-33b was not found to have a major impact on atherosclerosis, which is likely due to the low expression of this isoform in Mθs compared to miR-33a (Figure 7).
Figure 7.
Schematic representation of the role of miR-33a and miR-33b in atherogenesis
DISCUSSION
As a known regulator of cholesterol efflux and HDL-C metabolism, miR-33 is an attractive target for the treatment of cardiovascular disease, and several studies have found that antagonism of miR-33 can reduce atherosclerotic plaque burden (Karunakaran et al., 2015; Ouimet et al., 2015; Rayner et al., 2011b; Rotllan et al., 2013). Another study did not find reduced aortic lesions in anti-miR-33 treated mice (Marquart et al., 2013). However, the hypertriglyceridemia and hepatic steatosis observed in some long term anti-miR-33 studies (Allen et al., 2014; Goedeke et al., 2014), and the adverse metabolic effects reported in miR-33 knockout mice (Horie et al., 2013) have raised major concerns about the development of anti-miR-33 therapeutics for treatment of human patients. Much of this concern stems from an incomplete understanding of the mechanisms by which miR-33 impacts atherogenesis, as highlighted by the dual role of miR-33 regulating cholesterol efflux in Mθs and HDL biogenesis in the liver. This question is even more important due to the poor performance of CETP inhibitors and other HDL-C raising therapies in recent clinical trials (Rader and deGoma, 2014; Rader and Hovingh, 2014). If the effects of miR-33 are primarily due to its ability to raise circulating HDL-C levels, anti-miR-33 therapeutics may face many of the same issues found in these studies, while direct effects on the ability of arterial Mθs to efflux cholesterol may provide greater therapeutic value. Together, the findings laid forth in this manuscript help clarify many of the questions regarding how miR-33 regulates atherosclerotic plaque formation, which will be necessary for evaluating the possible therapeutic potential of miR-33 antagonists.
Similar to previous work in Apoe−/− mice (Horie et al., 2012), we observe that reconstitution of Ldlr−/− mice with bone marrow deficient in miR-33 reduces lipid accumulation in atherosclerotic plaques. However, the effects observed in our study are considerably more robust, with significant reductions also observed in total plaque size, necrotic core area, and the number of apoptotic cells. Since the primary function of ABCA1 and ABCG1 is the efflux of cholesterol onto poorly-lipidated ApoA1 and HDL particles respectively (Tall et al., 2008), these differences may be due to the low levels of HDL-C in Apoe−/− mice, which could limit the impact of elevating Mθ ABCA1 levels. These findings indicate that the ability of miR-33 to promote cholesterol efflux from arterial Mθs plays a more important role in its regulation of atherosclerosis than was suggested by previous work. Because these effects are independent of any changes in circulating HDL-C levels, these findings demonstrate the potential therapeutic value of anti-miR-33 therapies for promoting the removal of lipids from atherosclerotic plaques and thereby limiting plaque development.
Interestingly, we find that loss of miR-33 in Ldlr−/− mice results in a significant increase in BW, which is exacerbated by feeding of a WD. This predisposition to diet induced obesity is also accompanied by decreased insulin sensitivity and increased circulating levels of VLDL-C and LDL-C and TAGs. Together these changes likely offset the beneficial effects occurring in plaque Mθs, as there were no differences in plaque size or lipid accumulation in Ldlr−/− mice lacking miR-33. Unfortunately, these changes also impede our ability to address the specific impact of miR-33’s regulation of HDL biogenesis and bile acid metabolism in the liver. While BMT from WT mice into Ldlr−/− and DKO animals would allow us to determine how effects on the liver and other tissues impact plaque formation, it would not help with dissociating the effects on RCT from the changes observed in body weight, insulin resistance, and circulating lipids. These effects are contrary to what was previously reported in Apoe−/−mice lacking miR-33, where they did find reduced plaque size, did not observe any difference in circulating VLDL-C, LDL-C, or TAGs, and did not provide any data on BW or insulin sensitivity (Horie et al., 2012). Interestingly, a later report by the same group did show a dramatic increase in BW and insulin resistance in miR-33−/− mice when fed a high fat (60% saturated fat) diet (Horie et al., 2013). Further work is currently underway seeking to elucidate the specific mechanisms by which miR-33 regulates obesity and insulin resistance, as this will be important for the progress of any anti-miR-33 therapeutic efforts.
While most work on miR-33 has been done using mouse models, humans contain a second miR-33 isoform, miR-33b, which may also play an important role in atherosclerosis. In this work, we begin to assess the impact of miR-33 on atherogenesis by performing bone marrow transplants from miR-33bKI mice into the Ldlr−/− background. Addition of miR-33b to Mθs does not appear to have any impact on plaque formation or lipid accumulation. These results are likely due to the relatively low expression of miR-33b in Mθs compared to that of miR-33a. This finding is not specific to our miR-33bKI mice, as Mθs derived from human peripheral blood mononuclear cells also expressed much higher levels of miR-33a compared to miR-33b. These differences in miRNA expression vary between cell types suggesting that the role of miR-33b in the liver, where miR-33 is most highly expressed both in our mouse model and in human tissues (Najafi-Shoushtari et al., 2010), and other tissues may be considerably more important. Consistent with this, a previously described miR-33b knockin model was reported to have decreased expression of ABCA1 and other miR-33 target genes in primary hepatocytes (Horie et al., 2014). Furthermore, we find that expression of miR-33b in the liver is higher than that of miR-33a, which could be very interesting under conditions of obesity and insulin resistance, where the host gene of miR-33b, SREBP-1, is known to be dysregulated. Unfortunately, the poor survival rates and reduced body weight of homozygous miR-33bKI mice at birth and throughout early adulthood has precluded any meaningful exploration into how global addition of miR-33b impacts atherogenesis. Further work using adult inducible models or other techniques to overcome these developmental issues will be needed to properly address the role of miR-33b in atherosclerosis and other metabolic diseases.
A number of different functions of miR-33 have been described as possibly contributing to its regulation of atherosclerotic plaque formation. Among these, the ability of miR-33 to regulate Mθ cholesterol efflux and RCT is the most likely to directly impact the accumulation of lipids within arterial Mθs (Rayner et al., 2011b; Rayner et al., 2010). In this work, we demonstrate for the first time that loss of miR-33 increases the capacity of Mθs to efflux cholesterol in vivo. Additionally, we observe an additional function of miR-33 in regulating the uptake of apoptotic cells by Mθs in vitro, which could possibly also contribute to the changes seen in necrotic core size. Interestingly, another group has very recently reported similar changes in efferocytosis upon inhibition of miR-33, which were attributed to regulation of autophagy by miR-33 (Ouimet et al., 2017). However, neither the specific autophagic genes reported to be targeted by miR-33, nor most other genes related to autophagic function were found to be significantly altered in our RNA-seq analysis. Alternatively, MERTK protein levels were found to be reduced in miR-33 deficient Mθs, and miRNA prediction algorithms suggest that this may targeted by the passenger strand of miR-33, miR-33*. Finally, by using unbiased techniques for assessing global changes in lipid profiles, and mRNA expression, we provide a comprehensive profile of the factors influenced by miR-33 under normal and hyperlipidemic conditions, which provides further insight into the pathways and individual gene targets responsible for mediating the effects of miR-33.
Consistent with the increased capacity to efflux cholesterol previously observed, we find a dramatic reduction in the accumulation of all different species of cholesterol esters in miR-33 deficient Mθs under hyperlipidemic conditions. Our RNA-seq analysis in Mθs has revealed a number of genes that are differentially up and down regulated in response to loss of miR-33. Pathway analysis further revealed that many of these genes are involved in pathways that have previously been shown to be important for the development of atherosclerosis. An additional analysis, identifying genes that are altered under hyperlipidemic conditions, demonstrates that many of the genes whose expression was dysregulated in response to hyperlipidemic conditions were regulated in a reverse manner in cells lacking miR-33. These changes are likely due to the reduced lipid accumulation observed in miR-33 deficient Mθs, however a large proportion of the genes dysregulated in response to loss of miR-33 were not found to be altered in response to dyslipidemia, suggesting that these could be either directly or indirectly regulated by miR-33 independent of its effects on lipid content. Among the pathways found to be significantly overrepresented in this dataset were important pathways involved in regulation of metabolic function. Most of the genes in these pathways were significantly upregulated in DKO animals. These same pathways were largely repressed in Ldlr−/− mice compared to WT, suggesting that the reduced lipid accumulation in DKO animals is likely the primary factor responsible for the altered expression of these genes. Alternatively, most of the genes in pathways related to inflammation and ER stress (unfolded protein response) were downregulated both in DKO mice compared to Ldlr−/− and Ldlr−/− mice compared to WT, despite opposite changes in lipid content. Therefore, the alterations in these stress response pathways is likely independent of the overall changes in lipid content. While these alterations may be mediated through direct targeting of factors involved in regulation of these pathways, our in vitro assessment of Mθ polarization and ER stress mediated apoptosis did not reveal any differences between miR-33−/− and WT Mθs. Interestingly, we also find reduced levels of phosphatidylserine and phosphatidylethanolamine in miR-33 deficient Mθs. These changes are not as uniform or dramatic as the reductions in cholesterol esters, but may have important effects, as these lipid subspecies are known to play important roles in Mθ mobility, phagocytosis, and apoptosis (Greenberg et al., 2006; Marino and Kroemer, 2013; Shiratsuchi et al., 1998). Consistent with this, Lai L and colleagues have recently reported that absence of miR-33 in Mθs alters membrane microdomains, influencing the innate immune response (Lai et al., 2016). By overlaying our RNA-seq data with a network of genes known to interact with these lipid sub-species, we can identify a number of genes that are significantly dysregulated by loss of miR-33. Further predictive analysis demonstrates that many of these changes are predicted based on the observed alterations in these lipids. Moreover, the overall changes in this gene set are predicted to result in an inhibition of inflammatory response. This analysis indicates the changes in these specific lipid classes may contribute to the overall phenotypes observed in these mice, including effects on pathways like inflammatory response that were largely independent of the overall changes in lipid content. Further work looking at how loss of miR-33 impacts lipid rafts, membrane fluidity and the lipid composition of atherosclerotic plaques will provide additional insight into how these alterations may affect atherogenesis.
Overall, this work supports the promising therapeutic potential of miR-33 inhibitors for directly promoting the efflux of cholesterol from arterial Mθs, thereby limiting Mθ lipid accumulation and preventing further Mθ recruitment and activation. However, our findings also highlight the potential adverse effects of inhibiting miR-33 in other organs due to the obesity, insulin resistance, and hyperlipidemia observed in whole body miR-33−/− animals. These findings provide much needed insight into the mechanisms by which miR-33 regulates atherosclerotic plaque formation and metabolic function and suggest that targeted inhibition of miR-33 within atherosclerotic plaques may provide a more viable therapeutic option for limiting atherosclerotic plaque formation as observed in our BMT experiments. Further work elucidating the specific mechanisms by which miR-33 regulates Mθ functions, such as inflammation and efferocytosis, as well as whole body effects on obesity and IR, will further aid in assessing the therapeutic potential of miR-33 inhibition.
EXPERIMENTAL PROCEDURES
Further details and an outline of resources used in this work can be found in Supplemental Experimental Procedures. Briefly, RNA sequencing was performed through the Yale Center for Genome Analysis using an Illumina HiSeq 2000 platform (1 × 75bp read length). Trimmed reads were aligned using TopHat2, and transcript abundances and differences calculated using cuffdiff. Gene network and pathway analysis were carried out using Gene Set Enrichment Analysis software (Broad Institute), Qlucore Omics Explorer v 3.2 (Qlucore AB), and Ingenuity Pathway Analysis (Ingenuity Systems QIAGEN). Data are deposited in NCBI Gene Expression Omnibus (GSE103133). Animal studies were approved by the Institutional Animal Care and Use committee of Yale University School of Medicine. WT C57BL/6 and Ldlr−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Generation of miR-33−/− mice was accomplished with the assistance of Cyagen Biosciences Inc. Establishment of the miR-33bKI mouse model was accomplished in collaboration with Ingenious Targeting Laboratory. Accelerated atherosclerosis was induced by feeding mice for 12 weeks with a Western diet (WD) containing 1.25% cholesterol (D12108; Research Diets, Incorporated, New Brunswick, NJ, USA). Bone marrow transplant experiments, plaque characterization and measurements of metabolic function, circulating and cellular lipids and reverse cholesterol transport were carried out using established techniques. Statistical differences were measured using an unpaired two-sided Student’s t-test, or one-way ANOVA with Bonferroni correction for multiple comparisons. Normality was checked using the Kolmogorov-Smirnov test. A nonparametric test (Mann-Whitney) was used when data did not pass the normality test. A value of P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R35HL135820 to CF-H; R01HL105945 and R01HL135012 to YS; R01HL107794 to AB and F32DK10348902 to NP), the American Heart Association (16EIA27550005 to CF-H; 16GRNT26420047 to YS and 17SDG33110002 to NR), the American Diabetes Association (1-16-PMF-002 to AC-D), and the Foundation Leducq Transatlantic Network of Excellence in Cardiovascular Research MIRVAD (to CF-H). We would like to thank Sameet Mehta, and Rolando Garcia Milian for their assistance with the processing and analysis of RNA-seq data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
NP and CF-H conceived and designed the study and wrote the manuscript. NP, NR, AC-D, XZ, PP, MM, AB, DF and YS performed experiments and analyzed data.
References
- Allen RM, Marquart TJ, Albert CJ, Suchy FJ, Wang DQ, Ananthanarayanan M, Ford DA, Baldan A. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med. 2012;4:882–895. doi: 10.1002/emmm.201201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RM, Marquart TJ, Jesse JJ, Baldan A. Control of very low-density lipoprotein secretion by N-ethylmaleimide-sensitive factor and miR-33. Circ Res. 2014;115:10–22. doi: 10.1161/CIRCRESAHA.115.303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwari T, Joshi A, Mayr M. MicroRNAs in Cardiovascular Disease. J Am Coll Cardiol. 2016;68:2577–2584. doi: 10.1016/j.jacc.2016.09.945. [DOI] [PubMed] [Google Scholar]
- Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Ramirez CM, Goedeke L, Suarez Y. MicroRNAs in metabolic disease. Arterioscler Thromb Vasc Biol. 2013;33:178–185. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Suarez Y, Rayner KJ, Moore KJ. MicroRNAs in lipid metabolism. Curr Opin Lipidol. 2011;22:86–92. doi: 10.1097/MOL.0b013e3283428d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, MacDougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285:33652–33661. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedeke L, Salerno A, Ramirez CM, Guo L, Allen RM, Yin X, Langley SR, Esau C, Wanschel A, Fisher EA, et al. Long-term therapeutic silencing of miR-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO Mol Med. 2014;6:1133–1141. doi: 10.15252/emmm.201404046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedeke L, Vales-Lara FM, Fenstermaker M, Cirera-Salinas D, Chamorro-Jorganes A, Ramirez CM, Mattison JA, de Cabo R, Suarez Y, Fernandez-Hernando C. A regulatory role for microRNA 33* in controlling lipid metabolism gene expression. Mol Cell Biol. 2013;33:2339–2352. doi: 10.1128/MCB.01714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Baba O, Kuwabara Y, Chujo Y, Watanabe S, Kinoshita M, Horiguchi M, Nakamura T, Chonabayashi K, Hishizawa M, et al. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE−/− mice. J Am Heart Assoc. 2012;1:e003376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, Usami S, Izuhara M, Nakazeki F, Ide Y, et al. MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo. Sci Rep. 2014;4:5312. doi: 10.1038/srep05312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, Usami S, Izuhara M, Sowa N, Yahagi N, et al. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat Commun. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci U S A. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, et al. Macrophage Mitochondrial Energy Status Regulates Cholesterol Efflux and Is Enhanced by Anti-miR33 in Atherosclerosis. Circ Res. 2015;117:266–278. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, Azzam KM, Lin WC, Rai P, Lowe JM, Gabor KA, Madenspacher JH, Aloor JJ, Parks JS, Naar AM, et al. MicroRNA-33 Regulates the Innate Immune Response via ATP Binding Cassette Transporter-mediated Remodeling of Membrane Microdomains. The Journal of biological chemistry. 2016;291:19651–19660. doi: 10.1074/jbc.M116.723056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum JL, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Francl JM, Boehme S, Chiang JY. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology. 2013;58:1111–1121. doi: 10.1002/hep.26427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino G, Kroemer G. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 2013;23:1247–1248. doi: 10.1038/cr.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart TJ, Wu J, Lusis AJ, Baldan A. Anti-miR-33 therapy does not alter the progression of atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33:455–458. doi: 10.1161/ATVBAHA.112.300639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Ediriweera H, Afonso MS, Ramkhelawon B, Singaravelu R, Liao X, Bandler RC, Rahman K, Fisher EA, Rayner KJ, et al. microRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2017 doi: 10.1161/ATVBAHA.116.308916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K, et al. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125:4334–4348. doi: 10.1172/JCI81676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poller W, Dimmeler S, Heymans S, Zeller T, Haas J, Karakas M, Leistner DM, Jakob P, Nakagawa S, Blankenberg S, et al. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J. 2017 doi: 10.1093/eurheartj/ehx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader DJ, deGoma EM. Future of cholesteryl ester transfer protein inhibitors. Annu Rev Med. 2014;65:385–403. doi: 10.1146/annurev-med-050311-163305. [DOI] [PubMed] [Google Scholar]
- Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011a;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011b;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotllan N, Ramirez CM, Aryal B, Esau CC, Fernandez-Hernando C. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr−/− mice--brief report. Arterioscler Thromb Vasc Biol. 2013;33:1973–1977. doi: 10.1161/ATVBAHA.113.301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers V, Obad S, Petri A, McGarrah R, Lindholm MW, Black JC, Sinha S, Goody RJ, Lawrence MS, deLemos AS, et al. Pharmacological inhibition of a microRNA family in nonhuman primates by a seed-targeting 8-mer antimiR. Sci Transl Med. 2013;5:212ra162. doi: 10.1126/scitranslmed.3006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi A, Osada S, Kanazawa S, Nakanishi Y. Essential role of phosphatidylserine externalization in apoptosing cell phagocytosis by macrophages. Biochem Biophys Res Commun. 1998;246:549–555. doi: 10.1006/bbrc.1998.8663. [DOI] [PubMed] [Google Scholar]
- Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.