Water is an essential comonomer in a supramolecular polymer that is used as a recyclable, water-activated glue.
Abstract
Although the concept of structural water that is bound inside hydrophobic pockets and helps to stabilize protein structures is well established, water has rarely found a similar role in supramolecular polymers. Water is often used as a solvent for supramolecular polymerization, however without taking the role of a comonomer for the supramolecular polymer structure. We report a low–molecular weight monomer whose supramolecular polymerization is triggered by the incorporation of water. The presence of water molecules as comonomers is essential to the polymerization process. The supramolecular polymeric material exhibits strong adhesion to surfaces, such as glass and paper. It can be used as a water-activated glue, which can be released at higher temperatures and reused many times without losing its performance.
INTRODUCTION
Structural water molecules that are buried inside proteins behave very differently from bulk water and often are integral parts of the folded protein structures (1). Experimental and theoretical evidence clearly demonstrates that this structural water forms strong, structure-stabilizing hydrogen bonds with polar groups incorporated in the surrounding proteins. Therefore, water molecules perform two important roles. On the one hand, they tighten the protein structures through additional bonding interactions; however, on the other hand, they also contribute to making the protein structure more flexible because of their reversible noncovalent binding (2, 3). The concept and the importance of structural water as an essential constituent in protein folding are well acknowledged in biochemistry and cell biology (4).
Some fascinating examples about the application of structured water molecules as integral parts for the fabrication of supramolecular aggregates, including nanotubes (5), actuators (6), and polymers (7), have been reported, demonstrating the versatile role of water in material design and preparation. In the design of supramolecular polymers, an analogous concept of structural water appears to have been considered very rarely (8–10)—although water has been frequently used as a solvent for supramolecular polymerization (8, 9). Compared to conventional polymers, supramolecular polymers (10–13) self-assemble from low–molecular weight monomers (LMWMs) by noncovalent interactions, thus opening the opportunity to easily design and incorporate functional elements into polymeric chain structures (14, 15). Nevertheless, since the early example for supramolecular polymerization (16), studies use water molecules only as a solvent to disperse and solvate the monomers (17–20). Water itself has not yet been reported as an essential comonomer in supramolecular polymerization.
Here, we report an example of a supramolecular polymer containing water molecules taking the role of essential comonomers with great impact on the material properties and functions. The LMWM in this material is a triply benzo-21-crown-7 (B21C7)–substituted 1,3,5-benzenetricarboxamide (BTA) derivative (TC7; Fig. 1A) (21–24). Very much like structural water molecules in proteins, water molecules as comonomers efficiently induce and mediate unusual dynamics, flexibility, elasticity, and adhesive properties in the resulting functional supramolecular TC7-water copolymer.
Fig. 1. Water-activated supramolecular polymerization.
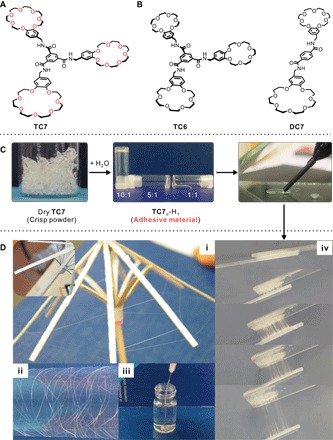
Chemical structures of (A) TC7 and (B) the control compounds TC6 and DC7. (C) Images of the TC7 samples with different water content [TC7n-H1 is used to abbreviate samples with TC7: water (H) ratio of n: 1 (w/w)]. (D) Fibers drawn from TC710-H1 (i and ii); fiber of TC73-H1 injected to hexane (rhodamine B was added to the glue to make it clearly visible) (iii); fiber generated by coating glass slides with TC75-H1 and then pulling them apart (at 80°C) (iv).
RESULTS
Water absorption behavior
When dry, TC7 is a fragile, nonsticky glass-like solid (Fig. 1C), and according to its powder x-ray diffraction (PXRD) pattern, the material is amorphous (fig. S15). The infrared (IR) spectrum of dry TC7 together with the known assembly motif of BTA (fig. S16) indicate that TC7 molecules self-assemble into one-dimensional supramolecular aggregates through amide-based H bonding (21, 23, 24). When dry TC7 is exposed to air under ambient condition [25°C, relative humidity (RH), 40%], it quickly absorbs water and rapidly converts into a highly viscous soft material (less than 1 min). During water absorption, TC7 gains at least 1.8% in weight and reaches an equilibrium state after ca. 10 min (fig. S18), as monitored by a microbalance. This behavior is unique because the structurally closely related controls TC6 and DC7 (Fig. 1B) show neither any weight growth nor the appearance of viscosity when exposed to air. More control compounds, with different crown ethers or cores, were further designed and applied (figs. S7 to S14). None of these control compounds display high viscosity and strong adhesion behavior when mixed with water, which shows BTA core-core hydrogen bonding to be as essential as the presence of B21C7 and the threefold symmetry of the compound. Other ring sizes (benzo-18-crown-6 and dibenzo-24-crown-8) or open-chain analogs do not show a similar adhesion behavior (see the Supplementary Information).
To test whether the water uptake is responsible for the drastic property changes, three TC7 samples with different water content were prepared (Fig. 1C): TC710-H1 (10:1, w/w), TC75-H1 (5:1, w/w), and TC71-H1 (1:1, w/w). As expected, water addition significantly changes the viscosity of the material: TC710-H1 displays a very high viscosity and exhibits shape persistence, TC75-H1 is a highly viscous glue-type liquid, and TC71-H1 is a transparent less-viscous liquid that slowly flows when the test tube is tilted. Thus, water uptake dramatically alters the macroscopic appearance and the viscosity of the TC7-H materials. This transition between the dry solid and the viscous material states is fully reversible, indicating that the hydration and dehydration processes are also reversible.
Rheological characterization provides quantitative information on the storage moduli (Gʹ) and loss moduli (G″) of TC7n-H1 (n = 5, 10; fig. S20) as functions of angular frequency at fixed strain (1.0 %). Both samples behave as viscoelastic liquids for which the Gʹ values are lower than the G″ values over the entire range of frequencies (25). In agreement with the macroscopic observation, TC710-H1 is mechanically stronger than TC75-H1: A Gʹ value of 0.5 kPa and a zero-shear viscosity of 105 mPa⋅s were measured at 25°C. These values decrease significantly when temperature increases from 25° to 40°C (fig. S22), exhibiting the thermosensitive nature of TC7-H materials.
Water addition also makes the resulting TC7-H materials easy to process. Thin and smooth fibers, with lengths up to several meters, were mechanically pulled from TC710-H1 (Fig. 1D, fig. S20, and movie S1). They are quite flexible and can easily be rolled up on a small wooden umbrella (Fig. 1D) or—with much stronger curvature—wrapped around a capillary of 0.3-mm diameter (movie S2), thus providing evidence for high–molecular weight supramolecular polymeric structures (26). Fibers deposited on surfaces stayed intact over more than 6 months without any obvious cracking or agglomeration. For TC73-H1, fibers and fibrous networks have also been successfully obtained by injection into hexane (movie S3). The material is sticky and bundles of parallel fibers can be pulled when the material is first deposited between two glass slides that are subsequently pulled apart (at 80°C; Fig. 1D), further proving good processability.
Structural elucidation of water molecules in polymers
To investigate the particular role of the water molecules incorporated in the TC7-H materials, attenuated total reflectance IR (ATR-IR) spectroscopy was performed (Fig. 2A and fig. S17). The spectrum of TC710-H1 shows two strong bands at 3294 and 3509 cm−1 in the spectral areas characteristic for complexed and free amide NH groups (some supramolecular systems with complexed amide groups exhibit peaks for the N–H stretch vibration around 3200 to 3300 cm−1) (23, 24, 27). Because overlap of O–H of water and N–H groups of TC7 may occur (28), D2O instead of H2O was applied. In the spectrum of TC710-D1, decreased intensities of bands at 3294 and at 3509 cm−1 are clearly visible (fig. S17), whereas a new band arises at 2505 cm−1, which can be assigned to the D–O stretch vibrations of D2O. The difference between the TC710-H1 and TC710-D1 samples indicates that the O–H band of H2O overlaps with the NH band. This indicates the existence of hydrogen-bonded water molecules (for example, for solid phase of water νasO–H ~ 3200 cm−1 and νsO–H ~ 3400 cm−1) (29). In principle, water could form H bonds with the polar O atom (in crown ether units) and/or the amide group (in cores). However, no noticeable H/D exchange between the NH groups and D2O has been observed in a TC710-D1 sample, and the proton signal of the NH group in the corresponding 1H nuclear magnetic resonance (NMR) spectrum remains visible (Fig. 2B and fig. S23). This speaks against interactions between water molecules and the amide NH protons in TC710-D1. That is, the water molecules in the TC710-H1 material form H bonds more or less exclusively with O atoms of crown ethers.
Fig. 2. The nature of water molecules in TC7-H materials.
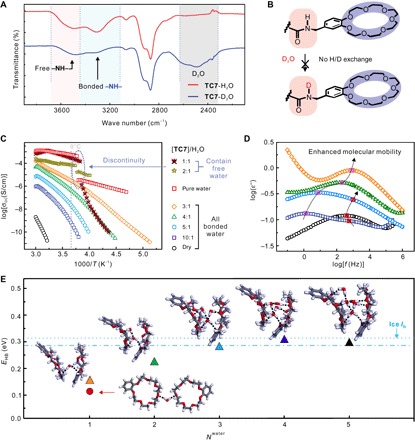
(A) ATR-IR spectra of TC7 samples prepared with H2O and D2O (TC710-H1 and TC710-D1). (B) H/D exchange experiments conducted on TC710-D1 adhesive (for details, see the Supplementary Materials). (C) The dependence of DC conductivity σdc versus 1/T for TC7-H materials with different water content. (D) Dielectric loss versus frequency for TC7-H materials with different water content at a temperature of −100°C: Frequency dependence of loss peaks due to hydrogen bonding between TC7 and water (maxima labeled in pink) and between two adjacent TC7 molecules (red labels). (E) Averaged hydrogen-bond strengths (EHB) of crown ether–water systems with different number of water molecules (Nwater) as obtained from the density functional theory (DFT) calculations: Some representative molecular structures are shown here (inserted chemical structures) and in fig. S26. The gray, red, and white spheres are carbon, oxygen, and hydrogen atoms, respectively. Hydrogen-bonds are indicated by short black-dashed lines. Blue horizontal–dashed and dot-dashed lines represent the theoretical and experimental EHB’s of bulk ice Ih, respectively.
Broadband dielectric spectroscopy (BDS) strongly supports the presence of water molecules as essential comonomers in the supramolecular polymer structure. The frequency dependence of the real part of the complex conductivity spectra of TC7n-H1 (n = 1, 2, 3, 4, 5, 10; fig. S26) corresponds to a typical conductivity-frequency behavior expected for semiconducting materials (fig. S27) (30). Their σdc values were obtained by fitting the Jonscher model and are displayed in Fig. 2C. For weight ratios of TC7n-H1 above n ≥ 3, the resulting σdc over 1/T plots do not exhibit any discontinuity at around the freezing temperature of bulk water (Fig. 2C), indicating that up to this weight ratio, the supramolecular polymer does not contain significant amounts of free water molecules with bulk water properties. On the contrary, both TC72-H1 and TC71-H1 display a clearly visible discontinuity in the σdc over 1/T plots very close to the temperature at which also water displays such a discontinuity. This clearly points to the presence of significant amounts of free water molecules within the materials that freeze at the temperature at which the discontinuity is observed. In good agreement with these findings, an in situ single crystal structure of frozen water (fig. S28) is only obtained from the high–water content sample TC71-H1 (30).
In addition, dielectric loss patterns were determined by BDS at −100°C. Figure 2D shows the frequency dependence of the dielectric loss at −100°C for TC7n-H1 (n ≥ 2). The dry TC7 sample displays a characteristic relaxation peak at a frequency around 103 Hz (maxima labeled in red), which is related to the molecular mobility of the TC7 molecules. In addition, a new relaxation peak emerges at ca. 1 Hz (maxima labeled in pink) for the TC710-H1 sample. This second peak can be assigned to the fluctuation of water molecules interacting with TC7. With increasing water content, the relaxation peak for the TC7-H2O interaction shifts to higher frequencies and increases in intensity and finally superimposes that of the TC7 molecules. Higher frequencies relate to higher molecular mobility, indicating weaker TC7-H2O interactions with increasing water content. This behavior implies a lower viscosity for the TC7-H materials with higher water content (31), which is consistent with the macroscopic behavior discussed above (TC710-H1 has a higher viscosity than that of TC75-H1; Fig. 1C).
Density functional theory simulations for hydrogen-bonded geometries
To further investigate how these water molecules connect to TC7, we performed DFT simulations for various hydrogen-bonded geometries and calculated the averaged EHB between crown ethers and water molecules, which are summarized in Fig. 2E and fig. S21. First, the model of crown ether units connected by one water molecule with a head-to-head geometry is considered, as indicated by the red circle dot. The EHB is only ~0.1 eV, much weaker than that in liquid or frozen water. If the crown ether units are connected face to face by water molecules, then EHB could gradually be enhanced as the number of Nwater increases. Some representative complex structures are shown in Fig. 2E, and more are displayed in fig. S21. In Fig. 2E, we find that the more copolymerized water molecules are, the higher the EHB is between crown ether units and center water molecules (upper triangles). When Nwater between two crown ether units reaches 4 to 5, the EHB increases to ~0.3 eV, close to the binding strength of hydrogen bonds in bulk ice Ih (blue horizontal lines in Fig. 2E) (32). We note that Nwater per crown ether molecule of ~2.5 (4 to 5 water molecules between two crown ether units) roughly corresponds to the weight ratio of TC710-H1. Further increasing Nwater cannot enhance EHB anymore. Instead, if more water molecules are introduced in this system, then water molecules will connect to each other or even form free bulk water clusters, as shown in fig. S29F (in this case, Nwater is 8, corresponding to TC72-H1).
On the basis of these findings from the BDS and the DFT simulations, we can conclude that the water molecules incorporated in the TC7n-H1 materials have two different roles: (i) For n ≥ 3—mixtures that correspond to low water content—the water molecules are not free but act as essential comonomers to form high–molecular weight supramolecular polymers through hydrogen bonding with crown ether oxygen atoms, thus cross-linking the TC7 stacks through hydrogen bonding. In line with the absence of H/D exchange reactions between incorporated D2O and the TC7 amide hydrogen atoms in TC710-H1, our structural model may involve the formation of stacks of TC7 molecules that are connected by amide-amide hydrogen bonding and π-stacking interactions, and these stacks are then cross-linked by water molecules (33). (ii) It is also demonstrated that the second role of incorporated water is that of water, which is not tightly bound to crown ether oxygen atoms and thus makes the polymeric structure flexible by acting as a type of “lubricant.” The higher water content, the more water molecules are available as lubricant water, and at high water content (n ≤ 2), free water (bulky water clusters) is present, which can even form small ice crystals below 0°C detectable by crystallography.
This adhesive material is a typical supramolecular copolymer containing two comonomers (water and TC7) (33), with the water-crown ether-based hydrogen bonding and BTA core–based hydrogen bonding as the main driving forces. On the basis of the polymerization mechanism, it is also a solvent-free supramolecular polymerization process with water as an integral part of the copolymer.
Adhesion properties
Although the material investigated here is intriguing from a fundamental point of view because of the special role of water as essential comonomer, its utility for potential applications makes it even more interesting. The high viscosity and the strong tendency to form OH–O hydrogen bonds between the crown ethers and the water molecules suggest that TC710-H1 might be a powerful adhesive when attached to OH-terminated hydrophilic surfaces, such as glass or paper (34–37).
TC710-H1 was deposited on a glass plate and processed by heating (for details, see the Supplementary Materials) to produce a uniform layer with a thickness of ca. 0.1 mm. Then, the second glass plate was pressed on that adhesive layer. The two plates adhered to each other immediately. This adhesion effect is stable for at least 24 months under ambient conditions (~25°C, 40 to 60 RH%) without a significant loss of the adhesive strength. Once two glass plates were adhered together, it is difficult to separate them. Weights up to 10 kg have been attached to one of the two slides, which were glued together on a 72-cm2 area. No separation of the two slides by shear forces has been observed (Fig. 3A and movie S4). Forces perpendicular to the plane of the adhesive layer do not separate glass plates either (Fig. 3D and figs. S30 and S31), when an area of 10 cm2 is used to glue the two slides to each other and a weight of 500 g is placed on the bottom slide. Similarly, TC710-H1 exhibits a strong and long-lasting adhesion with other hydrophilic surfaces, including silicon wafers and paper. For example, two pieces of paper strips were easily glued to each other several seconds after a very small amount (less than 10 mg) of TC710-H1 was placed on a 4.5-cm2 area, followed by pressing the second paper strip onto it. This sample easily carries 2 kg in weight without detaching at the adhesive layer (Fig. 3C).
Fig. 3. Application of TC710-H1 materials as adhesive materials.
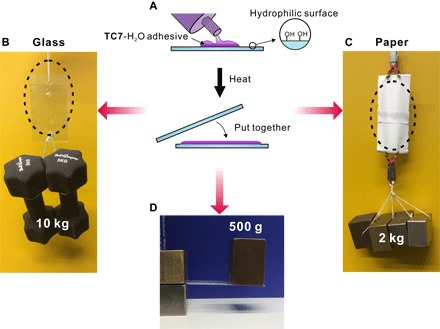
(A) A cartoon representation of the adhesion procedure. (B to D) Macroscopic adhesive behavior of TC710-H1 materials on hydrophilic surface. The adhesion areas are 9.0 × 8.0 cm2 (B), 4.5 × 1.0 cm2 (C), and 4.0 × 2.5 cm2 (D), respectively.
Pull-off adhesion tests were performed to quantitatively measure the adhesion strength of the TC710-H1 coating to glass surfaces (Fig. 4 and fig. S31) (27, 38, 39). At a pull rate of 200 psi/s (1.38 MPa/s), the average adhesion strength is 602 psi (4.15 MPa) at 25°C (Fig. 4B). This value is much higher than those of previously reported supramolecular polymer adhesives (19, 27). Commercially available PVA resulted in an adhesion strength of only 62 psi, only 1/10 of that of TC710-H1. With increasing temperature, the adhesion strength of our material decreases: At 40°, 50°, and 60°C, the corresponding adhesion strengths are 163, 92, and 60 psi (Fig. 4C), respectively. Consequently, the adhesion strength of TC710-H1 at 60°C is still comparable to that of the commercial PVA glue at room temperature.
Fig. 4. Measurements of pull-off adhesion strength.
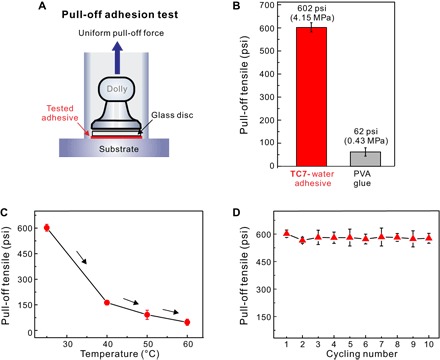
(A) Illustration of the pull-off adhesion test. (B) Comparison of the adhesion effect of TC710-H1 and commercially available adhesive poly(vinyl acetate) (PVA) glue at 25°C. (C) Adhesion strength of TC710-H1 at different temperatures. (D) Recycling tests of TC710-H1 at 25°C.
Tack tests of TC710-H1 confirm strong interactions among TC7 and water molecules (fig. S32). As the analysis criterion for the cohesive behavior, the maximum force is higher than 90 N at 25°C in the force-displacement diagram. TC710-H1 has pronounced “secondary” and “tertiary” maxima, indicating a tack behavior with pronounced stringing and high energy required for separation. High temperature (60°C) leads not only to a great decay in the maximum force but also to the disappearance of the “tertiary maximum,” indicating the weakening process of the cohesive behavior of TC710-H1 materials at elevated temperature. Pull-off adhesion tests were also performed at different conditions, especially at low RH% and low temperature. At 20 RH%, the average adhesion strengths are 512, 472, and 293 psi at 20°, −10°, and −20°C (39), respectively. In vacuum, TC710-H1 still shows strong adhesion behavior, with an average adhesion strength of 364 psi.
One might consider the decrease in adhesion strength with temperature as a disadvantage because it limits the temperature range of potential applications. However, this behavior can also be an advantage because the connection of the two pieces glued to each other with TC710-H1 can be more easily released at higher temperature. This makes the adhesive reusable (34, 37). To test the reusability, multiple cycles of pulling off the dolly, warming the material to 60°C, pressing the two glass slides together, and letting them rest for 20 min at room temperature were performed. After each cycle, another pulling test has been done. Even after 10 cycles, no substantial decrease has been observed in adhesion strength (Fig. 4D).
DISCUSSION
TC7 monomers themselves are stable under harsh conditions (for example, high temperature, high humidity, oxygen atmosphere, organic solvents atmospheres) and only starts to decompose above 350°C (fig. S33). When the TC710-H1 adhesive material was placed between two glass slides and then exposed to air for more than two and a half years, no decay in adhesive performance was observed.
In conclusion, the triply crown ether–substituted TC7 is interesting from a fundamental and from a point of view of potential applications. First, it represents an interesting supramolecular polymer in which water plays the role of an essential comonomer, whose incorporation causes dramatic property changes. Thus, water is important here far beyond being merely a solvent or being only responsible for hydrophobic effects that generate a driving force for supramolecular polymerization. Our experiments clearly indicate that the water molecules are hydrogen-bonded with the crown ether oxygen atoms to generate supramolecular polymers. Generally speaking, the incorporation of water in TC7-based materials produces an interesting adhesive material, which has a number of advantages: (i) Polymerization can occur at very high monomer concentrations, because even a small amount of water makes the material viscous, and solubility does not need to be considered. (ii) It is essentially a solvent-free polymerization (not only organic solvent-free), because all water molecules become constituents of supramolecular polymers. (iii) This adhesive material does not emit any toxic substances or unpleasant smell, because no organic solvent is required during the polymerization process or the preparation of the adhesive coating. (iv) The adhesion strength is high; nevertheless, the adhesive material can be reused by simply altering the temperature. Thus, the present work not only describes a successful resource-saving technology but also sheds new light on the fundamental importance of water in soft materials.
MATERIALS AND METHODS
All reagents were commercially available and used without further purification. Milli-Q water was used in all measurements. BC7-NH2 was prepared by a reported method (40). NMR spectra were recorded with a Bruker AVANCE III-400 spectrophotometer, using the deuterated solvent as the lock and residual solvent or TMS as the internal reference. PXRD data were collected in transmission mode on samples held on thin Mylar film in aluminum well plates on a Panalytical X’Pert PRO MPD equipped with a high-throughput screening XYZ stage, x-ray focusing mirror, and PIXcel detector using Ni-filtered Cu Kα radiation. Data were measured over the range of 5° to 50° in ~0.013° steps over 60 min. A Sartorius microbalance CP2P was used for water sorption measurements. Mass spectra were obtained using an electrospray ionization interface on an Agilent LC/MSD TOF system. The height of the adhesive coating was determined on a Leica microscope (Primo Vert). Scanning electron microscopy (SEM) experiments were performed on a high-resolution HITACHI SU 8030 scanning electron microscope. Fourier-transform IR (FT-IR) spectra were recorded on a Nicolet Avatar 320 FT-IR. The thermograms of the adhesive materials were measured using differential scanning calorimetry (DSC; MDSC2910, TA Instruments). In the DSC measurements, the average of heating and cooling scans was at a scan rate of 10°C/min. A Thermo Fisher Scientific RS6000 rheometer with a plate-plate geometry (diameter, 25 mm; gap, 1.5 mm) was used for the evaluation of Gʹ and G″. Small-angle x-ray scattering (SAXS) measurements were carried out on a SAXSess mc2 small- and wide-angle scattering system (Anton Paar GmbH) with a sealed copper tube at λCu-Kα = 0.1542 nm with the option of line and point collimation. Single crystal x-ray data were collected on a Bruker APEX2 with microfocus-sealed x-ray tube (Mo Kα radiation, λ = 0.71073 Å, Bruker APEX2 area detector). Dielectric spectroscopy was carried out in a frequency range from 10−1 to 106 Hz and between −100° and 60°C with a high-resolution ALPHA analyzer interfaced to an active sample head (Novocontrol). The measurements were carried out in parallel geometry. The temperature of the sample was controlled by a Quatro Novocontrol cryo-system with a stability of 0.1 K. To avoid the evaporation of water during the measurements, a sealed liquid parallel sample cell BDS 1308 (Novocontrol) was used. The adhesion strength measurements were performed on a pull-off adhesion tester (AT-A Automatic Adhesion Tester, PosiTest) (41). Tack tests were carried out on an Anton Paar Rheometer MCR102, with a plate-plate geometry (diameter, 25 mm; gap, 0.1 mm; and pull rate, 5 μm s−1). Thermogravimetric analysis (TGA) was carried out using a TA Instruments Q5000IR analyzer with an automated vertical overhead thermobalance. The samples were heated at a rate of 5.0°C/min.
Supplementary Material
Acknowledgments
We are grateful for the computational resources provided by the National Supercomputing Center in Changsha. We thank R. Haag for the discussions and Z. Zhong for the pull-off tests. Funding: S.D. thanks the Fundamental Research Funds for the Central Universities from Hunan University and the Alexander von Humboldt Foundation for financial support. Z.Q. acknowledges financial support from the Thousand Talents Program and Northwestern Polytechnical University of China (1800-16GH030121). C.A.S. is grateful to the Deutsche Forschungsgemeinschaft (CRC 765) for funding. Y.F. is supported by the National Natural Science Foundation of China under grant nos. 11604092 and 11634001. M.L. and C.J.S. acknowledge funding from the Engineering and Physical Sciences Research Council (EP/N004884/1) and the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013) through grant agreement no. 321156 (ERC-AG-PE5-ROBOT). Author contributions: S.D., Z.Q., and C.A.S. designed and directed the study. J.L. and A.S. performed the BDS. Y.F. performed the DFT calculation. M.L. and C.J.S. performed the crystal structure, PXRD, and TGA. L.C. performed the SAXS experiments. L.G. and W.C. performed the H/D experiments and pull-off adhesion tests. J.L. and J.S. performed the compound design, synthesis, and pull-off adhesion tests. S.D., Z.Q., and C.A.S. wrote the manuscript. All the authors commented on the paper. Competing interest: All authors declare that they have no competing interests. S.D. and Z.Q. claim responsibility for all the figures in the main text and the Supplementary Materials. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/11/eaao0900/DC1
Materials and Methods
scheme S1. Synthesis of TC7 and control compounds.
scheme S2. Pull-off test of control compounds.
fig. S1. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of TC7.
fig. S2. 13C NMR spectrum (100 MHz, CDCl3, 25°C) of TC7.
fig. S3. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of DC7.
fig. S4. 13C NMR spectrum (100 MHz, CDCl3, 25°C) of DC7.
fig. S5. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of TC6.
fig. S6. 13C NMR spectrum (125 MHz, CDCl3, 25°C) of TC6.
fig. S7. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of TC-open.
fig. S8. 13C NMR spectrum (125 MHz, CDCl3, 25°C) of TC-open.
fig. S9. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of TC8.
fig. S10. 1H NMR spectrum (125 MHz, CDCl3, 25°C) of TC8.
fig. S11. 13C NMR spectrum (400 MHz, CDCl3, 25°C) of TCB.
fig. S12. 1H NMR spectrum (125 MHz, CDCl3, 25°C) of TCB.
fig. S13. 1H NMR spectrum (500 MHz, CDCl3, 25°C) of TC7A.
fig. S14. 13C NMR spectrum (125 MHz, CDCl3, 25°C) of TC7A.
fig. S15. PXRD pattern of dry TC7.
fig. S16. IR spectrum of dry TC7.
fig. S17. IR spectra of TC710-H1 and TC710-D1.
fig. S18. Water absorbance curves of TC7 under ambient conditions (25°C, 40% RH).
fig. S19. DSC measurements of TC710-H1.
fig. S20. SEM images of TC710-H1 adhesive.
fig. S21. Storage (Gʹ) and loss (G″) moduli of TC710-H1 (red circles) and TC75-H1 (blue circles) at 25°C.
fig. S22. Rheological performance of TC7-H materials at different temperatures.
fig. S23. H/D exchange experiments of TC710-D1 adhesive.
fig. S24. SAXS of the TC710-H1 adhesive at 25°C.
fig. S25. SAXS of the TC71-H1 adhesive at 25°C.
fig. S26. The real part of the complex conductivity versus frequency of a TC75-H1 adhesive at the indicated temperatures.
fig. S27. [d log σdc/ dT]−1/2 versus T for TC7-H materials with different water contents.
fig. S28. H-bonding interactions between neighboring water molecules in the crystal structure of water (ice).
fig. S29. Detailed atomic structures of crown ether units connected by water molecules.
fig. S30. Tensile adhesion strength measurements of TC7-H adhesives.
fig. S31. Macroscopic tests of adhesion behavior of TC710-H1.
fig. S32. Tack tests of TC710-H1 adhesive at different temperature.
fig. S33. TGA measurements of TC7-H adhesive materials.
movie S1. Pulling a fiber from TC710-H1 adhesive material.
movie S2. Fibers pulled from TC710-H1 under optical microscopy.
movie S3. Injectable supramolecular adhesive from TC73-H1.
movie S4. Macroscopic TC710-H1 adhesion property.
REFERENCES AND NOTES
- 1.Chaplin M., Do we underestimate the importance of water in cell biology? Nat. Rev. Mol. Cell Biol. 7, 861–866 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Royer W. E., Pardanani A., Gibson Q. H., Peterson E. S., Friedman J. M., Ordered water molecules as key allosteric mediators in a cooperative dimeric hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 93, 14526–14531 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun T., Lin F.-H., Campbell R. L., Allingham J. S., Davies P. L., An antifreeze protein folds with an interior network of more than 400 semi-clathrate waters. Science 343, 795–798 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Ball P., Water as an active constituent in cell biology. Chem. Rev. 108, 74–108 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Johnson R. S., Yamazaki T., Kovalenko A., Fenniri H., Molecular basis for water-promoted supramolecular chirality inversion in helical rosette nanotubes. J. Am. Chem. Soc. 129, 5735–5743 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Arazoe H., Miyajima D., Akaike K., Araoka F., Sato E., Hikima T., Kawamoto M., Aida T., An autonomous actuator driven by fluctuations in ambient humidity. Nat. Mater. 15, 1084–1089 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Ma M., Guo L., Anderson D. G., Langer R., Bio-inspired polymer composite actuator and generator driven by water gradients. Science 339, 186–189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel E. A., del Barrio J., Loh X. J., Scherman O. A., Supramolecular polymeric hydrogels. Chem. Soc. Rev. 41, 6195–6214 (2012). [DOI] [PubMed] [Google Scholar]
- 9.A. Harada, Supramolecular Polymer Chemistry (Wiley-VCH, 2012). [Google Scholar]
- 10.Brunsveld L., Folmer B. J. B., Meijer E. W., Sijbesma R. P., Supramolecular polymers. Chem. Rev. 101, 4071–4098 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Aida T., Meijer E. W., Stupp S. I., Functional supramolecular polymers. Science 335, 813–817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan X., Wang F., Zheng B., Huang F., Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 41, 6042–6065 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Fiore G. L., Rowan S. J., Weder C., Optically healable polymers. Chem. Soc. Rev. 42, 7278–7288 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Korevaar P. A., George S. J., Markvoort A. J., Smulders M. M. J., Hilbers P. A. J., Schenning A. P. H. J., de Greef T. F. A., Meijer E. W., Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Kang J., Miyajima D., Mori T., Inoue Y., Itoh Y., Aida T., A rational strategy for the realization of chain-growth supramolecular polymerization. Science 347, 646–651 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Fouquey C., Lehn J.-M., Levelut A.-M., Molecular recognition directed self-assembly of supramolecular liquid crystalline polymers from complementary chiral components. Adv. Mater. 2, 254–257 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieg E., Bastings M. M. C., Besenius P., Rybtchinski B., Supramolecular polymers in aqueous media. Chem. Rev. 116, 2414–2477 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Burnworth M., Tang L., Kumpfer J. R., Duncan A. J., Beyer F. L., Fiore G. L., Rowan S. J., Weder C., Optically healable supramolecular polymers. Nature 472, 334–337 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Tayi A. S., Pashuck E. T., Newcomb C. J., McClendon M. T., Stupp S. I., Electrospinning bioactive supramolecular polymers from water. Biomacromolecules 15, 1323–1327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X., Tian H., Stimuli-responsive supramolecular polymers in aqueous solution. Acc. Chem. Res. 47, 1971–1981 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Cantekin S., de Greef T. F. A., Palmans A. R. A., Benzene-1,3,5-tricarboxamide: A versatile ordering moiety for supramolecular chemistry. Chem. Soc. Rev. 41, 6125–6137 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Zhang C., Li S., Zhang J., Zhu K., Li N., Huang F., Benzo-21-crown-7/secondary dialkylammonium salt [2]pseudorotaxane- and [2]rotaxane-type threaded structures. Org. Lett. 9, 5553–5556 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Stals P. J. M., Smulders M. M. J., Martín-Rapún R., Palmans A. R. A., Meijer E. W., Asymmetrically substituted benzene-1,3,5-tricarboxamides: Self-assembly and odd-even effects in the solid state and in dilute solution. Chemistry 15, 2071–2080 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Stals P. J. M., Everts J. C., de Bruijn R., Filot I. A. W., Smulders M. M. J., Martín-Rapún R., Pidko E. A., de Greef T. F. A., Palmans A. R. A., Meijer E. W., Dynamic supramolecular polymers based on benzene-1,3,5-tricarboxamides: The influence of amide connectivity on aggregate stability and amplification of chirality. Chemistry 16, 810–821 (2010). [DOI] [PubMed] [Google Scholar]
- 25.M. Tokita, K. Nishinari, Eds., Gel: Structures, Properties, and Functions: Fundamentals and Applications (Springer, 2009). [Google Scholar]
- 26.Gibson H. W., Yamaguchi N., Jones J. W., Supramolecular pseudorotaxane polymers from complementary pairs of homoditopic molecules. J. Am. Chem. Soc. 125, 3522–3533 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Courtois J., Baroudi I., Nouvel N., Degrandi E., Pensec S., Ducouret G., Chanéac C., Bouteiller L., Creton C., Supramolecular soft adhesive materials. Adv. Funct. Mater. 20, 1803–1811 (2010). [Google Scholar]
- 28.El-Eswed B. I., Zughul M. B., Derwish G. A. W., Infrared spectroscopic study of the role of water in crown ethers and their molecular complexes with 3- and 4-nitrophenol. J. Incl. Phenom. Macrocycl. Chem. 28, 245–258 (1997). [Google Scholar]
- 29.Rustenholtz A., Fulton J. L., Wai C. M., An FT-IR study of crown ether−water complexation in supercritical CO2. J. Phys. Chem. A 107, 11239–11244 (2003). [Google Scholar]
- 30.F. Kremer, A. Schönhals, Eds., Broadband Dielectric Spectroscopy (Springer, 2002). [Google Scholar]
- 31.Kyritsis A., Pissis P., Grammatikakis J., Dielectric relaxation spectroscopy in poly(hydroxyethyl acrylates)/water hydrogels. J. Polym. Sci. B Polym. Phys. 33, 1737–1750 (1995). [Google Scholar]
- 32.Hamada I., A van der Waals density functional study of ice Ih. J. Chem. Phys. 133, 214503 (2010). [DOI] [PubMed] [Google Scholar]
- 33.de Greef T. F. A., Meijer E. W., Materials science: Supramolecular polymers. Nature 453, 171–173 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Heinzmann C., Weder C., de Espinosa L. M., Supramolecular polymer adhesives: Advanced materials inspired by nature. Chem. Soc. Rev. 45, 342–358 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Lee H., Lee B. P., Messersmith P. B., A reversible wet/dry adhesive inspired by mussels and geckos. Nature 448, 338–341 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Zhao Q., Lee D. W., Ahn B. K., Seo S., Kaufman Y., Israelachvili J. N., Waite J. H., Underwater contact adhesion and microarchitecture in polyelectrolyte complexes actuated by solvent exchange. Nat. Mater. 15, 407–412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier G. P., Rapp M. V., Waite J. H., Israelachvili J. N., Butler A., Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 349, 628–632 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Lakrout H., Sergot P., Creton C., Direct observation of cavitation and fibrillation in a probe tack experiment on model acrylic pressure-sensitive-adhesives. J. Adhes. 69, 307–359 (1999). [Google Scholar]
- 39.D. A. Dillard, A. V. Pocius, Eds., The Mechanics of Adhesion (Elsevier Science, 2002). [Google Scholar]
- 40.Qi Z., de Molina P. M., Jiang W., Wang Q., Nowosinski K., Schulz A., Gradzielski M., Schalley C. A., Systems chemistry: Logic gates based on the stimuli-responsive gel–sol transition of a crown ether-functionalized bis(urea) gelator. Chem. Sci. 3, 2073–2082 (2012). [Google Scholar]
- 41.Paints and Varnishes — Pull-Off Test for Adhesion ISO 4624:2002 (2002).
- 42.Sheldrick G. M., SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheldrick G. M., Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H., OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009). [Google Scholar]
- 45.Kresse G., Hafner J., Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993). [DOI] [PubMed] [Google Scholar]
- 46.Kresse G., Hafner J., Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994). [DOI] [PubMed] [Google Scholar]
- 47.Klimeš J., Bowler D. R., Michaelides A., Chemical accuracy for the van der Waals density functional. J. Phys. Condens. Matter 22, 022201 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Yan X., Xu D., Chi X., Chen J., Dong S., Ding X., Yu Y., Huang F., A multiresponsive, shape-persistent, and elastic supramolecular polymer network gel constructed by orthogonal self-assembly. Adv. Mater. 24, 362–369 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Dong S., Yan X., Zheng B., Chen J., Ding X., Yu Y., Xu D., Zhang M., Huang F., A supramolecular polymer blend containing two different supramolecular polymers through self-sorting organization of two heteroditopic monomers. Chemistry 18, 4195–4199 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Wang F., Zhang J., Ding X., Dong S., Liu M., Zheng B., Li S., Wu L., Yu Y., Gibson H. W., Huang F., Metal coordination mediated reversible conversion between linear and cross-linked supramolecular polymers. Angew. Chem. Int. Ed. 49, 1090–1094 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Dong S., Gao L., Li J., Xu D., Zhou Q., Photo-responsive linear and cross-linked supramolecular polymers based on host–guest interactions. Polym. Chem. 4, 3968–3973 (2013). [Google Scholar]
- 52.Lopez A., Degrandi-Contraires E., Canetta E., Creton C., Keddie J. L., Asua J. M., Waterborne polyurethane-acrylic hybrid nanoparticles by miniemulsion polymerization: Applications in pressure-sensitive adhesives. Langmuir 27, 3878–3888 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/11/eaao0900/DC1
Materials and Methods
scheme S1. Synthesis of TC7 and control compounds.
scheme S2. Pull-off test of control compounds.
fig. S1. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of TC7.
fig. S2. 13C NMR spectrum (100 MHz, CDCl3, 25°C) of TC7.
fig. S3. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of DC7.
fig. S4. 13C NMR spectrum (100 MHz, CDCl3, 25°C) of DC7.
fig. S5. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of TC6.
fig. S6. 13C NMR spectrum (125 MHz, CDCl3, 25°C) of TC6.
fig. S7. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of TC-open.
fig. S8. 13C NMR spectrum (125 MHz, CDCl3, 25°C) of TC-open.
fig. S9. 1H NMR spectrum (400 MHz, CDCl3, 25°C) of TC8.
fig. S10. 1H NMR spectrum (125 MHz, CDCl3, 25°C) of TC8.
fig. S11. 13C NMR spectrum (400 MHz, CDCl3, 25°C) of TCB.
fig. S12. 1H NMR spectrum (125 MHz, CDCl3, 25°C) of TCB.
fig. S13. 1H NMR spectrum (500 MHz, CDCl3, 25°C) of TC7A.
fig. S14. 13C NMR spectrum (125 MHz, CDCl3, 25°C) of TC7A.
fig. S15. PXRD pattern of dry TC7.
fig. S16. IR spectrum of dry TC7.
fig. S17. IR spectra of TC710-H1 and TC710-D1.
fig. S18. Water absorbance curves of TC7 under ambient conditions (25°C, 40% RH).
fig. S19. DSC measurements of TC710-H1.
fig. S20. SEM images of TC710-H1 adhesive.
fig. S21. Storage (Gʹ) and loss (G″) moduli of TC710-H1 (red circles) and TC75-H1 (blue circles) at 25°C.
fig. S22. Rheological performance of TC7-H materials at different temperatures.
fig. S23. H/D exchange experiments of TC710-D1 adhesive.
fig. S24. SAXS of the TC710-H1 adhesive at 25°C.
fig. S25. SAXS of the TC71-H1 adhesive at 25°C.
fig. S26. The real part of the complex conductivity versus frequency of a TC75-H1 adhesive at the indicated temperatures.
fig. S27. [d log σdc/ dT]−1/2 versus T for TC7-H materials with different water contents.
fig. S28. H-bonding interactions between neighboring water molecules in the crystal structure of water (ice).
fig. S29. Detailed atomic structures of crown ether units connected by water molecules.
fig. S30. Tensile adhesion strength measurements of TC7-H adhesives.
fig. S31. Macroscopic tests of adhesion behavior of TC710-H1.
fig. S32. Tack tests of TC710-H1 adhesive at different temperature.
fig. S33. TGA measurements of TC7-H adhesive materials.
movie S1. Pulling a fiber from TC710-H1 adhesive material.
movie S2. Fibers pulled from TC710-H1 under optical microscopy.
movie S3. Injectable supramolecular adhesive from TC73-H1.
movie S4. Macroscopic TC710-H1 adhesion property.


